Oncogenic super-enhancer formation in tumorigenesis and its molecular mechanisms
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Super-enhancers (SEs) consist of a cluster of many enhancers bound to a great number of transcription factors. They are critical cis-regulatory elements that determine the identity
of various human cell types. During tumorigenesis, DNA mutations and indels, chromosomal rearrangements, three-dimensional chromatin structural changes, and viral infections mediate
oncogenic SE activation, and activated SEs have been found to regulate the expression of oncogenic genes. Inhibition specifically targeted to oncogenic SE assembly and activation provides a
novel powerful therapeutic strategy for various cancers. In this paper, we first introduce the current understanding of oncogenic SE assembly and activation and then summarize the pathogenic
factors and mechanism of oncogenic SE activation. Next, we elaborate on the oncogenic functions of SEs in cancers and the application of SEs as therapeutic targets. Finally, we turn our
focus to the use of SEs in basic research and clinical trials. SIMILAR CONTENT BEING VIEWED BY OTHERS SUPER-ENHANCERS AND NOVEL THERAPEUTIC TARGETS IN COLORECTAL CANCER Article Open access
11 March 2022 EPHA2 SUPER-ENHANCER PROMOTES TUMOR PROGRESSION BY RECRUITING FOSL2 AND TCF7L2 TO ACTIVATE THE TARGET GENE EPHA2 Article Open access 12 March 2021 ENHANCER REWIRING IN TUMORS:
AN OPPORTUNITY FOR THERAPEUTIC INTERVENTION Article 01 May 2021 INTRODUCTION The enhancer was first defined as a short DNA sequence from the Simian virus 40 genome that had a great ability
to enhance the transcription of its target genes in mammalian cells1. Since their discovery, enhancers have been increasingly studied, with a number of enhancers identified and their
structure and regulatory mechanism extensively clarified2. Transcription factors (TFs) bind to enhancers to recruit coactivators such as the mediator complex CREB-binding protein (CBP) and
p300 to alter the chromatin spatial structure, resulting in the interaction of TFs with enhancers, promoters or RNA polymerase2. Epigenetic modifications to histones and DNA have been proven
to be the main mediators of enhancer editing and maintenance. Histone modification is related to the active state of the enhancer; for example, monomethylation of histone H3 protein at
lysine 4 (H3K4me1) and acetylation at lysine 27 (H3K27ac) are correlated with functional enhancers3. Generally, enhancers are short noncoding DNA segments. They can be recognized by TFs and
activate transcription independent of enhancer position or orientation in the genome1. Enhancers are transcribed into enhancer RNAs (eRNAs), the expression level of which is associated with
the expression of genes nearby proximal enhancers, suggesting that enhancers play important roles in gene transcription4. In addition to the typical enhancers, there is another type of
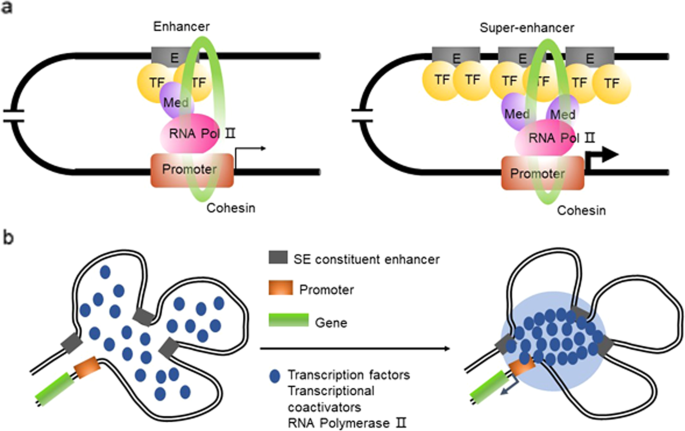
enhancer called super-enhancers (SE) or stretch enhancers, which frequently span several kilobases (averaging approximately 9 kb in length). SEs bind with abundant tissue-specific TFs in
various cells and master TFs, such as OCT4, SOX2, and Nanog, in embryonic stem cells5,6. Similar to typical enhancers, SEs are occupied by TFs, mediator complexes, chromatin regulators and
the RNA polymerase II (pol II) complex, but the density of these active molecules on SEs is several-fold that of typical enhancers. As a result, SEs can drive targeted gene transcription
more dramatically than typical enhancers5 (Fig. 1a). Moreover, SEs produce a higher level of eRNA (called seRNA) than is produced by a typical enhancer6. seRNA has extensive and far-reaching
significance in physiological, biochemical, and pathological processes7. SEs exhibit similar action mechanisms to typical enhancers. The interaction of an enhancer or RNA pol II with a
promoter is facilitated by an enhancer loaded with a cognate promoter to form a loop structure, and then, the basal RNA pol II transcription machinery is recruited to the promoter to
initiate downstream transcription8. SEs additively and synergistically influence each other, and constituent enhancers demonstrate a temporal and functional hierarchy9,10,11. Recently,
increasing evidence has revealed that SEs play vital roles in tumorigenesis and that SEs may be promising therapeutic targets for tumor treatment5. During tumorigenesis, DNA mutations and
indels12, chromosomal rearrangements13,14,15, three-dimensional (3D) chromatin structural changes16,17, and viral infections18,19 mediate the generation of oncogenic SEs that drive oncogene
transcription in the cells that acquire them15,20,21,22. In this paper, we elaborate on SE structure and activation modes, introduce SE regulatory mechanisms and explain their roles in the
initiation and development of tumors, and discuss therapeutic strategies targeting oncogenic SEs. SE CHARACTERISTICS AND IDENTIFICATION An SE is significantly different from a typical
enhancer (Fig. 1a). Currently, an SE is defined as a cluster of enhancers that spans a large region of the genome with a median size of 8.7 kb5. The components associated with enhancer
activity, such as mediator complexes, chromatin factors, H3K27ac and H3K4me1, histone acetyltransferases, p300 and CBP histone acetyltransferases, RNA pol II, and eRNA, are enriched in
association with SEs and exhibit increased chromatin accessibility6. SEs are characterized by the differential binding of tissue-specific TFs. The levels of master TFs such as OCT4, SOX2,
and Nanog are similar for typical enhancers and SEs, while Klf4 and Esrrb occupy SEs at significantly higher rates than they occupy typical enhancers5,6. Compared to typical enhancers, SEs
are more frequently bound by terminal transcription factors in the Wnt, TGF-β, and leukemia-inhibitory factor (LIF) signaling pathways, and SE-driven genes are much more sensitive than
typical enhancer-driven genes to perturbations in associated enhancer-binding transcriptional regulator genes5,11,21,22,23. Compared with typical enhancers, individual constituent enhancers
of SEs are capable of increasing transcriptional activation levels5. Some evidence indicates that constituent enhancers within an SE interact with each other additively or synergistically
and have nonredundant functions in gene regulation9,10,11, while the deletion of constituent enhancers may compromise the activity of other SE components9,11, leading to dysfunction of the
entire SE12. The formation of an SE is proposed in a schematic model (Fig. 1a). SEs have a high number of binding sites for TFs to which MEDs are recruited to alter the chromatin spatial
structure, resulting in the interaction of TFs with enhancers, promoters, or RNA pol24 (Fig. 1a). A phase separation model has been proposed to clarify the mechanisms underlying the
formation, function, and properties of SEs24,25 (Fig. 1b). Through a phase separation phenomenon similar to polymer condensation, heterogeneous mixtures of proteins and nucleic acids are
assembled into membrane-less organelle structures. Phase-separated biomolecule condensation is a mechanism by which biochemical reactions are compartmentalized and concentrated in cells26.
