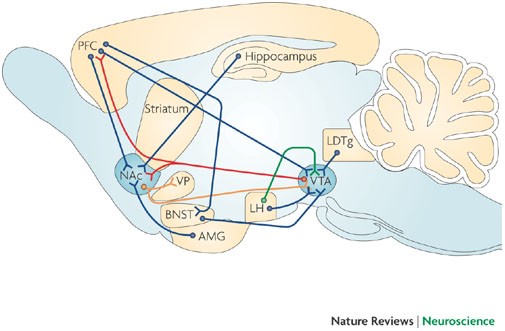
Synaptic plasticity and addiction
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

KEY POINTS * A major problem in the treatment of addiction is relapse, which is often caused by the powerful and long-lasting memories of the drug experience. * Drugs of abuse can hijack or
impair specific synaptic plasticity mechanisms in the mesolimbic dopamine system, which is central to reward processing in the brain. * Drugs of abuse or acute stress elicit long-term
potentiation (LTP) at excitatory synapses on dopamine cells in the ventral tegmental area (VTA). Morphine prevents a novel form of LTP at inhibitory synapses on the same dopamine cells. Both
changes are likely to increase dopamine cell firing. * Orexin, a neuropeptide implicated in arousal and feeding, enhances N-methyl-D-aspartate (NMDA) receptor-mediated synaptic responses in
dopamine cells leading to LTP of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor-mediated responses. The actions of orexin in the VTA might be important for several of
the behavioural adaptations caused by cocaine and, perhaps, other drugs of abuse. * At excitatory synapses on medium spiny neurons in the nucleus accumbens, cocaine causes a form of
long-term depression (LTD) that is due to the removal of synaptic AMPA receptors. It also impairs endocannabinoid-mediated LTD. In contrast, during withdrawal from chronic cocaine
administration, there appears to be an increase in excitatory synaptic transmission. Further work is necessary to determine whether other drugs of abuse have the same effects. * Other key
brain areas in which drugs of abuse affect synaptic function and plasticity include the bed nucleus of the stria terminalis and the amygdala. * There may be important differences in the
effects of drugs of abuse on synaptic function and plasticity depending on whether the drug is self-administered or not. It will be important in future work to use animal models that more
closely mimic the behaviour of human substance abusers. ABSTRACT Addiction is caused, in part, by powerful and long-lasting memories of the drug experience. Relapse caused by exposure to
cues associated with the drug experience is a major clinical problem that contributes to the persistence of addiction. Here we present the accumulated evidence that drugs of abuse can hijack
synaptic plasticity mechanisms in key brain circuits, most importantly in the mesolimbic dopamine system, which is central to reward processing in the brain. Reversing or preventing these
drug-induced synaptic modifications may prove beneficial in the treatment of one of society's most intractable health problems. Access through your institution Buy or subscribe This is
a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $189.00 per
year only $15.75 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DRUG
ADDICTION: FROM BENCH TO BEDSIDE Article Open access 12 August 2021 PLASTICITY OF SYNAPSES AND REWARD CIRCUIT FUNCTION IN THE GENESIS AND TREATMENT OF DEPRESSION Article 03 September 2022
RAPID HOMEOSTATIC PLASTICITY AND NEUROPSYCHIATRIC THERAPEUTICS Article Open access 22 August 2022 REFERENCES * Ramón y Cajal, S. La fine structure des centres nerveux. _Proc. R. Soc. Lond._
55, 444–468 (1894). Article Google Scholar * Bliss, T. V. P. & Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following
stimulation of the perforant path. _J. Physiol._ 232, 331–356 (1973). THE INITIAL, NOW CLASSIC, DESCRIPTION OF LTP IN THE HIPPOCAMPUS. Article CAS PubMed PubMed Central Google Scholar *
Malenka, R. C. & Bear, M. F. LTP and LTD: an embarrassment of riches. _Neuron_ 44, 5–21 (2004). Article CAS PubMed Google Scholar * Foeller, E. & Feldman, D. E. Synaptic basis
for developmental plasticity in somatosensory cortex. _Curr. Opin. Neurobiol._ 14, 89–95 (2004). Article CAS PubMed Google Scholar * Hyman, S. E. & Malenka, R. C. Addiction and the
brain: the neurobiology of compulsion and its persistence. _Nature Rev. Neurosci._ 2, 695–703 (2001). Article CAS Google Scholar * Kalivas, P. W. & Volkow, N. D. The neural basis of
addiction: a pathology of motivation and choice. _Am. J. Psychiatry_ 162, 1403–1413 (2005). Article PubMed Google Scholar * Montague, P. R., Hyman, S. E. & Cohen, J. D. Computational
roles for dopamine in behavioural control. _Nature_ 431, 760–767 (2004). Article CAS PubMed Google Scholar * Hyman, S. E., Malenka, R. C. & Nestler, E. J. Neural mechanisms of
addiction: the role of reward–related learning and memory. _Annu. Rev. Neurosci._ 29, 565–598 (2006). Article CAS PubMed Google Scholar * Kauer, J. A. Learning mechanisms in addiction:
synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. _Annu. Rev. Physiol._ 66, 447–475 (2004). Article CAS PubMed Google Scholar * Kelley, A. E.
