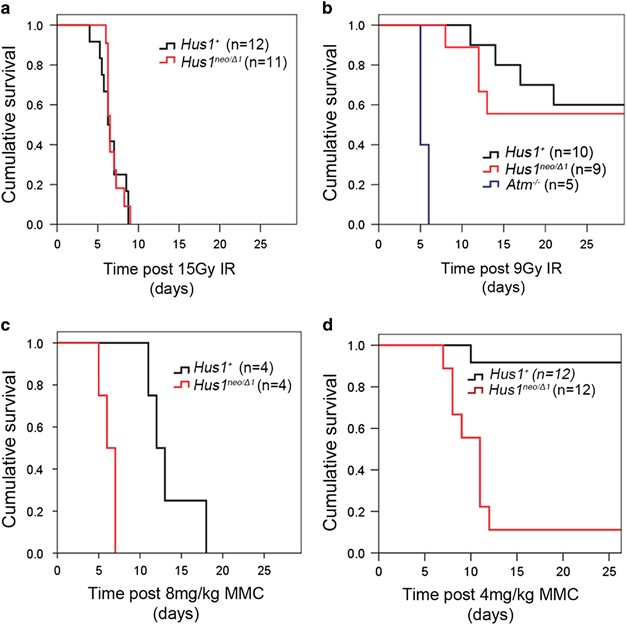
Hus1 regulates in vivo responses to genotoxic chemotherapies
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Cells are under constant attack from genotoxins and rely on a multifaceted DNA damage response (DDR) network to maintain genomic integrity. Central to the DDR are the ATM and ATR
kinases, which respond primarily to double-strand DNA breaks (DSBs) and replication stress, respectively. Optimal ATR signaling requires the RAD9A-RAD1-HUS1 (9-1-1) complex, a toroidal clamp
that is loaded at damage sites and scaffolds signaling and repair factors. Whereas complete ATR pathway inactivation causes embryonic lethality, partial _Hus1_ impairment has been
accomplished in adult mice using hypomorphic (_Hus1__neo_) and null _(Hus1__Δ1_) _Hus1_ alleles, and here we use this system to define the tissue- and cell type-specific actions of the
HUS1-mediated DDR _in vivo_. _Hus1__neo/Δ1_ mice showed hypersensitivity to agents that cause replication stress, including the crosslinking agent mitomycin C (MMC) and the replication
inhibitor hydroxyurea, but not the DSB inducer ionizing radiation. Analysis of tissue morphology, genomic instability, cell proliferation and apoptosis revealed that MMC treatment caused
severe damage in highly replicating tissues of mice with partial _Hus1_ inactivation. The role of the 9-1-1 complex in responding to MMC was partially ATR-independent, as a HUS1 mutant that
was proficient for ATR-induced checkpoint kinase 1 phosphorylation nevertheless conferred MMC hypersensitivity. To assess the interplay between the ATM and ATR pathways in responding to
replication stress _in vivo_, we used _Hus1/Atm_ double mutant mice. Whereas _Hus1__neo/neo_ and _Atm__−/−_ single mutant mice survived low-dose MMC similar to wild-type controls,
_Hus1__neo/neo__Atm__−/−_ double mutants showed striking MMC hypersensitivity, consistent with a model in which MMC exposure in the context of _Hus1_ dysfunction results in DSBs to which the
ATM pathway normally responds. This improved understanding of the inter-dependency between two major DDR mechanisms during the response to a conventional chemotherapeutic illustrates how
inhibition of checkpoint factors such as HUS1 may be effective for the treatment of ATM-deficient and other cancers. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 50 print issues and online access $259.00 per year only
$5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS RHNO1: AT THE CROSSROADS OF
DNA REPLICATION STRESS, DNA REPAIR, AND CANCER Article 06 August 2024 TDP1-INDEPENDENT PATHWAYS IN THE PROCESS AND REPAIR OF TOP1-INDUCED DNA DAMAGE Article Open access 22 July 2022
MECHANISM FOR LOCAL ATTENUATION OF DNA REPLICATION AT DOUBLE-STRAND BREAKS Article 19 February 2025 REFERENCES * Cimprich KA, Cortez D . ATR: an essential regulator of genome integrity. _Nat
Rev Mol Cell Biol_ 2008; 9: 616–627. Article CAS PubMed PubMed Central Google Scholar * McKinnon PJ . ATM and the molecular pathogenesis of ataxia telangiectasia. _Annu Rev Pathol_
2012; 7: 303–321. Article CAS PubMed Google Scholar * McKinnon PJ, Caldecott KW . DNA strand break repair and human genetic disease. _Annu Rev Genomics Hum Genet_ 2007; 8: 37–55. Article
CAS PubMed Google Scholar * Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F _et al_. Atm-deficient mice: a paradigm of ataxia telangiectasia. _Cell_ 1996; 86: 159–171.