These membraneless organelles rapidly exchange components within the cellular milieu, which is readily altered in response to environmental cues. Dynamic, synergistic, and multivalent
intermolecular interactions are associated with liquid–liquid phase separation27. A recent study showed that intrinsically disordered regions (IDRs) of BRD4 and MED1 can form phase-separated
droplets at sites of SE-mediated transcription, and MED1-IDR droplets can compartmentalize and concentrate the transcription apparatus to maintain their separation from nuclear extracts.
Thus, it is speculated that SE condensates facilitate the compartmentalization and concentration of the transcriptional components at specific genes through the phase-separating properties
of the IDRs in the TFs and cofactors25. Initiation of phase-separated condensate formation has also been associated with the activation domains in the master TFs OCT4 and GCN4 and mediator
complexes28. The absence of cohesin leads to the extensive fusion of SEs in the nucleus, which has been implicated in constraining SE–SE interactions29. These reports provide a new model for
elucidating transcriptional regulation and explaining the different aspects of SE biology24. In SE identification, high-throughput sequencing and next-generation sequencing (NGS)
technologies provide particularly powerful tools for genome-wide identification and enhancer/SE prediction. These approaches are primarily based on chromatin immunoprecipitation followed by
high-throughput sequencing (ChIP-seq)30, DNase I coupled to high-throughput sequencing (DNase-seq)31, and chromosome conformation capture (3C)32. On the basis of these tools, a series of
derived methods, such as ChIP-exo33, FAIRE-seq34, GRO-seq35, ChIA-PET36, ATAC-seq37, STARR-seq38, Hi-C (chromosome conformation capture coupled to sequencing)39, and HiChIP40, have also been
applied to identify enhancers/SEs. ChIP-seq is a high-resolution, low-noise, and high-coverage research method for the genome-wide analysis of histone modification, nucleosome localization,
and the distribution of transcription factor-binding sites30. ChIP-seq uses histone modification marks to identify molecules presumably associated with SEs, such as transcription factors,
the transcription cofactor p300, and the H3K27ac and H3K4me1 histone modifications5,6,30. DNase-seq is a biotechnology that uses high-throughput sequencing technology to analyze DNase I
hypersensitive sites in enzyme-sensitive regions for enhancer prediction31. The analysis of the mediator cohesin was carried out by ChIP-seq, and the interaction between chromatin can be
directly analyzed by 3C, 4C (circularized chromosome conformation capture), 5C (chromosome conformation capture carbon copy), or Hi-C technology, with the genes related to enhancers
determined at the same time13,32. The identification of genome-wide enhancers/SEs enables a more systematic and comprehensive study of enhancers/SEs in biological processes. FUNCTIONS OF
ONCOGENIC SES During tumorigenesis, tumor cells acquire specific SEs to promote oncogene expression, which mediates the dysregulation of signaling pathways6,12,22,41. These specific SEs are
known as oncogenic SEs. Oncogenic SEs were first identified in multiple myeloma cells and bind at high density to MED1 and BRD421. H3K27ac ChIP-seq data have been used to identify SEs in 18
human cancer cells, including cells from colorectal, prostate, pancreatic, breast, lung, liver, and cervical cancers and multiple myeloma, CML, T cell leukemia, lymphocytic leukemia, and
glioblastoma6. In recent years, several oncogenic SEs have been found in various cancers, including neuroblastoma, small-cell lung cancer, medulloblastoma, esophageal cancer, gastric
cancers, and melanoma42. Oncogenic SEs promote cell malignancy by increasing oncogene transcription11. Mechanistically, oncogenic SEs may activate the MAPK signaling pathway to inhibit
apoptosis and increase cell proliferation43. SEs also mediate the overexpression of the v-ets erythroblastosis virus E26 oncogene homolog (ERG), resulting in target gene expression to
promote cancer development44. In addition, oncogenic SEs increase the expression of CYP24A1, GJA5, SLAMF7, and ETV145. Nucleus translocation of SEs increases MYB expression in adenoid cystic
carcinoma (ACC), and SEs promote the expression of TERT in pheochromocytomas and paragangliomas46. CRC-associated SEs are enriched at transcription factor 4 (TCF4) binding sites11. ChIP-seq
analysis of CRC cells showed that TCF4 is a terminal TF in the Wnt pathway and occupies the c-MYC locus. TCF4 is a target of Wnt signaling that shows a strong H3K27Ac signal after cancer
cells acquire oncogenic SEs11. ChIP-seq analysis of H3K27Ac in MCF-7 cells indicated that the SE-targeted _ESR1_ gene encodes only estrogen receptor alpha (ERα); furthermore, this oncogenic
transcription factor can distinguish cancer subtypes through distinct signaling pathways. In ER-positive breast cancer cells, SE-targeted genes are enriched in processes involved in ERα
binding, whereas in triple-negative breast cancer cells, the SE-enriched sites are different from those enriched by oncogenic TFs6,47. The general function of SEs may involve channeling
oncogenic signaling pathways into gene expression programs that are required for sustaining cancer development11. FORMATION OF ONCOGENIC SES A large number of genome-wide studies have
revealed that disease-related somatic variations occur mainly in noncoding genomes and are often enriched in regulatory regions48,49. Germline and somatic cells appear to acquire SEs through
various mechanisms, including genomic deletions, duplications, translocations, insertions, inversions, and single-nucleotide polymorphisms (SNPs). These genetic alterations can disrupt
TF-binding sites in putative SEs, modify SE copy number, and change the genomic space, which lead to SE activation or inhibition, ultimately resulting in the deregulation of nearby target
genes13,16. In summary, novel oncogenic SEs may originate through a variety of mechanisms, including (1) mutations and genomic alterations12,50,51,52, (2) chromosomal
rearrangements14,15,23,41,53,54,55, (3) spatial alterations in SE location by 3D chromatin structural changes16,17, and (4) viral oncogenes18,19,56,57. DNA MUTATIONS AND INDELS RESULT IN THE
FORMATION OF ONCOGENIC SES The sequences comprising enhancer/SE DNA are mutated to alter promoter and enhancer/SE function. In T cell acute lymphoblastic leukemia (T-ALL), small insertions
of 2–18 bp in the noncoding intergenic region upstream of the _TAL1_ oncogene produce de novo binding sites for the transcription factor MYB, resulting in SE formation to drive TAL1
expression12. Binding to these de novo sites, MYB recruits CBP/p300 acetyltransferase and TAL1 transcription factor complexes to promote the formation of oncogenic SEs and drive key gene
expression in leukemogenesis (Fig. 2a). In addition to small insertions, SNPs are often found to initiate the activity of an oncogenic SE. For example, in neuroblastoma cells, the formation
