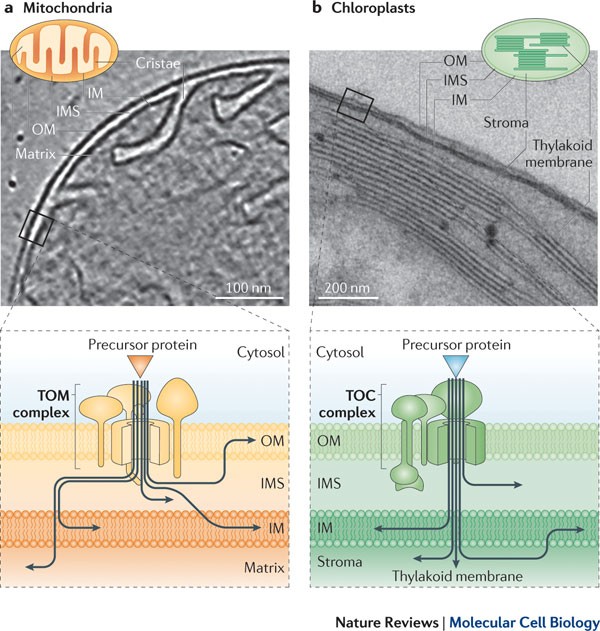
Common ground for protein translocation: access control for mitochondria and chloroplasts
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

KEY POINTS * The vast majority of mitochondrial and chloroplast proteins are cytosolically synthesized and have to be translocated into the organelle. * Precursor proteins contain amino
acid-based signals. These signals supply information allowing the proteins to target, and interact with, the cytosolic chaperones that provide guidance to organelles. * Translocases at the
outer membrane of mitochondria and chloroplasts form the general entry gate into both organelles. * The translocases of both organelles consist of three receptors, which bind to the
multitude of different precursor proteins and deliver them to a translocation pore formed by a protein with β-barrel structure. * Despite similarities with respect to their composition, the
translocons differ with respect to signal length requirement and their energizing of the translocation event. * The mode of translocation is also distinct between the translocation
machineries: mitochondrial import across the outer membrane is affinity-driven, whereas the passage of precursor proteins into chloroplasts is modulated by GTP binding and hydrolysis, and by
phosphorylation events. ABSTRACT Mitochondria and chloroplasts import the vast majority of their proteins across two membranes, and use translocases of the outer membrane as an entry gate.
These translocases interact with the incoming precursor protein and guiding chaperone factors. Within the translocon, precursor-protein receptors dock to a central component that mediates
both transfer through a cation-selective channel and initial sorting towards internal subcompartments. Despite these similarities, the mode of translocation differs between the two
organelles: in chloroplasts, GTP-binding and hydrolysis by the receptors is required for transport, whereas in mitochondria passage of the preprotein is driven by its increasing affinity for
the translocase subunits. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ARCHITECTURE OF CHLOROPLAST TOC–TIC TRANSLOCON SUPERCOMPLEX Article 26 January 2023 PEROXISOME BIOGENESIS INITIATED
BY PROTEIN PHASE SEPARATION Article 10 May 2023 IMPORT MECHANISM OF PEROXISOMAL PROTEINS WITH AN N-TERMINAL SIGNAL SEQUENCE Article Open access 09 May 2025 REFERENCES * Gray, M. W., Burger,
G. & Lang, B. F. Mitochondrial evolution. _Science_ 283, 1476–1481 (1999). CAS PubMed Google Scholar * McFadden, G. I. Endosymbiosis and evolution of the plant cell. _Curr. Opin.
Plant Biol._ 2, 513–519 (1999). CAS PubMed Google Scholar * Saraste, M. Oxidative phosphorylation at the _fin de siècle_. _Science_ 283, 1488–1493 (1999). CAS PubMed Google Scholar *
Lill, R. & Mühlenhoff, U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. _Annu. Rev. Biochem._ 77, 669–700 (2008). CAS PubMed Google
Scholar * López-Juez, E. Plastid biogenesis, between light and shadows. _J. Exp. Bot._ 58, 11–26 (2007). PubMed Google Scholar * Nelson, N. & Ben-Shem, A. The complex architecture of
oxygenic photosynthesis. _Nature Rev. Mol. Cell. Biol._ 5, 971–982 (2004). CAS Google Scholar * Sickmann, A. et al. The proteome of _Sacchoromyces cerevisiae_ mitochondria. _Proc. Natl
Acad. Sci. USA_ 103, 13207–13212 (2003). Google Scholar * van Wijk, K. J. Plastid proteomics. _Plant Physiol. Biochem._ 42, 963–977 (2004). CAS PubMed Google Scholar * Wickner, W. &
Schekman, R. Protein translocation across biological membranes. _Science_ 310, 1452–1456 (2005). CAS PubMed Google Scholar * Grudnik, P., Bange, G. & Sinning, I. Protein targeting by
the signal recognition particle. _Biol. Chem._ 390, 775–782 (2009). CAS PubMed Google Scholar * Schnell, D. J. & Hebert, D. N. Protein translocons: multifunctional mediators of
protein translocation across membranes. _Cell_ 112, 491–505 (2003). CAS PubMed Google Scholar * Stewart, M. Molecular mechanism of the nuclear protein import cycle. _Nature Rev. Mol. Cell
Biol._ 8, 195–208 (2007). CAS Google Scholar * Carrie, C., Giraud, E. & Whelan, J. Protein transport in organelles: dual targeting of proteins to mitochondria and chloroplasts. _FEBS
J._ 276, 1187–1195 (2009). CAS PubMed Google Scholar * Chacinska, A., Koehler, C. M., Milenkovic, D., Lithgow, T. & Pfanner, N. Importing mitochondrial proteins: machineries and
mechanisms. _Cell_ 138, 628–644 (2009). CAS PubMed PubMed Central Google Scholar * Neupert, W. & Herrmann, J. M. Translocation of proteins into mitochondria. _Annu. Rev. Biochem._
76, 723–749 (2007). CAS PubMed Google Scholar * Bruce, B. D. The paradox of plastid transit peptides: conservation of function despite divergence in primary structure. _Biochim. Biophys.
