Pi3k orchestration of the in vivo persistence of chimeric antigen receptor-modified t cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

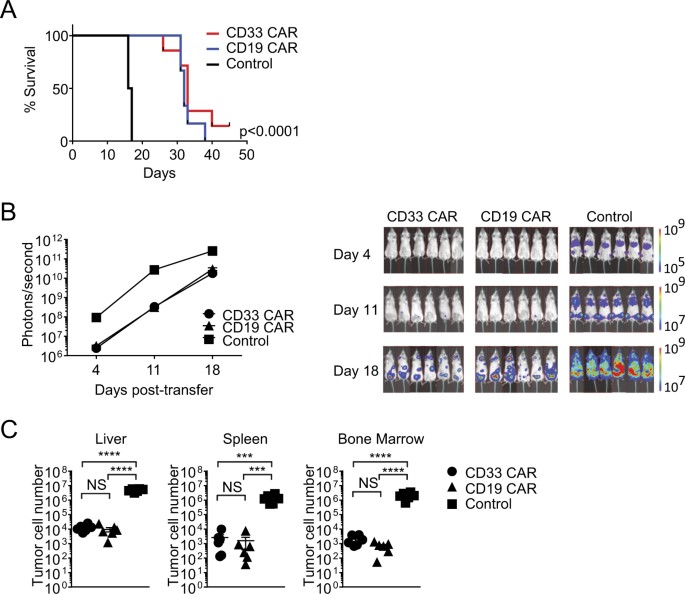
ABSTRACT In vivo persistence of chimeric antigen receptor (CAR)-modified T cells correlates with therapeutic efficacy, yet CAR-specific factors that support persistence are not well
resolved. Using a CD33-specific CAR in an acute myeloid leukemia (AML) model, we show how CAR expression alters T cell differentiation in a ligand independent manner. Ex vivo expanded CAR-T
cells demonstrated decreased naïve and stem memory populations and increased effector subsets relative to vector-transduced control cells. This was associated with reduced in vivo
persistence. Decreased persistence was not due to specificity or tumor presence, but to pre-transfer tonic signaling through the CAR CD3ζ ITAMs. We identified activation of the PI3K pathway
in CD33 CAR-T cells as responsible. Treatment with a PI3K inhibitor modulated the differentiation program of CAR-T cells, preserved a less differentiated state without affecting T cell
expansion, and improved in vivo persistence and reduced tumor burden. These results resolve mechanisms by which tonic signaling of CAR-T cells modulates their fate, and identifies a novel
pharmacologic approach to enhance the durability of CAR-T cells for immunotherapy. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ANTIGEN EXPERIENCE HISTORY DIRECTS DISTINCT FUNCTIONAL STATES OF
CD8+ CAR T CELLS DURING THE ANTILEUKEMIA RESPONSE Article Open access 02 January 2025 COUNTERACTING CAR T CELL DYSFUNCTION Article Open access 14 January 2021 DECADE-LONG LEUKAEMIA
REMISSIONS WITH PERSISTENCE OF CD4+ CAR T CELLS Article 02 February 2022 REFERENCES * Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502.
Article CAS Google Scholar * Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. Article CAS Google Scholar *
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–20. Article CAS Google
Scholar * Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and
multiple myeloma. Blood. 2013;122:3461–72. Article CAS Google Scholar * Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen
receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28:1596–605. Article CAS Google Scholar * Kenderian SS, Ruella M,
Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia.
2015;29:1637–47. Article CAS Google Scholar * O’Hear C, Heiber JF, Schubert I, Fey G, Geiger TL. Anti-CD33 chimeric antigen receptor targeting of acute myeloid leukemia. Haematologica.
2015;100:336–44. Article Google Scholar * Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent
cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. Article CAS Google Scholar * Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with
chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. Article CAS Google Scholar * Grupp SA,
Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New Engl J Med. 2013;368:1509–18. Article CAS Google
Scholar * Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl J Med. 2014;371:1507–17. Article
Google Scholar * Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients
with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. Article CAS Google Scholar * Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy
and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. Article Google Scholar * Savoldo B, Ramos CA, Liu E, Mims MP,
Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Investig. 2011;121:1822–6.
Article CAS Google Scholar * Zhao Z, Condomines M, van der Stegen SJ, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics
and persistence of CAR T Cells. Cancer Cell. 2015;28:415–28. Article CAS Google Scholar * Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric
antigen receptor-modified T cells derived from defined CD8 + and CD4 + subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. Article CAS Google Scholar * Xu Y,
Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood.
2014;123:3750–9. Article CAS Google Scholar * Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr., et al. Distinct signaling of coreceptors regulates specific
metabolism pathways and impacts memory development in CAR T Cells. Immunity. 2016;44:380–90. Article CAS Google Scholar * Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M,
et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. Article CAS Google Scholar * Sallusto F,
Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. Article CAS Google Scholar *
Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, et al. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med.
2017;214:1593–606. Article CAS Google Scholar * Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather
than central memory CD8 + T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106:17469–74. Article CAS Google Scholar * Wang X, Berger C, Wong CW, Forman SJ,
Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8 + T cells in immunodeficient mice. Blood. 2011;117:1888–98. Article CAS Google Scholar * Cieri N, Camisa B,
Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–84. Article CAS
Google Scholar * Kramer A, Green J, Pollard J Jr., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–30. Article Google Scholar * Kim
EH, Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front Immunol. 2013;4:20. PubMed PubMed Central Google Scholar * Araki K, Turner AP, Shaffer VO, Gangappa S,
Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–12. Article CAS Google Scholar * Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek
A, Choi KY, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–14. Article CAS Google Scholar * Kaech SM, Cui W.
Transcriptional control of effector and memory CD8 + T cell differentiation. Nat Rev Immunol. 2012;12:749–61. Article CAS Google Scholar * Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM,
Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. Article CAS Google Scholar * Flynn JK, Gorry PR. Stem memory T _c_ells
(TSCM)-their role in cancer and HIV immunotherapies. Clin Transl Immunol. 2014;3:e20. Article Google Scholar * Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al.
Generation of clinical-grade CD19-specific CAR-modified CD8 + memory stem cells for the treatment of human B-cell malignancies. Blood. 2016;128:519–28. Article CAS Google Scholar *
Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172:4661–5. Article CAS Google Scholar * Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known
knowns and the known unknowns. Trends Immunol. 2015;36:13–20. Article CAS Google Scholar * Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining
4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8 + T cell-mediated tumor eradication. Mol Ther. 2010;18:413–20. Article CAS Google Scholar * Delmore JE,
Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–17. Article CAS Google Scholar * Kagoya Y,
Nakatsugawa M, Yamashita Y, Ochi T, Guo T, Anczurowski M, et al. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J Clin Investig.
2016;126:3479–94. Article Google Scholar * Chapman NM, Chi H. mTOR links environmental signals to T Cell fate decisions. Front Immunol. 2014;5:686. PubMed Google Scholar * Wang R, Dillon
CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–82. Article CAS
Google Scholar * Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–26. Article
CAS Google Scholar * Co MS, Avdalovic NM, Caron PC, Avdalovic MV, Scheinberg DA, Queen C. Chimeric and humanized antibodies with specificity for the CD33 antigen. J Immunol.
1992;148:1149–54. CAS PubMed Google Scholar * Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM, et al. Construction and characterisation of a functional CD19 specific
single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol. 1997;34:1157–65. Article CAS Google Scholar * Kong D, Yamori T. Advances in development of
phosphatidylinositol 3-kinase inhibitors. Curr Med Chem. 2009;16:2839–54. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This research was supported by ALSAC/SJCRH, The
Assissi Foundation of Memphis, NCI Cancer Center Support Grant CA021765, and the Howard Hughes Medical Institute (JHB). The authors thank Richard Cross, Grieg Lennon, Parker Ingle, Tammar
Williams, and the SJCRH Blood Donor Center for assistance. AUTHOR INFORMATION Author notes * Carol E. O’Hear Present address: Genentech, Inc., South San Francisco, CA, USA AUTHORS AND
AFFILIATIONS * Department of Pathology, St. Jude Children’s Research Hospital, Memphis, TN, 38105, USA Wenting Zheng, Carol E. O’Hear, Rajshekhar Alli, Jacob H. Basham, Lindsay L. Jones
& Terrence L. Geiger * Department of Immunology, St. Jude Children’s Research Hospital, Memphis, TN, 38105, USA Hossam A. Abdelsamed & Ben Youngblood * Department of Computational
Biology, St. Jude Children’s Research Hospital, Memphis, TN, 38105, USA Lance E. Palmer Authors * Wenting Zheng View author publications You can also search for this author inPubMed Google
Scholar * Carol E. O’Hear View author publications You can also search for this author inPubMed Google Scholar * Rajshekhar Alli View author publications You can also search for this author
inPubMed Google Scholar * Jacob H. Basham View author publications You can also search for this author inPubMed Google Scholar * Hossam A. Abdelsamed View author publications You can also
search for this author inPubMed Google Scholar * Lance E. Palmer View author publications You can also search for this author inPubMed Google Scholar * Lindsay L. Jones View author
publications You can also search for this author inPubMed Google Scholar * Ben Youngblood View author publications You can also search for this author inPubMed Google Scholar * Terrence L.
Geiger View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Terrence L. Geiger. ETHICS DECLARATIONS CONFLICT OF
INTEREST The authors declare that they have no conflict of interest. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY MATERIALS RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Zheng, W., O’Hear, C., Alli, R. _et al._ PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. _Leukemia_ 32, 1157–1167
(2018). https://doi.org/10.1038/s41375-017-0008-6 Download citation * Received: 02 August 2017 * Revised: 07 December 2017 * Accepted: 13 December 2017 * Published: 02 February 2018 * Issue
Date: May 2018 * DOI: https://doi.org/10.1038/s41375-017-0008-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