of an SE at the _LMO1_ oncogene locus is dependent on the binding of GATA3 to a conserved GATA-binding site. An SNP located near the SE alters a conserved GATA-binding motif, changing it to
a TATA motif, which results in a significant reduction in SE activity and LMO1 expression52. In addition, SNPs disrupt SEs associated with tumor suppressor genes to promote tumorigenesis. A
meta-analysis of genome-wide association studies showed that the 15q15.1 risk locus of the _BMF_ (BCL2-modifying factor) gene carries chronic lymphocytic leukemia (CLL) susceptibility. The
SNP in the 15q15.1 risk locus generates SEs to regulate the proapoptotic gene _BMF_ and disrupts the binding of the TF RELA to the SE, leading to an increase in BCL2 antiapoptotic function
and the promotion of tumorigenesis51 (Fig. 2b). CHROMOSOMAL REARRANGEMENTS GENERATE ONCOGENIC SES Genomic rearrangements, inversions, translocations, and deletions move SEs from their
natural genomic context to oncogene regions, leading to SE activation. This phenomenon is known as “Super-enhancer hijacking” and has been reported in various cancers, including acute
myeloid leukemia (AML), neuroblastoma, medulloblastoma, and colorectal cancer13,14,15,41,53. One classic example is the inversion of a 9-kb fragment in AML cells that redirects an SE from
its role as a _GATA2_ tumor suppressor to an _EV1_ oncogene enhancer, leading to the downregulation of tumor suppressors and oncogene activation15. Another example of enhancer hijacking was
observed in ACC, a chromosomal translocation repositioning a distal SE to a location proximal to the _MYB_ gene, leading to high MYB expression55. Further 3C analysis confirmed chromatin
interactions between the MYB promoter and the aberrantly translocated SE. Furthermore, the translocated SE element was found to contain MYB-binding sites, which were actively bound by MYB
itself to form a positive feedback loop, further enhancing MYB expression. Most of the samples from a subgroup of medulloblastomas, especially those with highly expressed growth factor
independent 1 family proto-oncogenes (_GFI1_ and _GFI1B_), had recurrent structural variations that resulted in the relocation of _GFI1_ and _GFI1B_ into close proximity of foci occupied by
active SEs, initiating oncogenic activity41. A recent example of enhancer hijacking was that of hybrid SEs generated by _C19MC_–_TTYH1_ gene fusions that amplified the _C19MC_–LIN28A–MYCN
oncogenic circuit and drove the expression of embryo-restricted DNMT3B6 to promote a primitive malignant epigenetic state in embryonal tumors with multilayered rosettes58. In addition to
genomic rearrangements, copy number variations can also result in oncogenic SE activation. Somatic copy number and tissue-specific epigenetic analyses of 12 cancer cell types showed that
focal amplification of SEs near _KLF5, USP12, PARD6B, MYC_, and other cancer-related genes could drive aberrant expression of oncogenes54. Another study also showed that aberrant
amplification of the 350–2000 kb genomic region containing the _MYCN_ oncogene in neuroblastoma increased MYCN levels23. 3D STRUCTURAL CHANGES PRODUCE ONCOGENIC SE FORMATION Mammalian
genomes are partitioned into a series of topologically associating domains (TADs) with an average size of approximately 1 Mb, and these TADs are the structural and functional units of
chromosomes that function to spatially confine transcriptional regulatory circuits29,59. These TAD structures are invariant across diverse cell types and evolutionarily conserved in related
species. Chromatin interactions are more frequent in TADs than they are outside TADs. It is now clear that TADs have the function of constraining long-range enhancer–promoter interactions,
thereby insulating promoters from distal enhancers and SEs. Both genetic and epigenetic disruption of TAD boundaries allows new genes and enhancers/SEs to occupy spaces associated with
enhancer/SE hijacking, altering regulatory contacts and leading to cancer16,17,59 (Fig. 2c). TADs are formed by a chromatin loop architecture and are often involve the looping factors
CCCTC-binding factor (CTCF) and cohesin. The presence of CTCF-associated boundary elements prevents ectopic contacts and insulates TADs from neighboring enhancers. Recently, a cis-expression
structural alteration mapping algorithm was developed as a framework to systematically predict cancer-related gene overexpression. Through this approach, scientists have identified a TAD
boundary deletion event that leads to the spreading of active chromatin to an adjacently fused TAD and generates an SE element, which can increase the expression of the _IRS4_ gene in
sarcoma and squamous cancer cells53. In another example, tandem duplications at the _IGF2_ locus were found to extend over the intervening TAD boundary and to encompass an SE in colorectal
cancer cells. This finding showed that the tandem duplication of _IGF2_ and SE elements in the adjoining TAD led to de novo TAD formation and _IGF2_ overexpression53. Furthermore, CTCF- and
cohesion-binding sites acquire mutations in multiple cancer cell types. For example, CTCF- and cohesin-haploinsufficient mice are predisposed to cancer60. In T-ALL cells, CTCF-binding site
disruption leads to the activation of _TAL1_ and _LMO2_ by regulatory elements outside of the insulated loops, resulting in T cell transformation61. In addition to mutations in TAD
boundaries, epigenetic deregulation has also been demonstrated to be a mechanism for TAD disruption in gliomas62. It has been reported that increased methylation at the CTCF site and reduced
CTCF binding result in the partial disruption of the TAD structure, which leads to the activation of PDGFRA (an oncogenic driver)62. VIRAL INFECTION MEDIATES SE FORMATION Virus infection
induces SE formation to drive high-level transcription of some key genes involved in cell proliferation and survival. Viruses with oncogenic activity include Epstein–Barr virus (EBV), human
papilloma virus (HPV), human T cell leukemia virus (HTLV), and human hepatitis B virus. After it infects human B cells, EBV produces oncoproteins, including EBNA2, 3A, 3C, and EBNALP. These
oncoproteins activate NF-κB subunits and bind to SEs to drive the transcription of prosurvival and antiapoptotic genes such as _MYC, MIR155, IKZF3_, and _BCL2_, facilitating lymphoblastoid
cell line growth18,19. Further studies showed that EBV SEs (ESEs) were transcribed into eRNAs, which facilitated the transcriptional activation of the _MYC_ oncogene. Silencing MYC ESE eRNA
inhibited the growth of cells56. A recent study indicated that the high-risk HPV oncoprotein E6 activates cervical cancer SEs to promote tumorigenesis by targeting the histone demethylase
KDM5C57. Human lymphotropic virus type I (HTLV-I) frequently initiates adult T cell leukemia/lymphoma (ATLL). The proliferation of ATLL cells depends on BATF3 and IRF4, which cooperatively
