Oxidative stress and increased type-iv collagenase levels in bronchoalveolar lavage fluid from newborn babies
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Oxidative stress may increase lung permeability by up-regulation of matrix-metalloproteinase-9 (MMP-9), a type-IV collagenase that can disrupt alveolar basement membranes. We have
compared a marker of oxidative stress (protein carbonyl residues) with levels of MMP-9 and its inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1), in bronchoalveolar lavage samples
from newborn babies. Bronchoalveolar lavage samples (_n_ = 87, two from each time point) were taken in the first 6 postnatal days from 41 ventilated babies: 18 of <29 wk gestation, 10 of
29–36 wk, 9 term with persistent fetal circulation, and 4 term without lung disease. Respiratory disease severity at the time of bronchoalveolar lavage was assessed using the
arterial–alveolar oxygen tension ratio. One sample from each time point was used for the measurement of MMP-9 by zymography and TIMP-1 by ELISA. The second sample was used to measure
carbonyl group concentrations, also using an ELISA. Correlations were calculated between protein carbonyls, arterial–alveolar oxygen tension ratio, and MMP-9 and TIMP-1 concentrations.
Significant correlations were found between carbonyl concentrations and arterial–alveolar oxygen tension ratio (_r_ = −0.325, _p_ = 0.0031, _n_ = 81), MMP-9 (_r_ = 0.331, _p_ < 0.0029,
_n_ = 79), and TIMP-1 (_r_ = 0.436, _p_ < 0.0001, _n_ = 87). Worsening respiratory disease in newborn babies is associated with increased carbonyl concentrations in neonatal
bronchoalveolar lavage fluid, and these correlated with MMP-9 and TIMP-1 levels. Increased oxidative stress may damage the lung by increasing type-IV collagenase activity, causing disruption
of the extracellular matrix. SIMILAR CONTENT BEING VIEWED BY OTHERS MODULATION OF PULMONARY DESMOSOMES BY INHALER THERAPY IN PRETERM-BORN CHILDREN WITH BRONCHOPULMONARY DYSPLASIA Article
Open access 05 May 2023 L-CITRULLINE ATTENUATES LIPOPOLYSACCHARIDE-INDUCED INFLAMMATORY LUNG INJURY IN NEONATAL RATS Article 22 June 2023 EARLY MOLECULAR MARKERS OF VENTILATOR-ASSOCIATED
PNEUMONIA IN BRONCHOALVEOLAR LAVAGE IN PRETERM INFANTS Article Open access 07 September 2022 MAIN Newborn babies with lung disease are often exposed to high inspired oxygen concentrations to
maintain sufficient arterial oxygen saturation. In preterm babies this is most often owing to surfactant deficiency, but severe respiratory problems also occur in term babies, particularly
those with persistent fetal circulation (1). High oxygen concentrations increase the rate of oxygen free radical formation (reactive oxygen species), and these highly reactive molecules can
cause oxidative damage to proteins, DNA, lipids, cells, and tissues (2). Antioxidant defense mechanisms are poorly developed in the preterm baby and in term babies may be overcome by the
generation of excessive reactive oxygen species (3). Oxidative stress is a major contributing factor to the development of CLD (3). The link between oxidative stress and lung inflammation
has been extensively studied in animal models. Exposure of rats to 100% oxygen produces a lethal injury within 72 h characterized by increased permeability of the alveolar–capillary barrier
and interstitial and pulmonary edema. MMP-9 has been implicated in this hyperoxic lung damage (4, 5). TIMP-1 is the major inhibitor of MMP-9, and its expression is also increased by
hyperoxia (6). Conversely, TIMP-1 can also be oxidized, thereby rendering it ineffective (7). In this observational study, we have assessed whether oxidative stress in the newborn baby is
associated with increased BAL fluid concentrations of MMP-9 and TIMP-1. METHODS SUBJECTS. This was a prospective study of babies born in the Royal Maternity Hospital in Belfast between March