Memory and addiction: shared neural circuitry and molecular mechanisms. _Neuron_ 44, 161–179 (2004). Article CAS PubMed Google Scholar * Badiani, A. & Robinson, T. E. Drug-induced
neurobehavioral plasticity: the role of environmental context. _Behav. Pharmacol._ 15, 327–339 (2004). Article CAS PubMed Google Scholar * Kitamura, O., Wee, S., Specio, S. E., Koob, G.
F. & Pulvirenti, L. Escalation of methamphetamine self-administration in rats: a dose-effect function. _Psychopharmacology (Berl)_ 186, 48–53 (2006). Article CAS Google Scholar *
Morris, R. G. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. _Eur. J. Neurosci._ 23, 2829–2846 (2006). Article
CAS PubMed Google Scholar * Schenk, S., Valadez, A., Worley, C. M. & McNamara, C. Blockade of the acquisition of cocaine self-administration by the NMDA antagonist MK-801
(dizocilpine). _Behav. Pharmacol._ 4, 652–659 (1993). CAS PubMed Google Scholar * Kalivas, P. W. & Alesdatter, J. E. Involvement of NMDA receptor stimulation in the ventral tegmental
area and amygdala in behavioral sensitization to cocaine. _J. Pharmacol. Exp. Ther._ 267, 486–495 (1993). CAS PubMed Google Scholar * Harris, G. C., Wimmer, M., Byrne, R. &
Aston-Jones, G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. _Neuroscience_ 129, 841–847 (2004). Article
CAS PubMed Google Scholar * Harris, G. C. & Aston-Jones, G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment.
_Neuropsychopharmacology_ 28, 73–76 (2003). Article CAS PubMed Google Scholar * Karler, R., Calder, L. D., Chaudhry, I. A. & Turkanis, S. A. Blockade of “reverse tolerance” to
cocaine and amphetamine by MK-801. _Life Sciences_ 45, 599–606 (1989). Article CAS PubMed Google Scholar * Jeziorski, M., White, F. J. & Wolf, M. E. MK-801 prevents the development
of behavioral sensitization during repeated morphine administration. _Synapse_ 16, 137–147 (1994). Article CAS PubMed Google Scholar * Kim, H. S., Park, W. K., Jang, C. G. & Oh, S.
Inhibition by MK-801 of cocaine-induced sensitization, conditioned place preference, and dopamine-receptor supersensitivity in mice. _Brain. Res. Bull._ 40, 201–207 (1996). Article CAS
PubMed Google Scholar * Tzschentke, T. M. & Schmidt, W. J. N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. _Neurosci. Lett._
193, 37–40 (1995). Article CAS PubMed Google Scholar * Kalivas, P. W. & Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization
of motor activity. _Brain Res. Rev._ 16, 223–244 (1991). Article CAS PubMed Google Scholar * Robinson, T. E. & Berridge, K. C. The neural basis of drug craving: an
incentive-sensitization theory of addiction. _Brain Res. Rev._ 18, 247–291 (1993). Article CAS PubMed Google Scholar * Tong, Z. Y., Overton, P. G. & Clark, D. Chronic administration
of (+)-amphetamine alters the reactivity of midbrain dopaminergic neurons to prefrontal cortex stimulation in the rat. _Brain Res._ 674, 63–74 (1995). Article CAS PubMed Google Scholar *
Wolf, M. E. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. _Prog. Neurobiology_ 54, 1–42 (1998). Article Google Scholar * Everitt, B. J. &
Wolf, M. E. Psychomotor stimulant addiction: a neural systems perspective. _J. Neurosci._ 22, 3312–3320 (2002). Article CAS PubMed PubMed Central Google Scholar * Kalivas, P. W.
Glutamate systems in cocaine addiction. _Curr. Opin. Pharmacol._ 4, 23–29 (2004). Article CAS PubMed Google Scholar * Baler, R. D. & Volkow, N. D. Drug addiction: the neurobiology of
disrupted self-control. _Trends Mol. Med._ 12, 559–566 (2006). Article CAS PubMed Google Scholar * Malenka, R. C. & Nicoll, R. A. Long-term potentiation — a decade of progress?
_Science_ 285, 1870–1874 (1999). Article CAS PubMed Google Scholar * Lynch, M. A. Long-term potentiation and memory. _Physiol. Rev._ 84, 87–136 (2004). Article CAS PubMed Google
Scholar * Yuste, R. & Bonhoeffer, T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. _Annu. Rev. Neurosci._ 24, 1071–1089 (2001). Article CAS
PubMed Google Scholar * Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. _Nature_ 429, 761–766 (2004).
