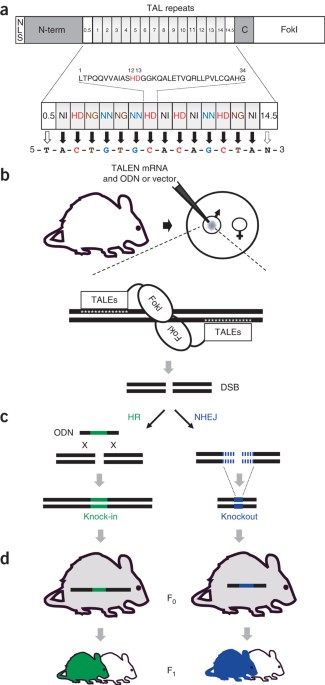
Generation of targeted mouse mutants by embryo microinjection of talen mrna
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Genetically engineered mice are instrumental for the analysis of mammalian gene function in health and disease. As classical gene targeting, which is performed in embryonic stem
(ES) cell cultures and generates chimeric mice, is a time-consuming and labor-intensive procedure, we recently used transcription activator–like (TAL) effector nucleases (TALENs) for
mutagenesis of the mouse genome directly in one-cell embryos. Here we describe a stepwise protocol for the generation of knock-in and knockout mice, including the selection of TALEN-binding
sites, the design and construction of TALEN coding regions and of mutagenic oligodeoxynucleotides (ODNs) and targeting vectors, mRNA production, embryo microinjection and the identification
of modified alleles in founder mutants and their progeny. After a setup time of 2–3 weeks of hands-on work for TALEN construction, investigators can obtain first founder mutants for genes of
choice within 7 weeks after embryo microinjections. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ESTABLISHMENT OF AN INTEGRATED AUTOMATED EMBRYONIC MANIPULATION SYSTEM FOR
PRODUCING GENETICALLY MODIFIED MICE Article Open access 03 June 2021 ELECTROPORATION AND GENETIC SUPPLY OF CAS9 INCREASE THE GENERATION EFFICIENCY OF CRISPR/CAS9 KNOCK-IN ALLELES IN C57BL/6J
MOUSE ZYGOTES Article Open access 21 October 2020 PRECISE ALLELE-SPECIFIC GENOME EDITING BY SPATIOTEMPORAL CONTROL OF CRISPR-CAS9 VIA PRONUCLEAR TRANSPLANTATION Article Open access 14
September 2020 REFERENCES * Capecchi, M.R. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. _Nat. Rev. Genet._ 6, 507–512 (2005). Article
CAS Google Scholar * Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. _Proc. Natl. Acad. Sci. USA_ 91,
6064–6068 (1994). Article CAS Google Scholar * Porteus, M.H. & Carroll, D. Gene targeting using zinc-finger nucleases. _Nat. Biotechnol._ 23, 967–973 (2005). Article CAS Google
Scholar * Meyer, M., de Angelis, M.H., Wurst, W. & Kuhn, R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. _Proc. Natl. Acad. Sci. USA_
107, 15022–15026 (2010). Article CAS Google Scholar * Meyer, M., Ortiz, O., Hrabé de Angelis, M., Wurst, W. & Kühn, R. Modeling disease mutations by gene targeting in one-cell mouse
embryos. _Proc. Natl. Acad. Sci. USA_ 109, 9354–9359 (2012). Article CAS Google Scholar * Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. _Science_
326, 1509–1512 (2009). Article CAS Google Scholar * Moscou, M.J. & Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. _Science_ 326, 1501 (2009). Article CAS
Google Scholar * Boch, J. & Bonas, U. _Xanthomonas_ AvrBs3 family-type III effectors: discovery and function. _Annu. Rev. Phytopathol._ 48, 419–436 (2010). Article CAS Google
Scholar * Bogdanove, A.J., Schornack, S. & Lahaye, T. TAL effectors: finding plant genes for disease and defense. _Curr. Opin. Plant Biol._ 13, 394–401 (2010). Article CAS Google
Scholar * Scholze, H. & Boch, J. TAL effectors are remote controls for gene activation. _Curr. Opin. Microbiol._ 14, 47–53 (2011). Article CAS Google Scholar * Deng, D. et al.
Structural basis for sequence-specific recognition of DNA by TAL effectors. _Science_ 335, 720–723 (2012). Article CAS Google Scholar * Mak, A.N.-S., Bradley, P., Cernadas, R.A.,
Bogdanove, A.J. & Stoddard, B.L. The crystal structure of TAL effector PthXo1 bound to its DNA target. _Science_ 335, 716–719 (2012). Article CAS Google Scholar * Miller, J.C. et al.
A TALE nuclease architecture for efficient genome editing. _Nat. Biotechnol._ 29, 143–148 (2011). Article CAS Google Scholar * Christian, M. et al. Targeting DNA double-strand breaks with
TAL effector nucleases. _Genetics_ 186, 757–761 (2010). Article CAS Google Scholar * Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based
constructs for DNA targeting. _Nucleic Acids Res._ 39, e82 (2011). Article CAS Google Scholar * Heyer, W.-D., Ehmsen, K.T. & Liu, J. Regulation of homologous recombination in
eukaryotes. _Annu. Rev. Genet._ 44, 113–139 (2010). Article CAS Google Scholar * Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway.
