Sorcin can trigger pancreatic cancer-associated new-onset diabetes through the secretion of inflammatory cytokines such as serpin e1 and ccl5
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT A rise in blood glucose is an early warning sign of underlying pancreatic cancer (PC) and may be an indicator of genetic events in PC progression. However, there is still a lack of
mechanistic research on pancreatic cancer-associated new-onset diabetes (PCAND). In the present study, we identified a gene _SRI_, which possesses a SNP with the potential to distinguish
PCAND and Type 2 diabetes mellitus (T2DM), by machine learning on the basis of the UK Biobank database. In vitro and in vivo, sorcin overexpression induced pancreatic β-cell dysfunction.
Sorcin can form a positive feedback loop with STAT3 to increase the transcription of serpin E1 and CCL5, which may directly induce β-cell dysfunction. In 88 biopsies, the expression of
sorcin was elevated in PC tissues, especially in PCAND samples. Furthermore, plasma serpin E1 levels are higher in peripheral blood samples from PCAND patients than in those from T2DM
patients. In conclusion, sorcin may be the key driver in PCAND, and further study on the sorcin-STAT3-serpin E1/CCL5 signaling axis may help us better understand the pathogenesis of PCAND
and identify potential biomarkers. SIMILAR CONTENT BEING VIEWED BY OTHERS BIOMARKER SCREENING USING INTEGRATED BIOINFORMATICS FOR THE DEVELOPMENT OF “NORMAL—IMPAIRED GLUCOSE INTOLERANCE—TYPE
2 DIABETES MELLITUS” Article Open access 24 February 2024 MECHANISM EXPLORATION AND BIOMARKER IDENTIFICATION OF GLYCEMIC DETERIORATION IN PATIENTS WITH DISEASES OF THE EXOCRINE PANCREAS
Article Open access 22 February 2024 MRPS6 MODULATES GLUCOSE-STIMULATED INSULIN SECRETION IN MOUSE ISLET CELLS THROUGH MITOCHONDRIAL UNFOLDED PROTEIN RESPONSE Article Open access 27
September 2023 INTRODUCTION Pancreatic cancer (PC) is a highly fatal disease with a 5-year cumulative survival rate of approximately 10% in the USA1,2. Early diagnosis of PC at a resectable
stage provides more treatment options and substantially improves patient survival3. Previous studies of pancreatic tumorigenesis have suggested that mutations in PC driver genes occur in a
specific order; activating mutations in _KRAS_ are present in low-grade pancreatic intraepithelial neoplasia (PanIN-1) lesions4 (94.1% mutation rate5), and inactivating mutations in _CDKN2A_
(17.0%), _TP53_ (63.9%) and _SMAD4_ (20.8%) occur thereafter and are found in transformed PanIN-2 and PanIN-3 lesions6,7. However, owing to the long duration PanIN-PC evolution and the lack
of specific marker gene mutations8, related early diagnostic strategies have not achieved significant clinical benefits. New-onset diabetes, especially in individuals aged over 50 years,
has been identified as an early warning sign of underlying PC. A case‒control study revealed that, on average, patients with pancreatic ductal adenocarcinoma (PDAC) develop hyperglycemia 36
to 30 months before their tumor diagnosis9, presenting a potential window of opportunity for early detection. Distinguishing new-onset diabetes from the more prevalent type 2 diabetes
mellitus (T2DM) is a prerequisite for targeted screening of this high-risk population. A recent study by Bao et al. suggested that pancreatic cancer-associated new-onset diabetes (PCAND) is
characterized primarily by reduced insulin secretory capacity resulting from β-cell dysfunction10. Insulin resistance, though also present in PCAND patients11, appears to be less severe than
that observed in patients with T2DM10. Recent studies have also identified a growing list of biomarkers associated with PCAND, including connexin-2612, vanin-1 (VNN-1) and matrix
metalloproteinase 9 (MMP-9)13,14, galectin-3 and S100A915, S-100A8 N-terminal peptide16, amylin17, the glucagon/insulin ratio18, insulin gene promoter polymorphisms19, adrenomedullin20,
islet amyloid polypeptide (IAPP)21, fatty acid binding protein-1 (FABP-1)22 and insulin-like growth factor-I23. However, the mechanistic link between PC and the pathogenesis of new-onset
diabetes remains largely unclear. The main challenge now is identifying the 1% of PCAND patients from common T2DM patients in the new-onset diabetes population24,25,26. To identify the key
regulator(s) involved in PCAND pathogenesis, we employed machine learning techniques to identify single nucleotide polymorphism (SNP) loci and their associated genes that possess
discriminatory power in distinguishing between PCAND and T2DM. Finally, we identified _SRI_ gene, which encodes a protein named sorcin (soluble resistance-related calcium binding protein)27.
Interestingly, we found that sorcin was significantly overexpressed in tumor samples from PDAC patients, especially in PCAND patients. In vitro _and_ in vivo, we found that sorcin
overexpression can impair pancreatic β-cells. We showed that sorcin forms a positive feedback loop with STAT3 and activates the transcription of inflammatory factors, such as CCL5 and serpin
E1. Finally, we preliminarily confirmed the potential of _SRI_ and its downstream serpin E1 in distinguishing PCAND from T2DM on the basis of an online database and small clinical cohorts.
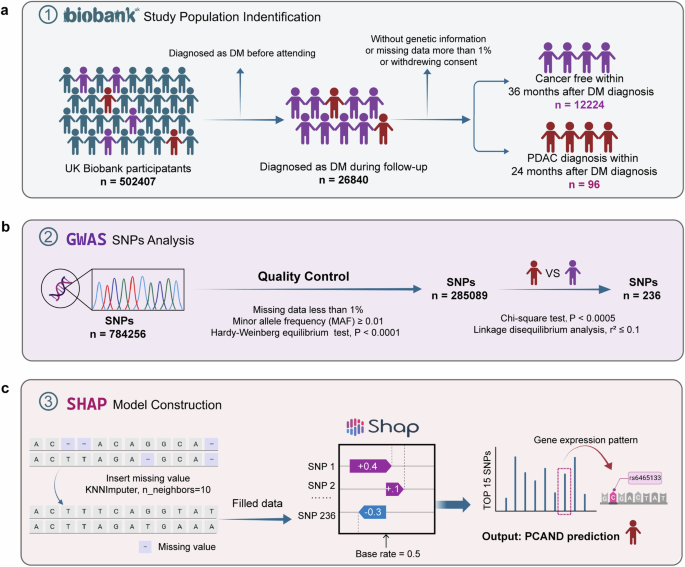
MATERIALS AND METHODS UK BIOBANK DATABASE STUDY DESIGN AND POPULATION The UK Biobank is an ongoing project to demonstrate the successful collection and sharing of linked genetic, physical
and clinical information at the population scale. Extensive genetic and clinical data have been collected for approximately 500,000 volunteers across the United Kingdom28. We identified
patients with cancer using the International Classification of Diseases codes (version 10, ICD-10) that were recorded in the national cancer registry on the basis of hospital admissions and
causes of death. T2DM cases were defined as having an ICD-10 code of E11.X. Only cases in which the individuals did not have T2DM or cancer at the date of the attending assessment center
were included in this research and subsequently followed up for incident T2DM and PDAC events. The participants were then split into groups according to the following criteria: PCAND if
diagnosed with PDAC within 24 months after the diagnosis of T2DM and T2DM if no cancer occurred during the follow-up, which was longer than 36 months after the diagnosis of T2DM. The
specific study screening flow chart is presented in Fig. 1a. The current research was conducted via the UK Biobank Resource under Application 91799. QUALITY CONTROL OF GENETIC DATA AND GWAS
ANALYSIS Genome-wide genetic data are available for 488,000 participants from the UK Biobank. We utilized genotype data from this dataset, which can be accessed at this link
(https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=263). To ensure data quality, we first extracted variants with less than 1% missing data. We subsequently extracted individuals with less
than 1% missing data and extracted variants with a minor allele frequency (MAF) ≥ 0.01. Next, we conducted a Hardy‒Weinberg equilibrium (HWE) test and calculated the _P_ values for all the
SNPs, extracting those with HWE _P_ values less than 0.0001. Ultimately, 285,089 SNPs and 12,320 individual samples were selected for subsequent GWAS, and the chi-square test was used as the
analytical method. We considered SNP loci with a _P_ value < 0.0005 as potentially significant loci in differentiating PCAND and T2DM and obtained 287 SNPs. We subsequently conducted
linkage disequilibrium (LD) analysis. We performed LD pruning to select only one representative SNP from each block. SNP pairs were considered independent if their correlation (r2) was less
than or equal to 0.1. After LD analysis, a total of 236 SNPs were selected. All data analyses were performed via PLINK 1.9 software. The flow chart is presented in Fig. 1b. MODEL
CONSTRUCTION To further select meaningful SNPs, we assessed the efficacy of SNPs in differentiating PCAND from T2DM. We presumed that missing data were distributed randomly and adopted the
K-nearest neighbors imputer algorithm (KNNImputer, n_neighbors=10) to manage the null values in the dataset. Subsequent to data imputation, the Shapley additive explanation (SHAP) values29
were leveraged to gain a deeper understanding of the significance of individual SNPs. Notably, SNPs exhibiting higher absolute SHAP values had greater value in individual predictions. In
this study, SHAP values were computed via a logistic regression model. An evaluation of the relative values revealed that the SHAP value showed a significant drop around 0.35; the top 15
SNPs were selected, and the corresponding genes were identified. Using the Gene Expression Profiling Interactive Analysis (GEPIA) website (http://gepia.cancer-pku.cn/), we investigated the
differential expression of these genes between pancreatic cancer and normal tissues and assessed the impact of these genes on pancreatic cancer prognosis. The flow chart is presented in Fig.