Article CAS PubMed PubMed Central Google Scholar * Lau, C. G. & Zukin, R. S. (2007). NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. _Nature Rev.
Neurosci._ 8, 413–426. Article CAS Google Scholar * Nicoll, R. A. & Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. _Nature Rev. Neurosci._ 6, 863–876 (2005).
Article CAS Google Scholar * Yeckel, M. F., Kapur, A. & Johnston, D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. _Nature Neurosci._ 2,
625–633 (1999). Article CAS PubMed Google Scholar * Contractor, A., Rogers, C., Maron, C., Henkemeyer, M., Swanson, G. T. & Heinemann S. F. Trans-synaptic Eph receptor-ephrin
signaling in hippocampal mossy fiber LTP. _Science_ 296, 1864–1869 (2002). Article CAS PubMed Google Scholar * Castillo, P. E., Schoch, S., Schmitz, F., Sudhof, T. C. & Malenka, R.
C. RIM1α is required for presynaptic long-term potentiation. _Nature_ 415, 327–330 (2002). Article CAS PubMed Google Scholar * Castillo, P. E. et al. Rab3A is essential for mossy fibre
long-term potentiation in the hippocampus. _Nature_ 388, 590–593 (1997). Article CAS PubMed Google Scholar * Carroll, R. C., Beattie, E. C., von Zastrow, M. & Malenka, R. C. Role of
AMPA receptor endocytosis in synaptic plasticity. _Nature Rev. Neurosci._ 2, 315–324 (2001). Article CAS Google Scholar * Selig, D. K., Hjelmstad, G. O., Herron, C., Nicoll, R. A. &
Malenka, R. C. Independent mechanisms for long-term depression of AMPA and NMDA responses. _Neuron_ 15, 417–426 (1995). Article CAS PubMed Google Scholar * Morishita, W., Marie, H. &
Malenka, R. C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. _Nature Neurosci._ 8, 1043–1050 (2005). Article CAS PubMed Google Scholar
* Ito, M. Long-term depression. _Annu. Rev. Neurosci._ 12, 85–102 (1989). Article CAS PubMed Google Scholar * Pfeiffer, B. E. & Huber, K. M. Current advances in local protein
synthesis and synaptic plasticity. _J. Neurosci._ 26, 7147–7150 (2006). Article CAS PubMed PubMed Central Google Scholar * Wilson, R. I. & Nicoll, R. A. Endocannabinoid signaling in
the brain. _Science_ 296, 678–682 (2002). Article CAS PubMed Google Scholar * Chevaleyre, V., Takahashi, K. A. & Castillo, P. E. Endocannabinoid-mediated synaptic plasticity in the
CNS. _Annu. Rev. Neurosci._ 29, 37–76 (2006). Article CAS PubMed Google Scholar * Chevaleyre, V. et al. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and
RIM1α. _Neuron_ 54, 801–812 (2007). Article CAS PubMed PubMed Central Google Scholar * Turrigiano, G. G. & Nelson, S. B. Homeostatic plasticity in the developing nervous system.
_Nature Rev. Neurosci._ 5, 97–107 (2004). Article CAS Google Scholar * Wierenga, C. J., Ibata, K. & Turrigiano, G. G. Postsynaptic expression of homeostatic plasticity at neocortical
synapses. _J. Neurosci._ 25, 2895–2905 (2005). Article CAS PubMed PubMed Central Google Scholar * Stellwagen, D. & Malenka, R. C. Synaptic scaling mediated by glial TNF-α. _Nature_
440, 1054–1059 (2006). Article CAS PubMed Google Scholar * Di Chiara, G. & Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the
mesolimbic system of freely moving rats. _Proc. Natl Acad. Sci. USA_ 85, 5274–5278 (1980). Article Google Scholar * Omelchenko, N. & Sesack, S. R. Glutamate synaptic inputs to ventral
tegmental area neurons in the rat derive primarily from subcortical sources. _Neuroscience_ 146, 1259–1274 (2007). Article CAS PubMed Google Scholar * Carr, D. B. & Sesack, S. R.
Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. _J. Neurosci._ 20,
3864–3873 (2000). Article CAS PubMed PubMed Central Google Scholar * Overton, P. G., Richards, C. D., Berry, M. S. & Clark, D. Long-term potentiation at excitatory amino acid
synapses on midbrain dopamine neurons. _Neuroreport_ 10, 221–226 (1999). Article CAS PubMed Google Scholar * Bonci, A. & Malenka, R. C. Properties and plasticity of excitatory
synapses on dopaminergic and GABAergic cells in the ventral tegmental area. _J. Neurosci._ 19, 3723–3730 (1999). Article CAS PubMed PubMed Central Google Scholar * Mansvelder, H. D.