drive ATLL-specific gene expression. The viral transcription factor HBZ is expressed in all ATLL cases, and HBZ binds to the BATF3 SE and regulates the expression of the BATF3 and MYC genes,
thereby contributing to ATLL cell proliferation63. ONCOGENIC SES MEDIATE THE ACTIVATION OF SIGNALING PATHWAYS AND THEIR MECHANISMS Some oncogenic SEs activate several pathways, including
Wnt11,64,65, TGF-β11,66, and LIF11,67, by regulating target genes. Moreover, oncogenic SEs are enriched in TF-binding sites that are associated with cancer signaling pathways11. These
findings support the idea that SEs act as platforms for integrating regulatory signals that trigger target gene expression. The Wnt pathway plays an important role in oncogenic SE mediation
of tumorigenesis. A previous study showed that Wnt pathway-related SEs were enriched in binding sites for TCF4 (a terminal TF in the Wnt pathway) in colorectal cancer cells driven by the
oncogenic Wnt pathway11. In a mouse model of basal cell carcinoma (BCC), a cell identity switch was enabled by a mostly permissive chromatin state accompanied by rapid Wnt pathway activation
and reprogramming of the associated SEs. Treatment of BCC with Wnt pathway inhibitors reduced the residual tumor burden and enhanced cell differentiation64. Wnt signaling collaborates with
chromatin architecture to post-transcriptionally dysregulate the expression of canonical cancer drivers. Recently obtained evidence has revealed that Wnt signaling and AHCTF1 promote
oncogenic MYC expression through SE-mediated genes65. The cancer cell-specific gating of MYC is regulated by AHCTF1 (also known as ELYS), which connects nucleoporins to oncogenic SEs _via_
β-catenin65. In addition, TGF-β and LIF signaling also play vital roles in the development of cancers. TGF-β signaling is particularly important for increased tumor aggression and
metastasis. In pancreatic cancer cells, the deletion of an SE in TGFBR2 significantly downregulated the expression of TGFBR2, resulting in impairment of the migration and EMT induced by
TGF-β66. LIF was identified as an essential factor under the control of a cancer-specific SE. Osteosarcoma cells treated with a LIF recombinant protein displayed upregulated metastasis. UTX
is a key activator of LIF transcription. GSK-J4, a UTX inhibitor, impaired SEs at the LIF gene locus, leading to LIF signaling pathway inhibition and subsequent defects 67. In addition to
the aforementioned signaling pathways, there are still many others that have important relationships with oncogenic SEs. Mutational RAS activity promotes oncogenic SE formation. The
inhibition of aberrant RAS signaling results in the loss of active enhancer marks, SE decommissioning, and decreased gene expression68. In addition, RAS signaling can directly modulate SE
function at enhancers by regulating the release of paused transcription. In rhabdomyosarcoma, for example, through the RAF–MEK–ERK MAPK pathway, oncogenic RAS inhibits myogenic
differentiation by reducing MYOG expression, which is mediated by ERK2-dependent promoter-proximal stalling of RNA pol II at the MYOG locus. MEK inhibition with trametinib results in the
loss of ERK2 at the MYOG promoter and the release of transcriptionally stalled MYOG expression, accompanied by the opening of chromatin and the establishment of SEs at myogenic-specific
genes69. THERAPEUTIC STRATEGIES TARGETING ONCOGENIC SES IN CANCER CELLS Increasing evidence has revealed that SEs play vital roles in tumorigenesis, and oncogenic SEs could be promising
therapeutic targets for cancer treatment. Inhibiting SE-driven oncogenic transcription is effective for therapy but presents significant challenges because transcription is a fundamental
biological process common to all living cells20. Therefore, transcription inhibitors must selectively target oncogenic transcription while inducing only minimal toxicity in normal cells.
Currently, there are two main kinds of small molecule inhibitors for targeting oncogenic SEs, BET inhibitors (BETis)70 and cyclin-dependent kinase (CDK) inhibitors71, which can selectively
kill cancer cells by inhibiting the transcription of oncogenic SEs. The former are competitive inhibitors of bromodomain and extraterminal domain (BET) family proteins (BRD2, BRD3, BRD4, and
BRDT), while the latter mainly target CDK7 and CDK9. Transcription initiation, pausing, and elongation proceed through ordered activation of regulatory and enzymatic cofactors. Active
oncogenic SEs enriched with H3K27ac marks can be recognized by BRD4, which interacts with the mediator coactivator complex, leading to the stepwise recruitment of CDK7 (a component of the
TFIIH general transcription factor complex) and CDK9 (a component of P-TEFb, i.e., positive transcription elongation factor b)72. CDK7 is thought to primarily control transcriptional
initiation by phosphorylating the RNA pol II C-terminal domain (CTD) at serine 5 (S5), serine 7 (S7), and TFIIE. CDK7 inhibition affects capping, pausing, elongation, and termination mostly
through the phosphorylation of CTD and CDK9 in the activated T-loop. Ultimately, CDK7 facilitates the recruitment of the histone methyltransferases SETD1A/B and SETD2 through CTD
phosphorylation and/or the activation of CDK9/P-TEFb71. CDK9 has been proven to control elongation. As a major CTD serine 2 (S2) kinase, CDK9 phosphorylates the NELF-E subunit of NELF and
the SPT5 subunit of DSIF, allowing the release of RNA pol II that is paused at the proximal promoter to induce productive elongation73. SMALL MOLECULE INHIBITORS TARGETING ONCOGENIC SES
Cancer cells hijack SEs to drive oncogene transcription, continuously promoting cell survival and proliferation. This aberrant SE-driven transcriptional event provides a new avenue for
anticancer therapy. Thus, several small molecule inhibitors have been developed, and their potential preclinical effects _in vivo_ and _in vitro_ have been observed47,74. Some of them showed
promising results in models established _in vitro_ but have had largely disappointing results in clinical trials75. The first-generation CDK inhibitors, referred to as “pan-CDK” inhibitors,
exhibited low affinity for CDKs and high cytotoxicity _in vivo_75. For example, flavopiridol, the most extensively investigated CDK inhibitor, can induce cell cycle arrest in the G1 and G2
phases in certain contexts and induce a cytotoxic response. Although it has broad-spectrum _in vitro_ activity, it was less active _in vivo_. Tumor lysis syndrome was reported in
approximately 40% of CLL patients treated with flavopiridol76. Thus, second-generation CDK inhibitors were developed with the aim of increased selectivity. BET inhibitors show a favorable
activity profile, and hematologic (mainly thrombocytopenia) and nonhematologic adverse events (gastrointestinal toxicities, fatigue, bilirubin increase, etc.) are reversible upon treatment
interruption70. Previous studies have shown that SE-driven genes have a higher sensitivity to chromatin/transcriptional regulator inhibition than traditional enhancer-driven genes11,23.
