Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) α1
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Through dynamic means, etiological factors, including chronic inflammation and insulin resistance have the potential to perpetuate metabolic incidences such as type 2 diabetes and obesity.
Abatement of such syndromes can be achieved by complex mechanisms initiated through bioactive compounds such as polyphenols derived from fruits. Using a whole-fruit approach, the effects of
dietary red raspberry, which is rich in polyphenols, on inflammatory responses and insulin resistance in the skeletal muscles of Mus musculus were studied along with the potential role of
AMP-activated protein kinase (AMPK) to act as a key mediator.
Wild-type (WT) mice and mice deficient in the catalytic subunit (α1) of AMPK (AMPKα1−/−) were fed with a high-fat diet (HFD) or HFD supplemented with raspberry (5% dry weight) for 10 weeks.
Factors involved in inflammatory responses, insulin signaling transduction, and mitochondrial biogenesis were evaluated.
Dietary raspberry reduced ectopic lipid storage, alleviated inflammation responses, improved whole-body insulin sensitivity, and promoted mitochondrial biogenesis in the skeletal muscle of
WT mice, but not AMPKα1−/− mice.
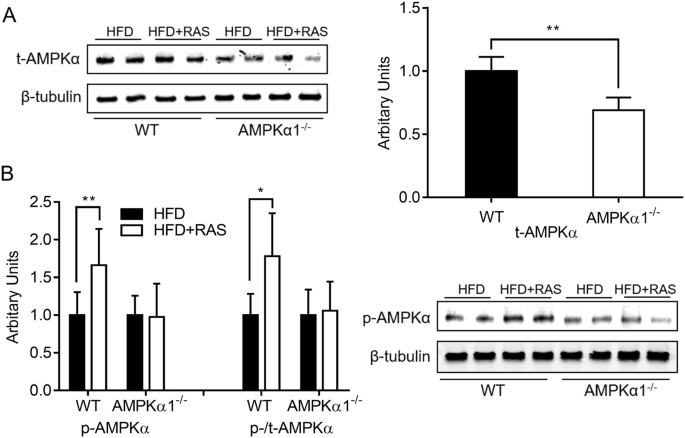
AMPKα1 is an important mediator for the beneficial effects of raspberry through alleviating inflammatory responses and sensitizing insulin signaling in skeletal muscle of HFD-fed mice.
Red raspberry is widely recognized for its high levels of vitamin C and bioactive polyphenols, including ellagitannins and anthocyanins, which have strong antioxidant capacities1. Several
animal studies have shown that supplementation of raspberry extracts exhibited beneficial effects for the prevention of obesity, inflammation and other metabolic diseases2, 3. However, the
impacts of dietary raspberry fruit on skeletal muscle insulin resistance and the underlying mechanisms remain largely unexplored.
Obesity induces ectopic lipid accumulation and desensitizes insulin signaling in skeletal muscle, thus resulting in systematic insulin resistance and type 2 diabetes4. AMP-activated protein
kinase (AMPK) is a key sensor of energy status in skeletal muscle through the control of glucose and fatty acid metabolism5. The structure of AMPK has been described as a heterotrimeric
complex comprised of the catalytic α-subunit and the regulatory β- and γ- subunits6. Activation of AMPK prevents obesity and associated metabolic diseases through the promotion of glucose
utilization, fatty acid oxidation, and mitochondrial biogenesis in skeletal muscle6. Dietary polyphenols, such as resveratrol, are strong activators of AMPK, which can then promote the
browning of white adipose and subsequently alleviate obesity7. Due to the high levels of polyphenols found in the red raspberry, it is postulated that AMPK plays an essential role in
mediating the beneficial effects of red raspberry on metabolic health.
The catalytic subunit of AMPK has 2 isoforms (α1 and α2). Although there is a compensatory mechanism between these two isoforms, their expression shows tissue-specific patterns8, 9 with
differential metabolic functions10, 11. The isoform α2 of AMPK is indispensable for increased glucose uptake by skeletal muscle induced by 5-aminoimidazole-4-carboxamide ribonucleotide
(AICAR) and hypoxia5, 12, 13. Meanwhile, the AMPKα1 isoform can be activated during skeletal muscle contraction14 and at low caffeine concentrations10. Indeed, AMPKα1 also plays an essential
role in myogenin expression and myogenesis15. Previous studies in our lab have shown the dominant expression of AMPKα1 in satellite cells, which when deleted, impeded muscle regeneration
after injury15. Deletion of AMPKα1 in macrophages during the transition from a proinflammatory (M1) to an anti-inflammatory (M2) phenotype impairs the resolution of inflammation and muscle
regeneration after injury16. Altogether, these studies suggested that AMPKα1 could mediate the alleviation of insulin resistance and metabolic syndromes in skeletal muscle of obese mice
consuming raspberry. Thus, we explored the influence of red raspberry on insulin sensitivity and inflammatory responses in skeletal muscles, along with the potential role of AMPKα1 to act as
a key mediator.
R26Cre/AMPKα1fl/fl mice were generated through the cross-breeding of AMPKα1fl/fl mice (Stock No: 014141, Jackson Lab, Bar Harbor, Maine) with tamoxifen-inducible R26-Cre mice (Stock No:
004847, Jackson Lab, Bar Harbor, Maine) at Washington State University. To induce the AMPKα1 knockout (AMPKα1−/−), 2-month-old male R26Cre/AMPKα1fl/fl mice were intraperitoneally injected
with tamoxifen (75 mg/kg body weight) for 4 continuous days17. AMPKα1fl/fl mice treated with tamoxifen were used as controls (Wild-type, WT). To minimize possible confounding changes,
dietary treatments started 3 days after the last tamoxifen injection15. All experimental procedures of animal use were performed according to the guidelines of National Institutes of Health
and approved by the Animal Use and Care Committee of Washington State University (Permit No. 04719).
Twelve wild-type and AMPKα1−/− mice, respectively, were randomly separated into two sub-groups and fed either a high-fat diet (HFD; 60% energy from fat, D12492; Research Diets, New
Brunswick, NJ, USA) or a HFD diet supplemented with freeze-dried raspberry (5% of dry feed weight, red raspberry powder). The concentration of the raspberry supplementation was determined by
preliminary studies in our lab18. Raspberry powder was prepared as previously described, which contains polyphenols at ~11 g gallic acid equivalent (GAE)/kg of dry weight, 4.24 ± 0.12%
protein, 1.91 ± 0.03% fat, 0.81 ± 0.02% ash, 16.14 ± 0.45% moisture, and the remaining to be mainly carbohydrates19. Mice were housed in a temperature-controlled environment (23 ± 2 °C,
alternating 12-h light/dark cycle) with ad libitum access to food and water. Feed intake and body weights were monitored weekly until the mice were killed 10 weeks later. Samples of blood,
the Gastrocnemius muscle (GA), and the Tibialis anterior muscle (TA) were rapidly isolated. TA were fixed in 4% paraformaldehyde for sectioning and staining, and GA were rapidly frozen in
liquid nitrogen and stored at −80 °C until further analyses.
Paraffin-embedded TA muscle sections (5-μm thick) were rehydrated through a series of incubations in xylene and ethanol solutions, and then used for Masson trichrome staining20. At least
four fields per section and four sections per sample were randomly selected for quantification of fat area and collagen area using the Image J 1.46r software (National Institutes of Health).
The average data per biological sample were used for calculations.
As previously described, total triacylglycerol determination was performed using the Folch method20, 21. The frozen GA muscle was powdered under liquid nitrogen and a 30 mg sample was
weighed. After adding 0.75 ml of chloroform-methanol 2:1 (v/v), the samples were left at 4 °C for 48 h. Then, 187.5 µl 0.9% NaCl was added and the mixture was kept at room temperature
overnight and then centrifuged at 10,000 × g for 5 min at 4 °C. The lower phase (20 µl) was transferred into a fresh tube and evaporated until dry for 1 h under the hood. Total
triacylglycerols were measured using a kit from Sigma following the manufacturer’s instructions (cat. no. TR0100). The results were displayed by dividing the total triacylglycerol content by
the initial muscle powder weight.
Total RNA was isolated using TRIzol reagent (Sigma, Saint Louis, MO, USA), followed by reverse-transcription to cDNA using the iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The
mRNA levels were measured by qRT-PCR carried out by the CFX RT-PCR detection system (Bio-Rad). After normalization to 18s rRNA content, relative mRNA expression was determined using the
method of 2-ΔΔCt22. Table 1 shows the primer sequences.
Immunoblotting analyses were performed as previously described using the Odyssey Infrared Image System (LI-COR Biosciences, Lincoln, NE, USA)15. Band densities of target proteins were
normalized to β-tubulin content. The following antibodies were purchased from Cell Signaling (Danver, MA, USA): AMPKα (no.2532), phospho-AMPKα at Thr172 (no. 2535), protein kinase B (AKT,
no.9272), phospho-AKT at Ser473 (no. 9271), protein kinase C (PKCθ, no.13643), phospho-PKCθ at Thr538 (no.9377), nuclear factor κB (NFκB) subunit p65 (no.8242), phospho-p65 at Ser536
(no.3033), c-Jun N-terminal kinases (JNK, no. 9252), phospho-JNK at Thr183/Tyr185 (no.9251) and cytochrome C (cyt C, no. 4280). IRDye 800CW goat anti-rabbit (no. 926-32211) and IRDye 680
goat anti-mouse (no. 926-68070) secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE, USA). For use, primary antibodies were diluted 1: 1000 using 1× TBST buffer (137 mM
Sodium Chloride, 20 mM Tris, 0.1% Tween-20, pH 7.6) with 5% BSA (Bovine Serum Albumin) and secondary antibodies were diluted 1: 10,000 using TBST buffer.
Within each genotype, the data were analyzed using unpaired two-tailed Student’s t test using SAS 9.0 (SAS Institute Inc., Cary, NC, USA). All the data were found normally distributed.
Results are expressed as mean ± s.d. A significant difference was considered as P