1998 and April 1999. The study was approved by the Research Ethics Committee of The Queen's University of Belfast, and parental consent was obtained before babies were enrolled. Babies
were eligible for study if they required intubation and mechanical ventilation within the first 6 d of life. Gestation was estimated by duration of amenorrhea combined with early prenatal
ultrasound measurement. Forty-one babies were studied, and 18 of them were born before 29 wk gestation, 10 were 29–36 wk, 9 were term babies with persistent fetal circulation, and 4 were
term babies without significant lung disease, ventilated for hypoxic ischemic encephalopathy, oversedation, congenital myopathy, or gastroschisis. The severity of the respiratory disease at
the time of BAL sampling was quantified using the a/A ratio. This was calculated from arterial blood gases using the formula a/A ratio = Po2/(94 × Fio2) − Pco2, where Po2 and Pco2 are
expressed in kPa and Fio2 (fraction of inspired oxygen) as a fraction of 1. The median a/A ratio using all arterial blood gases in the first 24 h of life was used to estimate initial
respiratory disease severity. In the 18 very preterm babies, CLD was defined as a requirement for supplemental oxygen to maintain oxygen saturation measured by pulse oximetry >92% when at
rest after the 36th week after conception. BAL. BAL was performed in a standardized way using a well-established technique (8). In summary, 1 mL/kg of sterile 0.9% saline was instilled
using a syringe _via_ a 5F feeding catheter, which had been placed through the endotracheal tube into the distal right main bronchus. The saline was instilled and immediately aspirated back
into the syringe. The sample was clarified by centrifugation at 1500 ×_g_ for 5 min at room temperature, and the supernatant was immediately frozen at −70°C for subsequent analysis. On each
occasion this was repeated twice, with the first sample used for measurement of MMP-9 and TIMP-1, and the second sample for measurement of carbonyl group concentrations. Serial BAL was
performed daily whenever possible from the time of intubation until extubation or the sixth day of life, whichever was soonest. Babies were not studied during the 12 h after a dose of
surfactant. MMP-9. MMP-9 concentrations were measured by zymography (9). SDS (7.5% SDS-PAGE) gels were copolymerized with 0.1% gelatin, and the proteins in 8 μL of BAL (diluted 1:5 in PBS)
were separated electrophoretically. The gels were then rinsed in Tris-Triton buffers to remove the SDS, incubated overnight in Tris-HCl, pH 7.6, containing 10 mM CaCl2 and 1% Triton, and
then stained with Coomassie blue. After destaining with a solution of 7.5% acetic acid and 5% methanol, the MMP activity was visualized as clear bands on a blue background. The intensity of
the bands was estimated using computerized image analysis and densitometry. If MMP-9 was undetectable, then the gels were rerun using undiluted BAL samples. The limit of detection was 0.5
ng/mL. Coefficients of variation were 4.5% (intragel) and 9.6% (intergel). When the measured MMP-9 concentration was below the detection limit of the assay, it was substituted with a value
equal to the detection limit for statistical analysis. TIMP-1. TIMP-1 (Amersham Pharmacia, Amersham, U.K.) was measured using a commercially available ELISA. The limit of detection was 1.56
ng/mL. Coefficients of variation were 4.8% (intraplate) and 7.9% (interplate). PROTEIN CARBONYLS. Carbonyl concentrations were determined using an in-house ELISA as described by Buss _et
al._(10). Briefly, after derivatization of carbonyl groups with dinitrophenylhydrazine, proteins were adsorbed to 96-well ELISA plates, captured with a commercially available
anti-dinitrophenylhydrazine antibody, and detected with a horseradish peroxidase/hydrogen peroxide, phenylenediamine system (10). The limit of detection was 0.28 nmol/mg protein.
