Maternal adipokines longitudinally measured across pregnancy and their associations with neonatal size, length, and adiposity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND/OBJECTIVES Maternal obesity impacts fetal growth as early as second trimester of pregnancy, yet little is known about the molecular mechanisms involved. We aimed to
examine associations between maternal adipokines throughout pregnancy and neonatal size by prepregnancy obesity status. METHODS In a prospective cohort of 2802 U.S. pregnant women from the
NICHD Fetal Growth Studies-Singleton Cohort (2009–2013), biospecimens were analyzed in a matched case−control subset of 321 women. Blood was collected at 10–14, 15–26 (fasting), 23–31, and
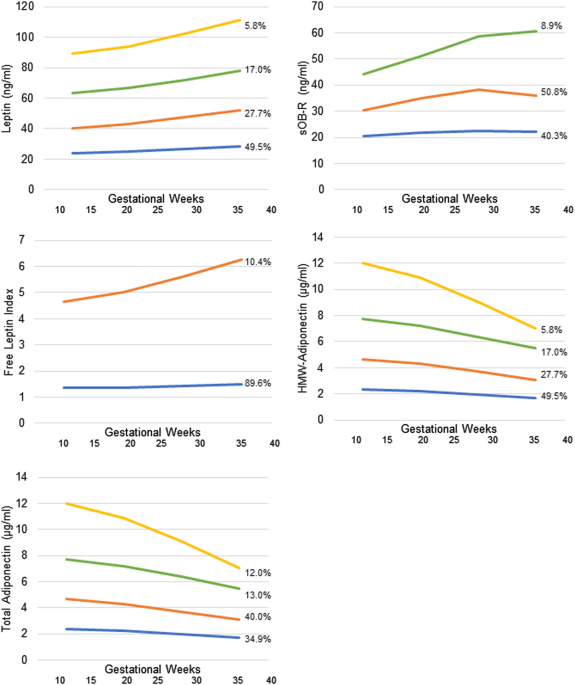
33–39 gestational weeks. Plasma leptin and soluble leptin receptor (sOB-R) and total and high-molecular-weight (HMW)-adiponectin were measured. Free leptin was calculated as leptin/sOB-R.
Birthweight was abstracted from medical records. Neonatal length and skinfolds were measured. RESULTS Leptin and sOB-R in late pregnancy tended to be positively and negatively associated
with neonatal length, respectively, while free leptin throughout pregnancy tended to be positively associated with length. Free leptin associations with neonatal length were differential by
obesity (i.e., inversely among women without obesity and positively among women with obesity). A per unit increase in free leptin at 33–39 weeks was associated with a shorter neonatal length
by −0.55 cm (95%CI, −0.83, −0.28) in women without obesity and longer length by 0.49 cm (95%CI, 0.34, 0.65) in women with obesity. HMW-adiponectin at 33–39 weeks was inversely associated
with neonatal length (_β_ = −1.29 cm; 95%CI, −1.74, −0.85) and skinfold thickness (_β_ = −1.46 mm; 95%CI, −1.58, −0.56) among women with obesity. Free leptin across pregnancy tended to be
negatively associated with neonatal skinfold thickness among women without obesity, while free leptin in early pregnancy was positively associated with skinfold thickness. CONCLUSIONS
Maternal adipokines were associated with multiple pathways that influence neonatal size including length and adiposity, which differed in timing across pregnancy and by prepregnancy obesity.
These findings provide new potential insights into mechanisms and timing by which maternal obesity may impact fetal growth. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per
year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ROLE OF
MATERNAL GLUCOSE METABOLISM IN THE ASSOCIATION BETWEEN MATERNAL BMI AND NEONATAL SIZE AND ADIPOSITY Article 08 November 2020 DETERMINANTS OF CORD BLOOD ADIPOKINES AND ASSOCIATION WITH
NEONATAL ABDOMINAL ADIPOSE TISSUE DISTRIBUTION Article Open access 04 December 2021 ALTERATIONS IN INFANT ADIPOKINE CONCENTRATIONS IN THE FIRST POSTNATAL WEEK WITH EXPOSURE TO DIABETES IN
PREGNANCY Article 22 March 2025 REFERENCES * Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a
systematic review and meta-analysis. PLoS ONE. 2013;8:e61627. Article CAS Google Scholar * Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, et al. Association of
maternal obesity with longitudinal ultrasonographic measures of fetal growth: findings rom the NICHD fetal growth studies—singletons. JAMA Pediatr. 2018;172:24–31. Article Google Scholar *
King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000;71:1218s–25s. Article CAS Google Scholar * Allison MB, Myers MG,Jr.. 20 years of leptin: connecting leptin
signaling to biological function. J Endocrinol. 2014;223:T25–35. Article CAS Google Scholar * Schaab M, Kratzsch J. The soluble leptin receptor. Best Pract Res Clin Endocrinol Metab.
2015;29:661–70. Article CAS Google Scholar * Misra VK, Straughen JK, Trudeau S. Maternal serum leptin during pregnancy and infant birth weight: the influence of maternal overweight and
obesity. Obes (Silver Spring). 2013;21:1064–9. Article CAS Google Scholar * Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol.
2006;194:1537–45. Article CAS Google Scholar * Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and
obesity-related diseases. Biomed Res Int. 2014;2014:658913. Article CAS Google Scholar * Hauguel-de Mouzon S, Catalano P. Adiponectin: are measurements clinically useful in pregnancy?
Diabetes Care. 2013;36:1434–6. Article CAS Google Scholar * Catov JM, Patrick TE, Powers RW, Ness RB, Harger G, Roberts JM. Maternal leptin across pregnancy in women with
small-for-gestational-age infants. Am J Obstet Gynecol. 2007;196:558 e1–8. Article CAS Google Scholar * Papastefanou I, Samolis S, Panagopoulos P, Tagia M, Bale C, Kouskoukis A, et al.
Correlation between maternal first trimester plasma leptin levels and birth weight among normotensive and preeclamptic women. J Matern Fetal Neonatal Med. 2010;23:1435–43. Article Google
Scholar * Tamura T, Goldenberg RL, Johnston KE, Cliver SP. Serum leptin concentrations during pregnancy and their relationship to fetal growth. Obstet Gynecol. 1998;91:389–95. Article CAS
Google Scholar * Nanda S, Akolekar R, Sarquis R, Mosconi AP, Nicolaides KH. Maternal serum adiponectin at 11 to 13 weeks of gestation in the prediction of macrosomia. Prenat Diagn.
2011;31:479–83. Article CAS Google Scholar * Valdes ER, Lattes KA, Munoz HS, Barja PY, Papapietro KV. First-trimester adiponectin and subsequent development of preeclampsia or fetal
growth restriction. Gynecol Obstet Invest. 2011;72:152–6. Article CAS Google Scholar * Wang J, Shang LX, Dong X, Wang X, Wu N, Wang SH, et al. Relationship of adiponectin and resistin
levels in umbilical serum, maternal serum and placenta with neonatal birth weight. Aust N Z J Obstet Gynaecol. 2010;50:432–8. Article Google Scholar * Godfrey K, Walker‐Bone K, Robinson S,
Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J Bone Miner Res.
2001;16:1694–703. Article CAS Google Scholar * Fewtrell MS, Cole TJ, Bishop NJ, Lucas A. Neonatal factors predicting childhood height in preterm infants: evidence for a persisting effect
of early metabolic bone disease? J Pediatr. 2000;137:668–73. Article CAS Google Scholar * Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness
of infants and young children born small-or large-for-gestational-age. Pediatrics. 1998;102:e60–e. Article CAS Google Scholar * Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin
P, De Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. Article CAS Google Scholar * Grewal J, Grantz KL, Zhang
C, Sciscione A, Wing DA, Grobman WA, et al. Cohort profile: NICHD fetal growth studies—singletons and twins. Int J Epidemiol. 2017;47:25–l. Article Google Scholar * Buck Louis GM, Grewal
J, Albert PS, Sciscione A, Wing DA, Grobman WA, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449e1–e41. Article Google
Scholar * Zhu Y, Mendola P, Albert PS, Bao W, Hinkle SN, Tsai MY, et al. Insulin-like growth factor axis and gestational diabetes mellitus: a longitudinal study in a multiracial cohort.
Diabetes. 2016;65:3495–504. Article CAS Google Scholar * World Health Organization. Training course on child growth assessment. Geneva: WHO; 2008. p. 17−25. * Ulijaszek SJ, Kerr DA.
Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–77. Article CAS Google Scholar * Johnson TS, Engstrom JL, Gelhar DK. Intra- and
interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr. 1997;24:497–505. Article CAS Google Scholar * de Onis M, Onyango AW, Van den Broeck
J, Chumlea WC, Martorell R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(1
Suppl):S27–36. Article Google Scholar * Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin
Nutr. 2002;76:1096–100. Article CAS Google Scholar * Weile B. Caliper skinfold measurements in newborns: analysis of a method. Biol Neonate. 1986;50:192–9. Article CAS Google Scholar *
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–64. * Samuelsen SO. A psudolikelihood
approach to analysis of nested case-control studies. Biometrika. 1997;84:379–94. Article Google Scholar * Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research.