Acta_ 1541, 2–21 (2001). CAS PubMed Google Scholar * Li, H.-M. & Chiu, C.-C. Protein transport into chloroplasts. _Annu. Rev. Plant Biol._ 61, 157–180 (2010). CAS PubMed Google
Scholar * Gakh, O., Cavadini, P. & Isaya, G. Mitochondrial processing peptidases. _Biochim. Biophys. Acta_ 1592, 63–77 (2002). CAS PubMed Google Scholar * Richter, S. & Lamppa,
G. K. Structural properties of the chloroplast stromal processing peptidase required for its function in transit peptide removal. _J. Biol. Chem._ 278, 39497–39502 (2003). CAS PubMed
Google Scholar * Huang, S., Taylor, N. L., Whelan, J. & Millar, A. H. Refining the definition of plant mitochondrial presequences through analysis of sorting signals, N-terminal
modifications, and cleavage motifs. _Plant Physiol._ 150, 1272–1285 (2009). CAS PubMed PubMed Central Google Scholar * Vögtle, F. N. et al. Global analysis of the mitochondrial
N-proteome identifies a processing peptidase critical for protein stability. _Cell_ 139, 428–439 (2009). PubMed Google Scholar * Martin, T. et al. A protein kinase family in _Arabidopsis_
phosphorylates chloroplast precursor proteins. _J. Biol. Chem._ 281, 40216–40223 (2006). CAS PubMed Google Scholar * Schleiff, E. & Klösgen, R. B. Without a little help from
'my' friends: direct insertion of proteins into chloroplast membranes? _Biochim. Biophys. Acta_ 1541, 22–33 (2001). CAS PubMed Google Scholar * van der Laan, M., Hutu, D. P.
& Rehling, P. On the mechanism of preprotein import by the mitochondrial presequence translocase. _Biochim. Biophys. Acta_ 1803, 732–739 (2010). CAS PubMed Google Scholar * Kutik, S.
et al. Dissecting membrane insertion of mitochondrial β-barrel proteins. _Cell_ 132, 1011–1024 (2008). CAS PubMed Google Scholar * Kleffmann, T. et al. The _Arabidopsis thaliana_
chloroplast proteome reveals pathway abundance and novel protein functions. _Curr. Biol._ 14, 354–362 (2004). CAS PubMed Google Scholar * Armbruster, U. et al. Chloroplast proteins
without cleavable transit peptides: rare exceptions or a major constituent of the chloroplast proteome? _Mol. Plant_ 2, 1325–1335 (2009). CAS PubMed Google Scholar * Hachiya, N. et al.
MSF, a novel cytoplasmic chaperone which functions in precursor targeting to mitochondria. _EMBO J._ 13, 5146–5154 (1994). CAS PubMed PubMed Central Google Scholar * May, T & Soll,
J. 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. _Plant Cell_ 12, 53–64 (2000). CAS PubMed PubMed Central Google Scholar * Yano, M., Terada, K.
& Mori, M. AIP is a mitochondrial import mediator that binds to both import receptor Tom20 and preproteins. _J. Cell. Biol._ 163, 45–56 (2003). CAS PubMed PubMed Central Google
Scholar * Young, J. C., Hoogenraad, N. J. & Hartl, F. U. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. _Cell_ 112, 41–50 (2003).
THIS STUDY SHOWED THE DELIVERY OF HSP70- AND HSP90-GUIDED PRECURSOR PROTEINS TO TOM70. CAS PubMed Google Scholar * Schemenewitz, A., Pollmann, S., Reinbothe, C. & Reinbothe, S. A
substrate-independent, 14-3-3 protein-mediated plastid import pathway of NADPH:protochlorophyllide oxidoreductase A. _Proc. Natl Acad. Sci. USA_ 104, 8538–8543 (2009). Google Scholar *
Qbadou, S. et al. The molecular chaperone Hsp90 delivers precursor proteins to the chloroplast import receptor Toc64. _EMBO J._ 25, 1836–1847 (2006). THIS WORK DEFINED THE PATHWAY THAT
TARGETS HSP70- AND HSP90-GUIDED PRECURSORS TO TOC64. CAS PubMed PubMed Central Google Scholar * Oecking, C. & Jaspert, T. Plant 14-3-3 proteins catch up with their mammalian
orthologs. _Curr. Opin. Plant Biol._ 12, 760–765 (2009). CAS PubMed Google Scholar * Beddoe, T. & Lithgow, T. Delivery of nascent polypeptides to the mitochondrial surface. _Biochim.