Coefficients of variation were 8.6% (intraplate) and 9.3% (interplate). PROTEIN ASSAY. Total protein concentrations in BAL fluid were quantified using the commercially available BioRad kit
(BioRad Laboratories LTD., U.K.). The detection limit was 2 μg/mL. MDA ASSAY. MDAs were determined by HPLC with fluorimetric detection after reaction with thiobarbituric acid (11). The limit
of detection was 0.03 μmol MDA/L. Coefficients of variation were 9.5% (intraassay) and 9.4% (interassay). DATA ANALYSIS. The distribution of the concentrations of MMP-9, TIMP-1, and protein
carbonyls were all highly negatively skewed but normally distributed when plotted logarithmically. Nonparametric tests were used throughout. Results were considered statistically
significant if _p_ < 0.05. To compute all statistics, StatView for Windows (v. 4.57, 1996, Abacus Concepts Inc., Berkeley, CA, U.S.A.) was used. For graphs, GraphPad Prism (v.2.01,
GraphPad Software Inc., San Diego, CA, U.S.A.) was used. RESULTS A total of 87 BAL samples from 41 babies were analyzed. The clinical characteristics of the babies and the number of BAL
samples from each of the groups are shown in Table 1. The median volume recovered after lavage was 0.7 (IQR, 0.5–1.2) mL. The term babies with persistent fetal circulation had the highest
requirements for supplemental oxygen and the lowest median a/A ratio on d 1 (_p_ < 0.0001). There was no statistically significant difference in the median day of sampling among the
groups (median, 3 d for both preterm groups, 4 d for term persistent fetal circulation, and 3.5 d for term normal;_p_ = 0.37). Protein carbonyl groups, MDAs, and TIMP-1 were detected in all
87 BAL samples. In eight cases there was insufficient volume of BAL fluid for both TIMP-1 and MMP-9 assays, thus MMP-9 was measured in only 79 samples. In 18 samples MMP-9 was undetectable
even when using undiluted BAL fluid. Simultaneous a/A ratios were available for 81 samples as six babies did not have blood gas analysis at the time of BAL. The results for protein
carbonyls, MDAs, MMP-9, TIMP-1, and TIMP-1/MMP-9 ratio for all subgroups are given in Tables 2 and 3. The results are shown as median and IQR. Protein carbonyl concentrations were
significantly different among the groups (Kruskal-Wallis one-way ANOVA, nanomoles per milligram;_p_ < 0.001 and nanomoles per milliliter;_p_ < 0.05). Worsening respiratory disease,
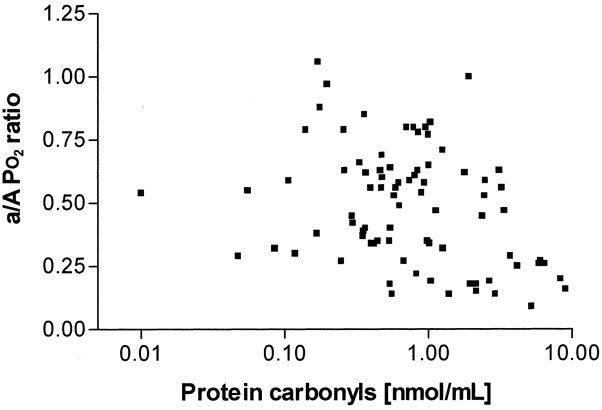
demonstrated by a lower a/A ratio, was associated with increased BAL fluid concentrations of protein carbonyls (_r_ = −0.325, _p_ = 0.0031, _n_ = 81;Fig. 1). There was a significant
correlation between protein carbonyl concentrations and MMP-9 (_r_ = 0.331, _p_ = 0.0031, _n_ = 79;Fig. 2) and TIMP-1 (_r_ = 0.436, _p_ < 0.0001, _n_ = 87;Fig. 3) concentrations in BAL
fluid. These correlations did not remain significant when protein carbonyl data were expressed as nanomoles per milligram of protein (_r_ = −0.109, −0.136, and −0.127 respectively; all _p_
> 0.05). Protein carbonyl concentrations did not correlate with changes in the ratio of MMP-9 to TIMP-1 concentrations in neonatal BAL fluid (_r_ = 0.144, _p_ = 0.206, _n_ = 79). MMP-9
and TIMP-1 concentrations were weakly, but significantly, correlated (_r_ = 0.318, _p_ = 0.005, _n_ = 79). In contrast to protein carbonyl concentrations, MDAs did not correlate
significantly with concentrations of MMP-9 (_r_ = 0.077, _p_ = 0.516, _n_ = 74) or TIMP-1 (_r_ = 0.048, _p_ = 0.671, _n_ = 81). MDAs also did not correlate with concentrations of protein
carbonyls (_r_ = 0.122, _p_ = 0.278, _n_ = 81). When analyzing the 44 BAL samples from the 18 very preterm babies in isolation, there was no significant correlation between carbonyl groups
and a/A ratio (_r_ = −0.132, _p_ = 0.394, _n_ = 44) or MMP-9 (_r_ = 0.300, _p_ = 0.067, _n_ = 38). There was, however, a significant correlation between carbonyl groups and TIMP-1 (_r_ =
0.407, _p_ = 0.0061, _n_ = 44). Eight babies in this group had CLD (24 BAL samples) and 10 did not (20 BAL samples). There was no significant difference in concentrations of protein
carbonyls, MDAs, MMP-9, and TIMP-1 in BAL fluid from this small group of babies with and without CLD, although the median MDA concentration was more than 3 × higher in babies who had CLD
compared with those who did not [0.38 (0.05–0.57) _versus_ 0.10 (0.03–0.38) μmol/L]. Additionally, a trend was apparent for increased concentrations of protein carbonyls [0.78 (0.45–1.14)
_versus_ 0.47 (0.35–0.59) nmol/mL, _p_ = 0.079]. DISCUSSION There is a clear link between oxidative stress and the lung inflammation that occurs during the development of CLD in the preterm
baby (3). The exact mechanisms of oxidative lung injury are not fully elucidated, and most studies of reactive oxygen species–mediated lung damage have used _in vitro_ systems or animal
models. Reactive oxygen species inhibit DNA synthesis, leading to diminished lung growth (12, 13). They oxidize both lipids (14) and proteins (15) in surfactant, rendering it less effective.