& McGehee, D. S. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. _Neuron_ 27, 349–357 (2000). Article CAS PubMed Google Scholar * Liu, Q. S., Pu, L.
& Poo, M. M. Repeated cocaine exposure _in vivo_ facilitates LTP induction in midbrain dopamine neurons. _Nature_ 437, 1027–1031 (2005). Article CAS PubMed PubMed Central Google
Scholar * Jones, S., Kornblum, J. L. & Kauer, J. A. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. _J. Neurosci._ 20, 5575–5580 (2000). Article CAS
PubMed PubMed Central Google Scholar * Thomas, M. T., Malenka, R. C. & Bonci, A. Modulation of long-term depression by dopamine in the mesolimbic system. _J. Neurosci._ 20, 5581–5586
(2000). Article CAS PubMed PubMed Central Google Scholar * Bellone, C. & Luscher, C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the
subunit composition of AMPA receptors. _Eur. J. Neurosci._ 21, 1280–1288 (2005). Article PubMed Google Scholar * Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. Single
cocaine exposure _in vivo_ induces long-term potentiation in dopamine neurons. _Nature_ 411, 583–587 (2001). Article CAS PubMed Google Scholar * Saal, D., Dong, Y., Bonci, A. &
Malenka, R. C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. _Neuron_ 37, 577–582 (2003). Article CAS PubMed Google Scholar * Faleiro, L. J., Jones,
S. & Kauer, J. A. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection.
_Neuropsychopharmacology_ 29, 2115–2125 (2004). REFERENCES 60–62 DEMONSTRATE THAT _IN VIVO_ ADMINISTRATION OF DIFFERENT CLASSES OF DRUGS OF ABUSE, AS WELL AS ACUTE STRESS, ELICIT LTP AT
EXCITATORY SYNAPSES ON MIDBRAIN DOPAMINE NEURONS. Article CAS PubMed Google Scholar * Marinelli, M. & Piazza, P. V. Interaction between glucocorticoid hormones, stress and
psychostimulant drugs. _Eur. J. Neurosci._ 16, 387–394 (2002). Article PubMed Google Scholar * Wallace, B. C. Psychological and environmental determinants of relapse in crack cocaine
smokers. _J. Subst. Abuse Treat._ 6, 95–106 (1989). Article CAS PubMed Google Scholar * Stewart, J. Stress and relapse to drug seeking: studies in laboratory animals shed light on
mechanisms and sources of long-term vulnerability. _Am. J. Addict._ 12, 1–17 (2003). Article CAS PubMed Google Scholar * Piazza, P. V. & Le Moal, M. The role of stress in drug
self-administration. _Trends Pharmacol. Sci._ 19, 67–74 (1998). Article CAS PubMed Google Scholar * Dong, Y. et al. Cocaine-induced potentiation of synaptic strength in dopamine neurons:
behavioral correlates in GluRA(−/−) mice. _Proc. Natl Acad. Sci. USA_ 101, 14282–14287 (2004). Article CAS PubMed PubMed Central Google Scholar * Malinow, R. & Malenka, R. C. AMPA
receptor trafficking and synaptic plasticity. _Annu. Rev. Neurosci._ 25, 103–126 (2002). Article CAS PubMed Google Scholar * Carlezon Jr, W. A. et al. Sensitization to morphine induced
by viral-mediated gene transfer. _Science_ 277, 812–814 (1997). DEMONSTRATION THAT VIRAL-MEDIATED EXPRESSION OF GLUR1 IN THE VENTRAL TEGMENTAL AREA ENHANCES THE LOCOMOTOR STIMULATORY AND
REWARDING ACTIONS OF MORPHINE. Article CAS Google Scholar * Borgland, S. L., Malenka, R. C. & Bonci, A. Acute and chronic cocaine-induced potentiation of synaptic strength in the
ventral tegmental area: electrophysiological and behavioral correlates in individual rats. _J. Neurosci._ 24, 7482–7490 (2004). Article CAS PubMed PubMed Central Google Scholar *
Neisewander, J. L. et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. _J. Neurosci._ 20, 798–805 (2000). Article
CAS PubMed PubMed Central Google Scholar * Pu, L., Liu, Q. S. & Poo, M. M. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. _Nature
Neurosci._ 9, 605–607 (2006). Article CAS PubMed Google Scholar * Lu, L., Dempsey, J., Liu, S. Y., Bossert, J. M. & Shaham, Y. A single infusion of brain-derived neurotrophic factor
into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. _J. Neurosci._ 24, 1604–1611 (2004). Article CAS PubMed PubMed Central Google
Scholar * Liu, S. J. & Zukin, R. S. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. _Trends Neurosci._ 30, 126–134 (2007). Article CAS PubMed Google Scholar
* Carlezon Jr, W. A. & Nestler, E. J. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? _Trends Neurosci._ 25, 610–615 (2002). Article Google
Scholar * Ju, W. et al. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. _Nature Neurosci._ 7, 244–253 (2004). Article CAS PubMed Google Scholar *
Clem, R. L. & Barth, A. Pathway-specific trafficking of native AMPARs by _in vivo_ experience. _Neuron_ 49, 663–670 (2006). Article CAS PubMed Google Scholar * Plant, K. et al.
Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. _Nature Neurosci._ 9, 602–604 (2006). Article CAS PubMed Google Scholar *
Adesnik, H. & Nicoll, R. A. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. _J. Neurosci._ 27, 4598–4602 (2007). Article CAS PubMed
PubMed Central Google Scholar * Bagal, A. A., Kao, J. P., Tang, C. M. & Thompson, S. M. Long-term potentiation of exogenous glutamate responses at single dendritic spines. _Proc. Natl
Acad. Sci. USA_ 102, 14434–14439 (2005). Article CAS PubMed PubMed Central Google Scholar * Bellone, C. & Luscher, C. Cocaine triggered AMPA receptor redistribution is reversed _in
vivo_ by mGluR-dependent long-term depression. _Nature Neurosci._ 9, 636–641 (2006). Article CAS PubMed Google Scholar * Mameli, M., Balland, B., Lujan, R. & Luscher, C. Rapid
synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. _Science_ 317, 530–533 (2007). REFERENCES 81 AND 82 PRESENT EVIDENCE THAT A NOVEL FORM OF MGLUR-LTD
REVERSES THE COCAINE-INDUCED LTP AT EXCITATORY SYNAPSES ON VENTRAL TEGMENTAL AREA DOPAMINE CELLS. Article CAS PubMed Google Scholar * de Lecea, L. et al. The hypocretins:
hypothalamus-specific peptides with neuroexcitatory activity. _Proc. Natl Acad. Sci. USA_ 95, 322–327 (1998). Article CAS PubMed PubMed Central Google Scholar * Sakurai, T. et al.
Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. _Cell_ 92, 573–585 (1998). Article CAS PubMed Google
Scholar * Harris, G. C. & Aston-Jones, G. Arousal and reward: a dichotomy in orexin function. _Trends Neurosci._ 29, 571–577 (2006). Article CAS PubMed Google Scholar * Fadel, J.
& Deutch, A. Y. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. _Neuroscience_ 111, 379–387 (2002). Article CAS
PubMed Google Scholar * Baldo, B. A., Daniel, R. A., Berridge, C. W. & Kelley, A. E. Overlapping distributions of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in
rat brain regions mediating arousal, motivation, and stress. _J. Comp. Neurol._ 464, 220–237 (2003). Article PubMed Google Scholar * Boutrel, B. Hypocretins: between desire and needs.
toward the understanding of a new hypothalamic brain pathway involved in motivation and addiction. _Med. Sci. (Paris)_ 22, 573–575 (2006). Article Google Scholar * Harris, G. C., Wimmer,
M. & Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. _Nature_ 437, 556–559 (2005). DEMONSTRATION THAT OREXIN NEURONS IN THE LATERAL HYPOTHALAMUS PLAY A
KEY ROLE IN THE REINSTATEMENT OF DRUG-SEEKING BEHAVIOUR AT LEAST IN PART DUE TO ACTIONS OF OREXIN A IN THE VENTRAL TEGMENTAL AREA. Article CAS PubMed Google Scholar * Narita, M. et al.
Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. _J. Neurosci._ 26, 398–405 (2006). Article CAS
PubMed PubMed Central Google Scholar * Borgland, S. L., Taha, S. A., Sarti, F., Fields, H. L. & Bonci, A. Orexin A in the VTA is critical for the induction of synaptic plasticity and
behavioral sensitization to cocaine. _Neuron_ 49, 589–601 (2006). DEMONSTRATION THAT OREXIN A ENHANCES NMDAR-MEDIATED SYNAPTIC CURRENTS IN VENTRAL TEGMENTAL AREA (VTA) DOPAMINE NEURONS AND
THAT ITS ACTIONS IN THE VTA ARE REQUIRED FOR BEHAVIOURAL SENSITIZATION TO COCAINE. Article CAS PubMed Google Scholar * Schilstrom, B. et al. Cocaine enhances NMDA receptor-mediated
currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. _J. Neurosci._ 26, 8549–8558 (2006). Article CAS PubMed PubMed Central
Google Scholar * Yim, C. Y. & Mogenson, G. J. Electrophysiological studies of neurons in the ventral tegmental area of Tsai. _Brain Res._ 181, 301–313 (1980). Article CAS PubMed
Google Scholar * Johnson, S. W. & North, R. A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. _J. Neurosci._ 12, 483–488 (1992). Article CAS PubMed
PubMed Central Google Scholar * Mansvelder, H. D., Keath, J. R. & McGehee, D. S. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. _Neuron_ 33, 905–919
(2002). Article CAS PubMed Google Scholar * Nugent, F. S., Penick, E. C. & Kauer, J. A. Opioids block long-term potentiation of inhibitory synapses. _Nature_ 446, 1086–1090 (2007).