Biophys. Acta_ 1592, 35–39 (2002). CAS PubMed Google Scholar * Bae, W. et al. AKR2A-mediated import of chloroplast outer membrane proteins is essential for chloroplast biogenesis. _Nature
Cell Biol._ 10, 220–227 (2008). THIS STUDY REPORTS THE IDENTIFICATION OF THE FACTOR THAT TARGETS PROTEINS TO THE CHLOROPLAST OUTER ENVELOPE. CAS PubMed Google Scholar * Dhanoa, P. K. et
al. Distinct pathways mediate the sorting of tail-anchored proteins to the plastid outer envelope. _PLoS ONE_ 5, e10098 (2010). PubMed PubMed Central Google Scholar * Shen, G. et al.
ANKYRIN REPEAT-CONTAINING PROTEIN 2A is an essential molecular chaperone for peroxisomal membrane-bound ASCORBATE PEROXIDASE3 in _Arabidopsis_. _Plant Cell_ 22, 811–831 (2010). CAS PubMed
PubMed Central Google Scholar * Kellems, R. E., Allison, V. F. & Butow, R. A. Cytoplasmic type 80S ribosomes associated with yeast mitochondria. IV. Attachment of ribosomes to the
outer membrane of isolated mitochondria. _J. Cell Biol._ 65, 1–14 (1975). CAS PubMed Google Scholar * Marc, P. et al. Genome-wide analysis of mRNAs targeted to yeast mitochondria. _EMBO
Rep._ 3, 159–164 (2002). CAS PubMed PubMed Central Google Scholar * Eliyahu, E. et al. Tom20 mediates localization of mRNAs to mitochondria in a translation-dependent manner. _Mol. Cell.
Biol._ 30, 284–294 (2010). CAS PubMed Google Scholar * Villarejo, A. et al. Evidence for a protein transported through the secretory pathway _en route_ to the higher plant chloroplast.
_Nature Cell Biol._ 7, 1224–1231 (2005). PubMed Google Scholar * Neuspiel, M. et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. _Curr.
Biol._ 18, 102–108 (2008). CAS PubMed Google Scholar * de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. _Nature_ 456, 605–610 (2008). PubMed
Google Scholar * Kormann, B. et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. _Science_ 325, 477–481 (2009). Google Scholar * Andersson, M. X., Goksör,
M. & Sandelius, A. S. Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. _J. Biol. Chem._ 282, 1170–1174
(2007). CAS PubMed Google Scholar * Oreb, M., Tews, I. & Schleiff, E. Policing Tic 'n' Toc, the doorway to chloroplasts. _Trends Cell Biol._ 18, 19–27 (2008). CAS PubMed
Google Scholar * Jarvis, P. Targeting of nucleus-encoded proteins to chloroplasts in plants. _New Phytol._ 179, 257–285 (2008). CAS PubMed Google Scholar * Dekker, P. J. T. et al.
Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. _Mol. Cell. Biol._ 18, 6515–6524 (1998). CAS PubMed PubMed
Central Google Scholar * Sherman, E. L., Go, N. E. & Nargang, F. E. Functions of the small proteins in the TOM complex of _Neurospora crassa_. _Mol. Biol. Cell_ 16, 4172–4182 (2005).
CAS PubMed PubMed Central Google Scholar * Dietmeier, K. et al. Tom5 functionally links mitochondrial preprotein receptors to the general import pore. _Nature_ 388, 195–200 (1997). CAS
PubMed Google Scholar * Becker, T. et al. Assembly of the mitochondrial protein import channel: role of Tom5 in two-stage interaction of Tom40 with the SAM complex. _Mol. Biol. Cell_ 21,
3106–3113 (2010). CAS PubMed PubMed Central Google Scholar * Jackson-Constan, D. & Keegstra, K. _Arabidopsis_ genes encoding components of the chloroplastic protein import apparatus.