Surfactant synthesis can also be inhibited by oxidative stress (16). Oxidation of α-1 proteinase inhibitor leads to its inactivation, which can predispose to excessive elastase activity
(17). Indeed, Speer _et al._(18) detected considerable free elastase activity in tracheal aspirate fluid in 42 of 140 ventilated neonates, with a pronounced increase in risk of pulmonary
interstitial emphysema for those with free elastase activity. There may also be other damaging effects on the proteins within the epithelial lining structures of the lung predisposing to
inflammation. Recently it has become clear that matrix metalloproteinases play a key role in lung inflammation and airways remodelling (19). Alveolar basement membranes are composed of
type-IV collagen, the major substrate of MMP-9. Preterm babies who subsequently exhibit CLD have higher MMP-9 levels in BAL fluid within the first 6 postnatal days compared with those who do
not have CLD (20). Disruption of the alveolar basement membrane results in the increased lung permeability observed in adults exposed to high oxygen concentrations (21) and in babies who
exhibit CLD (22). In animal studies, oxygen toxicity has been shown to increase both MMP-9 mRNA transcription (23) and lung MMP-9 levels (4, 5). Oxidative stress is difficult to quantify
directly as the very short half-lives of reactive oxygen species mean that they cannot easily be measured. However, using an artificial system, Gerber _et al._(24) found that there was an
enhanced generation of hydroxyl radicals in bronchoalveolar secretions from preterm infants compared with buffer, demonstrating the lack of a hydroxyl radical scavenger system in these
infants. In free radical modification of proteins, carbonyl groups are introduced to some amino acid residues, and the concentration of these has been used as a measure of protein oxidative
damage (25). Polyunsaturated fatty acids are modified by oxidation to peroxides, which can be quantified as MDAs (11). Measuring oxidation products in BAL fluid is ideal as it allows for the
balance between the amount of reactive oxygen species exposure and the antioxidant defenses. Measuring inspired oxygen exposure in isolation would not take into account the antioxidant
defenses that are probably more developed in the term babies. The amount of protein carbonylation on d 2–4 of life has previously been shown to be inversely proportional to gestational age
and is significantly increased in babies who have CLD, even when controlled for gestational age (26). The finding in this study that carbonyl group concentrations in neonatal BAL fluid are
correlated with the degree of respiratory disease severity is in keeping with these previous studies. The loss of significance when the protein carbonyl data are given as nanomoles per
milligram of protein can be explained on the basis of increasing microvascular permeability and protein leakage into the airways during the first few days of life in preterm infants with
severe respiratory distress syndrome (22). Recently, higher protein carbonyl concentrations in tracheal aspirates from preterm babies with a birthweight of <1500 g compared with those
>1500 g have been shown (27). Carbonyl concentrations in our study are overall higher than those of Buss _et al._(27). In our group of very preterm babies, carbonyl concentrations during
d 1–3 of life were significantly elevated compared with those found at d 4–6 of life. A relation between increased lipid peroxidation and the development of CLD is supported by the finding
of increased concentrations of pentane and ethane (products of lipid peroxidation) in exhaled air (28–30) and increased concentrations of lipid peroxidation products (MDAs) in tracheal
aspirates of babies who subsequently exhibited CLD (31). In this study no significant differences in MDA concentrations were found. However, the median MDA concentration in BAL fluid of