_Annu. Rev. Biochem._ 79, 181–211 (2010). Article CAS Google Scholar * Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. _Science_ 339, 819–823 (2013). Article CAS
Google Scholar * Mali, P. et al. RNA-guided human genome engineering via Cas9. _Science_ 339, 823–826 (2013). Article CAS Google Scholar * Wang, H. et al. One-step generation of mice
carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. _Cell_ 153, 910–918 (2013). Article CAS Google Scholar * Jinek, M. et al. A programmable dual-RNA-guided
DNA endonuclease in adaptive bacterial immunity. _Science_ 337, 816–821 (2012). Article CAS Google Scholar * Fu, Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas
nucleases in human cells. _Nat. Biotechnol._ 31, 822–826 (2013). Article CAS Google Scholar * Hsu, P.D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. _Nat. Biotechnol._
31, 827–832 (2013). Article CAS Google Scholar * Wefers, B. et al. Direct production of mouse disease models by embryo microinjection of TALENs and oligodeoxynucleotides. _Proc. Natl.
Acad. Sci. USA_ 110, 3782–3787 (2013). Article CAS Google Scholar * Hockemeyer, D. et al. Genetic engineering of human pluripotent cells using TALE nucleases. _Nat. Biotechnol._ 29,
731–734 (2011). Article CAS Google Scholar * Wang, H. et al. TALEN-mediated editing of the mouse Y chromosome. _Nat. Biotechnol._ 31, 530–532 (2013). Article CAS Google Scholar *
Doyle, E.L. et al. TAL effector-nucleotide targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. _Nucleic Acids Res._ 40, W117–W122 (2012). Article CAS Google
Scholar * Sander, J.D. et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. _Nat. Biotechnol._ 29, 697–698 (2011). Article CAS Google Scholar * Meckler,
J.F. et al. Quantitative analysis of TALE-DNA interactions suggests polarity effects. _Nucleic Acids Res._ 41, 4118–4128 (2013). Article CAS Google Scholar * Streubel, J., Blücher, C.,
Landgraf, A. & Boch, J. TAL effector RVD specificities and efficiencies. _Nat. Biotechnol._ 30, 593–595 (2012). Article CAS Google Scholar * Cong, L., Zhou, R., Kuo, Y.-C., Cunniff,
M. & Zhang, F. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. _Nat. Commun._ 3, 968 (2012). Article Google Scholar * Chen, F. et
al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. _Nat. Methods_ 8, 753–755 (2011). Article CAS Google Scholar * Wefers, B., Wurst, W. &
Kühn, R. Design and Generation of gene-targeting vectors. _Curr. Protoc. Mouse Biol._ 1, 199–211 (2011). PubMed Google Scholar * Hasty, P., Abuin, A. & Bradley, A. Gene targeting,
principles, and practice in mammalian cells. in _Gene Targeting: A Practical Approach_ (ed. Joyner, A.L.) 1–35 (Oxford University Press, 2000). * Hasty, P., Rivera-Pérez, J. & Bradley,
A. The length of homology required for gene targeting in embryonic stem cells. _Mol. Cell Biol._ 11, 5586–5591 (1991). Article CAS Google Scholar * Deng, C. & Capecchi, M.R.
Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. _Mol. Cell Biol._ 12, 3365–3371 (1992). Article CAS
Google Scholar * Reyon, D. et al. FLASH assembly of TALENs for high-throughput genome editing. _Nat. Biotechnol._ 30, 460–465 (2012). Article CAS Google Scholar * Briggs, A.W. et al.