_Plant Physiol._ 125, 1567–1576 (2001). CAS PubMed PubMed Central Google Scholar * Lister, R. et al. A transcriptomic and proteomic characterization of the _Arabidopsis_ mitochondrial
protein import apparatus and its response to mitochondrial dysfunction. _Plant Physiol._ 134, 777–789 (2004). CAS PubMed PubMed Central Google Scholar * Ivanova, Y., Smith, M. D., Chen,
K. & Schnell, D. J. Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. _Mol. Biol. Cell_ 15, 3379–3392 (2004). CAS PubMed
PubMed Central Google Scholar * Kubis, S. et al. Functional specialization amongst the _Arabidopsis_ Toc159 family of chloroplast protein import receptors. _Plant Cell_ 16, 2059–2077
(2004). REFERENCES 55 AND 56 DESCRIBE THE DIFFERENT FUNCTIONS OF THE DISTINCT TOC159 ISOFORMS. CAS PubMed PubMed Central Google Scholar * Inoue, H., Rounds, C. & Schnell, D. J. The
molecular basis for distinct pathways for protein import into _Arabidopsis_ chloroplasts. _Plant Cell_ 22, 1947–1960 (2010). CAS PubMed PubMed Central Google Scholar * Perry, A. J. et
al. Structure, topology and function of the translocase of the outer membrane of mitochondria. _Plant Physiol. Biochem._ 46, 265–274 (2008). CAS PubMed Google Scholar * Ahting, U. et al.
The TOMcore complex: the general import pore of the outer membrane of mitochondria. _J. Cell Biol._ 147, 959–968 (1999). CAS PubMed PubMed Central Google Scholar * Schleiff, E., Soll,
J., Küchler, M., Kühlbrandt, W. & Harrer, R. Characterization of the translocon of the outer envelope of chloroplasts. _J. Cell Biol._ 160, 541–551 (2003). CAS PubMed PubMed Central
Google Scholar * Künkele, K. P. et al. The preprotein translocation channel of the outer membrane of mitochondria. _Cell_ 93, 1009–1019 (1998). REFERENCES 60 AND 61 PROVIDE THE FIRST
STRUCTURAL AND FUNCTIONAL ANALYSIS OF PURIFIED TOM AND TOC COMPLEXES. PubMed Google Scholar * Model, K. et al. Protein translocase of the outer mitochondrial membrane: role of import
receptors in the structural organization of the TOM complex. _J. Mol. Biol._ 316, 657–666 (2002). CAS PubMed Google Scholar * Model, K., Meisinger, C. & Kühlbrandt, W. Cryo-electron
microscopy structure of a yeast mitochondrial preprotein translocase. _J. Mol. Biol._ 383, 1049–1057 (2008). CAS PubMed Google Scholar * van Wilpe, S. et al. Tom22 is a multifunctional
organizer of the mitochondrial preprotein translocase. _Nature_ 401, 485–489 (1999). THIS STUDY IDENTIFIED THE MULTIPLE FUNCTIONS OF TOM22 IN PRECURSOR-PROTEIN BINDING, MOLECULAR
ORGANIZATION OF THE TRANSLOCASE AND PORE REGULATION. CAS PubMed Google Scholar * Becker, T. et al. Preprotein recognition by the Toc complex. _EMBO J._ 23, 520–530 (2004). THE FIRST
MECHANISTIC ANALYSIS OF TRANSLOCATION STEPS IN THE TOC COMPLEX. CAS PubMed PubMed Central Google Scholar * Lee, J., Wang, F. & Schnell, D. J. Toc receptor dimerization participates
in the initiation of membrane translocation during protein import into chloroplasts. _J. Biol. Chem._ 284, 31130–31141 (2009). CAS PubMed PubMed Central Google Scholar * Jarvis, P. et
al. An _Arabidopsis_ mutant defective in the plastid general protein import apparatus. _Science_ 282, 100–103 (1998). THIS STUDY DESCRIBES THE PHENOTYPE AND ULTRASTRUCTURAL CHANGES OF THE
FIRST IDENTIFIED MUTANT OF THE TOC MACHINERY, THE _PPI1_ MUTANT. CAS PubMed Google Scholar * Oreb, M., Höfle, A., Mirus, O. & Schleiff, E. Phosphorylation regulates the assembly of
chloroplast import machinery. _J. Exp. Bot._ 59, 2309–2316 (2008). CAS PubMed PubMed Central Google Scholar * Aronsson, H., Combe, J., Patel, R. & Jarvis, P. _In vivo_ assessment of
the significance of phosphorylation of the _Arabidopsis_ chloroplast protein import receptor, atToc33. _FEBS Lett._ 580, 649–655 (2006). CAS PubMed Google Scholar * Oreb, M. et al.