DEMONSTRATION OF LTP OF INHIBITORY SYNAPSES ON VENTRAL TEGMENTAL AREA DOPAMINE NEURONS DUE TO A LONG-LASTING ENHANCEMENT OF GABA RELEASE TRIGGERED BY NMDAR-DEPENDENT RELEASE OF NITRIC OXIDE
FROM THE DOPAMINE NEURONS. EXPOSURE TO MORPHINE _IN VIVO_ BLOCKS THIS LTP BY INTERRUPTING THE SIGNALLING FROM NITRIC OXIDE TO GUANYLATE CYCLASE. Article CAS PubMed Google Scholar *
Martin, M., Chen, B. T., Hopf, F. W., Bowers, M. S. & Bonci, A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. _Nature Neurosci._ 9, 868–869
(2006). Article CAS PubMed Google Scholar * Cardinal, R. N. & Everitt, B. J. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. _Curr. Opin.
Neurobiol._ 14, 156–162 (2004). Article CAS PubMed Google Scholar * Kalivas, P. W., Volkow, N. & Seamans, J. Unmanageable motivation in addiction: a pathology in
prefrontal-accumbens glutamate transmission. _Neuron_ 45, 647–650 (2005). Article CAS PubMed Google Scholar * Pierce, R. C. & Kumaresan, V. The mesolimbic dopamine system: the final
common pathway for the reinforcing effect of drugs of abuse? _Neurosci. Biobehav. Rev._ 30, 215–238 (2006). Article CAS PubMed Google Scholar * Kombian, S. B. & Malenka, R. C.
Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. _Nature_ 368, 242–246 (1994). Article CAS PubMed Google Scholar * Schramm, N. L.,
Egli, R. E. & Winder, D. G. LTP in the mouse nucleus accumbens is developmentally regulated. _Synapse_ 45, 213–219 (2002). Article CAS PubMed Google Scholar * Yao, W. D. et al.
Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. _Neuron_ 41, 625–638 (2004). Article CAS PubMed Google Scholar * Robbe, D., Kopf, M.,
Remaury, A., Bockaert, J. & Manzoni, O. J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. _Proc. Natl Acad. Sci. USA_ 99, 8384–8388 (2002).
Article CAS PubMed PubMed Central Google Scholar * Hoffman, A. F., Oz, M., Caulder, T. & Lupica, C. R. Functional tolerance and blockade of long-term depression at synapses in the
nucleus accumbens after chronic cannabinoid exposure. _J. Neurosci._ 23, 4815–4820 (2003). Article CAS PubMed PubMed Central Google Scholar * Brebner, K. et al. Nucleus accumbens
long-term depression and the expression of behavioral sensitization. _Science_ 310, 1340–1343 (2005). Article CAS PubMed Google Scholar * Thomas, M. J., Beurrier, C., Bonci, A. &
Malenka, R. C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. _Nature Neurosci._ 4, 1217–1223 (2001). REFERENCES 106 AND 107
DEMONSTRATE THAT IN ANIMALS, WHICH HAD PREVIOUSLY BEEN EXPOSED TO COCAINE TO ELICIT SENSITIZATION, A SINGLE SUBSEQUENT DOSE OF COCAINE ELICITS LTD AT EXCITATORY SYNAPSES IN THE NUCLEUS
ACCUMBENS AND THAT PREVENTING THIS LTD _IN VIVO_ PREVENTS THE EXPRESSION OF BEHAVIORAL SENSITIZATION. Article CAS PubMed Google Scholar * Schramm-Sapyta, N. L., Olsen, C. M. &
Winder, D. G. Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. _Neuropsychopharmacology_ 31, 1444–1451 (2006). Article CAS PubMed Google
Scholar * Kourrich, S., Rothwell, P., Klug, J. & Thomas, M. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. _J. Neurosci._ 27, 7921–7928 (2007).
Article CAS PubMed PubMed Central Google Scholar * Robinson, T. E. & Kolb, B. Structural plasticity associated with exposure to drugs of abuse. _Neuropharmacology_ 47, S33–S46
(2004). Article CAS Google Scholar * Boudreau, A. C. & Wolf, M. E. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus
accumbens. _J. Neurosci._ 25, 9144–9151 (2005). Article CAS PubMed PubMed Central Google Scholar * Pierce, R. C., Bell, K., Duffy, P. & Kalivas, P. W. Repeated cocaine augments
excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. _J. Neurosci._ 16, 1550–1560 (1996). Article CAS PubMed PubMed Central
Google Scholar * Cornish, J. L. & Kalivas, P. W. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. _J. Neurosci._ 20, RC89 (2000). Article CAS
PubMed PubMed Central Google Scholar * Fourgeaud, L. et al. A single _in vivo_ exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. _J.