Treatment with the BETi JQ1 led to preferential loss of the BRD4 associated with SEs and consequent transcriptional elongation defects21. In other studies, heightened sensitivity was
potentially attributed to at least two complementary mechanisms: (1) the cooperativity of constituent enhancers and (2) the short half-lives of oncogenic TFs23,77. SEs enriched with master
TFs maintain TF expression _via_ feedforward loops, and SE depletion may result in reduced transcription. In _MYCN_-amplified neuroblastoma, THZ1 treatment led to preferential downregulation
of SE-associated genes, including _MYCN_, thus inhibiting the autoregulated suppression of MYCN-driven global transcription amplification23. BET INHIBITORS Thienotriazolodiazepines were
first characterized with antitumor activity and as inhibitors of acetylated histones that bind to bromodomain-containing proteins. A seminal report demonstrated that BETis could induce
terminal differentiation and apoptosis in preclinical NUT (nuclear protein in testis) midline carcinoma models78. Chromosomal translocation, involving the NUT gene fusing to the BET gene
BRD4, creates an in-frame BRD4–NUT oncogene, resulting in NUT midline carcinoma. Silencing of the BRD4–NUT fusion gene with BETis prevents the differentiation and proliferation of NUT
carcinoma cells78. In preclinical models of AML and multiple myeloma, BETis (I-BET151 and JQ1) were reported to have a strong inhibitory effect on tumor progression79,80. Recent reports have
also demonstrated that novel BETis have clear preclinical antitumor activity in a variety of solid tumors and hematologic cancers81. BETis target bromodomains and directly affect major
transcription factors and key tissue- or cancer-specific genes, such as _MYC_82, _AR_ and _TMPRSS2–ETS_ fusion genes83, _TERT_80, _BCL2_80, _FOSL1_84, _E2F2_85, _ITK_86, _IL7R_82, _CDK6_85,
_IRF4_87, and _IKZF1_80,87. In the BET family, BRD4 is considered to be an excellent target of BETis because of its important role in transcription. BETi-sensitive genes are associated with
adjacent SEs. The TFs YAP and TAZ play crucial roles in the recruitment of BRD4 to SEs, and the enhancers/SEs occupied by YAP/TAZ show a preferential loss of BRD4 and sensitivity to JQ1
treatment88. Several BETi compounds (Table 1) have entered phase I or II clinical trials. Although these studies are still in the initial stage, they provide a new direction for the clinical
treatment of cancers. TRANSCRIPTIONAL CDK INHIBITORS Historically, only three CDKs, CDK7, CDK9, and CDK8, were thought to be involved in the regulation of the transcription cycle. The
discovery of small molecule inhibitors has provided another potential approach for targeting oncogenic SEs. In addition to pan-CDK inhibitors, there are many inhibitors selectively targeting
CDK7 or CDK9, such as THZ1 and LDC067 (Table 1). THZ1 suppresses CDK7 by modifying cysteine 31289, with more than one-half of 1000 cancer cell lines showing IC50 values for THZ1 < 200
nM, leading to global transcriptional downregulation at high doses6. It was initially shown that sensitivity to THZ1 was conferred by targeting SE-driven RUNX1 in T-ALL89. Further reports
revealed that THZ1 can selectively target SE-driven transcriptional processes in preclinical cell and mouse tumor models, such as those of triple-negative breast cancer (TNBC)47,
MYCN-amplified neuroblastoma23, and small-cell lung cancer77. In AML preclinical models, CKIα inhibitors targeting CDK7 and CDK9 augment CKIα-induced p53 activation, suppress SE-driven
oncogenes, and induce apoptosis90. LDC067 is one of the first compounds found to have CDK9 selectivity, and it has been proven to have 55–230-fold greater selectivity for CDK9 than for the
other CDKs91. Treatment with LDC067 can inhibit transcription and induce apoptosis in HeLa cells and primary AML blasts91. CDK8 has been implicated in the transcription driven by
SE-controlled genes. Cortistatin A exhibits high affinity for CDK8 and CDK19 and has antiproliferative activity against multiple leukemia cell lines _in vitro_ and in AML mouse models92.
THZ531, a CDK12/13 inhibitor, synergizes with PARP inhibitors in models of Ewing sarcoma _in vitro_ and _in vivo_93. Although structural and biological validation of these inhibitors has not
been completed, their suppression of SE-associated transcriptional regulators provides a novel approach for targeting oncogenes. CONCLUDING REMARKS SEs play vital roles in transcriptional
regulation and have pathogenic ability, especially oncogenic ability, in a context-dependent manner. Despite compelling evidence that SEs regulate cell identity genes, there is still no
clear understanding of how SEs play regulatory roles. NGS technology recently provided a new means of mapping the genomic landscape in greater detail. ChIP-seq data and bioinformatics
algorithms are being utilized to identify genomic proximity and assign SEs to target genes. However, knowledge of the intrinsic structure of SEs and of their interactions with target genes
in three-dimensional space is still lacking; therefore, a comprehensive approach involving 5C and functional screening is needed. Other new technologies, including Hi-C, ATAC-seq, Hi-ChIP,
CUT&Tag, the CRISPR genome-editing tool, and single-cell sequencing technology, are also being used to reveal the mechanisms of SEs that regulate transcription and oncogenesis. From a
therapeutic standpoint, the discovery of SE targeting by JQ1 led to the development of first- and second-generation BET inhibitors. Since BETs were discovered, small molecule inhibitors
targeting individual SE components have shown great promise for clinical application. However, resistance to single-agent treatments of BETis94 and THZ195 has been reported in many
preclinical models. Therefore, future exploration of SEs will focus on clarifying how SE components regulate SE function and how to better utilize SEs in targeted therapy. REFERENCES *
Banerji, J., Rusconi, S. & Schaffner, W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. _Cell_ 27, 299–308 (1981). Article CAS PubMed Google Scholar *
Spitz, F. & Furlong, E. E. M. Transcription factors: from enhancer binding to developmental control. _Nat. Rev. Genet._ 13, 613–626 (2012). Article CAS PubMed Google Scholar *
Kundaje, A. et al. Integrative analysis of 111 reference human epigenomes. _Nature_ 518, 317–330 (2015). Article CAS PubMed PubMed Central Google Scholar * Leveille, N. et al.
Genome-wide profiling of p53-regulated enhancer RNAs uncovers a subset of enhancers controlled by a lncRNA. _Nat. Commun._ 6, 6520 (2015). Article CAS PubMed Google Scholar * Whyte, W.
A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. _Cell_ 153, 307–319 (2013). Article CAS PubMed PubMed Central Google Scholar *
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. _Cell_ 155, 934–947 (2013). Article CAS PubMed Google Scholar * Hah, N. et al. Inflammation-sensitive super
enhancers form domains of coordinately regulated enhancer RNAs. _Proc. Natl. Acad. Sci. U.S.A._ 112, E297–E302 (2015). Article CAS PubMed PubMed Central Google Scholar * Santos-Pereira,
J. M. & Aguilera, A. R loops: new modulators of genome dynamics and function. _Nat. Rev. Genet._ 16, 583–597 (2015). Article CAS PubMed Google Scholar * Shin, H. Y. et al. Hierarchy
within the mammary STAT5-driven Wap super-enhancer. _Nat. Genet._ 48, 904–911 (2016). Article CAS PubMed PubMed Central Google Scholar * Hay, D. et al. Genetic dissection of the
alpha-globin super-enhancer in vivo. _Nat. Genet._ 48, 895–903 (2016). Article CAS PubMed PubMed Central Google Scholar * Hnisz, D. et al. Convergence of developmental and oncogenic
signaling pathways at transcriptional super-enhancers. _Mol. Cell_ 58, 362–370 (2015). Article CAS PubMed PubMed Central Google Scholar * Mansour, M. R. et al. Oncogene regulation. An
oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. _Science_ 346, 1373–1377 (2014). Article CAS PubMed PubMed Central Google Scholar * Krijger,
P. H. & de Laat, W. Regulation of disease-associated gene expression in the 3D genome. _Nat. Rev. Mol. Cell Biol._ 17, 771–782 (2016). Article CAS PubMed Google Scholar * Valentijn,
L. J. et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. _Nat. Genet._ 47, 1411–1414 (2015). Article CAS PubMed Google Scholar * Groschel, S. et al.