Phospho-mimicry mutant of atToc33 affects early development of _Arabidopsis thaliana_. _FEBS Lett._ 581, 5945–5951 (2007). CAS PubMed Google Scholar * Saitoh, T. et al. Tom20 recognizes
mitochondrial presequences through dynamic equilibrium among multiple bound states. _EMBO J._ 26, 4777–4787 (2007). CAS PubMed PubMed Central Google Scholar * Mirus, O. & Schleiff,
E. The evolution of tetratricopeptide repeat domain containing receptors involved in protein translocation. _Endocytobiosis Cell Res._ 19, 31–50 (2009). Google Scholar * Wu, Y. & Sha,
B. Crystal structure of yeast outer membrane translocon member Tom70p. _Nature Struct. Mol. Biol._ 13, 589–593 (2006). CAS Google Scholar * Qbadou, S. et al. Toc64 — a preprotein-receptor
at the outer membrane with bipartide function. _J. Mol. Biol._ 367, 1330–1346 (2007). CAS PubMed Google Scholar * Chew, O. et al. A plant outer mitochondrial membrane protein with high
amino acid sequence identity to a chloroplast protein import receptor. _FEBS Lett._ 557, 109–114 (2004). CAS PubMed Google Scholar * Yamamoto, H. et al. Role of Tom70 in import of
presequence-containing mitochondrial proteins. _J. Biol. Chem._ 284, 31625–31646 (2009). Google Scholar * Hines, V. et al. Protein import into yeast mitochondria is accelerated by the outer
membrane protein MAS70. _EMBO J._ 9, 3191–3200 (1990). CAS PubMed PubMed Central Google Scholar * Aronsson, H. et al. Toc64/OEP64 is not essential for the efficient import of proteins
into chloroplasts in _Arabidopsis thaliana_. _Plant J._ 52, 53–68 (2007). CAS PubMed Google Scholar * Ramage, L., Junne, T., Hahne, K., Lithgow, T. & Schatz, G. Functional cooperation
of mitochondrial protein import receptors in yeast. _EMBO J._ 12, 4115–4123 (1993). CAS PubMed PubMed Central Google Scholar * Moczko, M. et al. Deletion of the receptor MOM19 strongly
impairs import of cleavable preproteins into _Sacchoromyces cerevisae_ mitochondria. _J. Biol. Chem._ 269, 9045–9051 (1994). CAS PubMed Google Scholar * Constan, D., Patel, R., Keegstra,
K. & Jarvis, P. An outer envelope membrane component of the plastid protein import apparatus plays an essential role in _Arabidopsis_. _Plant J._ 38, 93–106 (2004). CAS PubMed Google
Scholar * Kessler, F. & Schnell, D. J. Chloroplast biogenesis: diversity and regulation of the protein import apparatus. _Curr. Opin. Cell Biol._ 21, 494–500 (2009). CAS PubMed Google
Scholar * Yamano, K. et al. Tom20 and Tom22 share the common signal recognition pathway in mitochondrial protein import. _J. Biol. Chem._ 283, 3799–3807 (2008). CAS PubMed Google Scholar
* Brix, J., Dietmeier, K. & Pfanner, N. Differential recognition of preproteins by the purified cytosolic domains of the mitochondrial import receptors Tom20, Tom22 and Tom70. _J.
Biol. Chem._ 272, 20730–20735 (1997). CAS PubMed Google Scholar * Abe, Y. et al. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. _Cell_
100, 551–560 (2000). THIS STUDY DESCRIBES FOR THE FIRST TIME THE STRUCTURAL BASIS OF PRECURSOR RECOGNITION BY A RECEPTOR OF THE TOM TRANSLOCON. CAS PubMed Google Scholar * Keil, P. &
Pfanner, N. Insertion of MOM22 into mitochondrial outer membrane strictly depends on surface receptors. _FEBS Lett._ 321, 197–200 (1993). CAS PubMed Google Scholar * Wallas, T. R., Smith,
M. D., Sanchez-Nieto, S. & Schnell, D. J. The roles of Toc34 and Toc75 in targeting the Toc159 preprotein receptor to chloroplasts. _J. Biol. Chem._ 278, 44289–44297 (2003). CAS PubMed
Google Scholar * Bauer, J. et al. The major protein import receptor of plastids is essential for chloroplast biogenesis. _Nature_ 403, 203–207 (2000). CAS PubMed Google Scholar * Hill,
K. et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. _Nature_ 395, 516–521 (1998). CAS PubMed Google Scholar * Hinnah, S. C., Hill, K., Wagner,
R., Schlicher, T. & Soll, J. Reconstitution of a chloroplast protein import channel. _EMBO J._ 16, 7351–7360 (1997). REFERENCES 89 AND 90 PRESENT THE FIRST DESCRIPTION OF THE
RECONSTITUTION OF TOC75 AND TOM40, AND THEIR ELECTROPHYSIOLOGICAL CHARACTERIZATION. CAS PubMed PubMed Central Google Scholar * Hinnah, S. C., Wagner, R., Sveshnikova, N., Harrer, R.
& Soll, J. The chloroplast protein import channel Toc75: pore properties and interaction with transit peptides. _Biophys. J._ 83, 899–911 (2002). CAS PubMed PubMed Central Google
Scholar * Becker, L. et al. Preprotein translocase of the outer mitochondrial membrane: reconstituted Tom40 forms a characteristic TOM pore. _J. Mol. Biol._ 353, 1011–1020 (2005). CAS
PubMed Google Scholar * Poynor, M., Eckert, R. & Nussberger, S. Dynamics of the preprotein translocation channel of the outer membrane of mitochondria. _Biophys. J._ 95, 1511–1522
(2008). CAS PubMed PubMed Central Google Scholar * Schleiff, E. & McBride, H. The central matrix loop drives import of uncoupling protein 1 into mitochondria. _J. Cell Sci._ 113,
2267–2272 (2000). CAS PubMed Google Scholar * Wiedemann, N., Pfanner, N. & Ryan, M. T. The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into
mitochondria. _EMBO J._ 20, 951–960 (2001). CAS PubMed PubMed Central Google Scholar * Dolezal, P., Likic, V., Tachezy, J. & Lithgow, T. Evolution of the molecular machines for
protein import into mitochondria. _Science_ 313, 314–318 (2006). CAS PubMed Google Scholar * Bayrhuber, M. et al. Structure of the human voltage-dependent anion channel. _Proc. Natl Acad.