Article CAS PubMed Google Scholar * Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J _et al_. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. _Proc
Natl Acad Sci USA_ 1996; 93: 13084–13089. Article CAS PubMed PubMed Central Google Scholar * Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D . Targeted disruption of ATM
leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. _Genes Dev_ 1996; 10: 2411–2422. Article CAS PubMed Google Scholar * Kass EM,
Helgadottir HR, Chen C-C, Barbera M, Wang R, Westermark UK _et al_. Double-strand break repair by homologous recombination in primary mouse somatic cells requires BRCA1 but not the ATM
kinase. _Proc Natl Acad Sci USA_ 2013; 110: 5564–5569. Article CAS PubMed PubMed Central Google Scholar * Delacroix S, Wagner JM, Kobayashi M, Yamamoto K-I, Karnitz LM . The
Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. _Genes Dev_ 2007; 21: 1472–1477. Article CAS PubMed PubMed Central Google Scholar * Cotta-Ramusino C, McDonald
ER, Hurov K, Sowa ME, Harper JW, Elledge SJ . A DNA damage response screen identifies RHINO, a 9-1-1 and TopBP1 interacting protein required for ATR signaling. _Science_ 2011; 332:
1313–1317. Article CAS PubMed PubMed Central Google Scholar * Helt CE, Wang W, Keng PC, Bambara RA . Evidence that DNA damage detection machinery participates in DNA repair. _Cell
Cycle_ 2005; 4: 529–532. Article CAS PubMed Google Scholar * Wang W, Lindsey-Boltz LA, Sancar A, Bambara RA . Mechanism of stimulation of human DNA ligase I by the Rad9-rad1-Hus1
checkpoint complex. _J Biol Chem_ 2006; 281: 20865–20872. Article CAS PubMed Google Scholar * Friedrich-Heineken E, Toueille M, Tännler B, Bürki C, Ferrari E, Hottiger MO _et al_. The
two DNA clamps Rad9/Rad1/Hus1 complex and proliferating cell nuclear antigen differentially regulate Flap endonuclease 1 activity. _J Mol Biol_ 2005; 353: 980–989. Article CAS PubMed
Google Scholar * Kai M, Wang TSF . Checkpoint activation regulates mutagenic translesion synthesis. _Genes Dev_ 2003; 17: 64–76. Article CAS PubMed PubMed Central Google Scholar * Bai
H, Madabushi A, Guan X, Lu A-L . Interaction between human mismatch repair recognition proteins and checkpoint sensor Rad9-Rad1-Hus1. _DNA Repair_ 2010; 9: 478–487. Article CAS PubMed
PubMed Central Google Scholar * O'Driscoll M . Diseases associated with defective responses to DNA damage. _Cold Spring Harb Perspect Biol_ 2012; 4: a012773. Article PubMed PubMed
Central Google Scholar * Goodship J, Gill H, Carter J, Jackson A, Splitt M, Wright M . Autozygosity mapping of a Seckel Syndrome locus to chromosome 3q22.1-q24. _Am J Hum Genet_ 2000; 67:
498–503. Article CAS PubMed PubMed Central Google Scholar * O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA . A splicing mutation affecting expression of
ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. _Nature Genet_ 2003; 33: 497–501. Article CAS PubMed Google Scholar * Han L, Hu Z, Liu Y, Wang X, Hopkins
KM, Lieberman HB _et al_. Mouse Rad1 deletion enhances susceptibility for skin tumor development. _Mol Cancer_ 2010; 9: 67. Article PubMed PubMed Central Google Scholar * Jeon Y, Ko E,
Lee KY, Ko MJ, Park SY, Kang J _et al_. TopBP1 deficiency causes an early embryonic lethality and induces cellular senescence in primary cells. _J Biol Chem_ 2011; 286: 5414–5422. Article
CAS PubMed Google Scholar * Brown EJ, Baltimore D . ATR disruption leads to chromosomal fragmentation and early embryonic lethality. _Genes Dev_ 2000; 14: 397–402. CAS PubMed PubMed
Central Google Scholar * Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K _et al_. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage
checkpoint. _Genes Dev_ 2000; 14: 1448–1459. Article CAS PubMed PubMed Central Google Scholar * Weiss RS, Enoch T, Leder P . Inactivation of mouse Hus1 results in genomic instability
and impaired responses to genotoxic stress. _Genes Dev_ 2000; 14: 1886–1898. CAS PubMed PubMed Central Google Scholar * Hopkins KM, Auerbach W, Wang XY, Hande MP, Hang H, Wolgemuth DJ
_et al_. Deletion of mouse rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. _Mol Cell Biol_ 2004; 24: 7235–7248. Article CAS PubMed
PubMed Central Google Scholar * Murga M, Bunting S, Montaña MF, Soria R, Mulero F, Cañamero M _et al_. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging.
_Nature Genet_ 2009; 41: 891–898. Article CAS PubMed Google Scholar * Smith J, Tho LM, Xu N, Gillespie DA . The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. _Adv
Cancer Res_ 2010; 108: 73–112. Article CAS PubMed Google Scholar * Lam MH, Liu Q, Elledge SJ, Rosen JM . Chk1 is haploinsufficient for multiple functions critical to tumor suppression.
_Cancer Cell_ 2004; 6: 45–59. Article CAS PubMed Google Scholar * Gilad O, Nabet BY, Ragland RL, Schoppy DW, Smith KD, Durham AC _et al_. Combining ATR suppression with oncogenic Ras
synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. _Cancer Res_ 2010; 70: 9693–9702. Article CAS PubMed PubMed
Central Google Scholar * Maniwa Y, Yoshimura M, Bermudez VP, Yuki T, Okada K, Kanomata N _et al_. Accumulation of hRad9 protein in the nuclei of nonsmall cell lung carcinoma cells.