Neurosci._ 24, 6939–6945 (2004). Article CAS PubMed PubMed Central Google Scholar * Mato, S. et al. A single _in vivo_ exposure to δ9THC blocks endocannabinoid-mediated synaptic
plasticity. _Nature Neurosci._ 7, 585–596 (2004). REFERENCES 114 AND 115 DEMONSTRATE THAT A SINGLE _IN VIVO_ DOSE OF COCAINE OR THC ABOLISHES ENDOCANNABINOID-MEDIATED LTD IN THE NUCLEUS
ACCUMBENS. Article CAS PubMed Google Scholar * Mato, S., Robbe, D., Puente, N., Grandes, P. & Manzoni, O. J. Presynaptic homeostatic plasticity rescues long-term depression after
chronic δ9-tetrahydrocannabinol exposure. _J. Neurosci._ 25, 11619–11627 (2005). Article CAS PubMed PubMed Central Google Scholar * Goto, Y. & Grace, A. A. Dopamine-dependent
interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. _Neuron_ 47, 255–266 (2005). Article CAS PubMed Google
Scholar * Baker, D. A. et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. _Nature Neurosci._ 6, 743–749 (2003). Article CAS PubMed Google Scholar *
Szumlinski, K. K., Kalivas, P. W. & Worley, P. F. Homer proteins: implications for neuropsychiatric disorders. _Curr. Opin. Neurobiol._ 16, 251–257 (2006). Article CAS PubMed Google
Scholar * Sutton, M. A. et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. _Nature_ 421, 70–75 (2003). Article CAS PubMed Google Scholar * Kelz,
M. B. et al. Expression of the transcription factor δFosB in the brain controls sensitivity to cocaine. _Nature_ 401, 272–276 (1999). Article CAS PubMed Google Scholar * Todtenkopf, M.
S. et al. Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. _J. Neurosci._ 26, 11665–11669 (2006). REFERENCES 120–122 DEMONSTRATE THAT EXPRESSION OF AMPA RECEPTOR
SUBUNITS IN THE NUCLEUS ACCUMBENS INFLUENCE COCAINE-INDUCED BEHAVIOURS AS WELL AS THE REWARDING IMPACT OF ELECTRICAL STIMULATION IN THE MEDIAL FOREBRAIN BUNDLE. Article CAS PubMed PubMed
Central Google Scholar * Zhang, X. F., Hu, X. T. & White, F. J. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. _J. Neurosci._ 18,
488–498 (1998). Article PubMed PubMed Central Google Scholar * Hu, X. T., Basu, S. & White, F. J. Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity
of K+ channels in rat nucleus accumbens neurons. _J. Neurophysiol._ 92, 15971–15977 (2004). Article Google Scholar * Dong, Y. et al. CREB modulates excitability of nucleus accumbens
neurons. _Nature Neurosci._ 9, 475–477 (2006). Article CAS PubMed Google Scholar * Tsankova, N., Renthal, W., Kumar, A. & Nestler, E. J. Epigenetic regulation in psychiatric
disorders. _Nature Rev. Neurosci._ 8, 355–367 (2007). Article CAS Google Scholar * Delfs, J. M., Zhu, Y., Druhan, J. P. & Aston-Jones, G. Noradrenaline in the ventral forebrain is
critical for opiate withdrawal-induced aversion. _Nature_ 403, 430–434 (2000). Article CAS PubMed Google Scholar * Walker, J. R., Ahmed, S. H., Gracy, K. N. & Koob, G. F.
Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. _Brain Res._ 854, 85–92 (2000). Article
CAS PubMed Google Scholar * Weitlauf, C., Egli, R. E., Grueter, B. A. & Winder, D. G. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic
synapses in the dorsolateral bed nucleus of the stria terminalis. _J. Neurosci._ 24, 5741–5747 (2004). Article CAS PubMed PubMed Central Google Scholar * Dumont, E. C., Mark, G. P.,
Mader, S. & Williams, J. T. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. _Nature Neurosci._ 8, 413–414 (2005). DEMONSTRATION
THAT SELF-ADMINISTRATION OF COCAINE POTENTIATES EXCITATORY SYNAPTIC TRANSMISSION IN THE BED NUCLEUS OF THE STRIA TERMINALIS, BUT THAT PASSIVE ADMINISTRATION OF COCAINE OR FOOD DOES NOT.