Sci. USA_ 105, 15370–15375 (2008). CAS PubMed PubMed Central Google Scholar * Bredemeier, R. et al. Functional and phylogenetic properties of the pore-forming β-barrel transporters of
the Omp85 family. _J. Biol. Chem._ 282, 1882–1890 (2007). A DISSECTION OF THE ELECTROPHYSIOLOGICAL PROPERTIES OF SAM50-LIKE AND TOC75-LIKE OMP85 PROTEINS. CAS PubMed Google Scholar *
Bohnsack, M. T. & Schleiff, E. The evolution of protein targeting and translocation systems. _Biochim. Biophys. Acta_ 1803, 1115–1130 (2010). CAS PubMed Google Scholar * Eckart, K. et
al. A Toc75-like protein import channel is abundant in chloroplasts. _EMBO Rep._ 3, 557–562 (2002). CAS PubMed PubMed Central Google Scholar * Patel, R., Hsu, S. C., Bedard, J., Inoue,
K. & Jarvis, P. The Omp85-related chloroplast outer envelope protein OEP80 is essential for viability in _Arabidopsis_. _Plant. Physiol._ 148, 235–245 (2008). CAS PubMed PubMed Central
Google Scholar * Kim, S. et al. Structure and function of an essential component of the outer membrane protein assembly machine. _Science_ 317, 961–964 (2007). CAS PubMed Google Scholar
* Sánchez-Pulido, L., Devos, D., Genevrois, S., Vicente, M. & Valencia, A. POTRA: a conserved domain in the FtsQ family and a class of β-barrel outer membrane proteins. _Trends
Biochem. Sci._ 28, 523–526 (2003). PubMed Google Scholar * Koenig, P. et al. Conserved properties of polypeptide transport-associated (POTRA) domains derived from cyanobacterial Omp85. _J.
Biol. Chem._ 285, 18016–18024 (2010). CAS PubMed PubMed Central Google Scholar * Clantin, B. et al. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter
superfamily. _Science_ 317, 957–961 (2007). CAS PubMed Google Scholar * Ertel, F. et al. The evolutionarily related β-barrel polypeptide transporters from _Pisum sativum_ and _Nostoc_
PCC7120 contain two distinct functional domains. _J. Biol. Chem._ 280, 28281–28289 (2005). CAS PubMed Google Scholar * Dembowski, M., Künkele, K. P., Nargang, F. E., Neupert, W. &
Rapaport, D. Assembly of Tom6 and Tom7 into the TOM core complex of _Neurospora crassa_. _J. Biol. Chem._ 276, 17679–17685 (2001). CAS PubMed Google Scholar * Esaki, M. et al. Tom40
protein import channel binds to non-native proteins and prevents their aggregation. _Nature Struct. Mol. Biol._ 10, 988–994 (2003). CAS Google Scholar * Schmitt, S. et al. Role of Tom5 in
maintaining the structural stability of the TOM complex of mitochondria. _J. Biol. Chem._ 280, 14499–14506 (2005). CAS PubMed Google Scholar * Schleiff, E., Jelic, M. & Soll, J. A
GTP-driven motor moves proteins across the outer envelope of chloroplasts. _Proc. Natl Acad. Sci. USA_ 100, 4604–4609 (2003). CAS PubMed PubMed Central Google Scholar * Komiya, T. et al.