babies who subsequently exhibit CLD is almost 3 × higher than that in babies who do not have CLD. The difference was not statistically significant, but this relationship should be explored
in a larger study. Intriguingly, protein carbonyl concentrations did not correlate with concentrations of lipid peroxidation. Buss _et al._(27) reported a weak correlation between both
markers of oxidative stress in their group of preterm babies. In preterm babies surfactant lipids are a major source of lipids in the lungs. The immature lungs are just beginning to
synthesize surfactant (protein and lipid) within type II cells (32). Surfactant therapy in preterm babies may lead to similar lipid and MDA concentrations to those in term babies. We did not
correct our results for the dilution during the lavage procedure and have expressed our data per milliliter of lavage fluid as recommended by the European Respiratory Society Task Force on
BAL in children (33). Our procedure for collection of BAL fluid is more likely to sample the lower airways whereas most other studies have been performed on tracheal aspirates (22, 27, 31),
which sample the upper airways. In this study the total levels of MMP-9 and TIMP-1 have been measured. However, this does not necessarily reflect enzyme activity. MMP-9 is released as a
proenzyme, which is activated in the tissues by proteolytic cleavage (34). In the assay used in this study, total MMP-9 activity is measured, including the proenzyme and any MMP-9 that is
bound to TIMP-1. Similarly, the TIMP-1 ELISA measures all TIMP-1 present, including any that may be inactivated or bound to MMP-9. Our study suggests that oxidative stress to proteins
increases the expression of both MMP-9 and its main inhibitor, TIMP-1. This is in keeping with previously published animal studies (4, 5, 6). It does not, however, address the question of
whether oxidative stress modifies proteinase/antiproteinase balance. Oxygen toxicity also activates pro-MMP-3, a proteinase that activates the latent form of MMP-9 (35). Similarly, oxidative
stress can inactivate TIMP-1 (6). Although the ratio of MMP-9 to TIMP-1 levels did not alter with increasing oxygen toxicity, the methods used do not enable determination of changes in net
enzyme activity. In conclusion, we have shown a correlation between clinical respiratory disease severity and carbonyl concentrations in BAL fluid from newborn babies, both term and preterm,
taken within the first 6 d of life. This suggests that carbonyl concentrations can be used as a measure of oxidative stress in this population. We have also shown a significant correlation
between carbonyl concentrations and MMP-9 and TIMP-1 concentrations. This suggests that MMP-9 and TIMP-1 are up-regulated by oxidative stress in newborn babies, as they are in animal models.
Increased expression of type-IV collagenase during oxidative stress may result in disruption of the alveolar basement membrane, which leads to the increased capillary leakage and airways
remodelling observed in the early stages of CLD. ABBREVIATIONS * a/A ratio: arterial–alveolar oxygen tension ratio * BAL: bronchoalveolar lavage * CLD: chronic lung disease * IQR:
interquartile range * MDA: malondialdehyde * MMP-9: matrix metalloproteinase-9 * TIMP-1: tissue inhibitor of metalloproteinase-1 REFERENCES * Greenough A, Roberton NRC 1999 Acute respiratory
disease in the newborn. In: Rennie JM, Roberton NRC (eds) _Textbook of neonatology, 3rd Ed_. Churchill Livingstone, Edinburgh, 481–537. Google Scholar * Freeman BA, Crapo JD 1982 Free
radicals and tissue injury. _Lab Invest_ 47: 412–426 CAS PubMed Google Scholar * Saugstad OD 1997 Bronchopulmonary dysplasia and oxidative stress: are we closer to an understanding of the
pathogenesis of BPD?. _Acta Paediatr_ 86: 1277–1282 Article CAS PubMed Google Scholar * Pardo A, Selman M, Ridge K, Barrios R, Sznajder JI 1996 Increased expression of gelatinases and