Stat Med. 2001;20:1541–9. Article CAS Google Scholar * Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United
States national reference. BMC Pediatr. 2003;3:6. Article Google Scholar * Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in
interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167–75. Article Google Scholar * Nagin DS. Analyzing developmental trajectories: a semiparametric,
group-based approach. Psychol Methods. 1999;4:139. Article Google Scholar * Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, Kogevinas M. Leptin levels in cord blood and
anthropometric measures at birth: a systematic review and meta‐analysis. Paediatr Perinat Epidemiol. 2011;25:150–63. Article Google Scholar * Upadhyay J, Farr OM, Mantzoros CS. The role of
leptin in regulating bone metabolism. Metab-Clin Exp. 2015;64:105–13. Article CAS Google Scholar * Geary M, Pringle PJ, Persaud M, Wilshin J, Hindmarsh PC, Rodeck CH, et al. Leptin
concentrations in maternal serum and cord blood: relationship to maternal anthropometry and fetal growth. Br J Obstet Gynaecol. 1999;106:1054–60. Article CAS Google Scholar * Sørensen HT,
Sabroe S, Rothman KJ, Gillman M, Steffensen FH, Fischer P, et al. Birth weight and length as predictors for adult height. Am J Epidemiol. 1999;149:726–9. Article Google Scholar * Jones
IE, Williams SM, Goulding A. Associations of birth weight and length, childhood size, and smoking with bone fractures during growth: evidence from a birth cohort study. Am J Epidemiol.
2004;159:343–50. Article Google Scholar * Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. Review: developmental origins of osteoporotic fracture. Osteoporos Int.
2006;17:337–47. Article Google Scholar * Josefson JL, Zeiss DM, Rademaker AW, Metzger BE. Maternal leptin predicts adiposity of the neonate. Horm Res Paediatr. 2014;81:13–9. Article CAS
Google Scholar * Patenaude J, Lacerte G, Lacroix M, Guillemette L, Allard C, Doyon M, et al. Associations of maternal leptin with neonatal adiposity differ according to pregravid weight.
Neonatology. 2017;111:344–52. Article CAS Google Scholar * Valsamakis G, Papatheodorou DC, Naoum A, Margeli A, Papassotiriou I, Kapantais E, et al. Neonatal birth waist is positively
predicted by second trimester maternal active ghrelin, a pro-appetite hormone, and negatively associated with third trimester maternal leptin, a pro-satiety hormone. Early Hum Dev.
2014;90:487–92. Article CAS Google Scholar * Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol.
2003;179:293–9. Article CAS Google Scholar * Aye IL, Powell TL, Jansson T. Review: Adiponectin—the missing link between maternal adiposity, placental transport and fetal growth?
Placenta. 2013;34(Suppl):S40–5. Article CAS Google Scholar * Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: a population-based study. J Reprod
Med. 2007;52:1046–51. PubMed Google Scholar * Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev.
2017;18:350–69. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS FUNDING This research was supported by the _Eunice Kennedy Shriver_ National Institute of Child Health and
Human Development intramural funding as well as the American Recovery and Reinvestment Act funding (contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC,
HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z). AUTHOR CONTRIBUTIONS SNH had full access to all of the data in the study and takes
responsibility for the integrity of the data and the accuracy of the data analysis. Conception or design of the paper: SNH, CZ. Acquisition, analysis, or interpretation of data: All authors.
Statistical analysis: SNH, DL, JC. Obtaining funding: CZ. Administrative, technical, or material support: CZ, MYT. Critical revision of the manuscript for important intellectual content:
All authors. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development,
National Institutes of Health, Bethesda, MD, USA Stefanie N. Hinkle, Shristi Rawal, Danping Liu & Cuilin Zhang * Department of Nutritional Sciences, School of Health Professions,
Rutgers University, Newark, NJ, USA Shristi Rawal * Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA Danping Liu *
Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA Jinbo Chen * Department of Laboratory Medicine and
Pathology, University of Minnesota, Minneapolis, MN, USA Michael Y. Tsai Authors * Stefanie N. Hinkle View author publications You can also search for this author inPubMed Google Scholar *
Shristi Rawal View author publications You can also search for this author inPubMed Google Scholar * Danping Liu View author publications You can also search for this author inPubMed Google
Scholar * Jinbo Chen View author publications You can also search for this author inPubMed Google Scholar * Michael Y. Tsai View author publications You can also search for this author
inPubMed Google Scholar * Cuilin Zhang View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Cuilin Zhang. ETHICS
DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ELECTRONIC SUPPLEMENTARY MATERIAL HINKLE ET AL. SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hinkle, S.N., Rawal, S., Liu, D. _et al._ Maternal adipokines longitudinally measured across pregnancy and their associations
with neonatal size, length, and adiposity. _Int J Obes_ 43, 1422–1434 (2019). https://doi.org/10.1038/s41366-018-0255-2 Download citation * Received: 26 March 2018 * Revised: 14 August 2018
* Accepted: 19 August 2018 * Published: 21 November 2018 * Issue Date: July 2019 * DOI: https://doi.org/10.1038/s41366-018-0255-2 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