Interaction of mitochondrial targeting signals with acidic receptor domains along the protein import pathway: evidence for the 'acid chain' hypothesis. _EMBO J._ 17, 3886–3898
(1998). CAS PubMed PubMed Central Google Scholar * Bolliger, L., Junne, T., Schatz, G. & Lithgow, T. Acidic receptor domains on both sides of the outer membrane mediate translocation
of precursor proteins into yeast mitochondria. _EMBO J._ 14, 6318–6326 (1997). Google Scholar * Kouranov, A. & Schnell, D. J. Analysis of the interactions of preproteins with the
import machinery over the course of protein import into chloroplasts. _J. Cell Biol._ 29, 1677–1685 (1997). Google Scholar * Jelic, M., Soll, J. & Schleiff, E. Two Toc34 homologues with
different properties. _Biochemistry_ 42, 5906–5916 (2003). CAS PubMed Google Scholar * Smith, M. D. et al. atToc159 is a selective transit peptide receptor for the import of
nucleus-encoded chloroplast proteins. _J. Cell Biol._ 165, 323–334 (2004). THIS STUDY EXPLORES THE RECEPTOR FUNCTION OF TOC159. CAS PubMed PubMed Central Google Scholar * Lee, K. H.,
Kim, S. J., Lee, Y. J., Jin, J. B. & Hwang, I. The M domain of atToc159 plays an essential role in the import of proteins into chloroplasts and chloroplasts biogenesis. _J. Biol. Chem._
278, 36794–36805 (2003). CAS PubMed Google Scholar * Wang, F., Agne, B., Kessler, F. & Schnell, D. J. The role of GTP binding and hydrolysis at the atToc159 preprotein receptor during
protein import into chloroplasts. _J. Cell Biol._ 183, 87–99 (2008). CAS PubMed PubMed Central Google Scholar * Agne, B. et al. A Toc159 import receptor mutant, defective in hydrolysis
of GTP, supports preprotein import into chloroplasts. _J. Biol. Chem._ 284, 8670–8679 (2009). CAS PubMed PubMed Central Google Scholar * Becker, T. et al. Toc12, a novel subunit of the
intermembrane space preprotein translocon of chloroplasts. _Mol. Biol. Cell_ 15, 5130–5144 (2004). CAS PubMed PubMed Central Google Scholar * Matouschek, A. et al. Active unfolding of
precursor proteins during mitochondrial protein import. _EMBO J._ 16, 6727–6736 (1997). CAS PubMed PubMed Central Google Scholar * Gaume, B. et al. Unfolding of preproteins upon import
into mitochondria. _EMBO J._ 17, 6497–6507 (1998). CAS PubMed PubMed Central Google Scholar * Hageman, J. et al. Protein import into and sorting inside the chloroplast are independent
processes. _Plant Cell_ 2, 479–494 (1990). CAS PubMed PubMed Central Google Scholar * Bionda, T. et al. Chloroplast import signals: the length requirement for translocation _in vitro_
and _in vivo_. _J. Mol. Biol._ 402, 510–523 (2010). CAS PubMed Google Scholar * Sato, T., Esaki, M., Fernandez, J. M. & Endo, T. Comparison of the protein-unfolding pathways between
mitochondrial protein import and atomic-force microscopy measurements. _Proc. Natl Acad. Sci. USA_ 102, 17999–18004 (2005). THIS STUDY COMPARES THE MITOCHONDRIAL TRANSLOCATION ROUTES OF
N-TERMINAL- AND C-TERMINAL-FUSED SIGNALS WITH THE BIOPHYSICAL PROPERTIES OF THE PASSENGER. CAS PubMed PubMed Central Google Scholar * Oguro, T. et al. Structural stabilities of different
regions of the titin I27 domain contribute differently to unfolding upon mitochondrial protein import. _J. Mol. Biol._ 385, 811–819 (2009). CAS PubMed Google Scholar * Ruprecht, M. et
al. On the impact of precursor unfolding during protein import into chloroplasts. _Mol. Plant_ 3, 499–508 (2010). THIS STUDY COMPARES THE TRANSLOCATION ROUTE OF N-TERMINAL CHLOROPLAST
SIGNALS WITH THE BIOPHYSICAL PROPERTIES OF THE PASSENGER. CAS PubMed Google Scholar * Fulgosi, H. & Soll, J. The chloroplast protein import receptors Toc34 and Toc159 are
phosphorylated by distinct protein kinases. _J. Biol. Chem._ 277, 8934–8940 (2002). CAS PubMed Google Scholar * Agne, B. et al. The acidic A-domain of _Arabidopsis_ TOC159 occurs as a
hyperphosphorylated protein. _Plant Physiol._ 153, 1016–1030 (2010). CAS PubMed PubMed Central Google Scholar * Esaki, M. et al. Mitochondrial protein import. Requirement of presequence
elements and TOM components for precursor binding to the TOM complex. _J. Biol. Chem._ 279, 45701–45707 (2004). CAS PubMed Google Scholar * Gabriel, K., Egan, B. & Lithgow, T. Tom40,
the import channel of the mitochondrial outer membrane, plays an active role in sorting proteins. _EMBO J._ 22, 2380–2386 (2003). CAS PubMed PubMed Central Google Scholar * Moczko, M. et
al. The intermembrane space domain of mitochondrial Tom22 functions as a trans binding site for preproteins with N-terminal targeting sequences. _Mol. Cell. Biol._ 17, 6574–6584 (1997). CAS
PubMed PubMed Central Google Scholar * Chacinska, A. et al. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. _Cell_
120, 817–829 (2005). CAS PubMed Google Scholar * Chacinska, A. et al. Mitochondrial translocation contact sites: separation of dynamic and stabilizing elements of a TOM-TIM preprotein
supercomplex. _EMBO J._ 22, 5370–5381 (2003). CAS PubMed PubMed Central Google Scholar * Nielsen, E., Akita, M., Davila-Aponte, J. & Keegstra, K. Stable association of chloroplastic
precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. _EMBO J._ 16, 935–946 (1997). CAS PubMed PubMed