collagenase in rat lungs exposed to 100% oxygen. _Am J Respir Crit Care Med_ 154: 1067–1075 Article CAS PubMed Google Scholar * Pardo A, Barrios R, Maldonado V, Melendez J, Perez J, Ruiz
V, Segura-Valdez L, Sznajder JL, Selman M 1998 Gelatinases A and B are upregulated in rat lungs by subacute hyperoxia: pathogenetic implications. _Am J Pathol_ 153: 833–844 Article CAS
PubMed PubMed Central Google Scholar * Horowitz S, Dafni N, Shapiro DL, Holm BA, Notter RH, Quible DJ 1989 Hyperoxic exposure alters gene expression in the lung. Induction of the tissue
inhibitor of metalloproteinases mRNA and other mRNAs. _J Biol Chem_ 264: 7092–7095 CAS PubMed Google Scholar * Frears ER, Zhang Z, Blake DR, OConnell JP, Winyard PG 1996 Inactivation of
tissue inhibitor of metalloproteinase-1 by peroxynitrite. _FEBS Letts_ 381: 21–24 Article CAS Google Scholar * Kotecha S, Chan B, Azam N, Silverman M, Shaw RJ 1995 Increase in
interleukin-8 and soluble intercellular adhesion molecule-1 in broncho-alveolar lavage fluid from premature infants who develop chronic lung disease. _Arch Dis Child_ 72: F90–F96 Article
CAS Google Scholar * Kleiner DE, Stetler-Stevenson WG 1994 Quantitative zymography: detection of picogram quantities of gelatinases. _Anal Biochem_ 218: 325–329 Article CAS PubMed
Google Scholar * Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC 1997 Protein carbonyl measurement by a sensitive ELISA method. _Free Radic Biol Med_ 23: 361–366 Article CAS PubMed
Google Scholar * Young IS, Trimble ER 1991 Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. _Ann Clin Biochem_ 28: 504–508
Article PubMed Google Scholar * Han RNN, Buch S, Tseu I, Young J, Christie NA, Frndova H, Lye SJ, Post M, Tanswell AK 1996 Changes in structure, mechanics, and insulin-like growth factor
gene expression in the lungs of newborn rats exposed to air or 60% oxygen. _Pediatr Res_ 39: 921–929 Article CAS PubMed Google Scholar * Wilborn A, Evers LB, Canada AT 1996 Oxygen
toxicity to the developing lung of the mouse: role of reactive oxygen species. _Pediatr Res_ 40: 225–232 Article CAS PubMed Google Scholar * Bracci R 1997 Free oxygen radicals and
surfactant. _Biol Neonate_ 71( suppl 1): 23–27 Article CAS PubMed Google Scholar * Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S 1993 Mechanisms of
peroxynitrite-induced injury to lung surfactants. _Am J Physiol_ 265: L555–L564 CAS PubMed Google Scholar * Holm BA, Matalon S, Finkelstein JN, Notter RH 1988 Type II pneumocyte changes
during hyperoxic lung injury and recovery. _J Appl Physiol_ 65: 2672–2678 Article CAS PubMed Google Scholar * Ossanna PJ, Test ST, Matheson NR, Regiani S, Weiss SJ 1986 Oxidative
regulation of neutrophil elastase-alpha-1-proteinase inhibitor interactions. _J Clin Invest_ 77: 1939–1951 Article CAS PubMed PubMed Central Google Scholar * Speer CP, Ruess D, Harms K,
Herting E, Gefeller O 1993 Neutrophil elastase and acute pulmonary damage in neonates with severe respiratory distress syndrome. _Pediatrics_ 91: 794–799 CAS PubMed Google Scholar *
O'Connor CM, FitzGerald MX 1994 Matrix metalloproteases and lung disease. _Thorax_ 49: 602–609 Article CAS PubMed PubMed Central Google Scholar * Sweet DG, Pizzoti J, Wilbourn M,
Halliday HL, Warner JA 1999 Matrix metalloproteinase-9 (MMP-9) in the airways of infants at risk of developing chronic lung disease (CLD). _Eur Respir J_ 14( suppl 30): 248s Google Scholar
* Griffith DE, Holden WE, Morris JF, Min LK, Krishnamurthy GT 1986 Effects of common therapeutic concentrations of oxygen on lung clearance of 99mTc DTPA and bronchoalveolar lavage albumin
concentration. _Am Rev Respir Dis_ 134: 233–237 CAS PubMed Google Scholar * Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP 1994 Association of pulmonary inflammation and
increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high risk preterm
neonates. _Pediatrics_ 93: 712–718 CAS PubMed Google Scholar * Devaskar UP, Taylor W, Govindrajan R, Malicdem M, Heyman S, deMello DE 1994 Hyperoxia induces interstitial (type I) and