_Cancer_ 2005; 103: 126–132. Article CAS PubMed Google Scholar * Zhu A, Zhang CX, Lieberman HB . Rad9 has a functional role in human prostate carcinogenesis. _Cancer Res_ 2008; 68:
1267–1274. Article CAS PubMed PubMed Central Google Scholar * Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D _et al_. Identification of tumor-initiating cells in a
p53-null mouse model of breast cancer. _Cancer Res_ 2008; 68: 4674–4682. Article CAS PubMed PubMed Central Google Scholar * Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R,
Montaña MF _et al_. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. _Nat Struct Mol Biol_ 2011; 18: 1331–1335. Article CAS PubMed PubMed
Central Google Scholar * Schoppy DW, Ragland RL, Gilad O, Shastri N, Peters AA, Murga M _et al_. Oncogenic stress sensitizes murine cancers to hypomorphic suppression of ATR. _J Clin
Invest_ 2012; 122: 241–252. Article CAS PubMed Google Scholar * Kawasumi M, Lemos B, Bradner JE, Thibodeau R, Kim Y-S, Schmidt M _et al_. Protection from UV-induced skin carcinogenesis
by genetic inhibition of the ataxia telangiectasia and Rad3-related (ATR) kinase. _Proc Natl Acad Sci USA_ 2011; 108: 13716–13721. Article CAS PubMed PubMed Central Google Scholar * Luo
J, Solimini NL, Elledge SJ . Principles of cancer therapy: oncogene and non-oncogene addiction. _Cell_ 2009; 136: 823–837. Article CAS PubMed PubMed Central Google Scholar * Jackson
SP, Bartek J . The DNA-damage response in human biology and disease. _Nature_ 2009; 461: 1071–1078. Article CAS PubMed PubMed Central Google Scholar * Farmer H, McCabe N, Lord CJ, Tutt
ANJ, Johnson DA, Richardson TB _et al_. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. _Nature_ 2005; 434: 917–921. Article CAS PubMed Google Scholar *
Ellisen LW . PARP inhibitors in cancer therapy: promise, progress, and puzzles. _Cancer Cell_ 2011; 19: 165–167. Article CAS PubMed PubMed Central Google Scholar * Ma CX, Cai S, Li S,
Ryan CE, Guo Z, Schaff TW _et al_. Targeting Chk1 in p53-deficient triple-negative breast cancer is therapeutically beneficial in human-in-mouse tumor models. _J Clin Invest_ 2012; 122:
1541–1552. Article CAS PubMed PubMed Central Google Scholar * Origanti S, Cai S-R, Munir AZ, White LS, Piwnica-Worms H . Synthetic lethality of Chk1 inhibition combined with p53 and/or
p21 loss during a DNA damage response in normal and tumor cells. _Oncogene_ 2013; 32: 577–588. Article CAS PubMed Google Scholar * Reaper PM, Griffiths MR, Long JM, Charrier J-D,
Maccormick S, Charlton PA _et al_. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. _Nat Chem Biol_ 2011; 7: 428–430. Article CAS PubMed Google Scholar
* Hall AB, Newsome D, Wang Y, Boucher DM, Eustace B, Gu Y _et al_. Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. _Oncotarget_ 2014; 5:
5674–5685. Article PubMed PubMed Central Google Scholar * Bartek J, Mistrik M, Bartkova J . Thresholds of replication stress signaling in cancer development and treatment. _Nat Struct
Mol Biol_ 2012; 19: 5–7. Article CAS PubMed Google Scholar * Broustas CG, Lieberman HB . DNA damage response genes and the development of cancer metastasis. _Radiat Res_ 2014; 181:
111–130. Article CAS PubMed PubMed Central Google Scholar * Levitt PS, Liu H, Manning C, Weiss RS . Conditional inactivation of the mouse Hus1 cell cycle checkpoint gene. _Genomics_
2005; 86: 212–224. Article CAS PubMed Google Scholar * Levitt PS, Zhu M, Cassano A, Yazinski SA, Liu H, Darfler J _et al_. Genome maintenance defects in cultured cells and mice following
partial inactivation of the essential cell cycle checkpoint gene Hus1. _Mol Cell Biol_ 2007; 27: 2189–2201. Article CAS PubMed PubMed Central Google Scholar * Kinzel B, Hall J, Natt F,
