Alterations in vhl as potential biomarkers in renal-cell carcinoma
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Germ line mutations in the _VHL_ tumor-suppressor gene cause von Hippel–Lindau (VHL) disease, a hereditary neoplastic disease associated with clear-cell renal-cell carcinomas
(ccRCCs), central nervous system hemangioblastomas and pheochromocytomas. Disruption of _VHL_, by somatic mutation, hypermethylation of its promoter or chromosomal loss, is also seen in the
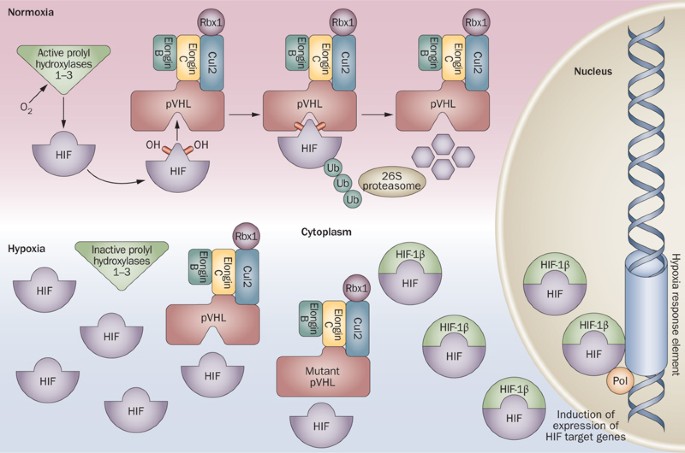
majority of cases of sporadic ccRCC. The protein product of _VHL_, pVHL, has multiple functions, the best-documented of which relates to its ability to target hypoxia-inducible factors
(HIFs) for polyubiquitination and proteasomal degradation through its role in substrate recognition as part of a ubiquitin ligase complex. Consequently, pVHL-defective ccRCCs overexpress
mRNAs that are under the transcriptional control of HIF. Drugs that modulate the downstream targets of the pVHL/HIF pathway, including sunitinib, sorafenib, temsirolimus and bevacizumab,
have proven benefit in treating ccRCC. In VHL disease, clear evidence supports strong genotype–phenotype correlations, but the situation in sporadic ccRCC is less clear. Data indicate that
_VHL_ alterations have a potential role as prognostic and predictive markers in ccRCC. Future clinical trials should prospectively define the _VHL_ alteration status of study participants so
that the true utility of such markers can be determined. KEY POINTS * Disruption of _VHL_, by somatic mutation, hypermethylation of its promoter or chromosomal loss, is seen in the majority
of cases of clear-cell renal-cell carcinoma * The best-documented function of pVHL involves targeting hypoxia-inducible factors (HIFs) for polyubiquitination and proteasomal degradation
through its role as the substrate recognition component of a ubiquitin ligase complex * Drugs that modulate downstream targets of the VHL/HIF pathway have proven efficacy in treating
clear-cell renal-cell carcinoma * HIF-independent functions of pVHL are important for its tumor-suppressor action: maintenance of the primary cilium, assembly of extracellular matrix,
control of microtubule dynamics, regulation of neuronal apoptosis and transcriptional regulation * Future clinical trials should prospectively define the _VHL_ mutation status of study
participants and include planned subgroup analyses to assess the importance of mutational status as a stratifying variable * Understanding the functional effects of pVHL alterations might
facilitate development of a classification system to differentiate high-risk from low-risk patients and identify those who could benefit from targeted therapies Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print
issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS GENOMIC PROFILING IN RENAL CELL CARCINOMA Article 19 June 2020 PBRM1, SETD2 AND BAP1 — THE TRINITY OF 3P IN CLEAR CELL RENAL CELL CARCINOMA Article 17 October 2022
WHOLE GENOME SEQUENCING REFINES STRATIFICATION AND THERAPY OF PATIENTS WITH CLEAR CELL RENAL CELL CARCINOMA Article Open access 15 July 2024 REFERENCES * Kaelin, W. G. Jr. Molecular basis of
the VHL hereditary cancer syndrome. _Nat. Rev. Cancer_ 2, 673–682 (2002). Article CAS PubMed Google Scholar * Maher, E. R. _ et al_. Clinical features and natural history of von