increases type IV collagenase mRNA expression and increases type I and IV collagenolytic activity in newborn rat lung. _Biol Neonate_ 66: 76–85 Article CAS PubMed Google Scholar * Gerber
CE, Bruchelt G, Stegmann H, Schwiensberg F, Speer CP 1999 Presence of bleomycin-detectable free iron in the alveolar system of preterm infants. _Biochem Biophys Res Commun_ 257: 218–222
Article CAS PubMed Google Scholar * Gladstone I, Levine R 1994 Oxidation of protein in neonatal lungs. _Pediatrics_ 93: 764–768 PubMed Google Scholar * Varsila E, Pesonen E, Andersson
S 1995 Early protein oxidation in the neonatal lung is related to development of chronic lung disease. _Acta Paediatr_ 84: 1296–1299 Article CAS PubMed Google Scholar * Buss IH, Darlow
BA, Winterbourn CC 2000 Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. _Pediatr Res_ 47: 640–645
Article CAS PubMed Google Scholar * Varsila E, Hallman M, Andersson S 1994 Free-radical-induced lipid peroxidation during the early neonatal period. _Acta Paediatr_ 83: 692–695 Article
CAS PubMed Google Scholar * Nycyk JA, Drury JA, Cooke RWL 1998 Breath pentane as a marker for lipid peroxidation and adverse outcome in preterm infants. _Arch Dis Child_ 79: F67–F69
Article CAS Google Scholar * Pitkänen OM, Hallman M, Andersson SM 1990 Correlation of free oxygen radical-induced lipid peroxidation with outcome in very low birth weight infants. _J
Pediatr_ 116: 760–764 Article PubMed Google Scholar * Schrod L, Neuhaus T, Speer CP, Girschick H 1997 Possible role of uric acid as an antioxidant in premature infants. _Biol Neonate_ 72:
102–111 Article CAS PubMed Google Scholar * Hislop A 1996 Foetal and postnatal anatomical development. In: Greenough A, Roberton NRC, Milner AD (eds) _Neonatal respiratory disorders_.
Arnold, Hodder Headline Group, London, 3–12. Google Scholar * ERS Task Force on Bronchoalveolar Lavage in Children 2000 Bronchoalveolar lavage in children. _Eur Respir J_ 15: 217–231 *
Davidson JM 1990 Biochemistry and turnover of lung interstitium. _Eur Respir J_ 3: 1048–1068 CAS PubMed Google Scholar * Nagase H 1997 Activation mechanisms of matrix metalloproteinases.
_Biol Chem_ 378: 151–160 CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Clinical Biochemistry, Institute of Clinical Science,
The Queen's University of Belfast, Belfast, BT12 6BJ, Northern Ireland, U.K. Bettina C Schock, Madeleine Ennis & Ian S Young * Child Health, Institute of Clinical Science, The
Queen's University of Belfast, Belfast, BT12 6BJ, Northern Ireland, U.K. David G Sweet & Henry L Halliday * School of Biological Sciences, University of Southampton, Biomedical
Sciences Building, Southampton, SO16 7PX, U.K. Jane A Warner Authors * Bettina C Schock View author publications You can also search for this author inPubMed Google Scholar * David G Sweet
View author publications You can also search for this author inPubMed Google Scholar * Madeleine Ennis View author publications You can also search for this author inPubMed Google Scholar *
Jane A Warner View author publications You can also search for this author inPubMed Google Scholar * Ian S Young View author publications You can also search for this author inPubMed Google
Scholar * Henry L Halliday View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Henry L Halliday. ADDITIONAL
INFORMATION Supported by a grant from the Northern Ireland Mother & Baby Action. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schock, B., Sweet,
D., Ennis, M. _et al._ Oxidative Stress and Increased Type-IV Collagenase Levels in Bronchoalveolar Lavage Fluid from Newborn Babies. _Pediatr Res_ 50, 29–33 (2001).
https://doi.org/10.1203/00006450-200107000-00008 Download citation * Received: 13 June 2000 * Accepted: 07 November 2000 * Issue Date: 01 July 2001 * DOI:
https://doi.org/10.1203/00006450-200107000-00008 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