Weiler J, Cohen D . Downregulation of Hus1 by antisense oligonucleotides enhances the sensitivity of human lung carcinoma cells to cisplatin. _Cancer_ 2005; 94: 1808–1814. Article Google
Scholar * Church D, Kerr R, Domingo E, Rosmarin D, Palles C, Maskell K _et al_. “Toxgnostics”: an unmet need in cancer medicine. _Nat Rev Cancer_ 2014; 14: 440–445. Article CAS PubMed
Google Scholar * Potten CS . A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. _Int J Radiat Biol_ 1990; 58: 925–973. Article CAS PubMed Google
Scholar * Flynn RL, Zou L . ATR: a master conductor of cellular responses to DNA replication stress. _Trends Biochem Sci_ 2011; 36: 133–140. Article CAS PubMed Google Scholar *
Kottemann MC, Smogorzewska A . Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. _Nature_ 2013; 493: 356–363. Article CAS PubMed PubMed Central Google Scholar * Weiss
RS, Matsuoka S, Elledge SJ, Leder P . Hus1 acts upstream of Chk1 in a mammalian DNA damage response pathway. _Current Biol_ 2002; 12: 73–77. Article CAS Google Scholar * Xu X, Guardiani
C, Yan C, Ivanov I . Opening pathways of the DNA clamps proliferating cell nuclear antigen and Rad9-Rad1-Hus1. _Nucleic Acids Res_ 2013; 41: 10020–10031. Article CAS PubMed PubMed Central
Google Scholar * Jansen JG, Fousteri MI, de Wind N . Send in the clamps: control of DNA translesion synthesis in eukaryotes. _Mol Cell_ 2007; 28: 522–529. Article CAS PubMed Google
Scholar * Shiotani B, Zou L . ATR signaling at a glance. _J Cell Sci_ 2009; 122: 301–304. Article CAS PubMed PubMed Central Google Scholar * Maréchal A, Zou L . DNA damage sensing by
the ATM and ATR kinases. _Cold Spring Harb Perspect Biol_ 2013; 5: a012716–a012716. Article PubMed PubMed Central Google Scholar * Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER,
Hurov KE, Luo J _et al_. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. _Science_ 2007; 316: 1160–1166. Article CAS PubMed Google Scholar *
Adams KE, Medhurst AL, Dart DA, Lakin ND . Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. _Oncogene_ 2006; 25:
3894–3904. Article CAS PubMed PubMed Central Google Scholar * Jazayeri A, Falck J, Lukas C, Bartek J, Smith GCM, Lukas J _et al_. ATM- and cell cycle-dependent regulation of ATR in
response to DNA double-strand breaks. _Nat Cell Biol_ 2006; 8: 37–45. Article CAS PubMed Google Scholar * Weiss RS, Leder P, Vaziri C . Critical role for mouse Hus1 in an S-phase DNA
damage cell cycle checkpoint. _Mol Cell Biol_ 2003; 23: 791–803. Article CAS PubMed PubMed Central Google Scholar * Balmus G, Zhu M, Mukherjee S, Lyndaker AM, Hume KR, Lee J _et al_.
Disease severity in a mouse model of ataxia telangiectasia is modulated by the DNA damage checkpoint gene Hus1. _Hum Mol Genet_ 2012; 21: 3408–3420. Article CAS PubMed PubMed Central
Google Scholar * Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW . Cancer genome landscapes. _Science_ 2013; 339: 1546–1558. Article CAS PubMed PubMed Central
Google Scholar * Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G _et al_. Deletion of the developmentally essential gene ATR in adult mice leads to age-related
phenotypes and stem cell loss. _Cell Stem Cell_ 2007; 1: 113–126. Article CAS PubMed PubMed Central Google Scholar * Yazinski SA, Westcott PMK, Ong K, Pinkas J, Peters RM, Weiss RS .
Dual inactivation of Hus1 and p53 in the mouse mammary gland results in accumulation of damaged cells and impaired tissue regeneration. _Proc Natl Acad Sci USA_ 2009; 106: 21282–21287.
Article CAS PubMed PubMed Central Google Scholar * Fokas E, Prevo R, Hammond EM, Brunner TB, McKenna WG, Muschel RJ . Targeting ATR in DNA damage response and cancer therapeutics.