A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. _Cell_ 157, 369–381 (2014). Article CAS PubMed Google Scholar * Spielmann, M.,
Lupiáñez, D. G. & Mundlos, S. Structural variation in the 3D genome. _Nat. Rev. Genet._ 19, 453–467 (2018). Article CAS PubMed Google Scholar * Furlong, E. E. M. & Levine, M.
Developmental enhancers and chromosome topology. _Science_ 361, 1341–1345 (2018). Article CAS PubMed PubMed Central Google Scholar * Gunnell, A. et al. RUNX super-enhancer control
through the Notch pathway by Epstein–Barr virus transcription factors regulates B cell growth. _Nucleic Acids Res._ 44, 4636–4650 (2016). Article CAS PubMed PubMed Central Google Scholar
* Zhou, H. et al. Epstein–Barr virus oncoprotein super-enhancers control B cell growth. _Cell Host Microbe_ 17, 205–216 (2015). Article CAS PubMed PubMed Central Google Scholar *
Bradner, J. E., Hnisz, D. & Young, R. A. Transcriptional addiction in cancer. _Cell_ 168, 629–643 (2017). Article CAS PubMed PubMed Central Google Scholar * Loven, J. et al.
Selective inhibition of tumor oncogenes by disruption of super-enhancers. _Cell_ 153, 320–334 (2013). Article CAS PubMed PubMed Central Google Scholar * Chapuy, B. et al. Discovery and
characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. _Cancer Cell_ 24, 777–790 (2013). Article CAS PubMed PubMed Central Google Scholar *
Chipumuro, E. et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. _Cell_ 159, 1126–1139 (2014). Article CAS PubMed PubMed Central
Google Scholar * Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. _Cell_ 169, 13–23 (2017). Article CAS
PubMed PubMed Central Google Scholar * Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. _Science_ 361, eaar3958 (2018). Article
PubMed PubMed Central CAS Google Scholar * Strom, A. R. et al. Phase separation drives heterochromatin domain formation. _Nature_ 547, 241–245 (2017). Article CAS PubMed PubMed
Central Google Scholar * Lin, Y., Protter, D. S., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. _Mol. Cell_ 60, 208–219
(2015). Article CAS PubMed PubMed Central Google Scholar * Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains.
_Cell_ 175, 1842–1855 (2018). Article CAS PubMed Google Scholar * Rao, S. S. P. et al. Cohesin loss eliminates all loop domains. _Cell_ 171, 305–320 (2017). Article CAS PubMed PubMed
Central Google Scholar * Visel, A. et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. _Nature_ 457, 854–858 (2009). Article CAS PubMed PubMed Central Google
Scholar * Boyle, A. P. et al. High-resolution mapping and characterization of open chromatin across the genome. _Cell_ 132, 311–322 (2008). Article CAS PubMed PubMed Central Google
Scholar * Jin, F. et al. A high-resolution map of the three-dimensional chromatin interactome in human cells. _Nature_ 503, 290–294 (2013). Article CAS PubMed PubMed Central Google
Scholar * Rhee, H. S. & Pugh, B. F. Comprehensive genome-wide protein–DNA interactions detected at single-nucleotide resolution. _Cell_ 147, 1408–1419 (2011). Article CAS PubMed
PubMed Central Google Scholar * Bell, O., Tiwari, V. K., Thoma, N. H. & Schubeler, D. Determinants and dynamics of genome accessibility. _Nat. Rev. Genet._ 12, 554–564 (2011). Article
CAS PubMed Google Scholar * Kim, T. K. et al. Widespread transcription at neuronal activity-regulated enhancers. _Nature_ 465, 182–187 (2010). Article CAS PubMed PubMed Central
Google Scholar * Fullwood, M. J. et al. An oestrogen-receptor-alpha-bound human chromatin interactome. _Nature_ 462, 58–64 (2009). Article CAS PubMed PubMed Central Google Scholar *
Zhao, X. et al. BCL2 amplicon loss and transcriptional remodeling drives ABT-199 resistance in B cell lymphoma models. _Cancer Cell_ 35, 752–766 (2019). Article CAS PubMed PubMed Central
Google Scholar * Arnold, C. D. et al. Genome-wide quantitative enhancer activity maps identified by STARR-seq. _Science_ 339, 1074–1077 (2013). Article CAS PubMed Google Scholar *
Mifsud, B. et al. Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. _Nat. Genet._ 47, 598–606 (2015). Article CAS PubMed Google Scholar * Mumbach, M.
R. et al. HiChIP: efficient and sensitive analysis of protein-directed genome architecture. _Nat. Methods_ 13, 919–922 (2016). Article CAS PubMed PubMed Central Google Scholar *
Northcott, P. A. et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. _Nature_ 511, 428–434 (2014). Article CAS PubMed PubMed Central Google Scholar * Sengupta,
S. & George, R. E. Super-enhancer-driven transcriptional dependencies in cancer. _Trends Cancer_ 3, 269–281 (2017). Article CAS PubMed PubMed Central Google Scholar * Nakamura, Y.
et al. Targeting of super-enhancers and mutant BRAF can suppress growth of BRAF -mutant colon cancer cells via repression of MAPK signaling pathway. _Cancer Lett._ 402, 100–109 (2017).
Article CAS PubMed Google Scholar * Babu, D. & Fullwood, M. J. Expanding the effects of ERG on chromatin landscapes and dysregulated transcription in prostate cancer. _Nat. Genet._
49, 1294–1295 (2017). Article CAS PubMed Google Scholar * Shen, Y. et al. Recombinant decorin fusion protein attenuates murine abdominal aortic aneurysm formation and rupture. _Sci.