1c. CELL CULTURE The AsPC-1, PANC-1, CFPAC-1, BxPC-3, Mia Paca-2, HPDE6, PANC-02, HEK293T and MIN6 cell lines were purchased from the American Type Culture Collection (ATCC). PANC-1,
CFPAC-1, Mia Paca-2, HPDE6 and PANC-02 cells were cultured in DMEM (Gibco, USA), and ASPC-1 and BxPC-3 cells were maintained in RPMI-1640 medium (Gibco, USA). MIN6 cells were cultured in
RPMI-1640 medium supplemented with 50 μM β-mercaptoethanol (Cienry, Zhejiang, China). Both DMEM and RPMI-1640 media were supplemented with 10% FBS (YEASEN, Shanghai, China) and 100 units/mL
penicillin and streptomycin (Cienry, Zhejiang, China). All the cells were cultured in a humidified incubator at 37 °C in a 5% CO2 atmosphere. The cells were passaged when 80–90% confluence
was reached, and the media were changed every 2 days. HUMAN CYTOKINE ARRAY A membrane-based antibody array (Proteome Profiler Human Cytokine Array Kit, R&D Systems, ARY005B) was used to
profile 36 soluble proteins, mostly cytokines and chemokines, in the conditioned medium from PANC-1 cells transfected with either _SRI_-siRNA or NC-siRNA. The complete list of proteins
represented in this antibody array can be found on the manufacturer’s website (https://www.rndsystems.com/products/proteome-profiler-human-cytokine-array-kit_ary005b). ANIMAL STUDY DESIGN To
verify the effect of _SRI_ expression in PC cells on islet function in vivo, four- to six-week-old female nude mice (_n_ = 24) were randomly divided into four groups. These mice received
subcutaneous injections of pancreatic cancer cell lines stably transduced with lentivirus (pCDH-_ovSRI_, pCDH-_shSRI_ and pCDH-_NC_; 1×106 cells in 100 μL of PBS for each line) or PBS (100
μL for each line). Blood glucose concentrations and body weights were measured every 4 days from the day after the first intraperitoneal injection. On the 24th day after subcutaneous
injection, the nude mice were fasted for 24 h and then sacrificed, after which their peripheral blood, tumor tissue and pancreatic tissue were collected. Peripheral blood was used to measure
fasting blood glucose and fasting insulin levels, and pancreatic tissue was used for immunofluorescence detection of insulin levels. To verify the damaging effects of the cytokines serpin
E1 and CCL5 on islets in vivo, four- to six-week-old female nude mice (_n_ = 30) were randomly divided into six groups. These mice received subcutaneous injections of pancreatic cancer cell
lines stably transduced with lentivirus (pCDH-_shSRI_ and pCDH-_NC_, 1×106 cells in 100 μL of PBS for each one) and received intratumoral injections of the cytokines serpin E1 and CCL5 (100
nM for each, recombinant protein from MedChemExpress, USA) or PBS (100 μL for each). The monitoring and sample testing methods used were the same as those described above. All animal
experiments were approved by the ethics committee of ZheJiang University, and the methods for in vivo studies were carried out in accordance with the approved guidelines. CLINICAL STUDY
DESIGN AND POPULATION Eighty-eight PDAC biopsies, consisting of samples from 32 patients without diabetes (pure PC), 28 patients with new-onset diabetes (PCAND, with diabetes diagnosed 24
months before the diagnosis of PDAC30), and 28 patients with long-standing T2DM (PC + T2DM, with diabetes diagnosed > 24 months before the diagnosis of PDAC), were obtained at the Second
Affiliated Hospital of Zhejiang University between January 2013 and December 2017. The diagnostic criteria for T2DM were in accordance with the American Diabetes Association31. All the
pancreatic biopsies were classified according to the American Joint Committee on Cancer (AJCC) Staging Manual, 6th Edition. Twenty-one peripheral blood samples, consisting of 8 PCAND cases
and 13 T2DM cases, were collected between January 2018 and January 2021. This study was approved by the ethics committees of Zhejiang University. This study was approved by the ethics
committees of the Second Affiliated Hospital of Zhejiang University (Approval Number: I2019001590). MEASUREMENT OF CYTOKINE LEVELS Peripheral blood samples from pancreatic cancer patients
with new-onset diabetes (_n_ = 8) and type 2 diabetes patients (_n_ = 13) were collected between January 2018 and January 2021 at the Second Affiliated Hospital of Zhejiang University. After
anticoagulant treatment and centrifugation at 3000 rpm for 10 min, the plasma concentrations of the cytokines CCL5 and serpin E1 were measured with an ELISA kit (CUSABIO, Wuhan, China)
according to the manufacturer’s instructions. STATISTICAL ANALYSIS The data were acquired from at least three independent experiments and are presented as the means ± SDs. All the
statistical analyses were performed in GraphPad Prism version 8.0.2. Unpaired Student’s t test was used for comparisons between two groups, and one-way ANOVA was used for comparisons among
multiple groups. Kaplan–Meier curves of overall survival were compared via the log-rank test. Correlation coefficients were calculated via the Pearson method. F values indicated variations
between groups. The higher the F value is, the greater the difference between groups; the significance of differences was assessed via the _P_ value, and _P_ < 0.05 was considered to
indicate statistical significance. Please see the Supplementary Information for details on the materials and processes used in this study. RESULTS THE SNP RS6465133 IN _SRI_ HAS THE
POTENTIAL TO DISTINGUISH PCAND FROM T2DM VIA MACHINE LEARNING Genomic studies can provide valuable insights into the underlying mechanisms of these phenotypic differences. By leveraging
extensive datasets available in large databases (UK Biobank), our objective was to identify difference in the genomic characteristics of PCAND and pure T2DM populations. A total of 12,320
individuals with new-onset diabetes were included in our study, 96 of whom were diagnosed with PCAND and 12,224 of whom were diagnosed with T2DM (Fig. 1a). According to quality control of
genetic data and GWAS, a total of 236 SNPs with significant differences between PCAND and T2DM were selected (Fig. 1b). After model construction via machine learning (Fig. 1c), we identified
the 15 most meaningful SNPs according to SHAP value (Fig. 2a). Among these 15 SNPs, 10 had corresponding genes (Fig. 2b). We assessed the gene expression patterns of these 10 genes via The
Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases and found that the expression of only the _SRI_ and _STK11_ genes was upregulated in PDAC samples compared with
normal pancreatic tissues (Fig. 2c-d). There was also a positive correlation between high _SRI_ expression and advanced TNM stages of PDAC (F value = 2.84, _P_ < 0.005), suggesting a
potential role in promoting tumor progression (Fig. 2e). Moreover, elevated expression of _SRI_ in PDAC was associated with poor overall survival and disease-free survival (Fig. 2f-g).