Article CAS PubMed Google Scholar * Grueter, B. A. et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed
nucleus of the stria terminalis is disrupted by cocaine administration. _J. Neurosci._ 26, 3210–3219 (2006). Article CAS PubMed PubMed Central Google Scholar * Sigurdsson, T., Doyere,
V., Cain, C. K. & LeDoux, J. E. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. _Neuropharmacology_ 52, 215–227 (2007). Article CAS PubMed
Google Scholar * Childress, A. R. et al. Limbic activation during cue-induced cocaine craving. _Am. J. Psychiatry_ 156, 11–18 (1999). Article CAS PubMed PubMed Central Google Scholar *
Fu, Y. et al. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. _J. Neurophysiol._ 97,
937–941 (2007). Article CAS PubMed Google Scholar * Richter, R. M. & Weiss, F. _In vivo_ CRF release in rat amygdala is increased during cocaine withdrawal in self-administering
rats. _Synapse_ 32, 254–261 (1999). Article CAS PubMed Google Scholar * Pollandt, S. et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor
at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. _Eur. J. Neurosci._ 24, 1733–1743 (2006). Article PubMed Google Scholar * Gong, S. et al. A gene expression
atlas of the central nervous system based on bacterial artificial chromosomes. _Nature_ 425, 917–925 (2003). Article CAS PubMed Google Scholar * Kreitzer, A. C. & Malenka, R. C.
Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. _Nature_ 445, 643–647 (2007). Article CAS PubMed Google Scholar * Day, M. et al.
Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. _Nature Neurosci._ 9, 251–259 (2006). Article CAS PubMed Google Scholar * Lee, K.
W. et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. _Proc. Natl Acad. Sci. USA_ 103, 3399–4304 (2006).
REFERENCES 138–140 USE BAC TRANSGENIC MICE TO DEMONSTRATE CELL SPECIFIC MODIFICATIONS OF MEDIUM SPINY NEURON SYNAPSES IN THE DORSAL AND VENTRAL STRIATUM FOLLOWING _IN VIVO_ MANIPULATIONS.
Article CAS PubMed PubMed Central Google Scholar * Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. _Nature_ 446, 633–639 (2007). Article CAS PubMed Google
Scholar * Zhang, F., Aravanis, A. M, Adamantidis, A., de Lecea, L. & Deisseroth, K. Circuit-breakers: optical technologies for probing neural signals and systems. _Nature Rev.
Neurosci._ 8, 577–581 (2007). Article CAS Google Scholar * Schultz W. Multiple dopamine functions at different time courses. _Annu. Rev. Neurosci._ 30, 259–288 (2007). Article CAS
PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Pharmacology, Physiology and Biotechnology, Brown University, Providence,
02912, Rhode Island, USA Julie A. Kauer * Department of Psychiatry and Behavioural Sciences, Nancy Pritzker Laboratory, Stanford University School of Medicine, Stanford, 94304, California,
USA Robert C. Malenka Authors * Julie A. Kauer View author publications You can also search for this author inPubMed Google Scholar * Robert C. Malenka View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Robert C. Malenka. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. GLOSSARY * Long-term potentiation (LTP). Activity-dependent strengthening of synaptic transmission that lasts at least one hour. * Long-term depression (LTD). Activity-dependent
weakening of synaptic transmission that lasts at least one hour. * Conditioned place preference A behavioural task during which a subject learns to associate the drug experience with a
specific physical environment. A subject will choose to spend more time in an environment in which it previously had a 'rewarding' experience and less time in an environment in
which it had an aversive experience. * Induction of synaptic plasticity Refers to the cellular mechanisms required for the events initiating or triggering LTP or LTD. * Excitatory
postsynaptic currents (EPSCs). Currents measured using electrophysiological recordings from a single neuron while electrically stimulating axons to release neurotransmitter. For the purposes
of this Review, EPSCs are glutamate-mediated. * Expression of synaptic plasticity Refers to the cellular mechanisms responsible for maintaining a change in synaptic strength, for example,
an increase in neurotransmitter release. * Occlusion The observation that synaptic stimulation produces no further LTP (or LTD) presumably because the underlying cellular mechanisms have
been maximally activated by some preceding stimulus. When LTP (or LTD) is absent, it is often difficult to determine whether it has been 'occluded' or blocked by inhibition or
inactivation of one or more essential cellular mechanisms. * Inhibitory postsynaptic currents (IPSCs). Currents measured using electrophysiological recordings from a single neuron while
electrically stimulating axons to release neurotransmitter. For the purposes of this Review, IPSCs are GABA-mediated. * Yoked design Experimental protocol in which a 'yoked'
control animal receives a drug administered by the investigator in a non-contingent manner, in the same amount and temporal pattern as an animal that is self-administering the drug. RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kauer, J., Malenka, R. Synaptic plasticity and addiction. _Nat Rev Neurosci_ 8, 844–858 (2007).
https://doi.org/10.1038/nrn2234 Download citation * Issue Date: November 2007 * DOI: https://doi.org/10.1038/nrn2234 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