Rep._ 7, 15857 (2017). Article PubMed PubMed Central CAS Google Scholar * Dwight, T. et al. TERT structural rearrangements in metastatic pheochromocytomas. _Endocr.-Relat. Cancer_ 25,
1–9 (2018). Article CAS PubMed Google Scholar * Wang, Y. et al. CDK7-dependent transcriptional addiction in triple-negative breast cancer. _Cell_ 163, 174–186 (2015). Article CAS
PubMed PubMed Central Google Scholar * Schaub, M. A., Boyle, A. P., Kundaje, A., Batzoglou, S. & Snyder, M. Linking disease associations with regulatory information in the human
genome. _Genome Res._ 22, 1748–1759 (2012). Article CAS PubMed PubMed Central Google Scholar * Maurano, M. T. et al. Systematic localization of common disease-associated variation in
regulatory DNA. _Science_ 337, 1190–1195 (2012). Article CAS PubMed PubMed Central Google Scholar * Zhang, X. et al. BRCA1 mutations attenuate super-enhancer function and chromatin
looping in haploinsufficient human breast epithelial cells. _Breast Cancer Res._ 21, 51 (2019). Article PubMed PubMed Central Google Scholar * Kandaswamy, R. et al. Genetic
predisposition to chronic lymphocytic leukemia is mediated by a BMF super-enhancer polymorphism. _Cell Rep._ 16, 2061–2067 (2016). Article CAS PubMed PubMed Central Google Scholar *
Oldridge, D. A. et al. Genetic predisposition to neuroblastoma mediated by a LMO1 super-enhancer polymorphism. _Nature_ 528, 418–421 (2015). Article CAS PubMed PubMed Central Google
Scholar * Weischenfeldt, J. et al. Pan-cancer analysis of somatic copy-number alterations implicates IRS4 and IGF2 in enhancer hijacking. _Nat. Genet._ 49, 65–74 (2017). Article CAS
PubMed Google Scholar * Zhang, X. et al. Identification of focally amplified lineage-specific super-enhancers in human epithelial cancers. _Nat. Genet._ 48, 176–182 (2016). Article CAS
PubMed Google Scholar * Drier, Y. et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. _Nat. Genet._ 48, 265–272 (2016). Article CAS PubMed
PubMed Central Google Scholar * Liang, J. et al. Epstein–Barr virus super-enhancer eRNAs are essential for MYC oncogene expression and lymphoblast proliferation. _Proc. Natl. Acad. Sci.
U.S.A._ 113, 14121–14126 (2016). Article CAS PubMed PubMed Central Google Scholar * Chen, X. et al. E6 protein expressed by high-risk HPV activates super-enhancers of the EGFR and c-MET
oncogenes by destabilizing the histone demethylase KDM5C. _Cancer Res._ 78, 1418–1430 (2018). Article CAS PubMed Google Scholar * Sin-Chan, P. et al. A C19MC-LIN28A-MYCN oncogenic
circuit driven by hijacked super-enhancers is a distinct therapeutic vulnerability in ETMRs: a lethal brain tumor. _Cancer Cell_ 36, 51–67 (2019). Article CAS PubMed Google Scholar *
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. _Nature_ 485, 376–380 (2012). Article CAS PubMed PubMed Central Google
Scholar * Viny, A. D. et al. Dose-dependent role of the cohesin complex in normal and malignant hematopoiesis. _J. Exp. Med._ 212, 1819–1832 (2015). Article CAS PubMed PubMed Central
Google Scholar * Hnisz, D. et al. Activation of proto-oncogenes by disruption of chromosome neighborhoods. _Science_ 351, 1454–1458 (2016). Article CAS PubMed PubMed Central Google
Scholar * Flavahan, W. A. et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. _Nature_ 529, 110–114 (2016). Article CAS PubMed Google Scholar * Nakagawa, M. et
al. Targeting the HTLV-I-regulated BATF3/IRF4 transcriptional network in adult T cell leukemia/lymphoma. _Cancer Cell_ 34, 286–297 (2018). Article CAS PubMed PubMed Central Google
Scholar * Biehs, B. et al. A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. _Nature_ 562, 429–433 (2018). Article CAS PubMed Google Scholar * Scholz,
B. A. et al. WNT signaling and AHCTF1 promote oncogenic MYC expression through super-enhancer-mediated gene gating. _Nat. Genet._ 51, 1723–1731 (2019). Article CAS PubMed Google Scholar
* Zhu, X. et al. A super-enhancer controls TGF-beta signaling in pancreatic cancer through downregulation of TGFBR2. _Cell Signal._ 66, 109470 (2019). Article PubMed Google Scholar * Lu,
B. et al. Epigenetic profiling identifies LIF as a super-enhancer-controlled regulator of stem cell-like properties in osteosarcoma. _Mol. Cancer Res._
https://doi.org/10.1158/1541-7786.MCR-19-0470 (2019). Article PubMed PubMed Central Google Scholar * Nabet, B. et al. Deregulation of the Ras-Erk signaling axis modulates the enhancer
landscape. _Cell Rep._ 12, 1300–1313 (2015). Article CAS PubMed PubMed Central Google Scholar * Yohe, M. E. et al. MEK inhibition induces MYOG and remodels super-enhancers in RAS-driven
rhabdomyosarcoma. _Sci. Trans. Med._ 10, eaan4470 (2018). Article CAS Google Scholar * Stathis, A. & Bertoni, F. BET proteins as targets for anticancer treatment. _Cancer Discov._ 8,
24–36 (2018). Article CAS PubMed Google Scholar * Galbraith, M. D., Bender, H. & Espinosa, J. M. Therapeutic targeting of transcriptional cyclin-dependent kinases. _Transcription_
10, 118–136 (2019). Article CAS PubMed Google Scholar * Hajmirza, A. et al. BET family protein BRD4: an emerging actor in NFkappaB signaling in inflammation and cancer. _Biomedicines_ 6,
16 (2018). Article PubMed Central CAS Google Scholar * Zhou, Q., Li, T. & Price, D. H. RNA polymerase II elongation control. _Annu. Rev. Biochem._ 81, 119–143 (2012). Article CAS
PubMed PubMed Central Google Scholar * Berthon, C. et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. _Lancet Haematol._ 3, e186–e195
(2016). Article PubMed Google Scholar * Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy.
_Nat. Rev. Drug Discov._ 14, 130–146 (2015). Article CAS PubMed PubMed Central Google Scholar * Bose, P., Simmons, G. L. & Grant, S. Cyclin-dependent kinase inhibitor therapy for
hematologic malignancies. _Expert Opin. Investig. Drugs_ 22, 723–738 (2013). Article CAS PubMed PubMed Central Google Scholar * Christensen, C. L. et al. Targeting transcriptional
addictions in small cell lung cancer with a covalent CDK7 inhibitor. _Cancer Cell_ 26, 909–922 (2014). Article CAS PubMed PubMed Central Google Scholar * Filippakopoulos, P. et al.
Selective inhibition of BET bromodomains. _Nature_ 468, 1067–1073 (2010). Article CAS PubMed PubMed Central Google Scholar * Zuber, J. et al. RNAi screen identifies Brd4 as a
therapeutic target in acute myeloid leukaemia. _Nature_ 478, 524–528 (2011). Article CAS PubMed PubMed Central Google Scholar * Delmore, J. E. et al. BET bromodomain inhibition as a
therapeutic strategy to target c-Myc. _Cell_ 146, 903–916 (2011). Article CAS Google Scholar * Ali, I., Choi, G. & Lee, K. BET inhibitors as anticancer agents: a patent review.