Hippel–Lindau disease. _Q. J. Med._ 77, 1151–1163 (1990). Article CAS PubMed Google Scholar * Gnarra, J. R. _ et al_. Post-transcriptional regulation of vascular endothelial growth
factor mRNA by the product of the _VHL_ tumor suppressor gene. _Proc. Natl Acad. Sci. USA_ 93, 10589–10594 (1996). Article CAS PubMed PubMed Central Google Scholar * Iliopoulos, O.,
Kibel, A., Gray, S. & Kaelin, W. G. Jr. Tumor suppression by the human von Hippel–Lindau gene product. _Nat. Med._ 1, 822–826 (1995). Article CAS PubMed Google Scholar * Schoenfeld,
A., Davidowitz, E. J. & Burk, R. D. A second major native von Hippel–Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. _Proc. Natl
Acad. Sci. USA_ 95, 8817–8822 (1998). Article CAS PubMed PubMed Central Google Scholar * Davidowitz, E. J., Schoenfeld, A. R. & Burk, R. D. VHL induces renal cell differentiation
and growth arrest through integration of cell–cell and cell–extracellular matrix signaling. _Mol. Cell. Biol._ 21, 865–874 (2001). Article CAS PubMed PubMed Central Google Scholar *
Lieubeau-Teillet, B. _ et al_. von Hippel–Lindau gene-mediated growth suppression and induction of differentiation in renal cell carcinoma cells grown as multicellular tumor spheroids.
_Cancer Res._ 58, 4957–4962 (1998). CAS PubMed Google Scholar * Pause, A., Lee, S., Lonergan, K. M. & Klausner, R. D. The von Hippel–Lindau tumor suppressor gene is required for cell
cycle exit upon serum withdrawal. _Proc. Natl Acad. Sci. USA_ 95, 993–998 (1998). Article CAS PubMed PubMed Central Google Scholar * Knudson, A. G. Jr. Mutation and cancer: statistical
study of retinoblastoma. _Proc. Natl Acad. Sci. USA_ 68, 820–823 (1971). Article PubMed PubMed Central Google Scholar * Foster, K. _ et al_. Somatic mutations of the von Hippel–Lindau
disease tumor suppressor gene in non-familial clear cell renal carcinoma. _Hum. Mol. Genet._ 3, 2169–2173 (1994). Article CAS PubMed Google Scholar * Gnarra, J. R. _ et al_. Mutations of
the _VHL_ tumor suppressor gene in renal carcinoma. _Nat. Genet._ 7, 85–90 (1994). Article CAS PubMed Google Scholar * Shuin, T. _ et al_. Frequent somatic mutations and loss of
heterozygosity of the von Hippel–Lindau tumor suppressor gene in primary human renal cell carcinomas. _Cancer Res._ 54, 2852–2855 (1994). CAS PubMed Google Scholar * Whaley, J. M. _ et
al_. Germ-line mutations in the von Hippel–Lindau tumor-suppressor gene are similar to somatic von Hippel–Lindau aberrations in sporadic renal cell carcinoma. _Am. J. Hum. Genet._ 55,
1092–1102 (1994). CAS PubMed PubMed Central Google Scholar * Cockman, M. E. _ et al_. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel–Lindau tumor suppressor
protein. _J. Biol. Chem._ 275, 25733–25741 (2000). Article CAS PubMed Google Scholar * Kamura, T. _ et al_. Activation of HIF-1α ubiquitination by a reconstituted von Hippel–Lindau (VHL)
tumor suppressor complex. _Proc. Natl Acad. Sci. USA_ 97, 10430–10435 (2000). Article CAS PubMed PubMed Central Google Scholar * Maxwell, P. H. _ et al_. The tumor suppressor protein
VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. _Nature_ 399, 271–275 (1999). Article CAS PubMed Google Scholar * Ohh, M. _ et al_. Ubiquitination of
hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel–Lindau protein. _Nat. Cell Biol._ 2, 423–427 (2000). Article CAS PubMed Google Scholar * Tanimoto,
K., Makino, Y., Pereira, T. & Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel–Lindau tumor suppressor protein. _EMBO J._ 19, 4298–4309 (2000).
Article CAS PubMed PubMed Central Google Scholar * Kaelin, W. G. The von Hippel–Lindau tumor suppressor protein: roles in cancer and oxygen sensing. _Cold Spring Harbor Symp. Quant.
Biol._ 70, 159–166 (2005). Article CAS PubMed Google Scholar * Kaelin, W. G. Jr. The von Hippel–Lindau protein, HIF hydroxylation, and oxygen sensing. _Biochem. Biophys. Res. Commun._ 3
38, 627–638 (2005). Article Google Scholar * Kaelin, W. G. Jr. The von Hippel–Lindau tumor suppressor protein: an update. _Methods Enzymol._ 43 5, 371–383 (2007). Article Google Scholar
* Kaelin, W. G. Jr. The von Hippel–Lindau tumor suppressor protein and clear cell renal carcinoma. _Clin. Cancer Res._ 13 (Pt 2), 680s–684s (2007). * Kaelin, W. G. Jr. The von Hippel–Lindau
tumor suppressor protein: O2 sensing and cancer. _Nat. Rev. Cancer_ 8, 865–873 (2008). Article CAS PubMed Google Scholar * Kaelin, W. G. Von Hippel–Lindau disease. _Annu. Rev. Pathol._
2, 145–173 (2007). Article CAS PubMed Google Scholar * Nyhan, M. J., O'Sullivan, G. C. & McKenna, S. L. Role of the _VHL_ (von Hippel–Lindau) gene in renal cancer: a
multifunctional tumor suppressor. _Biochem. Soc. Trans._ 36 (Pt 3), 472–478 (2008). Article CAS PubMed Google Scholar * Frew, I. J. & Krek, W. Multitasking by pVHL in tumor
suppression. _Curr. Opin. Cell Biol._ 19, 685–690 (2007). Article CAS PubMed Google Scholar * Folkman, J. Tumor angiogenesis: therapeutic implications. _N. Engl. J. Med._ 285, 1182–1186
(1971). Article CAS PubMed Google Scholar * Cébe-Suarez, S., Zehnder-Fjällman, A. & Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. _Cell. Mol.
Life Sci._ 6 3, 601–615 (2006). Article Google Scholar * Bastien, L. _ et al_. Targeted therapies in metastatic renal cancer in 2009. _BJU Int._ 103, 1334–1342 (2009). Article CAS PubMed
Google Scholar * Atkins, M. B. _ et al_. Innovations and challenges in renal cancer: summary statement from the Third Cambridge Conference. _Cancer_ 115 (Suppl.), 2247–2251 (2009).
Article PubMed Google Scholar * Kaelin, W. G. Jr. Treatment of kidney cancer: insights provided by the VHL tumor-suppressor protein. _Cancer_ 115 (Suppl.), 2262–2272 (2009). Article CAS
PubMed Google Scholar * Motzer, R. J., Bacik, J., Murphy, B. A., Russo, P. & Mazumdar, M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against
advanced renal cell carcinoma. _J. Clin. Oncol._ 20, 289–296 (2002). Article CAS PubMed Google Scholar * Slamon, D. J. _ et al_. Use of chemotherapy plus a monoclonal antibody against
HER2 for metastatic breast cancer that overexpresses HER2. _N. Engl. J. Med._ 344, 783–792 (2001). Article CAS PubMed Google Scholar * Lievre, A. _ et al_. _KRAS_ mutation status is
predictive of response to cetuximab therapy in colorectal cancer. _Cancer Res._ 66, 3992–3995 (2006). Article CAS PubMed Google Scholar * Lynch, T. J. _ et al_. Activating mutations in
the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. _N. Engl. J. Med._ 350, 2129–2139 (2004). Article CAS PubMed Google Scholar *
Ong, K. R. _ et al_. Genotype–phenotype correlations in von Hippel–Lindau disease. _Hum. Mutat._ 28, 143–149 (2007). Article CAS PubMed Google Scholar * Forman, J. R., Worth, C. L.,
Bickerton, G. R., Eisen, T. G. & Blundell, T. L. Structural bioinformatics mutation analysis reveals genotype–phenotype correlations in von Hippel–Lindau disease and suggests molecular
mechanisms of tumorigenesis. _Proteins_ 77, 84–96 (2009). Article CAS PubMed Google Scholar * Clifford, S. C. _ et al_. Contrasting effects on HIF-1α regulation by disease-causing pVHL
mutations correlate with patterns of tumorigenesis in von Hippel–Lindau disease. _Hum. Mol. Genet._ 10, 1029–1038 (2001). Article CAS PubMed Google Scholar * Molina, A. M. & Motzer,
R. J. Current algorithms and prognostic factors in the treatment of metastatic renal cell carcinoma. _Clin. Genitourin. Cancer_ 6 (Suppl. 1), S7–S13 (2008). Article CAS PubMed Google
Scholar * Knauth, K., Bex, C., Jemth, P. & Buchberger, A. Renal cell carcinoma risk in type 2 von Hippel–Lindau disease correlates with defects in pVHL stability and HIF-1α
interactions. _Oncogene_ 25, 370–377 (2006). Article CAS PubMed Google Scholar * Li, L. _ et al_. Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and
type 2B _VHL_ mutations. _Mol. Cell Biol._ 27, 5381–5392 (2007). Article CAS PubMed PubMed Central Google Scholar * Pastore, Y. D. _ et al_. Mutations in the _VHL_ gene in sporadic
apparently congenital polycythemia. _Blood_ 101, 1591–1595 (2003). Article CAS PubMed Google Scholar * Ang, S. O. _ et al_. Disruption of oxygen homeostasis underlies congenital Chuvash
polycythemia. _Nat. Genet._ 32, 614–621 (2002). Article CAS PubMed Google Scholar * Banks, R. E. _ et al_. Genetic and epigenetic analysis of von Hippel–Lindau (_VHL_) gene alterations
and relationship with clinical variables in sporadic renal cancer. _Cancer Res._ 66, 2000–2011 (2006). Article CAS PubMed Google Scholar * Brauch, H. _ et al_. _VHL_ alterations in human
clear cell renal cell carcinoma: association with advanced tumor stage and a novel hot spot mutation. _Cancer Res._ 60, 1942–1948 (2000). CAS PubMed Google Scholar * Giménez-Bachs, J. M.
_ et al_. VHL protein alterations in sporadic renal cell carcinoma. _Clin. Oncol. (R. Coll. Radiol.)_ 19, 784–789 (2007). Article Google Scholar * Kim, J. H. _ et al_. Somatic VHL
alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. _Oncol. Rep._ 13, 859–864 (2005). CAS PubMed Google Scholar * Kondo, K. _ et al_. Comprehensive
mutational analysis of the _VHL_ gene in sporadic renal cell carcinoma: relationship to clinicopathological parameters. _Genes Chromosomes Cancer_ 34, 58–68 (2002). Article CAS PubMed
Google Scholar * Ma, X., Yang, K., Lindblad, P., Egevad, L. & Hemminki, K. _VHL_ gene alterations in renal cell carcinoma patients: novel hotspot or founder mutations and linkage
disequilibrium. _Oncogene_ 20, 5393–5400 (2001). Article CAS PubMed Google Scholar * Nickerson, M. L. _ et al_. Improved identification of von Hippel–Lindau gene alterations in clear
cell renal tumors. _Clin. Cancer Res._ 14, 4726–4734 (2008). Article CAS PubMed PubMed Central Google Scholar * Patard, J. J. _ et al_. Low CAIX expression and absence of _VHL_ gene
mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. _Int. J. Cancer_ 123, 395–400 (2008). Article CAS PubMed PubMed Central Google
Scholar * Schraml, P. _ et al_. _VHL_ mutations and their correlation with tumor cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. _J.
Pathol._ 196, 186–193 (2002). Article CAS PubMed Google Scholar * Smits, K. M. _ et al_. Genetic and epigenetic alterations in the von Hippel–Lindau gene: the influence on renal cancer
prognosis. _Clin. Cancer Res._ 14, 782–787 (2008). Article CAS PubMed Google Scholar * Suzuki, H. _ et al_. Mutational state of von Hippel–Lindau and adenomatous polyposis coli genes in
renal tumors. _Oncology_ 5 4, 252–257 (1997). Article Google Scholar * van Houwelingen, K. P. _ et al_. Prevalence of von Hippel–Lindau gene mutations in sporadic renal cell carcinoma:
results from The Netherlands cohort study. _BMC Cancer_ 5, 57 (2005). Article PubMed PubMed Central Google Scholar * Yao, M. _ et al_. _VHL_ tumor suppressor gene alterations associated
with good prognosis in sporadic clear-cell renal carcinoma. _J. Natl Cancer Inst._ 94, 1569–1575 (2002). Article CAS PubMed Google Scholar * Choyke, P. L. _ et al_. The natural history
of renal lesions in von Hippel–Lindau disease: a serial CT study in 28 patients. _AJR Am. J. Roentgenol._ 15 9, 1229–1234 (1992). Article Google Scholar * Neumann, H. P. _ et al_.
Prevalence, morphology and biology of renal cell carcinoma in von Hippel–Lindau disease compared to sporadic renal cell carcinoma. _J. Urol._ 160, 1248–1254 (1998). Article CAS PubMed
Google Scholar * Gallou, C. _ et al_. Genotype–phenotype correlation in von Hippel–Lindau families with renal lesions. _Hum. Mutat._ 24, 215–224 (2004). Article CAS PubMed Google Scholar
* Yang, K., Lindblad, P., Egevad, L. & Hemminki, K. Novel somatic mutations in the _VHL_ gene in Swedish archived sporadic renal cell carcinomas. _Cancer Lett._ 141, 1–8 (1999).
Article PubMed Google Scholar * Gordan, J. D. _ et al_. HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. _Cancer Cell_ 14, 435–446
(2008). Article CAS PubMed PubMed Central Google Scholar * Smaldone, M. C. & Maranchie, J. K. Clinical implications of hypoxia inducible factor in renal cell carcinoma. _Urol.
Oncol._ 27, 238–245 (2009). Article CAS PubMed Google Scholar * Kondo, K., Kim, W. Y., Lechpammer, M. & Kaelin, W. G. Jr. Inhibition of HIF2α is sufficient to suppress pVHL-defective
tumor growth. _PLoS Biol._ 1, E83 (2003). Article PubMed PubMed Central Google Scholar * Zimmer, M., Doucette, D., Siddiqui, N. & Iliopoulos, O. Inhibition of hypoxia-inducible
factor is sufficient for growth suppression of _VHL__−/−_ tumors. _Mol. Cancer Res._ 2, 89–95 (2004). CAS PubMed Google Scholar * Kondo, K., Klco, J., Nakamura, E., Lechpammer, M. &
Kaelin, W. G. Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel–Lindau protein. _Cancer Cell_ 1, 237–246 (2002). Article CAS PubMed Google Scholar * Maranchie,
J. K. _ et al_. The contribution of VHL substrate binding and HIF-1α to the phenotype of VHL loss in renal cell carcinoma. _Cancer Cell_ 1, 247–255 (2002). Article CAS PubMed Google
Scholar * Hoffman, M. A. _ et al_. von Hippel–Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. _Hum. Mol. Genet._ 10, 1019–1027 (2001). Article
CAS PubMed Google Scholar * Hu, C. J., Wang, L. Y., Chodosh, L. A., Keith, B. & Simon, M. C. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene
regulation. _Mol. Cell Biol._ 23, 9361–9374 (2003). Article CAS PubMed PubMed Central Google Scholar * Raval, R. R. _ et al_. Contrasting properties of hypoxia-inducible factor 1
(HIF-1) and HIF-2 in von Hippel–Lindau-associated renal cell carcinoma. _Mol. Cell Biol._ 25, 5675–5686 (2005). Article CAS PubMed PubMed Central Google Scholar * Zimmer, M. _ et al_.
Small-molecule inhibitors of HIF-2α translation link its 5'UTR iron-responsive element to oxygen sensing. _Mol. Cell._ 32, 838–848 (2008). Article CAS PubMed PubMed Central Google
Scholar * Bui, M. H. _ et al_. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. _Clin. Cancer
Res._ 9, 802–811 (2003). CAS PubMed Google Scholar * Choueiri, T. K. _ et al_. von Hippel–Lindau gene status and response to vascular endothelial growth factor targeted therapy for
metastatic clear cell renal cell carcinoma. _J. Urol._ 180, 860–866 (2008). Article CAS PubMed Google Scholar * Rini, B. I. _ et al_. Clinical response to therapy targeted at vascular
endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and von Hippel–Lindau gene status. _BJU Int._ 98, 756–762 (2006). Article CAS PubMed Google
Scholar * Gad, S. _ et al_. Somatic von Hippel–-Lindau (_VHL_) gene analysis and clinical outcome under anti-angiogenic treatment in metastatic renal cell carcinoma: preliminary results.
_Targeted Oncology_ 2, 3–6 (2007). Article Google Scholar * Hutson, T. E. _ et al_. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with
pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. _J. Clin. Oncol._ 26 (Suppl.), 5046 (2008). Article Google Scholar * Cho, D. _ et al_. Potential histologic and molecular
predictors of response to temsirolimus in patients with advanced renal cell carcinoma. _Clin. Genitourin. Cancer_ 5, 379–385 (2007). Article CAS PubMed Google Scholar * Atkins, M. _ et
al_. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. _Clin. Cancer Res._ 11, 3714–3721 (2005). Article CAS PubMed Google Scholar * Beroukhim,
R. _ et al_. Patterns of gene expression and copy-number alterations in von Hippel–Lindau disease-associated and sporadic clear cell carcinoma of the kidney. _Cancer Res._ 69, 4674–4681
(2009). Article CAS PubMed PubMed Central Google Scholar * Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. _Oncology_ 69 (Suppl. 3), 4–10 (2005). Article CAS PubMed
Google Scholar * Poon, E., Harris, A. L. & Ashcroft, M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. _Expert Rev. Mol. Med._ 11, e26 (2009). Article PubMed Google
Scholar * Koh, M. Y. & Powis, G. HAF: the new player in oxygen-independent HIF-1α degradation. _Cell Cycle_ 8, 1359–1366 (2009). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS The authors thank the Cambridge Biomedical Research Centre, Cambridge, UK, for their support. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cancer Research UK
Cambridge Research Institute, Li Ka Shing Centre, Hills Road, CB2 0RE, Cambridge, UK Lucy Gossage * Department of Oncology, University of Cambridge, Box 193, Addenbrookes Hospital, CB2 0QQ,
Cambridge, UK Tim Eisen Authors * Lucy Gossage View author publications You can also search for this author inPubMed Google Scholar * Tim Eisen View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Lucy Gossage. ETHICS DECLARATIONS COMPETING INTERESTS L. Gossage declares no competing interests. T. Eisen
declares that he has received honoraria and is a member of the advisory boards for Amgen, Astra Zeneca, Aveo, Bayer, Bristol-Myers, GlaxoSmithKline, Immatics, Pfizer and Wyeth, and has
received grant or research funding from Astra Zeneca, Bayer and Pfizer. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 Classification systems for genetic mutations. (PDF 355 kb) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gossage, L., Eisen, T. Alterations in _VHL_ as potential biomarkers in renal-cell carcinoma. _Nat Rev Clin Oncol_
7, 277–288 (2010). https://doi.org/10.1038/nrclinonc.2010.42 Download citation * Published: 06 April 2010 * Issue Date: May 2010 * DOI: https://doi.org/10.1038/nrclinonc.2010.42 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