However, _STK11_ expression was not associated with PDAC stage or prognosis. We utilized a dual-luciferase reporter gene system to validate the activity of the SNP rs6465133 as a
transcriptional enhancer, as it is present within an intron. The results revealed that, compared with the wild-type plasmid, the mutant plasmid presented greater luciferase activity
(Supplementary Fig. 1a-b). To validate these findings in an independent cohort, we examined biopsies obtained from 88 patients diagnosed with PDAC. Sorcin expression was significantly
upregulated in tumor tissues compared with adjacent normal tissues (scores of sorcin levels via IHC, PC tissues vs. paired adjacent normal tissues: 6.49 ± 1.68 vs. 2.18 ± 1.02 (_n_ = 88),
_P_ < 0.0001) (Fig. 2h). However, the expression levels of sorcin were similar in tumor samples of different TNM stages in our cohort (Fig. 2i), probably because the TNM stage is a
macroscopic and anatomy-dependent system that may not reflect the cancerous behavior of pancreatic cancer. Otherwise, when the patients were categorized on the basis of sorcin expression in
their tumor samples, the high _SRI_ group and the low _SRI_ group had similar median survival times (16 months in the high _SRI_ group vs. 18 months in the low _SRI_ group), although the
rate of death seemed to decrease for patients in the low _SRI_ group once they reached 30 months after surgery (Fig. 2j). PC CELLS INHIBIT INSULIN SECRETION IN MIN6 CELLS IN A
SORCIN-DEPENDENT MANNER IN VITRO Mounting evidence suggests that PCAND is a paraneoplastic phenomenon caused by paracrine factors secreted by cancer or stroma cells32,33, some of which have
been shown to impinge on β-cells and inhibit insulin secretion14,20,34. To investigate how sorcin upregulation may lead to islet dysfunction in PCAND, we utilized in vitro cell cultures to
mimic the interactions between pancreatic cancer and islet tissue (Fig. 3a). All five PC cell lines we tested (PANC-1, CFPAC-1, BxPC-3, Mia Paca-2, and AsPC-1) recapitulated the elevated
expression of sorcin found in patient tumor samples compared with the normal pancreatic duct cell line HPDE6 (Fig. 3b, c). The same expression patterns were observed in two published
external datasets, GSE138437 and GSE166165 (Supplementary Fig. 2a, b). Previous studies have shown that insulin-secreting cell lines, such as MIN6 and INS-1, exhibit impaired
glucose-stimulated insulin secretion (GSIS) when cocultured with PC cells or treated with conditioned media from PC cells14,20,34. To assess whether high expression of sorcin is required for
this process, we performed a knockdown experiment using small-interfering RNAs (siRNAs). Three PC cell lines with particularly high sorcin expression, PANC-1, AsPC-1 and CFPAC-1, were
transfected with either siRNA against _SRI_ (_SRI_-siRNA) or negative control siRNA (_NC_-siRNA). The knockdown efficiency of _SRI_-siRNA was estimated to be approximately 15%-40% via
Western blotting (Fig. 3d and Supplementary Fig. 2c). MIN6 cells exposed to conditioned media collected from _NC_-siRNA-transfected PC cells (CM-_NC_-siRNA) presented a suppressed GSIS
response (Fig. 3e and Supplementary Fig. 2d, e), decreased insulin content (Fig. 3f–h and Supplementary Fig. 2f-h) and decreased expression of transcripts related to insulin synthesis and
secretion35,36,37,38,39 (Fig. 3i–l and Supplementary Fig. 2i–l), as quantified by qRT‒PCR. This phenomenon was accompanied by decreased viability (Fig. 3m, n and Supplementary Fig. 2m, n)
and increased apoptosis in MIN6 cells (Fig. 3o, p and Supplementary Fig. 2o, p). The deleterious effects of conditioned media on MIN6 cells were partially rescued when the expression of
sorcin in the PC cells was knocked down by _SRI_-siRNA (CM-_SRI_-siRNA in Fig. 3c–o and Supplementary Fig. 2c–p). Together, these results suggest that sorcin is involved in the production of
paracrine factors by PC cells, which can negatively impact MIN6 cell viability and the ability of MIN6 cells to synthesize and release insulin. PC CELLS INHIBIT INSULIN SECRETION IN
PANCREATIC Β-CELLS IN A SORCIN-DEPENDENT MANNER IN VIVO To further elucidate the potential regulatory role of high expression of _SRI_ in the damage inflicted by PC cells on islet cells in
vivo, we devised a comprehensive research framework (Fig. 4a). We employed the lentivirus transduction technique to generate PC cell lines with either overexpression (pCDH-_ovSRI_) or
knockdown (pCDH-_shSRI_) of the _SRI_ gene, using an empty plasmid (pCDH-_NC_) as a negative control. The efficiency of overexpression and knockdown was verified by Western blotting (Fig.
4b). Diverging from conventional models employing pancreatic orthotopic tumors associated with PCAND34, we opted for a subcutaneous tumor approach to circumvent direct pancreatic injury, and
PBS was subcutaneously injected as a blank control. The body weights and blood glucose levels of the nude mice were continuously monitored after subcutaneous injection of pancreatic cancer
cells, and the results revealed no significant differences in body weight among the four groups (Supplementary Fig. 3), suggesting the absence of cachexia in the subcutaneous tumor model.
However, there were no significant differences in blood glucose levels between the groups (Fig. 4c). This outcome may be attributed to the fact that we did not enforce absolute fasting prior
to blood glucose testing. We sacrificed the mice and collected plasma, subcutaneous tumor tissue, and pancreatic tissue when the tumor size was within the tolerable range of that of nude
mice and fasted them for 24 h before sacrifice. Interestingly, we found that nude mice bearing pCDH-_ovSRI_ tumors had higher fasting blood glucose levels (Fig. 4d) and lower fasting insulin
levels (Fig. 4e) than those bearing pCDH-_shSRI_ tumors. Moreover, the immunofluorescence results revealed that the islets in the visual field of the pCDH-_ovSRI_ group almost disappeared,
whereas the islet morphology and insulin signal in the pCDH-_shSRI_ group were similar to those in the PBS group (Fig. 4f). The findings from our in vivo experiments indicate that pancreatic
islet damage can also occur as a consequence of nonadjacent subcutaneous tumors, suggesting the involvement of blood-mediated processes. However, the precise mediators responsible for this
phenomenon require further investigation and exploration. In addition, we observed that the size of the subcutaneous tumors was positively correlated with the _SRI_ expression level (Fig.
