Colchicine’s effects on metabolic and inflammatory molecules in adults with obesity and metabolic syndrome: results from a pilot randomized controlled trial
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT OBJECTIVE Recent clinical trials have demonstrated that colchicine may have metabolic and cardiovascular and benefits in at-risk patients; however, the mechanisms through which
colchicine may improve outcomes are still unclear. We sought to examine colchicine’s effects on circulating inflammatory and metabolic molecules in adults with obesity and metabolic syndrome
(MetS). METHODS Blood samples were collected pre- and post-intervention during a double-blind randomized controlled trial in which 40 adults with obesity and MetS were randomized to
colchicine 0.6 mg or placebo twice-daily for 3 months. Serum samples were analyzed for 1305 circulating factors using the SomaScan Platform. The Benjamini–Hochberg procedure was used to
adjust the false discovery rate (FDR) for multiple testing. RESULTS At baseline, age (48.0 ± 13.8 vs. 44.7 ± 10.3 years) and BMI (39.8 ± 6.4 vs. 41.8 ± 8.2 kg/m2) were not different between
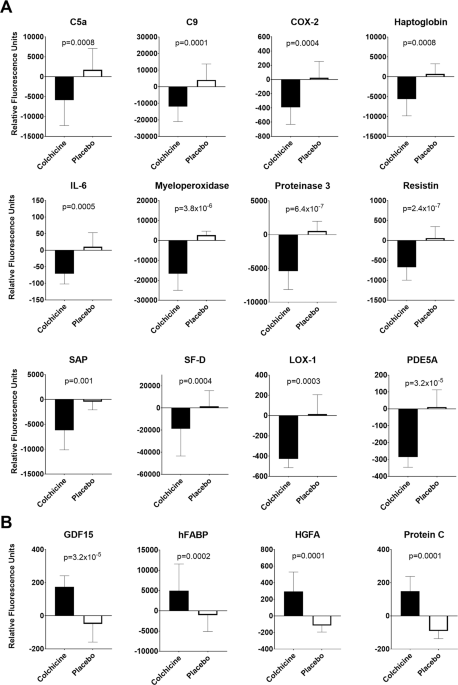
groups. After controlling for the FDR, 34 molecules were significantly changed by colchicine. Colchicine decreased concentrations of multiple inflammatory molecules, including C-reactive
protein, interleukin 6, and resistin, in addition to vascular-related proteins (e.g., oxidized low-density lipoprotein receptor, phosphodiesterase 5A). Conversely, relative to placebo,
colchicine significantly increased concentrations of eight molecules including secreted factors associated with metabolism and anti-thrombosis. CONCLUSIONS In adults with obesity, colchicine
significantly affected concentrations of proteins involved in the innate immune system, endothelial function and atherosclerosis, uncovering new mechanisms behind its cardiometabolic
effects. Further research is warranted to investigate whether colchicine’s IL-6 suppressive effects may be beneficial in COVID-19. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per
year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Wilson PW, D’Agostino RB, Parise H,
Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. Article CAS Google Scholar * Sattar N, Gaw
A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland
Coronary Prevention Study. Circulation. 2003;108:414–9. Article CAS Google Scholar * Nishida M, Moriyama T, Ishii K, Takashima S, Yoshizaki K, Sugita Y, et al. Effects of IL-6,
adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta. 2007;384:99–104. Article CAS Google Scholar * Saklayen MG. The global epidemic of the metabolic
syndrome. Curr Hypertens Rep. 2018;20:12. Article Google Scholar * Demidowich AP, Levine JA, Onyekaba GI, Khan SM, Chen KY, Brady SM, et al. Effects of colchicine in adults with metabolic
syndrome: a pilot randomized controlled trial. Diabetes Obesity Metab. 2019;21:1642–51. Article CAS Google Scholar * Wang L, Sawhney M, Zhao Y, Carpio GR, Fonseca V, Shi L. Association
between colchicine and risk of diabetes among the veterans affairs population with gout. Clin Ther. 2015;37:1206–15. Article CAS Google Scholar * Thompson PL, Nidorf SM. Colchicine: an
affordable anti-inflammatory agent for atherosclerosis. Curr Opin Lipidol. 2018;29:467–73. Article CAS Google Scholar * Martinez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill
C, et al. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc. 2015;4:e002128. Article Google
Scholar * Demidowich AP, Wolska A, Wilson SR, Levine JA, Sorokin AV, Brady SM, et al. Colchicine’s effects on lipoprotein particle concentrations in adults with metabolic syndrome: a
secondary analysis of a randomized controlled trial. J Clin Lipidol. 2019;13:1016–22. Article Google Scholar * Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA, Schneider DJ, Gold L, et al.
Nucleic acid ligands with protein-like side chains: modified aptamers and their use as diagnostic and therapeutic agents. Mol Ther Nucleic Acids. 2014;3:e201. Article CAS Google Scholar *
Cheung F, Fantoni G, Conner M, Sellers BA, Kotliarov Y, Candia J, et al. Web tool for navigating and plotting SomaLogic ADAT files. J Open Res Softw. 2017;20:5. * Adela R, Banerjee SK.
GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. 2015;2015:490842. Article Google Scholar * Kempf T, Eden M, Strelau
J, Naguib M, Willenbockel C, Tongers J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury.
Circ Res. 2006;98:351–60. Article CAS Google Scholar * Nakayasu ES, Syed F, Tersey SA, Gritsenko MA, Mitchell HD, Chan CY, et al. Comprehensive proteomics analysis of stressed human
islets identifies GDF15 as a target for type 1 diabetes intervention. Cell Metab. 2020;31:363–74. Article CAS Google Scholar * Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross
JA, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578:444–8. Article CAS Google Scholar * Weng J, Koch P, Shimada T, Mitchison TJ.
Hepatokine induction by colchicine prevents systemic inflammation via activating PTPN6 inhibitory signaling. In: ASCB Annual Meeting, Washington, DC. Mol Biol Cell 2019; 30: M184: Mol Biol
Cell. * Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. Article CAS Google Scholar * Yan
M, Mehta JL, Hu C. LOX-1 and obesity. Cardiovasc Drugs Ther. 2011;25:469–76. Article CAS Google Scholar * Leung YY, Yao Hui LL, Kraus VB. Colchicine-Update on mechanisms of action and
therapeutic uses. Semin Arthritis and Rheum. 2015;45:341–50. Article CAS Google Scholar * Fernandez-Real JM, Valdes S, Manco M, Chico B, Botas P, Campo A, et al. Surfactant protein d, a
marker of lung innate immunity, is positively associated with insulin sensitivity. Diabetes Care. 2010;33:847–53. Article CAS Google Scholar * Pedersen BK. IL-6 signalling in exercise and
disease. Biochem Soc Trans. 2007;35:1295–7. Article CAS Google Scholar * Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin
resistance in mice. Diabetes. 2003;52:2784–9. Article CAS Google Scholar * Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like
IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84. Article CAS Google Scholar * Muniyappa R, Gubbi S.
COVID-19 pandemic, corona viruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–41. * Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al.
COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. Article CAS Google Scholar * Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F,
et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020. * Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA,
Angelidis C, Giotaki SG, et al. The GReek study in the Effects of Colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol. 2020. *
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–505. Article CAS
Google Scholar * Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–10. Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to thank the NIH Clinical Center nurses of 5NW, the Metabolic Unit, and OP9 for their dedication and assistance in
the carrying out of the clinical study. CHI CONSORTIUM Huizhi Zhou4, Rongye Shi4, Poorani Subramanian4, John Tsang4, Yasmine Belkaid4 FUNDING 1ZIAHD000641, with supplemental funding from an
NICHD Division of Intramural Research Director’s Award. This research was also supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious
Diseases, and the trans-NIH Center for Human Immunology. AUTHOR INFORMATION Author notes * Andrew P. Demidowich Present address: Johns Hopkins Community Physicians at Howard County General
Hospital, Johns Hopkins Medicine, Columbia, MD, 21044, USA AUTHORS AND AFFILIATIONS * Section on Growth and Obesity, Division of Intramural Research, Eunice Kennedy Shriver National
Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD, 20892-1103, USA Andrew P. Demidowich, Jordan A. Levine, Tushar P. Patel & Jack
A. Yanovski * Office of the Clinical Director, NICHD, NIH, Bethesda, MD, 20892, USA Andrew P. Demidowich * Department of Endocrinology, Diabetes and Metabolism, Johns Hopkins School of
Medicine, Baltimore, MD, 21205, USA Andrew P. Demidowich * Center for Human Immunology (CHI), National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD, 20892, USA Richard
Apps, Foo K. Cheung, Jinguo Chen, Giovanna Fantoni, Huizhi Zhou, Rongye Shi, Poorani Subramanian, John Tsang & Yasmine Belkaid Authors * Andrew P. Demidowich View author publications You
can also search for this author inPubMed Google Scholar * Jordan A. Levine View author publications You can also search for this author inPubMed Google Scholar * Richard Apps View author
publications You can also search for this author inPubMed Google Scholar * Foo K. Cheung View author publications You can also search for this author inPubMed Google Scholar * Jinguo Chen
View author publications You can also search for this author inPubMed Google Scholar * Giovanna Fantoni View author publications You can also search for this author inPubMed Google Scholar *
Tushar P. Patel View author publications You can also search for this author inPubMed Google Scholar * Jack A. Yanovski View author publications You can also search for this author inPubMed
Google Scholar CONSORTIA CHI CONSORTIUM * Huizhi Zhou * , Rongye Shi * , Poorani Subramanian * , John Tsang * & Yasmine Belkaid CORRESPONDING AUTHOR Correspondence to Andrew P.
Demidowich. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors have no conflict of interest to disclose. JAY receives grant support for unrelated studies sponsored by Rhythm
Pharmaceuticals Inc., and by Soleno Therapeutics Inc. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. Members of the CHI Consortium are listed below Acknowledgements. SUPPLEMENTARY INFORMATION SUPPLEMENTAL TABLE 1 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Demidowich, A.P., Levine, J.A., Apps, R. _et al._ Colchicine’s effects on metabolic and inflammatory molecules in adults with obesity and metabolic
syndrome: results from a pilot randomized controlled trial. _Int J Obes_ 44, 1793–1799 (2020). https://doi.org/10.1038/s41366-020-0598-3 Download citation * Received: 23 March 2020 *
Revised: 03 May 2020 * Accepted: 07 May 2020 * Published: 27 May 2020 * Issue Date: August 2020 * DOI: https://doi.org/10.1038/s41366-020-0598-3 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