Iterative capped assembly: rapid and scalable synthesis of repeat-module DNA such as TAL effectors from individual monomers. _Nucleic Acids Res._ 40, e117 (2012). Article CAS Google
Scholar * Kim, H. et al. Surrogate reporters for enrichment of cells with nuclease-induced mutations. _Nat. Methods_ 8, 941–943 (2011). Article CAS Google Scholar * Perez-Pinera, P.,
Ousterout, D.G., Brown, M.T. & Gersbach, C.A. Gene targeting to the ROSA26 locus directed by engineered zinc-finger nucleases. _Nucleic Acids Res._ 40, 3741–3752 (2012). Article CAS
Google Scholar * Adenot, P.G., Mercier, Y., Renard, J.P. & Thompson, E.M. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential
transcriptional activity in pronuclei of 1-cell mouse embryos. _Dev. Camb. Engl._ 124, 4615–4625 (1997). CAS Google Scholar * Sung, Y.H. et al. Knockout mice created by TALEN-mediated gene
targeting. _Nat. Biotechnol._ 31, 23–24 (2013). Article CAS Google Scholar * Tesson, L. et al. Knockout rats generated by embryo microinjection of TALENs. _Nat. Biotechnol._ 29, 695–696
(2011). Article CAS Google Scholar * Davies, B. et al. Site-specific mutation of the Zic2 locus by microinjection of TALEN mRNA in mouse CD1, C3H and C57BL/6J oocytes. _PLoS ONE_ 8,
e60216 (2013). Article CAS Google Scholar * Qiu, Z. et al. High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. _Nucleic Acids Res._
41, e120 (2013). * Southern, E. Southern blotting. _Nat. Protoc._ 1, 518–525 (2006). Article CAS Google Scholar * Liew, M. et al. Genotyping of single-nucleotide polymorphisms by
high-resolution melting of small amplicons. _Clin. Chem._ 50, 1156–1164 (2004). Article CAS Google Scholar * Ittner, L.M. & Götz, J. Pronuclear injection for the production of
transgenic mice. _Nat. Protoc._ 2, 1206–1215 (2007). Article CAS Google Scholar * Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. _Manipulating the Mouse Embryo_ (Cold
Spring Harbour Laboratory Press, 2003). * Sanjana, N.E. et al. A transcription activator-like effector toolbox for genome engineering. _Nat. Protoc._ 7, 171–192 (2012). Article CAS Google
Scholar * Schmid-Burgk, J.L., Schmidt, T., Kaiser, V., Höning, K. & Hornung, V. A ligation-independent cloning technique for high-throughput assembly of transcription activator–like
effector genes. _Nat. Biotechnol._ 31, 76–81 (2013). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the European Union within the EUCOMMTools
project (HEALTH-F4-2010-261492 to W.W.), by the German Ministry of Education and Research within the DIGTOP project (01GS0858 to W.W. and R.K.) of the German National Genome Research Network
(NGFN)-Plus program and by the Indian Council of Agricultural Research (no.29-1/2009-EQR/Edn to S.K.P.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Developmental Genetics,
Helmholtz Zentrum München, German Research Center for Environmental Health, Munich, Germany Benedikt Wefers, Sudeepta K Panda, Oskar Ortiz, Christina Brandl, Svenja Hensler, Jens Hansen,
Wolfgang Wurst & Ralf Kühn * Technische Universität München, Freising-Weihenstephan, Germany Sudeepta K Panda, Christina Brandl, Svenja Hensler, Wolfgang Wurst & Ralf Kühn *
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Munich, Germany Wolfgang Wurst * Max Planck Institute of Psychiatry, Munich, Germany Wolfgang Wurst Authors * Benedikt Wefers
View author publications You can also search for this author inPubMed Google Scholar * Sudeepta K Panda View author publications You can also search for this author inPubMed Google Scholar *
Oskar Ortiz View author publications You can also search for this author inPubMed Google Scholar * Christina Brandl View author publications You can also search for this author inPubMed
Google Scholar * Svenja Hensler View author publications You can also search for this author inPubMed Google Scholar * Jens Hansen View author publications You can also search for this
author inPubMed Google Scholar * Wolfgang Wurst View author publications You can also search for this author inPubMed Google Scholar * Ralf Kühn View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS B.W., S.K.P., O.O., C.B. and R.K. performed the research and analyzed the data; B.W., S.K.P., O.O., C.B., S.H. and R.K. wrote the
manuscript; J.H. designed the TALEN_designer_ tools and webpage; W.W. and R.K. supervised the research. CORRESPONDING AUTHOR Correspondence to Ralf Kühn. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 GENOTYPING: EXEMPLARY RESULTS FOR DIRECT SEQUENCING OF PCR
PRODUCTS. Representative chromatograms of heterozygous mutants harboring a single nucleotide substitution (marked in red) (A) or an indel (B) resulting in two superimposed traces.
SUPPLEMENTARY FIGURE 2 GENOTYPING: EXEMPLARY RESULTS OF A T7 ENDONUCLEASE I ASSAY. Wild-type controls (wt) harbor only the full length PCR product (open triangle), whereas heterozygous
mutants (het) show the additional presence of two digestion products (filled triangles) obtained by the cleavage of heteroduplex molecules within the TALEN target region. SUPPLEMENTARY
FIGURE 3 GENOTYPING: EXEMPLARY RESULTS FOR HRMA ANALYSIS. Representative melting curves of PCR products amplified from a wild-type control (grey) and a mutant founder mouse (red).
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 Genotyping: exemplary results for direct sequencing of PCR products. (PDF 155 kb) SUPPLEMENTARY FIGURE 2 Genotyping: exemplary results of a
T7 endonuclease I assay. (PDF 148 kb) SUPPLEMENTARY FIGURE 3 Genotyping: exemplary results for HRMA analysis. (PDF 27 kb) SUPPLEMENTARY DATA (PDF 889 kb) PRONUCLEAR MICROINJECTION This movie
demonstrates the microinjection into the male pronucleus of a mouse one-cell embryo, fixed with a holding pipette. (AVI 2868 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Wefers, B., Panda, S., Ortiz, O. _et al._ Generation of targeted mouse mutants by embryo microinjection of TALEN mRNA. _Nat Protoc_ 8, 2355–2379 (2013).
https://doi.org/10.1038/nprot.2013.142 Download citation * Published: 31 October 2013 * Issue Date: December 2013 * DOI: https://doi.org/10.1038/nprot.2013.142 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative
