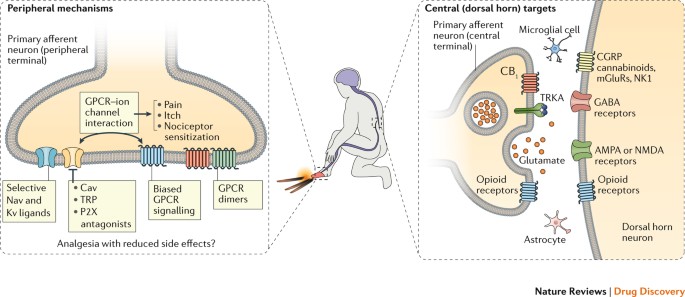
Breaking barriers to novel analgesic drug development
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

KEY POINTS * Pain is the primary reason why people seek medical care; more than 40% of the US population is affected by chronic pain. * Opioids, which are the most commonly used and often
the most effective class of analgesics, produce tolerance, dependence and constipation, and are associated with major abuse liabilities. The respiratory depression associated with high doses
has led to a catastrophic increase in the number of drug overdose deaths in the United States. * Several new or previously overlooked targets are gaining significant attention. In the field
of G-protein-coupled receptors (GPCRs), these include new ligands targeting opioid receptor heteromers, different opioid receptor subtypes and biased agonists. Non-opioid GPCRs currently
being pursued include cannabinoid receptor 2 (CB2), angiotensin type 2 receptor (AT2R) and chemokine receptors. * Various academic and industry groups are pursuing ion channel strategies by
targeting sodium, potassium and calcium channels — specifically, certain Nav1.7, Nav1.8 and voltage-dependent calcium channel (Cavs) ligands are showing particular promise in early
preclinical and clinical trials. * Several enzyme targets that modulate pain pathways are also being pursued. * Despite considerable efforts, there have been several high-profile failures of
novel analgesics in the clinic. * Barriers that need to be overcome to develop efficacious analgesics include issues related to the lack of predictability of preclinical models in certain
contexts, the translation of pathways from animal models to humans, exaggerated placebo effects and issues with clinical trial design. ABSTRACT Acute and chronic pain complaints, although
common, are generally poorly served by existing therapies. This unmet clinical need reflects a failure to develop novel classes of analgesics with superior efficacy, diminished adverse
effects and a lower abuse liability than those currently available. Reasons for this include the heterogeneity of clinical pain conditions, the complexity and diversity of underlying
pathophysiological mechanisms, and the unreliability of some preclinical pain models. However, recent advances in our understanding of the neurobiology of pain are beginning to offer
opportunities for developing novel therapeutic strategies and revisiting existing targets, including modulating ion channels, enzymes and G-protein-coupled receptors. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio
journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per
year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DISCOVERY AND
VALIDATION OF BIOMARKERS TO AID THE DEVELOPMENT OF SAFE AND EFFECTIVE PAIN THERAPEUTICS: CHALLENGES AND OPPORTUNITIES Article 15 June 2020 PATHOLOGY OF PAIN AND ITS IMPLICATIONS FOR
THERAPEUTIC INTERVENTIONS Article Open access 08 June 2024 DEZOCINE AS A POTENT ANALGESIC: OVERVIEW OF ITS PHARMACOLOGICAL CHARACTERIZATION Article 04 November 2021 CHANGE HISTORY * _ 23
JUNE 2017 In the original published article, the ligands RB-64 and PZM21 have been shown as attributed to Trevena in table 1 in the 'biased GPCR ligands' row. This error has been
corrected in the HTML and PDF versions of the article. _ * _ 06 OCTOBER 2017 The compounds APD371,LY2828360, S-777469 and KHK6188 were incorrectly referred to as inhibitors of the
cannabinoid receptors CB1 and CB2 in Table 1, when they are cannabinoid receptor agonists. In addition, KHK6188 is not currently in a Phase 2 clinical trial for neuropathic pain as stated in
Table 1 and development of this agent has been discontinued. The error has been corrected in the html and pdf versions online. _ REFERENCES * Dubois, M. Y., Gallagher, R. M. & Lippe, P.
M. Pain medicine position paper. _Pain Med._ 10, 972–1000 (2009). PubMed Google Scholar * Johannes, C. B., Le, T. K., Zhou, X., Johnston, J. A. & Dworkin, R. H. The prevalence of
chronic pain in United States adults: results of an Internet-based survey. _J. Pain_ 11, 1230–1239 (2010). PubMed Google Scholar * Volkow, N. D. & McLellan, A. T. Opioid abuse in
chronic pain — misconceptions and mitigation strategies. _N. Engl. J. Med._ 374, 1253–1263 (2016). THIS REVIEW HIGHLIGHTS COMMON MISCONCEPTIONS ABOUT ABUSE-RELATED LIABILITIES OF
PRESCRIPTION OPIOIDS AND PROPOSES STRATEGIES THAT COULD HELP TO MITIGATE THESE RISKS. CAS PubMed Google Scholar * Decosterd, I. & Woolf, C. J. Spared nerve injury: an animal model of
persistent peripheral neuropathic pain. _Pain_ 87, 149–158 (2000). IN THIS STUDY, THE AUTHORS DESCRIBE A TECHNIQUE FOR MODELLING PERIPHERAL NEUROPATHIC PAIN IN LABORATORY RODENTS. CAS
PubMed Google Scholar * Honore, P. et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory
neurons. _Neuroscience_ 98, 585–598 (2000). CAS PubMed Google Scholar * Costigan, M. et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1.
_Brain_ 133, 2519–2527 (2010). THIS RESEARCH ARTICLE DESCRIBES A PUTATIVE HUMAN PAIN GENE THAT COULD INFORM THE SELECTION OF NOVEL DRUG TARGETS FOR PATIENTS WITH NEUROPATHIC PAIN. IT MAY
HELP TO EXPLAIN WHY SOME, BUT NOT ALL, PEOPLE WITH NERVE INJURY PROGRESS TO CHRONIC PAIN. PubMed PubMed Central Google Scholar * von Hehn, C. A., Baron, R. & Woolf, C. J.
Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. _Neuron_ 73, 638–652 (2012). THIS WORK DESCRIBES HOW THE VARIABLE EXPRESSION OF SENSORY NERVE INJURY SYMPTOMS CAN
PROVIDE INSIGHTS INTO THE UNDERLYING PATHOPHYSIOLOGICAL MECHANISMS AND GUIDANCE FOR THE DEVELOPMENT OF PERSONALIZED PAIN THERAPIES. CAS PubMed PubMed Central Google Scholar * Ji, R. R.,
Xu, Z. Z. & Gao, Y. J. Emerging targets in neuroinflammation-driven chronic pain. _Nat. Rev. Drug Discov._ 13, 533–548 (2014). HERE, THE AUTHORS DISCUSS EMERGING NEUROINFLAMMATORY PAIN
TARGETS AND DESCRIBE POTENTIAL THERAPEUTIC OPPORTUNITIES TO TARGET EXCESSIVE NEUROINFLAMMATION. CAS PubMed PubMed Central Google Scholar * Latremoliere, A. & Woolf, C. J. Central
sensitization: a generator of pain hypersensitivity by central neural plasticity. _J. Pain_ 10, 895–926 (2009). THIS WORK DESCRIBES THE MECHANISMS AND TRIGGERS THAT UNDERLIE THE INITIATION
AND MAINTENANCE OF CENTRAL SENSITIZATION, AND HOW THEY ARE ALTERED BY CHANGES IN THE PROPERTIES AND EXPRESSION PATTERNS OF GLUTAMATE RECEPTORS. PubMed PubMed Central Google Scholar *
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. _Cell_ 139, 267–284 (2009). IN THIS PIECE, THE AUTHORS REVIEW THE BIOLOGICAL
UNDERPINNINGS OF SOMATOSENSATION AT THE CIRCUIT, CELLULAR AND SUBCELLULAR LEVELS, WITH PARTICULAR EMPHASIS ON PAIN-RELATED RECEPTORS AND MECHANISMS. CAS PubMed PubMed Central Google
Scholar * Ossipov, M. H., Dussor, G. O. & Porreca, F. Central modulation of pain. _J. Clin. Invest._ 120, 3779–3787 (2010). THIS REVIEW EXPLORES EVIDENCE THAT CENTRAL MODULATORY
CIRCUITS CAN DRAMATICALLY CHANGE THE SUBJECTIVE EXPERIENCE OF PAINFUL STIMULI. CAS PubMed PubMed Central Google Scholar * Vardeh, D., Mannion, R. J. & Woolf, C. J. Toward a
mechanism-based approach to pain diagnosis. _J. Pain_ 17, T50–T69 (2016). HERE, THE AUTHORS PROPOSE THAT IDENTIFYING SPECIFIC MECHANISMS THAT UNDERLIE CHRONIC PAIN COULD PROVIDE THE BASIS
FOR A PERSONALIZED-MEDICINE APPROACH TO ANALGESIA. PubMed PubMed Central Google Scholar * Woolf, C. J. Overcoming obstacles to developing new analgesics. _Nat. Med._ 16, 1241–1247 (2010).
THIS ARTICLE DISCUSSES THE MANY COMPLEXITIES THAT HAVE MADE THE DEVELOPMENT OF NEW ANALGESICS SO CHALLENGING. CAS PubMed Google Scholar * Andrews, N. A. et al. Ensuring transparency and
minimization of methodologic bias in preclinical pain research: PPRECISE considerations. _Pain_ 157, 901–909 (2016). HERE, MEMBERS OF THE PRECLINICAL PAIN RESEARCH CONSORTIUM FOR
INVESTIGATING SAFETY AND EFFICACY (PPRECISE) WORKING GROUP PROPOSE NEW VOLUNTARY STANDARDS OF SCIENTIFIC RIGOUR AND TRANSPARENT REPORTING TO PROMOTE MORE-EFFICIENT ADVANCEMENT OF THE SEARCH
FOR NEW PAIN TREATMENTS. PubMed Google Scholar * Singla, N. et al. Assay sensitivity of pain intensity versus pain relief in acute pain clinical trials: ACTTION systematic review and
meta-analysis. _J. Pain_ 16, 683–691 (2015). THIS META-ANALYSIS FOUND THAT FOR PRECLINICAL ACUTE PAIN TRIALS, READOUTS OF TOTAL PAIN RELIEF MAY BE MORE SENSITIVE TO TREATMENT THAN SUMMED
PAIN INTENSITY DIFFERENCES. PubMed Google Scholar * Finnerup, N. B. et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. _Lancet Neurol._ 14,
162–173 (2015). CAS PubMed PubMed Central Google Scholar * Costigan, M., Scholz, J. & Woolf, C. J. Neuropathic pain: a maladaptive response of the nervous system to damage. _Annu.
Rev. Neurosci._ 32, 1–32 (2009). CAS PubMed PubMed Central Google Scholar * Patapoutian, A., Tate, S. & Woolf, C. J. Transient receptor potential channels: targeting pain at the
source. _Nat. Rev. Drug Discov._ 8, 55–68 (2009). CAS PubMed PubMed Central Google Scholar * Woolf, C. J. Pain: morphine, metabolites, mambas, and mutations. _Lancet Neurol._ 12, 18–20
(2013). PubMed Google Scholar * Woolf, C. J. & Salter, M. W. Neuronal plasticity: increasing the gain in pain. _Science_ 288, 1765–1769 (2000). IN THIS REVIEW, THE AUTHORS
CONCEPTUALIZE HOW PLASTICITY IN ASCENDING SENSORY PATHWAYS MAY ELICIT PAIN HYPERSENSITIVITY BY INCREASING SIGNAL GAIN. CAS PubMed Google Scholar * Chiu, I. M., von Hehn, C. A. &
Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. _Nat. Neurosci._ 15, 1063–1067 (2012). CAS PubMed PubMed Central Google Scholar
* Woolf, C. J. What is this thing called pain? _J. Clin. Invest._ 120, 3742–3744 (2010). CAS PubMed PubMed Central Google Scholar * Julius, D. & Basbaum, A. I. Molecular mechanisms
of nociception. _Nature_ 413, 203–210 (2001). HERE, THE AUTHORS DESCRIBE MOLECULAR MECHANISMS OF PRIMARY AFFERENT NEURONS, THEIR MODALITY SENSITIVITIES AND VARIOUS TRANSDUCERS, PEPTIDES,
LIPIDS AND GROWTH FACTORS THAT SIGNAL PAIN AND MEDIATE PAIN-RELATED SIGNALS. CAS PubMed Google Scholar * Michaud, K., Bombardier, C. & Emery, P. Quality of life in patients with
rheumatoid arthritis: does abatacept make a difference? _Clin. Exp. Rheumatol._ 25, S35–S45 (2007). CAS PubMed Google Scholar * Drenth, J. P. & Waxman, S. G. Mutations in
sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. _J. Clin. Invest._ 117, 3603–3609 (2007). CAS PubMed PubMed Central Google Scholar * Woolf, C. J. Central
sensitization: implications for the diagnosis and treatment of pain. _Pain_ 152, S2–S15 (2011). PubMed Google Scholar * Hains, B. C. et al. Upregulation of sodium channel Nav1.3 and
functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. _J. Neurosci._ 23, 8881–8892 (2003). CAS PubMed PubMed Central
Google Scholar * Nassar, M. A. et al. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. _Mol. Pain_ 2, 33 (2006). PubMed PubMed Central Google
Scholar * Dong, X. W. et al. Small interfering RNA-mediated selective knockdown of NaV1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats.
_Neuroscience_ 146, 812–821 (2007). CAS PubMed Google Scholar * Jarvis, M. F. et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and
inflammatory pain in the rat. _Proc. Natl Acad. Sci. USA_ 104, 8520–8525 (2007). CAS PubMed Google Scholar * Ekberg, J. et al. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron
specific sodium channels and chronic pain behavior without motor deficits. _Proc. Natl Acad. Sci. USA_ 103, 17030–17035 (2006). CAS PubMed Google Scholar * Gold, M. S. et al.
Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. _J. Neurosci._ 23, 158–166 (2003). CAS PubMed PubMed Central Google Scholar * Joshi, S. K. et al. Involvement of the
TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. _Pain_ 123, 75–82 (2006). CAS PubMed Google Scholar * Roza, C., Laird, J. M.,
Souslova, V., Wood, J. N. & Cervero, F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. _J.
Physiol._ 550, 921–926 (2003). CAS PubMed PubMed Central Google Scholar * Fritch, P. C. et al. Novel KCNQ2/Q3 agonists as potential therapeutics for epilepsy and neuropathic pain. _J.
Med. Chem._ 53, 887–896 (2010). CAS PubMed Google Scholar * Dost, R., Rostock, A. & Rundfeldt, C. The anti-hyperalgesic activity of retigabine is mediated by KCNQ potassium channel
activation. _Naunyn Schmiedebergs Arch. Pharmacol._ 369, 382–390 (2004). CAS PubMed Google Scholar * Lee, S. Pharmacological inhibition of voltage-gated Ca2+ channels for chronic pain
relief. _Curr. Neuropharmacol._ 11, 606–620 (2013). CAS PubMed PubMed Central Google Scholar * Stemkowski, P. L., Noh, M. C., Chen, Y. & Smith, P. A. Increased excitability of
medium-sized dorsal root ganglion neurons by prolonged interleukin-1β exposure is K+ channel dependent and reversible. _J. Physiol._ 593, 3739–3755 (2015). CAS PubMed PubMed Central
Google Scholar * Zogopoulos, P., Vasileiou, I., Patsouris, E. & Theocharis, S. E. The role of endocannabinoids in pain modulation. _Fundam. Clin. Pharmacol._ 27, 64–80 (2013). CAS
PubMed Google Scholar * Gutenstein, H. & Akil, H. in _Goodman and Gilman's Pharmacological Basis of Therapeutics_ Ch. 21 (eds Brunton, L., Lazo, J. & Parker, K.) 547–590 (The
McGraw Hill companies, 2006). CHAPTER 21 OF THIS AUTHORITATIVE PHARMACOLOGY TEXT PROVIDES A THOROUGH OVERVIEW OF OPIOID ANALGESIC PHARMACOLOGY. Google Scholar * Fries, D. S. in _Principles
of Medicinal Chemistry_ Ch. 14 (eds Foye, W. O., Lemke, T. L. & Williams,D. A.) 247–269 (William & Wilkins, 1995). CHAPTER 14 OF THIS ESSENTIAL MEDICINAL CHEMISTRY SOURCE DESCRIBES
THE MEDICINAL CHEMISTRY OF COMMON OPIOID AND ANTI-INFLAMMATORY ANALGESICS. Google Scholar * Lesniak, A. & Lipkowski, A. W. Opioid peptides in peripheral pain control. _Acta Neurobiol.
Exp. (Wars.)_ 71, 129–138 (2011). Google Scholar * Navratilova, E. et al. Positive emotions and brain reward circuits in chronic pain. _J. Comp. Neurol._ 524, 1646–1652 (2016). PubMed
PubMed Central Google Scholar * Navratilova, E. & Porreca, F. Reward and motivation in pain and pain relief. _Nat. Neurosci._ 17, 1304–1312 (2014). CAS PubMed PubMed Central Google
Scholar * Kolodny, A. et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. _Annu. Rev. Public Health_ 36, 559–574 (2015). PubMed Google
Scholar * Cassidy, T. A., DasMahapatra, P., Black, R. A., Wieman, M. S. & Butler, S. F. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent
opioid formulation. _Pain Med._ 15, 440–451 (2014). PubMed Google Scholar * Kunins, H. V. Abuse-deterrent opioid formulations: part of a public health strategy to reverse the opioid
epidemic. _JAMA Intern. Med._ 175, 987–988 (2015). PubMed Google Scholar * Chilcoat, H. D., Coplan, P. M., Harikrishnan, V. & Alexander, L. Decreased diversion by doctor-shopping for a
reformulated extended release oxycodone product (OxyContin). _Drug Alcohol Depend._ 165, 221–228 (2016). PubMed Google Scholar * Coplan, P. M. et al. The effect of an abuse-deterrent
opioid formulation on opioid abuse-related outcomes in the post-marketing setting. _Clin. Pharmacol. Ther._ 100, 275–286 (2016). CAS PubMed PubMed Central Google Scholar * Cicero, T. J.,
Ellis, M. S. & Surratt, H. L. Effect of abuse-deterrent formulation of OxyContin. _N. Engl. J. Med._ 367, 187–189 (2012). CAS PubMed Google Scholar * Cicero, T. J., Ellis, M. S.
& Kasper, Z. A. A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. _Pain_ 157, 1232–1238 (2016). CAS
PubMed Google Scholar * Walsh, S. L., Strain, E. C., Abreu, M. E. & Bigelow, G. E. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in
humans. _Psychopharmacology (Berl.)_ 157, 151–162 (2001). CAS Google Scholar * Pallasch, T. J. & Gill, C. J. Butorphanol and nalbuphine: a pharmacologic comparison. _Oral Surg. Oral
Med. Oral Pathol._ 59, 15–20 (1985). CAS PubMed Google Scholar * Webster, L., Menzaghi, F. & Spencer, R. CR845, a novel peripherally-acting kappa opioid receptor agonist, has low
abuse potential compared with pentazocine. _J. Pain_ 16, S81 (2015). Google Scholar * Spahn, V. et al. A nontoxic pain killer designed by modeling of pathological receptor conformations.
_Science_ 355, 966–969 (2017). CAS PubMed Google Scholar * Milligan, G. The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization. _Mol. Pharmacol._ 84,
158–169 (2013). CAS PubMed PubMed Central Google Scholar * Rozenfeld, R. & Devi, L. A. Receptor heteromerization and drug discovery. _Trends Pharmacol. Sci._ 31, 124–130 (2010). CAS
PubMed PubMed Central Google Scholar * Yekkirala, A. S. Two to tango: GPCR oligomers and GPCR–TRP channel interactions in nociception. _Life Sci._ 92, 438–445 (2013). CAS PubMed
Google Scholar * Yekkirala, A. S., Kalyuzhny, A. E. & Portoghese, P. S. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by
bivalent ligands. _ACS Chem. Biol._ 8, 1412–1416 (2013). CAS PubMed PubMed Central Google Scholar * Lenard, N. R., Daniels, D. J., Portoghese, P. S. & Roerig, S. C. Absence of
conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. _Eur. J. Pharmacol._ 566, 75–82
(2007). CAS PubMed Google Scholar * Aceto, M. D. et al. MDAN-21: a bivalent opioid ligand containing mu-agonist and delta-antagonist pharmacophores and its effects in rhesus monkeys.
_Int. J. Med. Chem._ 2012, 327257 (2012). PubMed PubMed Central Google Scholar * Daniels, D. J. et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between
pharmacophores in a bivalent ligand series. _Proc. Natl Acad. Sci. USA_ 102, 19208–19213 (2005). CAS PubMed Google Scholar * Le Naour, M. et al. Bivalent ligands that target mu opioid
(MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance. _J. Med. Chem._ 56, 5505–5513 (2013). CAS PubMed Google Scholar * Akgun, E. et al. Ligands that interact
with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. _Proc. Natl Acad. Sci. USA_ 110, 11595–11599 (2013). CAS PubMed Google Scholar * Akgun,
E. et al. Inhibition of inflammatory and neuropathic pain by targeting a mu opioid receptor/chemokine receptor5 heteromer (MOR-CCR5). _J. Med. Chem._ 58, 8647–8657 (2015). CAS PubMed
PubMed Central Google Scholar * Yekkirala, A. S. et al. N-Naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. _Proc. Natl Acad. Sci.
USA_ 108, 5098–5103 (2011). CAS PubMed Google Scholar * Chakrabarti, S., Liu, N. J. & Gintzler, A. R. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates
female-specific opioid analgesia. _Proc. Natl Acad. Sci. USA_ 107, 20115–20119 (2010). CAS PubMed Google Scholar * Ding, H. et al. A novel orvinol analog, BU08028, as a safe opioid
analgesic without abuse liability in primates. _Proc. Natl Acad. Sci. USA_ 113, E5511–E5518 (2016). CAS PubMed Google Scholar * Pasternak, G. W. & Pan, Y. X. Mu opioids and their
receptors: evolution of a concept. _Pharmacol. Rev._ 65, 1257–1317 (2013). CAS PubMed PubMed Central Google Scholar * Marrone, G. F. et al. Truncated mu opioid GPCR variant involvement
in opioid-dependent and opioid-independent pain modulatory systems within the CNS. _Proc. Natl Acad. Sci. USA_ 113, 3663–3668 (2016). CAS PubMed Google Scholar * Majumdar, S. et al.
Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. _Proc. Natl Acad. Sci. USA_ 108, 19778–19783
(2011). CAS PubMed Google Scholar * Wieskopf, J. S. et al. Broad-spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene.
_Pain_ 155, 2063–2070 (2014). CAS PubMed PubMed Central Google Scholar * Liu, X. Y. et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by
opioids. _Cell_ 147, 447–458 (2011). CAS PubMed PubMed Central Google Scholar * White, K. L. et al. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique
spectrum of activities _in vivo_. _J. Pharmacol. Exp. Ther._ 352, 98–109 (2015). PubMed PubMed Central Google Scholar * Tang, W., Strachan, R. T., Lefkowitz, R. J. & Rockman, H. A.
Allosteric modulation of beta-arrestin-biased angiotensin II type 1 receptor signaling by membrane stretch. _J. Biol. Chem._ 289, 28271–28283 (2014). CAS PubMed PubMed Central Google
Scholar * Drake, M. T. et al. β-Arrestin-biased agonism at the β2-adrenergic receptor. _J. Biol. Chem._ 283, 5669–5676 (2008). CAS PubMed Google Scholar * Wisler, J. W., Xiao, K.,
Thomsen, A. R. & Lefkowitz, R. J. Recent developments in biased agonism. _Curr. Opin. Cell Biol._ 27, 18–24 (2014). IN THIS REVIEW, THE AUTHORS DESCRIBE HOW DIFFERENT LIGANDS CAN INDUCE
DISTINCT RECEPTOR CONFORMATIONS AT THE SAME GPCR TO ELICIT UNIQUE DOWNSTREAM SIGNALLING PROFILES. THEY PROVIDE SUPPORT FOR HOW THESE PROPERTIES COULD BE EXPLOITED TO DEVELOP ANALGESICS WITH
DIFFERENT OR REDUCED SIDE-EFFECT PROFILES. CAS PubMed Google Scholar * Shukla, A. K. et al. Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane
receptors. _Proc. Natl Acad. Sci. USA_ 105, 9988–9993 (2008). CAS PubMed Google Scholar * Whalen, E. J., Rajagopal, S. & Lefkowitz, R. J. Therapeutic potential of beta-arrestin- and G
protein-biased agonists. _Trends Mol. Med._ 17, 126–139 (2011). CAS PubMed Google Scholar * Zidar, D. A., Violin, J. D., Whalen, E. J. & Lefkowitz, R. J. Selective engagement of G
protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. _Proc. Natl Acad. Sci. USA_ 106, 9649–9654 (2009). CAS PubMed Google Scholar * Gesty-Palmer, D. et
al. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. _Sci. Transl Med._ 1, 1ra1 (2009). PubMed PubMed
Central Google Scholar * Strachan, R. T. et al. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). _J. Biol. Chem._ 289,
14211–14224 (2014). CAS PubMed PubMed Central Google Scholar * Violin, J. D., Crombie, A. L., Soergel, D. G. & Lark, M. W. Biased ligands at G-protein-coupled receptors: promise and
progress. _Trends Pharmacol. Sci._ 35, 308–316 (2014). CAS PubMed Google Scholar * Soergel, D. G. et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces
on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. _Pain_ 155, 1829–1835 (2014). CAS PubMed Google Scholar
* Soergel, D. G. et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. _J. Clin. Pharmacol._ 54, 351–357 (2014). CAS PubMed Google
Scholar * Manglik, A. et al. Structure-based discovery of opioid analgesics with reduced side effects. _Nature_ 537, 185–190 (2016). CAS PubMed PubMed Central Google Scholar * Tabrizi,
M. A., Baraldi, P. G., Borea, P. A. & Varani, K. Medicinal chemistry, pharmacology, and potential therapeutic benefits of cannabinoid CB2 receptor agonists. _Chem. Rev._ 116, 519–560
(2016). Google Scholar * Han, S., Thatte, J., Buzard, D. J. & Jones, R. M. Therapeutic utility of cannabinoid receptor type 2 (CB2) selective agonists. _J. Med. Chem._ 56, 8224–8256
(2013). CAS PubMed Google Scholar * Nevalainen, T. Recent development of CB2 selective and peripheral CB1/CB2 cannabinoid receptor ligands. _Curr. Med. Chem._ 21, 187–203 (2014). CAS
PubMed Google Scholar * Anand, U. et al. Mechanisms underlying clinical efficacy of angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: clinical tissue and _in
vitro_ studies. _Mol. Pain_ 11, 38 (2015). PubMed PubMed Central Google Scholar * Danser, A. H. & Anand, P. The angiotensin II type 2 receptor for pain control. _Cell_ 157, 1504–1506
(2014). CAS PubMed Google Scholar * Rice, A. S. et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic
neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. _Lancet_ 383, 1637–1647 (2014). CAS PubMed Google Scholar * Anand, U. et al. Angiotensin II type 2
receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. _Eur. J. Pain_ 17, 1012–1026 (2013). CAS
PubMed Google Scholar * Smith, M. T., Woodruff, T. M., Wyse, B. D., Muralidharan, A. & Walther, T. A small molecule angiotensin II type 2 receptor (AT2R) antagonist produces analgesia
in a rat model of neuropathic pain by inhibition of p38 mitogen-activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. _Pain Med._ 14, 1557–1568 (2013).
PubMed Google Scholar * Marion, E. et al. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. _Cell_ 157, 1565–1576 (2014). CAS PubMed Google
Scholar * Lemmens, S., Brone, B., Dooley, D., Hendrix, S. & Geurts, N. Alpha-adrenoceptor modulation in central nervous system trauma: pain, spasms, and paralysis — an unlucky triad.
_Med. Res. Rev._ 35, 653–677 (2015). CAS PubMed Google Scholar * Giovannitti, J. A. Jr, Thoms, S. M. & Crawford, J. J. Alpha-2 adrenergic receptor agonists: a review of current
clinical applications. _Anesth. Prog._ 62, 31–39 (2015). PubMed PubMed Central Google Scholar * Mori, K. et al. Effects of norepinephrine on rat cultured microglial cells that express
alpha1, alpha2, beta1 and beta2 adrenergic receptors. _Neuropharmacology_ 43, 1026–1034 (2002). CAS PubMed Google Scholar * Lavand'homme, P. M. & Eisenach, J. C. Perioperative
administration of the alpha2-adrenoceptor agonist clonidine at the site of nerve injury reduces the development of mechanical hypersensitivity and modulates local cytokine expression. _Pain_
105, 247–254 (2003). CAS PubMed Google Scholar * Feng, X. et al. Intrathecal administration of clonidine attenuates spinal neuroimmune activation in a rat model of neuropathic pain with
existing hyperalgesia. _Eur. J. Pharmacol._ 614, 38–43 (2009). CAS PubMed Google Scholar * Wei, H. & Pertovaara, A. Spinal and pontine alpha2-adrenoceptors have opposite effects on
pain-related behavior in the neuropathic rat. _Eur. J. Pharmacol._ 551, 41–49 (2006). CAS PubMed Google Scholar * Schnabel, A., Meyer-Friessem, C. H., Reichl, S. U., Zahn, P. K. &
Pogatzki-Zahn, E. M. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. _Pain_ 154, 1140–1149 (2013). CAS
PubMed Google Scholar * Schnabel, A. et al. Efficacy and safety of intraoperative dexmedetomidine for acute postoperative pain in children: a meta-analysis of randomized controlled trials.
_Paediatr. Anaesth._ 23, 170–179 (2013). PubMed Google Scholar * Melik Parsadaniantz, S., Rivat, C., Rostene, W. & Reaux-Le Goazigo, A. Opioid and chemokine receptor crosstalk: a
promising target for pain therapy? _Nat. Rev. Neurosci._ 16, 69–78 (2015). PubMed Google Scholar * Abbadie, C. et al. Chemokines and pain mechanisms. _Brain Res. Rev._ 60, 125–134 (2009).
CAS PubMed Google Scholar * Reaux-Le Goazigo, A., Van Steenwinckel, J., Rostene, W. & Melik Parsadaniantz, S. Current status of chemokines in the adult CNS. _Prog. Neurobiol._ 104,
67–92 (2013). CAS PubMed Google Scholar * Van Steenwinckel, J. et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain
after peripheral nerve injury. _J. Neurosci._ 31, 5865–5875 (2011). CAS PubMed PubMed Central Google Scholar * Xie, F., Wang, Y., Li, X., Chao, Y. C. & Yue, Y. Early repeated
administration of CXCR4 antagonist AMD3100 dose-dependently improves neuropathic pain in rats after L5 spinal nerve ligation. _Neurochem. Res._ 41, 2289–2299 (2016). CAS PubMed Google
Scholar * Talbot, S., Foster, S. L. & Woolf, C. J. Neuroimmunity: physiology and pathology. _Annu. Rev. Immunol._ 34, 421–447 (2016). CAS PubMed Google Scholar * Szabo, I. et al.
Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. _Proc. Natl Acad. Sci. USA_ 99, 10276–10281 (2002). CAS PubMed
Google Scholar * Szabo, I. et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. _J. Leukoc. Biol._
74, 1074–1082 (2003). CAS PubMed Google Scholar * Grimm, M. C. et al. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. _J.
Exp. Med._ 188, 317–325 (1998). CAS PubMed PubMed Central Google Scholar * Pello, O. M. et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for
immune response regulation. _Eur. J. Immunol._ 38, 537–549 (2008). CAS PubMed Google Scholar * Rivat, C. et al. Src family kinases involved in CXCL12-induced loss of acute morphine
analgesia. _Brain Behav. Immun._ 38, 38–52 (2014). CAS PubMed Google Scholar * Zhao, C. M. et al. Spinal MCP-1 contributes to the development of morphine antinociceptive tolerance in
rats. _Am. J. Med. Sci._ 344, 473–479 (2012). PubMed Google Scholar * Zhang, N., Rogers, T. J., Caterina, M. & Oppenheim, J. J. Proinflammatory chemokines, such as C-C chemokine ligand
3, desensitize mu-opioid receptors on dorsal root ganglia neurons. _J. Immunol._ 173, 594–599 (2004). CAS PubMed Google Scholar * Ye, D. et al. Activation of CXCL10/CXCR3 signaling
attenuates morphine analgesia: involvement of Gi protein. _J. Mol. Neurosci._ 53, 571–579 (2014). CAS PubMed Google Scholar * Rittner, H. L. et al. Pain control by CXCR2 ligands through
Ca2+-regulated release of opioid peptides from polymorphonuclear cells. _FASEB J._ 20, 2627–2629 (2006). CAS PubMed Google Scholar * Wilson, N. M., Jung, H., Ripsch, M. S., Miller, R. J.
& White, F. A. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. _Brain Behav. Immun._ 25, 565–573 (2011). CAS PubMed Google Scholar * Kalliomaki, J. et al. A
randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. _Pain_ 154, 761–767 (2013). PubMed Google Scholar * Padi, S. S.
et al. Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. _Pain_ 153, 95–106
(2012). CAS PubMed Google Scholar * Szallasi, A., Cortright, D. N., Blum, C. A. & Eid, S. R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist
proof-of-concept. _Nat. Rev. Drug Discov._ 6, 357–372 (2007). CAS PubMed Google Scholar * Habib, A. M., Wood, J. N. & Cox, J. J. Sodium channels and pain. _Handb. Exp. Pharmacol._
227, 39–56 (2015). CAS PubMed Google Scholar * Waxman, S. G. et al. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use.
_Lancet Neurol._ 13, 1152–1160 (2014). CAS PubMed Google Scholar * Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. _Nature_ 389, 816–824
(1997). CAS PubMed Google Scholar * Nilius, B. & Szallasi, A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine.
_Pharmacol. Rev._ 66, 676–814 (2014). PubMed Google Scholar * Moran, M. M., McAlexander, M. A., Biro, T. & Szallasi, A. Transient receptor potential channels as therapeutic targets.
_Nat. Rev. Drug Discov._ 10, 601–620 (2011). CAS PubMed Google Scholar * Carnevale, V. & Rohacs, T. TRPV1: a target for rational drug design. _Pharmaceuticals (Basel)_ 9, E52 (2016).
Google Scholar * Lehto, S. G. et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluorom ethyl)phenyl)-acrylamide (AMG8562), a
novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. _J. Pharmacol. Exp. Ther._ 326, 218–229 (2008). CAS PubMed Google Scholar *
Watabiki, T. et al. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. _J. Pharmacol. Exp. Ther._
336, 743–750 (2011). CAS PubMed Google Scholar * Chiche, D., Brown, W. & Walker, P. NEO6860, a novel modality selective TRPV1 antagonist: results from a phase I, double-blind,
placebo-controlled study in healthy subjects. _J. Pain_ 17, S79 (2016). Google Scholar * Gao, Y., Cao, E., Julius, D. & Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of
ligand and lipid action. _Nature_ 534, 347–351 (2016). CAS PubMed PubMed Central Google Scholar * Cao, L. et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory
neurons and patients with inherited erythromelalgia. _Sci. Transl Med._ 8, 335ra56 (2016). PubMed Google Scholar * Dib-Hajj, S. D., Yang, Y., Black, J. A. & Waxman, S. G. The NaV1.7
sodium channel: from molecule to man. _Nat. Rev. Neurosci._ 14, 49–62 (2013). CAS PubMed Google Scholar * Cox, J. J. et al. An SCN9A channelopathy causes congenital inability to
experience pain. _Nature_ 444, 894–898 (2006). CAS PubMed Google Scholar * Cox, J. J. et al. Congenital insensitivity to pain: novel SCN9A missense and in-frame deletion mutations. _Hum.
Mut._ 31, E1670–E1686 (2010). CAS PubMed Google Scholar * Nassar, M. A. et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain.
_Proc. Natl Acad. Sci. USA_ 101, 12706–12711 (2004). CAS PubMed Google Scholar * Minett, M. S. et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and
sympathetic neurons. _Nat. Commun._ 3, 791 (2012). PubMed PubMed Central Google Scholar * Gingras, J. et al. Global Nav1.7 knockout mice recapitulate the phenotype of human congenital
indifference to pain. _PLoS ONE_ 9, e105895 (2014). PubMed PubMed Central Google Scholar * Bagal, S. K., Marron, B. E., Owen, R. M., Storer, R. I. & Swain, N. A. Voltage gated sodium
channels as drug discovery targets. _Channels (Austin)_ 9, 360–366 (2015). Google Scholar * Sun, S., Cohen, C. J. & Dehnhardt, C. M. Inhibitors of voltage-gated sodium channel Nav1.7:
patent applications since 2010. _Pharm. Pat. Anal._ 3, 509–521 (2014). CAS PubMed Google Scholar * Focken, T. et al. Discovery of aryl sulfonamides as isoform-selective inhibitors of
NaV1.7 with efficacy in rodent pain models. _ACS Med. Chem. Lett._ 7, 277–282 (2016). CAS PubMed PubMed Central Google Scholar * McCormack, K. et al. Voltage sensor interaction site for
selective small molecule inhibitors of voltage-gated sodium channels. _Proc. Natl Acad. Sci. USA_ 110, E2724–E2732 (2013). CAS PubMed Google Scholar * Butt, M. et al. Morphologic,
stereologic, and morphometric evaluation of the nervous system in young cynomolgus monkeys (_Macaca fascicularis_) following maternal administration of tanezumab, a monoclonal antibody to
nerve growth factor. _Toxicol. Sci._ 142, 463–476 (2014). CAS PubMed Google Scholar * Wainger, B. J. et al. Modeling pain _in vitro_ using nociceptor neurons reprogrammed from
fibroblasts. _Nat. Neurosci._ 18, 17–24 (2015). CAS PubMed Google Scholar * Talbot, S. et al. Silencing nociceptor neurons reduces allergic airway inflammation. _Neuron_ 87, 341–354
(2015). CAS PubMed PubMed Central Google Scholar * Bauer, C. S. et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain
is inhibited by the alpha2delta ligand pregabalin. _J. Neurosci._ 29, 4076–4088 (2009). CAS PubMed PubMed Central Google Scholar * Lawrence, J. Nav1.7: a new channel for pain treatment.
_Pharm. J._ http://dx.doi.org/10.1211/PJ.2016.20200841 (2016). * Bowman, C. J. et al. Developmental toxicity assessment of tanezumab, an anti-nerve growth factor monoclonal antibody, in
cynomolgus monkeys (_Macaca fascicularis_). _Reprod. Toxicol._ 53, 105–118 (2015). CAS PubMed Google Scholar * Murray, J. K. et al. Single residue substitutions that confer voltage-gated
sodium ion channel subtype selectivity in the NaV1.7 inhibitory peptide GpTx-1. _J. Med. Chem._ 59, 2704–2717 (2016). CAS PubMed Google Scholar * Weiss, J. et al. Loss-of-function
mutations in sodium channel Nav1.7 cause anosmia. _Nature_ 472, 186–190 (2011). CAS PubMed PubMed Central Google Scholar * Minett, M. S. et al. Endogenous opioids contribute to
insensitivity to pain in humans and mice lacking sodium channel Nav1.7. _Nat. Commun._ 6, 8967 (2015). CAS PubMed PubMed Central Google Scholar * Kort, M. E. et al. Subtype-selective
NaV1.8 sodium channel blockers: identification of potent, orally active nicotinamide derivatives. _Bioorg. Med. Chem. Lett._ 20, 6812–6815 (2010). CAS PubMed Google Scholar * Bagal, S. K.
et al. Recent progress in sodium channel modulators for pain. _Bioorg. Med. Chem. Lett._ 24, 3690–3699 (2014). CAS PubMed Google Scholar * Wilson, M. J. et al. μ-Conotoxins that
differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. _Proc. Natl Acad. Sci. USA_ 108, 10302–10307 (2011). CAS PubMed
Google Scholar * Rush, A. M., Cummins, T. R. & Waxman, S. G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. _J. Physiol._ 579, 1–14
(2007). CAS PubMed Google Scholar * Dib-Hajj, S. D., Black, J. A. & Waxman, S. G. NaV1.9: a sodium channel linked to human pain. _Nat. Rev. Neurosci._ 16, 511–519 (2015). CAS PubMed
Google Scholar * Goral, R. O., Leipold, E., Nematian-Ardestani, E. & Heinemann, S. H. Heterologous expression of NaV1.9 chimeras in various cell systems. _Pflugers Arch._ 467,
2423–2435 (2015). CAS PubMed Google Scholar * Owsianik, G., Talavera, K., Voets, T. & Nilius, B. Permeation and selectivity of TRP channels. _Annu. Rev. Physiol._ 68, 685–717 (2006).
CAS PubMed Google Scholar * Hellwig, N. et al. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. _J. Biol. Chem._ 279, 34553–34561 (2004). CAS PubMed Google
Scholar * Meyers, J. R. et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. _J. Neurosci._ 23, 4054–4065 (2003). CAS PubMed PubMed Central
Google Scholar * Binshtok, A. M., Bean, B. P. & Woolf, C. J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. _Nature_ 449, 607–610 (2007). CAS
PubMed Google Scholar * Puopolo, M. et al. Permeation and block of TRPV1 channels by the cationic lidocaine derivative QX-314. _J. Neurophysiol._ 109, 1704–1712 (2013). CAS PubMed PubMed
Central Google Scholar * Brenneis, C. et al. Bupivacaine-induced cellular entry of QX-314 and its contribution to differential nerve block. _Br. J. Pharmacol._ 171, 438–451 (2014). CAS
PubMed Google Scholar * Virginio, C., MacKenzie, A., Rassendren, F. A., North, R. A. & Surprenant, A. Pore dilation of neuronal P2X receptor channels. _Nat. Neurosci._ 2, 315–321
(1999). CAS PubMed Google Scholar * Khakh, B. S., Bao, X. R., Labarca, C. & Lester, H. A. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. _Nat.
Neurosci._ 2, 322–330 (1999). CAS PubMed Google Scholar * Yan, Z., Li, S., Liang, Z., Tomic, M. & Stojilkovic, S. S. The P2X7 receptor channel pore dilates under physiological ion
conditions. _J. Gen. Physiol._ 132, 563–573 (2008). CAS PubMed PubMed Central Google Scholar * Binshtok, A. M. et al. Coapplication of lidocaine and the permanently charged sodium
channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. _Anesthesiology_ 111, 127–137 (2009). CAS PubMed PubMed Central Google Scholar * Roberson, D. P.,
Binshtok, A. M., Blasl, F., Bean, B. P. & Woolf, C. J. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. _Br. J.
Pharmacol._ 164, 48–58 (2011). CAS PubMed PubMed Central Google Scholar * Leffler, A. et al. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent
sensory neurons. _J. Clin. Invest._ 118, 763–776 (2008). PubMed PubMed Central Google Scholar * Leffler, A., Lattrell, A., Kronewald, S., Niedermirtl, F. & Nau, C. Activation of TRPA1
by membrane permeable local anesthetics. _Mol. Pain_ 7, 62 (2011). CAS PubMed PubMed Central Google Scholar * Roberson, D. P. et al. Activity-dependent silencing reveals functionally
distinct itch-generating sensory neurons. _Nat. Neurosci._ 16, 910–918 (2013). CAS PubMed PubMed Central Google Scholar * Zamponi, G. W., Striessnig, J., Koschak, A. & Dolphin, A. C.
The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. _Pharmacol. Rev._ 67, 821–870 (2015). CAS PubMed PubMed Central
Google Scholar * Zamponi, G. W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. _Nat. Rev. Drug Discov._ 15, 19–34 (2016). IN THIS REVIEW, ZAMPONI
DISCUSSES VARIOUS CHALLENGES AND OPPORTUNITIES FOR USING CALCIUM CHANNELS AS DRUG TARGETS FOR NEUROLOGICAL DISORDERS. CAS PubMed Google Scholar * Abbadie, C. et al. Analgesic effects of a
substituted N-triazole oxindole (TROX-1), a state-dependent, voltage-gated calcium channel 2 blocker. _J. Pharmacol. Exp. Ther._ 334, 545–555 (2010). CAS PubMed Google Scholar * Patel,
R. et al. Electrophysiological characterization of activation state-dependent CaV2 channel antagonist TROX-1 in spinal nerve injured rats. _Neuroscience_ 297, 47–57 (2015). CAS PubMed
PubMed Central Google Scholar * Shao, P. P. et al. Aminopiperidine sulfonamide Cav2.2 channel inhibitors for the treatment of chronic pain. _J. Med. Chem._ 55, 9847–9855 (2012). CAS
PubMed Google Scholar * Lipscombe, D. & Andrade, A. Calcium channel CaVα1 splice isoforms — tissue specificity and drug action. _Curr. Mol. Pharmacol._ 8, 22–31 (2015). CAS PubMed
PubMed Central Google Scholar * Bourinet, E. et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. _EMBO J._ 24, 315–324
(2005). CAS PubMed Google Scholar * Choi, S. et al. Attenuated pain responses in mice lacking CaV3.2 T-type channels. _Genes Brain Behav._ 6, 425–431 (2007). CAS PubMed Google Scholar
* Jarvis, M. F. et al. A peripherally acting, selective T-type calcium channel blocker, ABT-639, effectively reduces nociceptive and neuropathic pain in rats. _Biochem. Pharmacol._ 89,
536–544 (2014). CAS PubMed Google Scholar * Wallace, M., Duan, R., Liu, W., Locke, C. & Nothaft, W. A. Randomized, double-blind, placebo-controlled, crossover study of the T-type
calcium channel blocker ABT-639 in an intradermal capsaicin experimental pain model in healthy adults. _Pain Med._ 17, 551–560 (2015). PubMed Google Scholar * Ziegler, D., Duan, W. R., An,
G., Thomas, J. W. & Nothaft, W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral
neuropathic pain. _Pain_ 156, 2013–2020 (2015). CAS PubMed PubMed Central Google Scholar * Francois, A. et al. State-dependent properties of a new T-type calcium channel blocker enhance
CaV3.2 selectivity and support analgesic effects. _Pain_ 154, 283–293 (2013). CAS PubMed Google Scholar * Xu, J. et al. A mixed Ca2+ channel blocker, A-1264087, utilizes peripheral and
spinal mechanisms to inhibit spinal nociceptive transmission in a rat model of neuropathic pain. _J. Neurophysiol._ 111, 394–404 (2014). CAS PubMed Google Scholar * Zhu, C. Z. et al.
Mechanistic insights into the analgesic efficacy of A-1264087, a novel neuronal Ca2+ channel blocker that reduces nociception in rat preclinical pain models. _J. Pain_ 387, e1–e14 (2014).
Google Scholar * Scott, V. E. et al. A-1048400 is a novel, orally active, state-dependent neuronal calcium channel blocker that produces dose-dependent antinociception without altering
hemodynamic function in rats. _Biochem. Pharmacol._ 83, 406–418 (2012). CAS PubMed Google Scholar * Marsh, B., Acosta, C., Djouhri, L. & Lawson, S. N. Leak K+ channel mRNAs in dorsal
root ganglia: relation to inflammation and spontaneous pain behaviour. _Mol. Cell. Neurosci._ 49, 375–386 (2012). CAS PubMed Google Scholar * Pollema-Mays, S. L., Centeno, M. V., Ashford,
C. J., Apkarian, A. V. & Martina, M. Expression of background potassium channels in rat DRG is cell-specific and down-regulated in a neuropathic pain model. _Mol. Cell. Neurosci._ 57,
1–9 (2013). CAS PubMed Google Scholar * Zheng, Q. et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain
in a rat model. _Pain_ 154, 434–448 (2013). CAS PubMed Google Scholar * Laumet, G. et al. G9a is essential for epigenetic silencing of K+ channel genes in acute-to-chronic pain
transition. _Nat. Neurosci._ 18, 1746–1755 (2015). CAS PubMed PubMed Central Google Scholar * Lu, R. et al. Slack channels expressed in sensory neurons control neuropathic pain in mice.
_J. Neurosci._ 35, 1125–1135 (2015). PubMed PubMed Central Google Scholar * Lyu, C. et al. G protein-gated inwardly rectifying potassium channel subunits 1 and 2 are down-regulated in rat
dorsal root ganglion neurons and spinal cord after peripheral axotomy. _Mol. Pain_ 11, 44 (2015). PubMed PubMed Central Google Scholar * Maljevic, S. & Lerche, H. Potassium channels:
a review of broadening therapeutic possibilities for neurological diseases. _J. Neurol._ 260, 2201–2211 (2013). CAS PubMed Google Scholar * Tsantoulas, C. & McMahon, S. B. Opening
paths to novel analgesics: the role of potassium channels in chronic pain. _Trends Neurosci._ 37, 146–158 (2014). CAS PubMed PubMed Central Google Scholar * Tsantoulas, C. Emerging
potassium channel targets for the treatment of pain. _Curr. Opin. Support. Palliat. Care_ 9, 147–154 (2015). PubMed Google Scholar * Wickenden, A. D. & McNaughton-Smith, G. Kv7
channels as targets for the treatment of pain. _Curr. Pharm. Des._ 15, 1773–1798 (2009). CAS PubMed Google Scholar * Wu, Y. J. et al. Discovery of
(S,E)-3-(2-fluorophenyl)-N-(1-(3-(pyridin-3-yloxy)phenyl)ethyl)-acrylamide as a potent and efficacious KCNQ2 (Kv7.2) opener for the treatment of neuropathic pain. _Bioorg. Med. Chem. Lett._
23, 6188–6191 (2013). CAS PubMed Google Scholar * Zheng, Y. et al. Activation of peripheral KCNQ channels relieves gout pain. _Pain_ 156, 1025–1035 (2015). CAS PubMed PubMed Central
Google Scholar * Mathie, A. & Veale, E. L. Two-pore domain potassium channels: potential therapeutic targets for the treatment of pain. _Pflugers Arch._ 467, 931–943 (2015). CAS PubMed
Google Scholar * Wang, H. S. et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. _Science_ 282, 1890–1893 (1998). CAS PubMed Google Scholar * Pan,
Z. et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. _J. Neurosci._ 26, 2599–2613 (2006). CAS PubMed PubMed Central
Google Scholar * Cooper, E. C. Made for “anchorin”: Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons. _Semin. Cell Dev. Biol._ 22, 185–192
(2011). CAS PubMed Google Scholar * Blackburn-Munro, G. & Jensen, B. S. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic
pain. _Eur. J. Pharmacol._ 460, 109–116 (2003). CAS PubMed Google Scholar * Li, H. et al. Antinociceptive efficacy of retigabine in the monosodium lodoacetate rat model for osteoarthritis
pain. _Pharmacology_ 95, 251–257 (2015). CAS PubMed Google Scholar * Rose, K. et al. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. _Pain_ 152,
742–754 (2011). CAS PubMed PubMed Central Google Scholar * King, C. H., Lancaster, E., Salomon, D., Peles, E. & Scherer, S. S. Kv7.2 regulates the function of peripheral sensory
neurons. _J. Comp. Neurol._ 522, 3262–3280 (2014). CAS PubMed PubMed Central Google Scholar * King, C. H. & Scherer, S. S. Kv7.5 is the primary Kv7 subunit expressed in C-fibers. _J.
Comp. Neurol._ 520, 1940–1950 (2012). CAS PubMed PubMed Central Google Scholar * Feliciangeli, S., Chatelain, F. C., Bichet, D. & Lesage, F. The family of K2P channels: salient
structural and functional properties. _J. Physiol._ 593, 2587–2603 (2015). CAS PubMed PubMed Central Google Scholar * Busserolles, J., Tsantoulas, C., Eschalier, A. & Lopez Garcia,
J. A. Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. _Pain_ 157 (Suppl. 1), S7–S14 (2016). PubMed Google Scholar * Devilliers, M. et al. Activation of
TREK-1 by morphine results in analgesia without adverse side effects. _Nat. Commun._ 4, 2941 (2013). PubMed Google Scholar * Bagriantsev, S. N. et al. A high-throughput functional screen
identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. _ACS Chem. Biol._ 8, 1841–1851 (2013). CAS PubMed PubMed Central Google Scholar * Rodrigues, N.
et al. Synthesis and structure-activity relationship study of substituted caffeate esters as antinociceptive agents modulating the TREK-1 channel. _Eur. J. Med. Chem._ 75, 391–402 (2014).
CAS PubMed Google Scholar * Bocksteins, E. & Snyders, D. J. Electrically silent Kv subunits: their molecular and functional characteristics. _Physiology (Bethesda)_ 27, 73–84 (2012).
CAS Google Scholar * Bocksteins, E. Kv5, Kv6, Kv8, and Kv9 subunits: no simple silent bystanders. _J. Gen. Physiol._ 147, 105–125 (2016). CAS PubMed PubMed Central Google Scholar *
Tsantoulas, C. et al. Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury. _J. Neurosci._ 32, 17502–17513 (2012). CAS
PubMed PubMed Central Google Scholar * Tsantoulas, C. et al. Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. _Exp. Neurol._ 251,
115–126 (2014). CAS PubMed PubMed Central Google Scholar * Bocksteins, E. et al. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG
neurons. _Am. J. Physiol. Cell Physiol._ 296, C1271–C1278 (2009). CAS PubMed PubMed Central Google Scholar * Stas, J. I., Bocksteins, E., Labro, A. J. & Snyders, D. J. Modulation of
closed-state inactivation in Kv2.1/Kv6.4 heterotetramers as mechanism for 4-AP induced potentiation. _PLoS ONE_ 10, e0141349 (2015). PubMed PubMed Central Google Scholar * Knabl, J. et
al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. _Nature_ 451, 330–334 (2008). CAS PubMed Google Scholar * Bonin, R. P. & De Koninck, Y. Restoring
ionotropic inhibition as an analgesic strategy. _Neurosci Lett._ 557, 43–51 (2013). CAS PubMed Google Scholar * Klinger, F. et al. δ subunit-containing GABAA receptors are preferred
targets for the centrally acting analgesic flupirtine. _Br. J. Pharmacol._ 172, 4946–4958 (2015). CAS PubMed PubMed Central Google Scholar * Zeilhofer, H. U., Ralvenius, W. T. &
Acuna, M. A. Restoring the spinal pain gate: GABAA receptors as targets for novel analgesics. _Adv. Pharmacol._ 73, 71–96 (2015). CAS PubMed Google Scholar * Tegeder, I. et al. GTP
cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. _Nat. Med._ 12, 1269–1277 (2006). CAS PubMed Google Scholar * Latremoliere, A. et al. Reduction of
neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. _Neuron_ 86, 1393–1406 (2015). CAS PubMed PubMed Central Google Scholar * Chidley, C., Haruki,
H., Pedersen, M. G., Muller, E. & Johnsson, K. A yeast-based screen reveals that sulfasalazine inhibits tetrahydrobiopterin biosynthesis. _Nat. Chem. Biol._ 7, 375–383 (2011). CAS
PubMed Google Scholar * Chandrasekhar, S. et al. Identification and characterization of novel microsomal prostaglandin E synthase-1 inhibitors for analgesia. _J. Pharmacol. Exp. Ther._
356, 635–644 (2016). CAS PubMed Google Scholar * Jin, Y. et al. Pharmacodynamic comparison of LY3023703, a novel microsomal prostaglandin E synthase 1 inhibitor, with celecoxib. _Clin.
Pharmacol. Ther._ 99, 274–284 (2016). CAS PubMed Google Scholar * Wagner, K., Yang, J., Inceoglu, B. & Hammock, B. D. Soluble epoxide hydrolase inhibition is antinociceptive in a
mouse model of diabetic neuropathy. _J. Pain_ 15, 907–914 (2014). CAS PubMed PubMed Central Google Scholar * Inceoglu, B. et al. Endoplasmic reticulum stress in the peripheral nervous
system is a significant driver of neuropathic pain. _Proc. Natl Acad. Sci. USA_ 112, 9082–9087 (2015). CAS PubMed Google Scholar * Sisignano, M. et al. 5,6-EET is released upon neuronal
activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. _J. Neurosci._ 32, 6364–6372 (2012). CAS PubMed PubMed Central Google Scholar * Brenneis,
C. et al. Soluble epoxide hydrolase limits mechanical hyperalgesia during inflammation. _Mol. Pain_ 7, 78 (2011). CAS PubMed PubMed Central Google Scholar * Lam, D. K., Dang, D., Zhang,
J., Dolan, J. C. & Schmidt, B. L. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. _J. Neurosci._ 32, 14178–14183 (2012). CAS PubMed PubMed Central
Google Scholar * Christianson, C. A. et al. Spinal matrix metalloproteinase 3 mediates inflammatory hyperalgesia via a tumor necrosis factor-dependent mechanism. _Neuroscience_ 200, 199–210
(2012). CAS PubMed Google Scholar * Berta, T. et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. _J. Clin. Invest._ 124, 1173–1186 (2014).
CAS PubMed PubMed Central Google Scholar * Clark, A. K., Grist, J., Al-Kashi, A., Perretti, M. & Malcangio, M. Spinal cathepsin S and fractalkine contribute to chronic pain in the
collagen-induced arthritis model. _Arthritis Rheum._ 64, 2038–2047 (2012). CAS PubMed Google Scholar * Vicuna, L. et al. The serine protease inhibitor SerpinA3N attenuates neuropathic
pain by inhibiting T cell-derived leukocyte elastase. _Nat. Med._ 21, 518–523 (2015). CAS PubMed PubMed Central Google Scholar * Landry, R. P., Jacobs, V. L., Romero-Sandoval, E. A.
& DeLeo, J. A. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: _in vitro_ evidence for differential responses in human and rodent
microglia and macrophages. _Exp. Neurol._ 234, 340–350 (2012). CAS PubMed Google Scholar * Ji, R. R. Mitogen-activated protein kinases as potential targets for pain killers. _Curr. Opin.
Investig. Drugs_ 5, 71–75 (2004). PubMed Google Scholar * Ostenfeld, T. et al. A randomized, placebo-controlled trial of the analgesic efficacy and safety of the p38 MAP kinase inhibitor,
losmapimod, in patients with neuropathic pain from lumbosacral radiculopathy. _Clin. J. Pain_ 31, 283–293 (2015). PubMed Google Scholar * Smith, M. T., Wyse, B. D. & Edwards, S. R.
Small molecule angiotensin II type 2 receptor (AT2R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding, and efficacy in rats. _Pain
Med._ 14, 692–705 (2013). PubMed Google Scholar * Bevan, S., Quallo, T. & Andersson, D. A. TRPV1. _Handb. Exp. Pharmacol._ 222, 207–245 (2014). CAS PubMed Google Scholar * Girardin,
F. Membrane transporter proteins: a challenge for CNS drug development. _Dialogues Clin. Neurosci._ 8, 311–321 (2006). PubMed PubMed Central Google Scholar * Huggins, J. P., Smart, T.
S., Langman, S., Taylor, L. & Young, T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which
modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. _Pain_ 153, 1837–1846 (2012). CAS PubMed Google Scholar *
Keith, J. M. et al. Preclinical characterization of the FAAH inhibitor JNJ-42165279. _ACS Med. Chem. Lett._ 6, 1204–1208 (2015). CAS PubMed PubMed Central Google Scholar * Pawsey, S. et
al. Safety, tolerability and pharmacokinetics of FAAH inhibitor V158866: a double-blind, randomised, placebo-controlled phase I study in healthy volunteers. _Drugs R. D_ 16, 181–191 (2016).
CAS PubMed PubMed Central Google Scholar * Cajanus, K. et al. Effect of endocannabinoid degradation on pain: role of FAAH polymorphisms in experimental and postoperative pain in women
treated for breast cancer. _Pain_ 157, 361–369 (2016). CAS PubMed Google Scholar * Woolf, C. J., Safieh-Garabedian, B., Ma, Q. P., Crilly, P. & Winter, J. Nerve growth factor
contributes to the generation of inflammatory sensory hypersensitivity. _Neuroscience_ 62, 327–331 (1994). CAS PubMed Google Scholar * Gimbel, J. S. et al. Long-term safety and
effectiveness of tanezumab as treatment for chronic low back pain. _Pain_ 155, 1793–1801 (2014). CAS PubMed Google Scholar * Bramson, C. et al. Exploring the role of tanezumab as a novel
treatment for the relief of neuropathic pain. _Pain Med._ 16, 1163–1176 (2015). PubMed Google Scholar * Schnitzer, T. J. & Marks, J. A. A systematic review of the efficacy and general
safety of antibodies to NGF in the treatment of OA of the hip or knee. _Osteoarthritis Cartilage_ 23 (Suppl. 1), S8–S17 (2015). PubMed Google Scholar * Hochberg, M. C. et al. When is
osteonecrosis not osteonecrosis? Adjudication of reported serious adverse joint events in the tanezumab clinical development program. _Arthritis Rheumatol._ 68, 382–391 (2016). CAS PubMed
Google Scholar * Sorge, R. E. et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. _Nat. Methods_ 11, 629–632 (2014). CAS PubMed Google
Scholar * Baron, R. et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. _Pain_ 158, 261–272 (2016). PubMed Central Google Scholar *
Mogil, J. S., Davis, K. D. & Derbyshire, S. W. The necessity of animal models in pain research. _Pain_ 151, 12–17 (2010). IN THIS REVIEW, MOGIL AND COLLEAGUES DESCRIBE THE CRUCIAL ROLE
OF ANIMAL MODELS IN THE DEVELOPMENT OF CLINICAL ANALGESICS AND PROPOSE ADVANCEMENTS IN ANIMAL MODELS OF PAIN THAT COULD IMPROVE THE RELEVANCE AND TRANSLATABILITY OF PRECLINICAL STUDIES.
PubMed Google Scholar * Whiteside, G. T., Pomonis, J. D. & Kennedy, J. D. An industry perspective on the role and utility of animal models of pain in drug discovery. _Neurosci. Lett._
557, 65–72 (2013). CAS PubMed Google Scholar * Hill, R. NK1 (substance P) receptor antagonists — why are they not analgesic in humans? _Trends Pharmacol. Sci._ 21, 244–246 (2000). CAS
PubMed Google Scholar * Sorge, R. E. et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. _Nat. Neurosci._ 18, 1081–1083 (2015). CAS PubMed
PubMed Central Google Scholar * Edwards, R. R., Dworkin, R. H., Sullivan, M. D., Turk, D. C. & Wasan, A. D. The role of psychosocial processes in the development and maintenance of
chronic pain. _J. Pain_ 17, T70–T92 (2016). PubMed PubMed Central Google Scholar * Smith, S. M. et al. Pain intensity rating training: results from an exploratory study of the ACTTION
PROTECCT system. _Pain_ 157, 1056–1064 (2016). PubMed Google Scholar * Dodd, S., Dean, O. M., Vian, J. & Berk, M. A. Review of the theoretical and biological understanding of the
nocebo and placebo phenomena. _Clin. Ther._ 39, 469–476 (2017). PubMed Google Scholar * Paice, J. A. et al. AAPT diagnostic criteria for chronic cancer pain conditions. _J. Pain_ 18,
233–246 (2017). PubMed Google Scholar * Smith, S. M. et al. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials:
IMMPACT considerations. _J. Pain_ http://dx.doi.org/10.1016/j.jpain.2017.02.429 (2017). * Edwards, R. R. et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT
recommendations. _Pain_ 157, 1851–1871 (2016). CAS PubMed PubMed Central Google Scholar * Viscusi, E. R. et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the
mu-opioid receptor, for the intravenous treatment of acute pain. _Pain_ 157, 264–272 (2016). CAS PubMed Google Scholar * Lu, Z. et al. Mediation of opioid analgesia by a truncated
6-transmembrane GPCR. _J. Clin. Invest._ 125, 2626–2630 (2015). PubMed PubMed Central Google Scholar * Moriconi, A. et al. Targeting the minor pocket of C5aR for the rational design of an
oral allosteric inhibitor for inflammatory and neuropathic pain relief. _Proc. Natl Acad. Sci. USA_ 111, 16937–16942 (2014). CAS PubMed Google Scholar * Altun, A. et al. Attenuation of
morphine antinociceptive tolerance by cannabinoid CB1 and CB2 receptor antagonists. _J. Physiol. Sci._ 65, 407–415 (2015). CAS PubMed Google Scholar * Haugh, O., Penman, J., Irving, A. J.
& Campbell, V. A. The emerging role of the cannabinoid receptor family in peripheral and neuro-immune interactions. _Curr. Drug Targets_ 17, 1834–1840 (2016). CAS PubMed Google
Scholar * Deliu, E. et al. The lysophosphatidylinositol receptor GPR55 modulates pain perception in the periaqueductal gray. _Mol. Pharmacol._ 88, 265–272 (2015). CAS PubMed PubMed
Central Google Scholar * Kort, M. E. & Kym, P. R. TRPV1 antagonists: clinical setbacks and prospects for future development. _Prog. Med. Chem._ 51, 57–70 (2012). CAS PubMed Google
Scholar * Manitpisitkul, P. et al. Safety, tolerability and pharmacokinetic and pharmacodynamic learnings from a double-blind, randomized, placebo-controlled, sequential group
first-in-human study of the TRPV1 antagonist, JNJ-38893777, in healthy men. _Clin. Drug Investig._ 35, 353–363 (2015). CAS PubMed Google Scholar * Quiding, H. et al. TRPV1 antagonistic
analgesic effect: a randomized study of AZD1386 in pain after third molar extraction. _Pain_ 154, 808–812 (2013). CAS PubMed Google Scholar * De Petrocellis, L. & Moriello, A. S.
Modulation of the TRPV1 channel: current clinical trials and recent patents with focus on neurological conditions. _Recent Pat. CNS Drug Discov._ 8, 180–204 (2013). CAS PubMed Google
Scholar * Ghosh, S. et al. Full fatty acid amide hydrolase inhibition combined with partial monoacylglycerol lipase inhibition: augmented and sustained antinociceptive effects with reduced
cannabimimetic side effects in mice. _J. Pharmacol. Exp. Ther._ 354, 111–120 (2015). CAS PubMed PubMed Central Google Scholar * Grim, T. W. et al. Combined inhibition of FAAH and COX
produces enhanced anti-allodynic effects in mouse neuropathic and inflammatory pain models. _Pharmacol. Biochem. Behav._ 124, 405–411 (2014). CAS PubMed PubMed Central Google Scholar *
Starowicz, K. et al. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism.
_PLoS ONE_ 8, e60040 (2013). CAS PubMed PubMed Central Google Scholar * Schlosburg, J. E. et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the
endocannabinoid system. _Nat. Neurosci._ 13, 1113–1119 (2010). CAS PubMed PubMed Central Google Scholar * Luz, J. G. et al. Crystal structures of mPGES-1 inhibitor complexes form a basis
for the rational design of potent analgesic and anti-inflammatory therapeutics. _J. Med. Chem._ 58, 4727–4737 (2015). CAS PubMed Google Scholar * Leclerc, P. et al. Characterization of a
human and murine mPGES-1 inhibitor and comparison to mPGES-1 genetic deletion in mouse models of inflammation. _Prostaglandins Other Lipid Mediat._ 107, 26–34 (2013). CAS PubMed Google
Scholar * Sjogren, T. et al. Crystal structure of microsomal prostaglandin E2 synthase provides insight into diversity in the MAPEG superfamily. _Proc. Natl Acad. Sci. USA_ 110, 3806–3811
(2013). PubMed Google Scholar * Hellio le Graverand, M. P. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat
(SD-6010), in patients with symptomatic osteoarthritis of the knee. _Ann. Rheum. Dis._ 72, 187–195 (2013). PubMed Google Scholar * Wozniak, K. M. et al. The orally active glutamate
carboxypeptidase II inhibitor E2072 exhibits sustained nerve exposure and attenuates peripheral neuropathy. _J. Pharmacol. Exp. Ther._ 343, 746–754 (2012). CAS PubMed Google Scholar *
Vornov, J. J. et al. Pharmacokinetics and pharmacodynamics of the glutamate carboxypeptidase II inhibitor 2-MPPA show prolonged alleviation of neuropathic pain through an indirect mechanism.
_J. Pharmacol. Exp. Ther._ 346, 406–413 (2013). CAS PubMed PubMed Central Google Scholar * Huang, J. L., Chen, X. L., Guo, C. & Wang, Y. X. Contributions of spinal D-amino acid
oxidase to bone cancer pain. _Amino Acids_ 43, 1905–1918 (2012). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors acknowledge generous funding support from the
following US National Institutes of Health grants: NS039518 and NS038253 (US National Institute of Neurological Disorders and Stroke (NINDS) to C.J.W); NS072040 and NS036855 (NINDS to
B.P.B.); and DA041912 (US National Institute on Drug Abuse to A.S.Y.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurobiology, Harvard Medical School, Boston
Children's Hospital, 300 Longwood Avenue, Boston, 02115, Massachusetts, USA Ajay S. Yekkirala, David P. Roberson, Bruce P. Bean & Clifford J. Woolf * FM Kirby Neurobiology Center
Boston Children's Hospital, 300 Longwood Avenue, Boston, 02115, Massachusetts, USA Ajay S. Yekkirala, David P. Roberson & Clifford J. Woolf * Blue Therapeutics, Harvard Innovation
Launch Lab, 114 Western Avenue, Allston, 02134, Massachusetts, USA Ajay S. Yekkirala & David P. Roberson Authors * Ajay S. Yekkirala View author publications You can also search for this
author inPubMed Google Scholar * David P. Roberson View author publications You can also search for this author inPubMed Google Scholar * Bruce P. Bean View author publications You can also
search for this author inPubMed Google Scholar * Clifford J. Woolf View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence
to Ajay S. Yekkirala or Clifford J. Woolf. ETHICS DECLARATIONS COMPETING INTERESTS A.S.Y and D.P.R. are co-founders of Blue Therapeutics in Allston, Massachusetts, USA, which is focused on
developing non-addictive painkillers targeting G-protein-coupled receptor heteromers. A.S.Y. holds a patent on an analgesic agent. B.P.B. is a co-founder of Flex Pharma in Boston,
Massachusetts, USA, and co-holds patents on using charged sodium channel blockers for pain relief and other indications. C.J.W is co-founder and scientific advisor to Quartet Medicine in
Cambridge, Massachusetts, USA, which is focused on developing treatments for chronic pain and inflammation targeting the tetrahydrobiopterin pathway; he is also a consultant and stock holder
for Abide Therapeutics in San Diego, California, USA, and holds several patents related to methods and approaches for studying and treating pain. RELATED LINKS DATABASES ClinicalTrials.gov
FURTHER INFORMATION Arena Pharmaceuticals press release on APD371 BioTuesdays — Xenon still committed to TV-45070 for neuropathic pain Cara Therapeutics — Kappa Opioid Receptor Agonists
CNV2197944 phase II trial announcement press release CR845 phase IIa results press release CR845 phase III patient recruitment press release DEX-IN phase II efficacy press release DEX-IN
phase II side effects press release US Centers for Disease Control and Prevention — Overdose Xenon and Genentech collaboration press release Z944 phase Ib results press release POWERPOINT
SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR TABLE 1 POWERPOINT SLIDE FOR TABLE 2 POWERPOINT SLIDE FOR TABLE 3 GLOSSARY * Analgesics Pharmacological
agents or ligands that produce analgesia. * Tolerance A state in which the drug no longer produces the same effect and a higher dose is therefore needed. * Dependence An adaptive state that
develops when a pharmacological agent is used repeatedly and leads to withdrawal on cessation of the drug regimen. * Hyperalgesia Enhanced nociceptive response to a noxious stimulus, leading
to greater discomfort than before. * Allodynia Nociceptive response elicited even to previously non-noxious stimuli. * Phenocopy When an organism shows phenotypic characteristics that
reflect a different genotype from its own. * Neuropathic pain A condition leading to pain due to damage or disease of nervous system tissues. * Central sensitization A condition of the
nervous system in which neurons in the central nervous system are in a state of prolonged increase in excitability and synaptic efficacy, coupled with the loss of inhibitory activity. *
Analgesia A lack of, or insensibility to, pain. * Nociception Sensory neuronal responses to noxious or damaging stimuli that attribute the sensation of pain. * Depersonalization A state in
which an individual's thoughts, feelings and emotions seem to not belong to them. * Antinociception Inhibition of sensory neuronal response to noxious stimuli that leads to reduction of
pain sensation. * Ligand bias Occurs when a ligand shows selectivity or preference to a particular signal transduction mechanism for a target receptor. Also called 'functional
selectivity'. * Psychotomimetic effects A state of psychosis of the mind leading to delusions, hallucinations, and so on that are precipitated by a pharmacological agent or ligand. *
Post-herpetic neuralgia Pain caused by nerve damage due to infection with varicella zoster virus. * Withdrawal Symptoms such as anxiety and shaking that develop on cessation of a drug that
has been used repeatedly. * Trigeminal neuralgia A painful condition caused by disease affecting, dysfunction of or damage to the trigeminal nerve. * Anosmia Loss of the sense of smell. *
Allosteric modulators Ligands that alter the activity of an agonist, antagonist or inverse agonist of a target by binding to a site distinct from the active site. * Phenotypic screens
Unbiased screening strategies in which the functional output is a pre-determined alteration of phenotype. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Yekkirala, A., Roberson, D., Bean, B. _et al._ Breaking barriers to novel analgesic drug development. _Nat Rev Drug Discov_ 16, 545–564 (2017). https://doi.org/10.1038/nrd.2017.87 Download
citation * Published: 09 June 2017 * Issue Date: August 2017 * DOI: https://doi.org/10.1038/nrd.2017.87 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