_Cancer Treat Rev_ 2014; 40: 109–117. Article CAS PubMed Google Scholar * Brooks K, Oakes V, Edwards B, Ranall M, Leo P, Pavey S _et al_. A potent Chk1 inhibitor is selectively cytotoxic
in melanomas with high levels of replicative stress. _Oncogene_ 2013; 32: 788–796. Article CAS PubMed Google Scholar * Tang Y, Dai Y, Grant S, Dent P . Enhancing CHK1 inhibitor
lethality in glioblastoma. _Cancer Biol Ther_ 2012; 13: 379–8867. Article CAS PubMed PubMed Central Google Scholar * la Torre de J, Gil-Moreno A, García A, Rojo F, Xercavins J, Salido E
_et al_. Expression of DNA damage checkpoint protein Hus1 in epithelial ovarian tumors correlates with prognostic markers. _Int J Gynecol Pathol_ 2008; 27: 24–32. Article Google Scholar *
Broustas CG, Zhu A, Lieberman HB . Rad9 protein contributes to prostate tumor progression by promoting cell migration and anoikis resistance. _J Biol Chem_ 2012; 287: 41324–41333. Article
CAS PubMed PubMed Central Google Scholar * Lyndaker AM, Lim PX, Mleczko JM, Diggins CE, Holloway JK, Holmes RJ _et al_. Conditional inactivation of the DNA damage response gene Hus1 in
mouse testis reveals separable roles for components of the RAD9-RAD1-HUS1 complex in meiotic chromosome maintenance. _PLoS Genet_ 2013; 9: e1003320. Article CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS We thank Amy Lyndaker, Steve Jackson and Yaron Galanti for helpful discussions and comments on the manuscript; Natasha Karp for help with
statistical analysis and the staff of the Cornell Lab Animal Services and CARE programs for excellent animal care. This work was supported by a National Institutes of Health grant R01
CA108773 to RSW; a Cornell University College of Veterinary Medicine Graduate Research Assistantship to GB; a Cornell University College of Veterinary Medicine Clinical Fellowship to KRH;
and a National Center for Research Resources grant (S10RR023781) for instrumentation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical Sciences, Cornell University,
Ithaca, NY, USA G Balmus, P X Lim, A Oswald, K R Hume, A Cassano, J Pierre, A Hill, T Southard & R S Weiss * Department of Clinical Sciences, Cornell University, Ithaca, NY, USA K R Hume
* Department of Microbiology and Immunology, Cornell University, Ithaca, NY, USA W Huang & A August * Department of Population Medicine and Diagnostic Sciences, Cornell University,
Ithaca, NY, USA T Stokol Authors * G Balmus View author publications You can also search for this author inPubMed Google Scholar * P X Lim View author publications You can also search for
this author inPubMed Google Scholar * A Oswald View author publications You can also search for this author inPubMed Google Scholar * K R Hume View author publications You can also search
for this author inPubMed Google Scholar * A Cassano View author publications You can also search for this author inPubMed Google Scholar * J Pierre View author publications You can also
search for this author inPubMed Google Scholar * A Hill View author publications You can also search for this author inPubMed Google Scholar * W Huang View author publications You can also
search for this author inPubMed Google Scholar * A August View author publications You can also search for this author inPubMed Google Scholar * T Stokol View author publications You can
also search for this author inPubMed Google Scholar * T Southard View author publications You can also search for this author inPubMed Google Scholar * R S Weiss View author publications You
can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to R S Weiss. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of
interest. ADDITIONAL INFORMATION Supplementary Information accompanies this paper on the Oncogene website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 7352 KB) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Balmus, G., Lim, P., Oswald, A. _et al._ HUS1 regulates _in vivo_ responses to genotoxic chemotherapies. _Oncogene_
35, 662–669 (2016). https://doi.org/10.1038/onc.2015.118 Download citation * Received: 01 July 2014 * Revised: 08 March 2015 * Accepted: 10 March 2015 * Published: 27 April 2015 * Issue
Date: 04 February 2016 * DOI: https://doi.org/10.1038/onc.2015.118 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