4g-i), suggesting that _SRI_ may also be associated with the proliferation of pancreatic cancer cells. SORCIN-OVEREXPRESSING PC CELLS RELEASE CCL5 AND SERPIN E1 TO INHIBIT INSULIN SECRETION
IN MIN6 CELLS To further elucidate the mechanism by which conditioned media from PC cells impact β-cells, we performed experiments to identify the paracrine factors released by PC cells
under the regulation of sorcin. The pancreatic tumor microenvironment is known to be rich in inflammatory cytokines that support tumor growth40,41 and contribute to β-cell dysfunction and
apoptosis42. To assess the possibility that sorcin-overexpressing PC cells release inflammatory cytokines, we used a human cytokine array to analyze the cytokine profile in the supernatants
(conditioned media) collected from PANC-1 cells with and without sorcin knockdown. Five cytokines were significantly downregulated in the _SRI_-siRNA group (Fig. 5a, b). Among them, only
_CCL5_ and _SERPIN E1_ were consistently downregulated in all five PC cell lines following sorcin knockdown (Fig. 5c and Supplementary Fig. 4a–d). Furthermore, we observed a significant
increase in the mRNA levels of _CCL5_ and _SERPIN E1_ after the SRI gene was overexpressed in Mia Paca-2 and BxPC-3 cells (Supplementary Fig. 4e, f). Recombinant CCL5 and serpin E1 proteins
inhibited the GSIS response in MIN6 cells in a dose-dependent manner (Fig. 5d, e), confirming the role of these inflammatory cytokines in disrupting β-cell insulin secretion. Moreover,
treatment with CCL5 and serpin E1 for prolonged durations ( > 48 h for CCL5 and > 12 h for serpin E1) led to an increase in p38 mitogen-activated protein kinase (MAPK) activation in
MIN6 cells (Fig. 5f, g), which has been associated with β-cell apoptosis43 and may also underlie the apoptotic phenotype induced by conditioned media from sorcin-overexpressing PC cells
(CM-_NC_-siRNA in Figs. 3m–p and 5h). Notably, PC-induced p38 activation in MIN6 cells was attenuated when sorcin expression was knocked down (CM-_SRI_-siRNA in Fig. 5h). Thus, we identified
CCL5 and serpin E1 as key components of PC cell secretions that disrupt β-cell functions and identified p38 as a potential downstream target of these inflammatory cytokines in β-cells. To
validate the in vivo effects of the secretion of the inflammatory cytokines downstream of SRI (CCL5 and serpin E1) on islets in situ, we subcutaneously implanted pCDH-_NC_ and pCDH-_shSRI_
pancreatic cancer cells into nude mice. Following tumor formation, we administered intratumoral injections of CCL5, serpin E1, or PBS (Fig. 5i). Prior to sacrifice, we fasted the mice for 24
h when the tumor size remained within the tolerable range for nude mice and subsequently collected plasma, subcutaneous tumor tissue, and pancreatic tissue. As anticipated, in both sets of
nude mice (those harboring pCDH-_NC_ and those harboring pCDH-_shSRI_ pancreatic cancer cells), intratumoral administration of CCL5 and serpin E1 led to elevated fasting blood glucose levels
(Fig. 5j) and reduced fasting insulin levels (Fig. 5k) compared with those in the PBS group. Concurrently, immunofluorescence findings demonstrated that intratumoral injection of CCL5 or
serpin E1 diminished insulin signaling within the pancreatic islets in situ and decreased PDX1 signaling, which is linked to insulin synthesis (Fig. 5n). Furthermore, we noted increased
sizes of subcutaneous tumors after intratumoral injection of CCL5 or serpin E1, which was particularly evident in the pCDH-shSRI groups (Fig. 5l, m), indicating the potential collaborative
effects of SRI and downstream inflammatory cytokines on the proliferation of pancreatic cancer cells. SORCIN UPREGULATES CCL5 AND SERPIN E1 EXPRESSION BY FORMING A POSITIVE FEEDBACK LOOP
WITH STAT3 Thus far, we have shown that the overexpression of sorcin in PC cells leads to increased secretion of CCL5 and serpin E1, which act on nearby β-cells. Since sorcin itself is not
known to be a transcription factor, we speculated that it may interact with one or more transcription factors to upregulate CCL5 and serpin E1 expression in PC cells. Indeed, sorcin has been
reported to interact with signal transducer and activator of transcription 3 (STAT3) in mouse hepatocytes44. In PC cells, sorcin and STAT3 colocalize (Fig. 6a) and can be
coimmunoprecipitated as a protein complex (Fig. 6b, c). Specifically, following transfection with the pcDNA-_SRI_-FLAG plasmid, immunoprecipitation analysis revealed the presence of STAT3,
along with its phosphorylated form, in complex with sorcin protein (Fig. 6c). The phosphorylation level of STAT3 appeared to be dictated by the expression level of sorcin, and the level of
phospho-STAT3 (p-STAT3) was increased after sorcin overexpression in both the nucleus and cytoplasm (Fig. 6d). In PANC-1 and AsPC-1 cells, the p-STAT3 level increased with pcDNA-_SRI_-FLAG
transfection in a concentration-dependent manner (Fig. 6e) and decreased with siRNA-mediated sorcin knockdown (Fig. 6f). Similarly, PC cell lines with elevated sorcin expression (Fig. 3b-c)
presented higher levels of p-STAT3 than did the normal pancreatic duct epithelial cell line HPDE6 (Fig. 6g). In PDAC tumor tissues from human patients, sorcin was highly expressed in the
cytoplasm of PC cells, while p-STAT3, an activated transcription factor, was enriched in the nucleus (Supplementary Fig. 5a). Interestingly, when STAT3 expression was knocked down by siRNAs
in PANC-1 cells, sorcin expression was also largely diminished (Fig. 6h), suggesting that STAT3, in turn, increased the expression level of sorcin. Thus, the synergistic interactions between
sorcin and STAT3 form a positive feedback loop (Fig. 6i), resulting in the sustained overexpression of sorcin and the activation of STAT3 in PC cells. To further confirm that the
sorcin-STAT3 loop is responsible for increasing the transcription of CCL5 and serpin E1 in PC cells, we examined the effect of STAT3 knockdown on _CCL5_ and _SERPIN E1_ transcript levels. In
AsPC-1 and CFPAC-1 cells, STAT3 knockdown via three different siRNAs resulted in the downregulation of _CCL5_ and _SERPIN E1_ transcripts (Fig. 6j, k and Supplementary Fig. 5b), similar to
what we observed with sorcin knockdown (Fig. 5c and Supplementary Fig. 4a–d). In PANC-1 cells, on the other hand, the impact of STAT3 knockdown on _CCL5_ mRNA levels varied with different
siRNAs, while all three _STAT3_-siRNAs led to a slight but significant decrease in _SERPIN E1_ mRNA. This discrepancy is perhaps not surprising, considering that STAT3 knockdown not only
affects gene targets directly downstream of the sorcin-STAT3 loop (Fig. 6k) but also disrupts the interactions between STAT3 and other proteins45. IN THE CLINICAL COHORT, _SRI_
DIFFERENTIATED BETWEEN PCAND AND T2DM, AND DOWNSTREAM SERPIN E1 MAY BE A POTENTIAL BIOMARKER In our previous cohort of 88 patients with PDAC, patients were further classified into three
groups on the basis of their diabetes status: those with no diabetes (pure PC), those with PCAND, and those with long-term diabetes (PC + T2DM). Notably, a greater level of sorcin expression
was detected in PCAND tumor tissues than in PC + T2DM tumor tissues (sorcin IHC scores, PCAND vs. PC + T2DM: 7.10 ± 1.71 (_n_ = 28) vs. 5.85 ± 1.67 (_n_ = 28), _P_ = 0.008; pure PC vs.
PCAND: 6.51 ± 1.51 (_n_ = 32) vs. 7.10 ± 1.71 (_n_ = 28), _P_ = 0.136) (Fig. 7a, b). The area under the curve (AUC) for sorcin in differentiating between PCAND patients and PC + T2DM
patients was 0.675 (_P_ = 0.02423, 95% CI 0.5358-0.8150) (Fig. 7c). Furthermore, fasting blood glucose levels in patients with pure PC and PCAND before pancreatectomy were positively
correlated with sorcin expression levels (Pearson correlation coefficient between sorcin IHC scores and fasting blood glucose level _r_ = 0.281, _P_ = 0.0326) (Fig. 7d), which was not
observed in PC + T2DM patients (Pearson correlation coefficient between sorcin IHC scores and fasting blood glucose level, _r_ = -0.0572, _P_ = 0.7722) (Fig. 7e), suggesting a potential link
between the upregulation of sorcin and islet dysfunction specific to PCAND. Interestingly, in rare instances in which the pancreatic tissue section included both the PDAC tumor and the
adjacent islets, high sorcin levels in PDAC tumors coincided with low insulin levels and PDX1 levels in tumor-adjacent islets (Supplementary Fig. 6a, b). However, since this phenomenon was
observed in only two available sections, the conclusion may not be solid and only partially suggests that decreased insulin secretion is likely responsible for the increased fasting blood
glucose level in patients with high sorcin expression. According to the TCGA and GTEx data, both _CCL5_ and _SERPIN E1_ were expressed at significantly higher levels in pancreatic cancer
tissues (T, _n_ = 179) than in nearby normal pancreatic tissues (N, _n_ = 171) (Fig. 7f, i). Among PDAC patients, high _SERPIN E1_ expression was associated with a poor prognosis (Fig. 7j,
k). The differences between the high _CCL5_ and low _CCL5_ groups was not statistically significant, although the overall and disease-free survival rates did appear to be greater for
patients in the low _CCL5_ group after 20 months (Fig. 7g, h). To assess the performance of CCL5 and serpin E1 as potential biomarkers for PCAND, we measured their concentrations in the
peripheral blood of patients diagnosed with either PCAND (_n_ = 8) or T2DM (_n_ = 13). There was no difference in CCL5 expression between the two groups (Fig. 7l). This may be related to the
fact that another important source of CCL5 is adipose tissue, which has a relatively high concentration in T2DM patients, resulting in its insufficient potential as a biomarker of
PCAND46,47. However, the level of serpin E1 was significantly greater in PCAND than in pure T2DM (Fig. 7m). In this small cohort, serpin E1 achieved an AUROC of 0.8364 in differentiating
between PCAND and pure T2DM (_P_ = 0.0113, 95% CI 0.6415–1.000) (Fig. 7n), demonstrating its potential utility as a biomarker for PCAND. DISCUSSION Pancreatic cancer (PC), a devastating
disease characterized by late diagnosis, limited treatment success and a dismal prognosis, remains a major medical challenge. A rise in blood glucose is one of the early warning signs of
underlying PC and may be an indicator of genetic events in PC progression. Considering the convenience and popularity of blood glucose monitoring, one of the keys to early diagnosis of PC is
to identify the small subset (1%) of PCAND patients among the new-onset diabetes population as early as possible48,49,50. An improved understanding of the molecular mechanisms and signaling
pathways underlying its specific pathogenesis is needed to support progress in PCAND detection. The SHAP technique is a method used to interpret the optimal model output, and it has been
used to select important features for clinical prediction models in some studies51,52. A recent study used machine learning and SHAP technology to predict patients with new-onset diabetes at
risk of PC53. However, this study focused only on clinical indicators and did not consider genomic factors. In this study, we employed machine learning techniques to identify SNPs and their
corresponding genes that may be used to distinguish PCAND from T2DM. We further mapped a novel sorcin-STAT3-serpin E1/CCL5 signaling axis in PC cells, which explains how early
presymptomatic PC may coincide with new-onset diabetes in some patients54. Sorcin and STAT3 form a positive feedback loop to increase the transcription of serpin E1 and CCL5. These
inflammatory cytokines released by PC cells can impair nearby islet β-cells, likely by activating the p38 signaling pathway. In addition, in biopsies obtained from 88 PDAC patients, we
detected elevated expression of sorcin in pancreatic cancer tissues, especially in PCAND. These results suggest that sorcin may be the key driver in PCAND and that aberrant activation of the
sorcin-STAT3-serpin E1/CCL5 signaling axis likely underlies PCAND pathogenesis. While exploring the driver mechanism of _SRI_ in PCAND, we identified potential bidirectional crosstalk
between PCAND pathogenesis and inflammation, which is likely regulated by the sorcin-STAT3-serpin E1/CCL5 signaling axis. Interestingly, the signaling axis we describe here shares a common
critical node, STAT3, with the inflammatory pathway downstream of KRAS, whose mutations are the most common genetic abnormality in PC6. Given the long duration (over 10 years for PC
development)55 and lack of specificity of _KRAS_ mutation in the detection of PC progression8, screening or diagnostic use of these mutations in the clinic is limited. In this study, we
found that fasting blood glucose levels in pure PC and PCAND patients before pancreatectomy were positively correlated with sorcin expression levels. Therefore, the increase in blood glucose
driven by _SRI_ gene could be due to the externalization of PCAND, which typically manifests 2–3 years prior to the diagnosis of PC25, most likely during the progression from PanIN-3 to PC
(Fig. 8)5,56,57,58,59,60,61. These results further support the notion that early screening strategies based on _SRI_ gene may be better than those based on _KRAS_ and other oncogenes that
are mutated in the early PanIN stage. Previous research has suggested that sorcin acts as a protective factor in β-cells in T2DM62. However, our in vitro research revealed that PC-derived
sorcin plays a negative role in β-cell function and can induce inflammatory damage. Unlike T2DM with adipocyte-derived inflammatory cytokines63, PC has a specific inflammatory tumor
microenvironment (TME)64. This study indicated that the increased secretion of serpin E1 and CCL5 induced by the sorcin-STAT3 interaction may in turn contribute to the formation of an
inflammatory TME65, alongside _KRAS_-associated inflammatory signaling66. Furthermore, on the basis of large-scale cohorts from the UK Biobank, we confirmed that the _SRI_-based model is
superior to models based on other driver genes, such as _KRAS_, in differentiating PCAND from T2DM and that the combination of _SRI_, _KRAS_, and _CDKN2A_ with a clinical model can further
increase the efficiency. On the other hand, on the basis of a small cohort of PCAND and T2DM patients, the concentration of serpin E1 in peripheral blood samples showed decent diagnostic
performance. We are aware that this is a preliminary study that has several limitations, such as the sample size of the clinical cohorts. As a next step, we believe that a larger-scale
validation study with a longitudinal sampling scheme should be carried out in the future. In summary, GWAS analysis and machine learning based on a large-scale database identified a SNP
(rs6465133) in the _SRI_ gene whose frequency was significantly different between the PCAND and T2DM populations. Further biological experiments revealed a novel sorcin-STAT3-serpin E1/CCL5
signaling axis as a key driver of PCAND pathogenesis. The convergence of sorcin and KRAS signaling on STAT3 suggests potential bidirectional crosstalk, which should be considered when
selecting targeted therapies for PC involving these pathways. Our results also suggest that plasma serpin E1 may be a potential biomarker for PCAND. Further studies on the molecules
downstream of the sorcin pathway may yield valuable clues for the early diagnosis of PC. DATA AVAILABILITY All data are available in the main text or the supplementary materials. CHANGE
HISTORY * _ 25 NOVEMBER 2024 The original online version of this article was revised: In this article, Jiali Gong, Xiawei Li, Zengyu Feng, and Jianyao Lou should have been denoted as equally
contributing authors. The original article has been corrected. _ * _ 28 NOVEMBER 2024 A Correction to this paper has been published: https://doi.org/10.1038/s12276-024-01363-3 _ REFERENCES
* Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. Pancreatic cancer. _Lancet_ 395, 2008–2020, https://doi.org/10.1016/S0140-6736(20)30974-0 (2020). Article PubMed CAS Google
Scholar * Stoffel, E. M., Brand, R. E. & Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. _Gastroenterology_
164, 752–765, https://doi.org/10.1053/j.gastro.2023.02.012 (2023). Article PubMed Google Scholar * Poruk, K. E., Firpo, M. A., Adler, D. G. & Mulvihill, S. J. Screening for pancreatic
cancer: why, how, and who? _Ann Surg_ 257, 17–26, https://doi.org/10.1097/SLA.0b013e31825ffbfb (2013). Article PubMed Google Scholar * Kanda, M. et al. Presence of somatic mutations in
most early-stage pancreatic intraepithelial neoplasia. _Gastroenterology_ 142, 730–773, https://doi.org/10.1053/j.gastro.2011.12.042 (2012). Article PubMed CAS Google Scholar * Waddell,
N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. _Nature_ 518, 495–501, https://doi.org/10.1038/nature14169 (2015). Article PubMed PubMed Central CAS
Google Scholar * Makohon-Moore, A. & Iacobuzio-Donahue, C. A. Pancreatic cancer biology and genetics from an evolutionary perspective. _Nat Rev Cancer_ 16, 553–565,
https://doi.org/10.1038/nrc.2016.66 (2016). Article PubMed PubMed Central CAS Google Scholar * Waters, A. M. & Der, C. J. KRAS: The critical driver and therapeutic target for
pancreatic cancer. _Cold Spring Harb Perspect Med_ 8, a031435, https://doi.org/10.1101/cshperspect.a031435 (2018). Article PubMed PubMed Central CAS Google Scholar * Siveke, J. T. et
al. Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. _Cancer Cell_ 12, 266–279,
https://doi.org/10.1016/j.ccr.2007.08.002 (2007). Article PubMed CAS Google Scholar * Sharma, A., Smyrk, T. C., Levy, M. J., Topazian, M. A. & Chari, S. T. Fasting blood glucose
levels provide estimate of duration and progression of pancreatic cancer before diagnosis. _Gastroenterology_ 155, 490–500.e492, https://doi.org/10.1053/j.gastro.2018.04.025 (2018). Article
PubMed CAS Google Scholar * Bao, J. et al. Pancreatic cancer-associated diabetes mellitus is characterized by reduced β-cell secretory capacity, rather than insulin resistance. _Diab
Res Clin Pr_ 185, 109223, https://doi.org/10.1016/j.diabres.2022.109223 (2022). Article CAS Google Scholar * Permert, J. et al. Improved glucose metabolism after subtotal pancreatectomy
for pancreatic cancer. _Br J Surg_ 80, 1047–1050, https://doi.org/10.1002/bjs.1800800841 (1993). Article PubMed CAS Google Scholar * Pfeffer, F. et al. Expression of connexin26 in islets
of Langerhans is associated with impaired glucose tolerance in patients with pancreatic adenocarcinoma. _Pancreas_ 29, 284–290, https://doi.org/10.1097/00006676-200411000-00007 (2004).
Article PubMed CAS Google Scholar * Huang, H. et al. Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression
profiles. _Am J Gastroenterol Suppl_ 105, 1661–1669, https://doi.org/10.1038/ajg.2010.32 (2010). Article CAS Google Scholar * Kang, M. et al. VNN1, a potential biomarker for pancreatic
cancer-associated new-onset diabetes, aggravates paraneoplastic islet dysfunction by increasing oxidative stress. _Cancer Lett_ 373, 241–250, https://doi.org/10.1016/j.canlet.2015.12.031
(2016). Article PubMed CAS Google Scholar * Liao, W.-C. et al. Galectin-3 and S100A9: Novel diabetogenic factors mediating pancreatic cancer–associated diabetes. _Diab Care_ 42, 1752,
https://doi.org/10.2337/dc19-0217 (2019). Article CAS Google Scholar * Basso, D. et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: A diabetes cause? _Clin Chim Acta_ 372,
120–128, https://doi.org/10.1016/j.cca.2006.03.027 (2006). Article PubMed CAS Google Scholar * Ding, X., Flatt, P. R., Permert, J. & Adrian, T. E. Pancreatic cancer cells selectively
stimulate islet beta cells to secrete amylin. _Gastroenterology_ 114, 130–138, https://doi.org/10.1016/s0016-5085(98)70641-9 (1998). Article PubMed CAS Google Scholar * Kolb, A. et al.
Glucagon/insulin ratio as a potential biomarker for pancreatic cancer in patients with new-onset diabetes mellitus. _Cancer Biol Ther_ 8, 1527–1533, https://doi.org/10.4161/cbt.8.16.9006
(2009). Article PubMed CAS Google Scholar * Krechler, T. et al. Polymorphism -23HPhI in the promoter of insulin gene and pancreatic cancer: a pilot study. _Neoplasma_ 56, 26–32,
https://doi.org/10.4149/neo_2009_01_26 (2009). Article PubMed CAS Google Scholar * Javeed, N. et al. Pancreatic cancer-derived exosomes cause paraneoplastic β-cell dysfunction. _Clin
Cancer Res_ 21, 1722–1733, https://doi.org/10.1158/1078-0432.CCR-14-2022 (2015). Article PubMed CAS Google Scholar * Permert, J. et al. Islet amyloid polypeptide in patients with
pancreatic cancer and diabetes. _N Engl J Med_ 330, 313–318, https://doi.org/10.1056/NEJM199402033300503 (1994). Article PubMed CAS Google Scholar * Sharaf, R. N. et al. Computational
prediction and experimental validation associating FABP-1 and pancreatic adenocarcinoma with diabetes. _BMC Gastroenterol_ 11, 5–5, https://doi.org/10.1186/1471-230X-11-5 (2011). Article
PubMed PubMed Central Google Scholar * Basso, D. et al. Insulin-like growth factor-I, interleukin-1 alpha and beta in pancreatic cancer: role in tumor invasiveness and associated
diabetes. _Int J Clin Lab Res._ 25, 40–43, https://doi.org/10.1007/BF02592575 (1995). Article PubMed CAS Google Scholar * Abbruzzese, J. L. et al. The interface of pancreatic cancer with
diabetes, obesity, and inflammation: research gaps and opportunities: summary of a national institute of diabetes and digestive and kidney diseases workshop. _Pancreas_ 47, 516–525,
https://doi.org/10.1097/MPA.0000000000001037 (2018). Article PubMed PubMed Central Google Scholar * Chari, S. T. et al. Pancreatic cancer-associated diabetes mellitus: prevalence and
temporal association with diagnosis of cancer. _Gastroenterology_ 134, 95–101, https://doi.org/10.1053/j.gastro.2007.10.040 (2008). Article PubMed CAS Google Scholar * Sharma, A. et al.
Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. _Gastroenterology_ 155, 730–739.e3, https://doi.org/10.1053/j.gastro.2018.05.023 (2018). Article PubMed
Google Scholar * Meyers, M. B., Schneider, K. A., Spengler, B. A., Chang, T. D. & Biedler, J. L. Sorcin (V19), a soluble acidic calcium-binding protein overproduced in
multidrug-resistant cells. Identification of the protein by anti-sorcin antibody. _Biochem Pha_ 36, 2373–2380, https://doi.org/10.1016/0006-2952(87)90606-x (1987). Article CAS Google
Scholar * Editoral. UK Biobank data on 500,000 people paves way to precision medicine. _Nature_ 562, 163–164, https://doi.org/10.1038/d41586-018-06950-9 (2018). * Xue, B. et al. Use of
machine learning to develop and evaluate models using preoperative and intraoperative data to identify risks of postoperative complications. _JAMA Netw Open_ 4, e212240,
https://doi.org/10.1001/jamanetworkopen.2021.2240 (2021). Article PubMed PubMed Central Google Scholar * Sharma, S., Tapper, W. J., Collins, A. & Hamady, Z. Z. R. Predicting
Pancreatic Cancer in the UK biobank cohort using polygenic risk scores and diabetes mellitus. _Gastroenterology_ 162, 1665–1674, https://doi.org/10.1053/j.gastro.2022.01.016 (2022). Article
PubMed CAS Google Scholar * American Diabetes Association. Diagnosis and classification of diabetes mellitus. _Diabetes Care_ 36, S67–S74, https://doi.org/10.2337/dc13-S067 (2013). *
Sah, R. P., Nagpal, S. J. S., Mukhopadhyay, D. & Chari, S. T. New insights into pancreatic cancer-induced paraneoplastic diabetes. _Nat Rev Gastroenterol Hepatol_ 10, 423–433,
https://doi.org/10.1038/nrgastro.2013.49 (2013). Article PubMed PubMed Central CAS Google Scholar * Perera, C. J. et al. Role of pancreatic stellate cell-derived exosomes in pancreatic
cancer-related diabetes: a novel hypothesis. _Cancers (Basel)_ 13, 5224, https://doi.org/10.3390/cancers13205224 (2021). Article PubMed CAS Google Scholar * Pang, W. et al. Pancreatic
cancer-derived exosomal microRNA-19a induces β-cell dysfunction by targeting ADCY1 and EPAC2. _Int J Biol Sci_ 17, 3622–3633, https://doi.org/10.7150/ijbs.56271 (2021). Article PubMed
PubMed Central CAS Google Scholar * Talchai, C., Xuan, S., Lin, H. V., Sussel, L. & Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. _Cell_
150, 1223–1234, https://doi.org/10.1016/j.cell.2012.07.029 (2012). Article PubMed PubMed Central CAS Google Scholar * Zhang, C. et al. MafA is a key regulator of glucose-stimulated
insulin secretion. _Mol Cell Biol_ 25, 4969–4976, https://doi.org/10.1128/MCB.25.12.4969-4976.2005 (2005). Article PubMed PubMed Central CAS Google Scholar * Zhao, L. et al. The islet
beta cell-enriched MafA activator is a key regulator of insulin gene transcription. _J Biol Chem_ 280, 11887–11894, https://doi.org/10.1074/jbc.M409475200 (2005). Article PubMed CAS
Google Scholar * Fujimoto, K. & Polonsky, K. S. Pdx1 and other factors that regulate pancreatic beta-cell survival. _Diab Obes Metab_ 11(Suppl 4), 30–37,
https://doi.org/10.1111/j.1463-1326.2009.01121.x (2009). Article CAS Google Scholar * Chandra, V. et al. RFX6 regulates insulin secretion by modulating Ca2+ homeostasis in human β cells.
_Cell reports_ 9, 2206–2218, https://doi.org/10.1016/j.celrep.2014.11.010 (2014). Article PubMed CAS Google Scholar * Greer, J. B. & Whitcomb, D. C. Inflammation and pancreatic
cancer: an evidence-based review. _Curr Opin Pharm_ 9, 411–418, https://doi.org/10.1016/j.coph.2009.06.011 (2009). Article CAS Google Scholar * Padoan, A., Plebani, M. & Basso, D.
Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. _Int J Mol Sci_ 20, 676, https://doi.org/10.3390/ijms20030676 (2019). Article PubMed PubMed Central CAS
Google Scholar * Donath, M. Y. et al. Mechanisms of β-cell death in type 2 diabetes. _Diabetes_ 54, S108, https://doi.org/10.2337/diabetes.54.suppl_2.S108 (2005). Article PubMed CAS
Google Scholar * Wei, X., Gu, N., Feng, N., Guo, X. & Ma, X. Inhibition of p38 mitogen-activated protein kinase exerts a hypoglycemic effect by improving β cell function via inhibition
of β cell apoptosis in db/db mice. _J Enzym Inhib Med Chem_ 33, 1494–1500, https://doi.org/10.1080/14756366.2018.1477138 (2018). Article CAS Google Scholar * Li, X. et al. Negative
regulation of hepatic inflammation by the soluble resistance-related calcium-binding protein signal transducer and activator of transcription 3. _Front Immunol_ 8, 709,
https://doi.org/10.3389/fimmu.2017.00709 (2017). Article PubMed PubMed Central CAS Google Scholar * Zou, S. et al. Targeting STAT3 in cancer immunotherapy. _Mol Cancer_ 19, 145,
https://doi.org/10.1186/s12943-020-01258-7 (2020). Article PubMed PubMed Central CAS Google Scholar * Pan, X., Kaminga, A. C., Wen, S. W. & Liu, A. Chemokines in prediabetes and
type 2 diabetes: a meta-analysis. _Front Immunol_ 12, 622438, https://doi.org/10.3389/fimmu.2021.622438 (2021). Article PubMed PubMed Central CAS Google Scholar * Chan, P.-C. et al.
Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity. _Clin Sci (Lond)_ 136, 121–137,
https://doi.org/10.1042/CS20210959 (2022). Article PubMed CAS Google Scholar * Singhi, A. D., Koay, E. J., Chari, S. T. & Maitra, A. Early detection of pancreatic cancer:
opportunities and challenges. _Gastroenterology_ 156, 2024–2040, https://doi.org/10.1053/j.gastro.2019.01.259 (2019). Article PubMed Google Scholar * Chari, S. T. et al. Probability of
pancreatic cancer following diabetes: a population-based study. _Gastroenterology_ 129, 504–511, https://doi.org/10.1016/j.gastro.2005.05.007 (2005). Article PubMed Google Scholar *
Owens, D. K. et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. _JAMA_ 322, 438–444, https://doi.org/10.1001/jama.2019.10232
(2019). Article PubMed Google Scholar * Hathaway, Q. A. et al. Machine-learning to stratify diabetic patients using novel cardiac biomarkers and integrative genomics. _Cardiovasc
Diabetol_ 18, 78, https://doi.org/10.1186/s12933-019-0879-0 (2019). Article PubMed PubMed Central CAS Google Scholar * Ma, M. et al. Predicting the molecular subtype of breast cancer
and identifying interpretable imaging features using machine learning algorithms. _Eur Radio_ 32, 1652–1662, https://doi.org/10.1007/s00330-021-08271-4 (2022). Article CAS Google Scholar
* Khan, S. & Bhushan, B. Machine Learning Predicts Patients With New-onset Diabetes at Risk of Pancreatic ancer. _J Clin Gastroenterol_ 58, 681–691,
https://doi.org/10.1097/MCG.0000000000001897 (2023). Article CAS Google Scholar * Chung, H. H., Lim, K. S. & Park, J. K. Clinical clues of pre-symptomatic pancreatic ductal
adenocarcinoma prior to its diagnosis: a retrospective review of CT Scans and laboratory tests. _Clin Pr_ 12, 70–77, https://doi.org/10.3390/clinpract12010008 (2022). Article Google Scholar
* Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. _Nature_ 467, 1114–1117, https://doi.org/10.1038/nature09515 (2010). Article PubMed
PubMed Central CAS Google Scholar * Jones, S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. _Science_ 321, 1801–1806,
https://doi.org/10.1126/science.1164368 (2008). Article PubMed PubMed Central CAS Google Scholar * Andrew, V. et al. Pancreatic cancer genomes reveal aberrations in axon guidance
pathway genes. _Nature_ 491, 399–405, https://doi.org/10.1038/nature11547 (2012). Article CAS Google Scholar * Mark, S J. et al. Clinical implications of genomic alterations in the tumour
and circulation of pancreatic cancer patients. _Nat Commun_ 6, 7686, https://doi.org/10.1038/ncomms8686 (2015). Article Google Scholar * Waters, A. M. & Der, C. J. KRAS: The Critical
Driver and Therapeutic Target for Pancreatic Cancer. _Cold Spring Harb. perspect. med_ 8, a031435, https://doi.org/10.1101/cshperspect.a031435 (2018). Article PubMed PubMed Central CAS
Google Scholar * Witkiewicz, A. K. et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. _Nat Commun_ 6, 6744,
https://doi.org/10.1038/ncomms7744 (2015). Article PubMed CAS Google Scholar * Khan, M. A. et al. Molecular Drivers of Pancreatic Cancer Pathogenesis: Looking Inward to Move Forward.
_Int J Mol Sci_ 18, 779, https://doi.org/10.3390/ijms18040779 (2017). Article PubMed PubMed Central CAS Google Scholar * Marmugi, A. et al. Sorcin links pancreatic β-cell lipotoxicity
to ER Ca2+ Stores. _Diabetes_ 65, 1009–1021, https://doi.org/10.2337/db15-1334 (2016). Article PubMed PubMed Central CAS Google Scholar * Bruun, J. M. et al. Regulation of adiponectin
by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. _Am J Physiol Endocrinol Metab_ 285, E527–E533, https://doi.org/10.1152/ajpendo.00110.2003 (2003). Article
PubMed CAS Google Scholar * Ho, W. J., Jaffee, E. M. & Zheng, L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. _Nat Rev Clin Oncol_ 17,
527–540, https://doi.org/10.1038/s41571-020-0363-5 (2020). Article PubMed PubMed Central Google Scholar * Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 Axis in Cancer
Progression. _Cancers (Basel)_ 12, 1765, https://doi.org/10.3390/cancers12071765 (2020). Article PubMed CAS Google Scholar * Kitajima, S., Thummalapalli, R. & Barbie, D. A.
Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. _Semin Cell Dev Biol_ 58, 127–135, https://doi.org/10.1016/j.semcdb.2016.06.009 (2016). Article PubMed PubMed
Central CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Yanwei Li, Guifeng Xiao, Wei Yin, and Zhaoxiaonan Lin from the Core Facilities, Zhejiang University School of
Medicine, for their technical support. AUTHOR INFORMATION Author notes * These authors contributed equally: Jiali Gong, Xiawei Li, Zengyu Feng, Jianyao Lou AUTHORS AND AFFILIATIONS * Second
Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China Jiali Gong, Xiawei Li, Zengyu Feng, Jianyao Lou, Kaiyue Pu, Yongji Sun, Yizhao Zhou, Meihua Shangguan,
Wenjie Lu, Xin Dong, Jian Wu, Hong Zhu, Qiaojun He & Yulian Wu * Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education, Cancer Institute, Second
Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China Jiali Gong, Xiawei Li, Zengyu Feng, Jianyao Lou, Kaiyue Pu, Yongji Sun, Yizhao Zhou, Meihua Shangguan,
Wenjie Lu, Xin Dong & Yulian Wu * Cancer Center, Zhejiang University, Hangzhou, Zhejiang, China Jiali Gong, Xiawei Li, Zengyu Feng, Jianyao Lou, Kaiyue Pu, Yongji Sun, Yizhao Zhou,
Meihua Shangguan, Wenjie Lu, Xin Dong & Yulian Wu * Department of Surgery, Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, Zhejiang, China Jiali Gong, Yongji
Sun, Sien Hu, Tianyu Song & Yulian Wu * Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA, USA Xiawei Li * School of Public Health and Eye Center The Second
Affiliated Hospital, Zhejiang University, Hangzhou, China Kai Zhang * School of Public Health, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China Jian Wu * Institute of
Wenzhou, Zhejiang University, Wenzhou, Zhejiang, China Jian Wu * Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, College of Pharmaceutical Sciences, Zhejiang University,
Hangzhou, Zhejiang, China Hong Zhu & Qiaojun He * Center for Drug Safety Evaluation and Research of Zhejiang University, Hangzhou, Zhejiang, China Hong Zhu & Qiaojun He * Innovation
Institute for Artificial Intelligence in Medicine and Liangzhu Laboratory, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, China Hongxia Xu Authors * Jiali Gong View
author publications You can also search for this author inPubMed Google Scholar * Xiawei Li View author publications You can also search for this author inPubMed Google Scholar * Zengyu Feng
View author publications You can also search for this author inPubMed Google Scholar * Jianyao Lou View author publications You can also search for this author inPubMed Google Scholar *
Kaiyue Pu View author publications You can also search for this author inPubMed Google Scholar * Yongji Sun View author publications You can also search for this author inPubMed Google
Scholar * Sien Hu View author publications You can also search for this author inPubMed Google Scholar * Yizhao Zhou View author publications You can also search for this author inPubMed
Google Scholar * Tianyu Song View author publications You can also search for this author inPubMed Google Scholar * Meihua Shangguan View author publications You can also search for this
author inPubMed Google Scholar * Kai Zhang View author publications You can also search for this author inPubMed Google Scholar * Wenjie Lu View author publications You can also search for
this author inPubMed Google Scholar * Xin Dong View author publications You can also search for this author inPubMed Google Scholar * Jian Wu View author publications You can also search for
this author inPubMed Google Scholar * Hong Zhu View author publications You can also search for this author inPubMed Google Scholar * Qiaojun He View author publications You can also search
for this author inPubMed Google Scholar * Hongxia Xu View author publications You can also search for this author inPubMed Google Scholar * Yulian Wu View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Qiaojun He, Hongxia Xu or Yulian Wu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gong, J., Li, X., Feng, Z. _et al._ Sorcin can trigger pancreatic cancer-associated new-onset diabetes through the secretion of inflammatory
cytokines such as serpin E1 and CCL5. _Exp Mol Med_ 56, 2535–2547 (2024). https://doi.org/10.1038/s12276-024-01346-4 Download citation * Received: 18 November 2023 * Revised: 28 July 2024 *
Accepted: 19 August 2024 * Published: 08 November 2024 * Issue Date: November 2024 * DOI: https://doi.org/10.1038/s12276-024-01346-4 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