Central Google Scholar * Akita, M., Nielsen, E. & Keegsta, K. Identification of protein complexes in the chloroplastic envelope membranes via chemical cross-linking. _J. Cell Biol._
136, 983–994 (1997). REFERENCES 133, 134, 135 DESCRIBE THE INITIAL IDENTIFICATION OF THE TOM–TIM23 AND TOC–TIC SUPERCOMPLEXES. CAS PubMed PubMed Central Google Scholar * Yi, L. &
Dalbey, R. E. Oxa1/Alb3/YidC system for insertion of membrane proteins in mitochondria, chloroplasts and bacteria. _Mol. Membr. Biol._ 22, 101–111 (2005). CAS PubMed Google Scholar *
Herrmann, J. M. & Köhl, R. Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria. _J. Cell Biol._ 176, 559–563 (2007). CAS PubMed PubMed Central
Google Scholar * Soll, J. & Schleiff, E. Protein import into chloroplasts. _Nature Rev. Mol. Cell Biol._ 5, 198–208 (2004). CAS Google Scholar * Kovacheva, S., Bédard, J., Wardle, A.,
Patel, R. & Jarvis, P. Further _in vivo_ studies on the role of the molecular chaperone, Hsp93, in plastid protein import. _Plant J._ 50, 364–379 (2007). CAS PubMed Google Scholar *
Shi, L.-X. & Theg, S. M. A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss _Physcomitrella patens_. _Plant Cell_ 22, 205–220 (2010). CAS
PubMed PubMed Central Google Scholar * Su, P.-H. & Li, H. Stromal Hsp70 is important for protein translocation into pea and _Arabidopsis_ chloroplasts. _Plant Cell_ 22, 1516–1531
(2010). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS T.B. thanks N. Pfanner for support. The work was supported by Baden-Württemberg Stiftung (T.B.), the
Deutsche Forschungsgemeinschaft (DFG) in the frame of the Sonderforschungsbereich SFB746 (T.B.), the Volkswagen-foundation (E.S.) and the DFG in the frame of the Sonderforschungsbereich
SFB807/P17 (E.S.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biosciences, Goethe University, Cluster of Excellence 'Macromolecular Complexes', Centre of Membrane
Proteomics, Molecular Cell Biology of Plants, Max-von-Laue Str. 9, Frankfurt, D-60438, Germany Enrico Schleiff * Institut für Biochemie und Molekularbiologie, Zentrum für Biochemie und
Molekulare Zellforschung, Universität Freiburg, Freiburg, 79104, Germany Thomas Becker Authors * Enrico Schleiff View author publications You can also search for this author inPubMed Google
Scholar * Thomas Becker View author publications You can also search for this author inPubMed Google Scholar ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
financial interests. RELATED LINKS RELATED LINKS FURTHER INFORMATION Thomas Becker's homepage Enrico Schleiff's homepage GLOSSARY * Endosymbiosis Endosymbiosis is the process in
which a free-living bacteria — the ancestral endosymbiont — was enclosed by a cell and, during evolution, became integrated into the cellular network. By transfer of most of its genetic
content to the host, the nucleus lost its independence and became an organelle. * Thylakoid membrane A component of chloroplasts, the thylakoid membrane is a specialized membranous
compartment where photosynthesis occurs. * Oxygenic photosynthesis Oxygenic photosynthesis is the conversion of carbon dioxide and water into organic compounds, especially sugars, and oxygen
by the thylakoid and stromal enzymes, including the photosystems. * Amphiphilic α-helix An amphiphilic α-helix is a helix in which one side is composed of hydrophobic amino acids and the
other of hydrophilic amino acids. * β-barrel proteins β-barrel proteins are membrane proteins that are typically found in the outer membrane of mitochondria, of chloroplasts and of
Gram-negative bacteria. These proteins form a membrane-inserted barrel composed of β-strands. * 14-3-3 proteins Proteins that are expressed in eukaryotic cells and that bind preferentially
to phosphorylated regions in diverse proteins involved in signal transduction and protein translocation. * Tetratricopeptide repeat (TPR). A structural motif, found in a wide variety of
proteins, that is composed of 34 amino acids. TPRs are involved in intra- and inter-molecular interactions. * Ankyrin Ankyrin repeats are structurally but not functionally conserved units of
33 amino acids that consist of two α-helices separated by a loop, and comprise one of the most common structural motifs identified in bacterial, archaeal and eukaryotic proteins. * ERMES
complex (Endoplasmic reticulummitochondria encounter structure complex). This is the complex that tethers mitochondria and the endoplasmic reticulum. It is composed of the two mitochondrial
membrane proteins Mdm34 and Mdm10, the integral endoplasmic reticulum membrane protein Mmm1 and the peripheral protein Mdm12. * Chemical crosslinking Chemical crosslinking is the
introduction of synthetic bonds that link two proteins in close proximity by chemical molecules — for example, by maleimide, which reacts with the thiol group of cysteines. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schleiff, E., Becker, T. Common ground for protein translocation: access control for mitochondria and chloroplasts.
_Nat Rev Mol Cell Biol_ 12, 48–59 (2011). https://doi.org/10.1038/nrm3027 Download citation * Published: 08 December 2010 * Issue Date: January 2011 * DOI: https://doi.org/10.1038/nrm3027
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