_Recent Pat. Anti-Cancer Drug Discov._ 12, 340–364 (2017). Article CAS Google Scholar * Bernasconi, E. et al. Preclinical evaluation of the BET bromodomain inhibitor BAY 1238097 for the
treatment of lymphoma. _Br. J. Haematol._ 178, 936–948 (2017). Article CAS PubMed Google Scholar * Faivre, E. J. et al. Exploitation of castration-resistant prostate cancer transcription
factor dependencies by the novel BET inhibitor ABBV-075. _Mol. Cancer Res._ 15, 35–44 (2017). Article CAS PubMed Google Scholar * Baker, E. K. et al. BET inhibitors induce apoptosis
through a MYC independent mechanism and synergise with CDK inhibitors to kill osteosarcoma cells. _Sci. Rep._ 5, 10120 (2015). Article CAS PubMed PubMed Central Google Scholar *
Riveiro, M. E. et al. OTX015 (MK-8628), a novel BET inhibitor, exhibits antitumor activity in non-small cell and small cell lung cancer models harboring different oncogenic mutations.
_Oncotarget_ 7, 84675–84687 (2016). Article PubMed PubMed Central Google Scholar * Rhyasen, G. W. et al. AZD5153: a novel bivalent BET bromodomain inhibitor highly active against
hematologic malignancies. _Mol. Cancer Ther._ 15, 2563–2574 (2016). Article CAS PubMed Google Scholar * Siu, K. T. et al. Preclinical activity of CPI-0610, a novel small-molecule
bromodomain and extra-terminal protein inhibitor in the therapy of multiple myeloma. _Leukemia_ 31, 1760–1769 (2017). Article CAS PubMed Google Scholar * Zanconato, F. et al.
Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. _Nat. Med._ 24, 1599–1610 (2018). Article CAS PubMed PubMed Central Google Scholar * Kwiatkowski, N. et
al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. _Nature_ 511, 616–620 (2014). Article CAS PubMed PubMed Central Google Scholar * Minzel, W. et al. Small
molecules co-targeting CKIalpha and the transcriptional kinases CDK7/9 control AML in preclinical models. _Cell_ 175, 171–185 (2018). Article CAS PubMed PubMed Central Google Scholar *
Albert, T. K. et al. Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor. _Br. J. Pharm._ 171, 55–68 (2014). Article
CAS Google Scholar * Pelish, H. E. et al. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. _Nature_ 526, 273–276 (2015). Article CAS PubMed PubMed
Central Google Scholar * Iniguez, A. B. et al. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. _Cancer Cell_ 33, 202–216 (2018). Article CAS PubMed
PubMed Central Google Scholar * Fong, C. Y. et al. BET inhibitor resistance emerges from leukaemia stem cells. _Nature_ 525, 538–542 (2015). Article CAS PubMed PubMed Central Google
Scholar * Gao, Y. et al. Overcoming resistance to the THZ series of covalent transcriptional CDK inhibitors. _Cell Chem. Biol._ 25, 135–142 (2018). Article CAS PubMed Google Scholar *
Bhagwat, A. S. et al. BET bromodomain inhibition releases the mediator complex from select cis-regulatory elements. _Cell Rep._ 15, 519–530 (2016). Article CAS PubMed PubMed Central
Google Scholar * Hottinger, A. F. et al. Dose optimization of MK-8628 (OTX015), a small molecule inhibitor of bromodomain and extra-terminal (BET) proteins, in patients (pts) with recurrent
glioblastoma (GB). _J. Clin. Oncol._ 34, e14123 (2016). Article Google Scholar * Henssen, A. et al. Targeting MYCN-driven transcription by BET-bromodomain inhibition. _Clin. Cancer Res._
22, 2470–2481 (2016). Article CAS PubMed Google Scholar * Boi, M. et al. The BET bromodomain inhibitor OTX015 affects pathogenetic pathways in preclinical B-cell tumor models and
synergizes with targeted drugs. _Clin. Cancer Res._ 21, 1628–1638 (2015). Article CAS PubMed Google Scholar * Abramson, J. S. et al. BET inhibitor CPI-0610 is well tolerated and induces
responses in diffuse large B-cell lymphoma and follicular lymphoma: preliminary analysis of an ongoing Phase 1 study. _Blood_ 126, 1491 (2015). Article Google Scholar * Guo, N. H., Zheng,
J. F., Zi, F. M. & Cheng, J. I-BET151 suppresses osteoclast formation and inflammatory cytokines secretion by targetting BRD4 in multiple myeloma. _Biosci. Rep._ 39, 12 (2019). Google
Scholar * Postel-Vinay, S. et al. First-in-human phase I dose escalation study of the Bromodomain and Extra-Terminal motif (BET) inhibitor BAY 1238097 in subjects with advanced
malignancies. _Eur. J. Cancer_ 69, S7–S8 (2016). Article Google Scholar * Eliades, P. et al. High MITF expression is associated with super-enhancers and suppressed by CDK7 inhibition in
melanoma. _J. Investig. Dermatol._ 138, 1582–1590 (2018). Article CAS PubMed Google Scholar * Jiang, Y. Y. et al. Targeting super-enhancer-associated oncogenes in oesophageal squamous
cell carcinoma. _Gut_ 66, 1358–1368 (2017). Article CAS PubMed Google Scholar * Hu, S. et al. Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. _Cancer
Res._ 79, 3479–3491 (2019). Article PubMed Google Scholar * Gerlach, D. et al. The novel BET bromodomain inhibitor BI 894999 represses super-enhancer-associated transcription and
synergizes with CDK9 inhibition in AML. _Oncogene_ 37, 2687–2701 (2018). Article CAS PubMed PubMed Central Google Scholar * Kennedy, A. L. et al. Functional, chemical genomic, and
super-enhancer screening identify sensitivity to cyclin D1/CDK4 pathway inhibition in Ewing sarcoma. _Oncotarget_ 6, 30178–30193 (2015). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported in part by the National Natural Science Foundation of China (81872226 and 81502346), Natural Science Foundation of Hunan Province
(2018JJ6131 and 2019JJ40175), Changsha Science and Technology Project (kg1801107), and Research Projects of Hunan Health Commission (B2019084). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Hunan Key Laboratory of Oncotarget Gene and Clinical Laboratory, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha,
410013, China Qunying Jia, Yuan Tan, Yuejin Li & Faqing Tang * Department of Otolaryngology, The Second People’s Hospital of Foshan, Foshan, 528000, Guangdong, China Shuhua Chen Authors
* Qunying Jia View author publications You can also search for this author inPubMed Google Scholar * Shuhua Chen View author publications You can also search for this author inPubMed Google
Scholar * Yuan Tan View author publications You can also search for this author inPubMed Google Scholar * Yuejin Li View author publications You can also search for this author inPubMed
Google Scholar * Faqing Tang View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Faqing Tang. ETHICS DECLARATIONS
CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jia, Q., Chen, S., Tan, Y. _et al._ Oncogenic super-enhancer formation in tumorigenesis and its molecular mechanisms. _Exp Mol Med_ 52,
713–723 (2020). https://doi.org/10.1038/s12276-020-0428-7 Download citation * Received: 19 December 2019 * Revised: 08 March 2020 * Accepted: 23 March 2020 * Published: 07 May 2020 * Issue
Date: May 2020 * DOI: https://doi.org/10.1038/s12276-020-0428-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
