Comparison of visceral fat measurement by dual-energy x-ray absorptiometry to computed tomography in hiv and non-hiv
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND/OBJECTIVES Individuals with HIV are susceptible to visceral fat accumulation, which confers an increased risk of cardiometabolic disease. Advanced software to ascertain
visceral fat content from dual-energy X-ray absorptiometry (DXA) has not been validated among this population. We sought to compare DXA with computed tomography (CT) in the measurement of
visceral fat cross-sectional area (VAT) in HIV and non-HIV using Bland–Altman analyses. SUBJECTS/METHODS Data were combined from five previously conducted studies of individuals with HIV
(_n_ = 313) and controls without HIV (_n_ = 144) in which paired DXA and CT scans were available. In cross-sectional analyses, DXA-VAT was compared with CT-VAT among participants with and
without HIV. In longitudinal analyses, changes in VAT over time were compared between DXA and CT among participants with and without HIV receiving no intervention over 12 months and among
individuals with HIV receiving tesamorelin—a medication known to reduce VAT—over 6 months. RESULTS In HIV, DXA underestimated VAT compared with CT among individuals with increased visceral
adiposity. The measurement bias was −9 ± 47 cm2 overall, but became progressively larger with greater VAT (_P_ < 0.0001), e.g., −61 ± 58 cm2 among those with VAT ≥ 200 cm2. Sex-stratified
analyses revealed that the relationship between VAT and measurement bias was especially pronounced in men (_P_ < 0.0001). Longitudinally, DXA underestimated changes in VAT, particularly
among those at the extremes of VAT gain or loss (_P_ < 0.0001). In contrast to the cross-sectional findings, the tendency for DXA to underestimate longitudinal changes in VAT was evident
in both men and women. Analogous findings were seen among controls in cross-sectional and longitudinal analyses. CONCLUSIONS DXA underestimated VAT relative to CT in men with and without
HIV, who had increased visceral adiposity. DXA also underestimated changes in VAT over time in men and women, irrespective of HIV status. DXA-VAT should be used with caution among both HIV
and non-HIV-infected populations. SIMILAR CONTENT BEING VIEWED BY OTHERS ACCURACY OF DUAL-ENERGY X-RAY ABSORPTIOMETRY FOR ASSESSING LONGITUDINAL CHANGE IN VISCERAL ADIPOSE TISSUE IN PATIENTS
WITH CORONARY ARTERY DISEASE Article 13 May 2021 AGE- AND SEX-SPECIFIC VISCERAL FAT REFERENCE CUTOFFS AND THEIR ASSOCIATION WITH CARDIO-METABOLIC RISK Article 20 January 2021 ESTIMATED
VISCERAL ADIPOSITY IS ASSOCIATED WITH RISK OF CARDIOMETABOLIC CONDITIONS IN A POPULATION BASED STUDY Article Open access 27 April 2021 INTRODUCTION Independent of body mass index (BMI), fat
distribution is an important determinant of cardiometabolic health1. Specifically, visceral fat has been associated with hypertension2,3, dyslipidemia3,4, glucose intolerance3,4, and
coronary artery disease5,6. On the other hand, subcutaneous fat has been generally regarded as a benign storage depot3,4,7. Among individuals with HIV, visceral fat accumulation is commonly
observed, even at a normal or overweight BMI8,9. Moreover, consonant with findings from the general population, HIV-related changes in fat distribution have been shown to confer an increased
risk of cardiovascular disease10,11,12. In HIV and in the general population, there is a critical need for a safe, affordable, and accurate method to quantify visceral fat. Such an approach
would allow for the routine clinical use of visceral fat measurement to stratify cardiovascular risk and to monitor the efficacy of metabolic interventions. The gold standard for abdominal
adipose tissue measurement is computed tomography (CT)13. In this regard, visceral fat cross-sectional area at the L4 vertebral level (CT-VAT) has been widely adopted due to its strict
relation to skeletal landmarks and thus high degree of reproducibility (_r_ = 0.99)13,14. However, as CT involves radiation exposure and is costly, alternative means to assess visceral fat
have been sought15. Dual-energy X-ray absorptiometry (DXA) is an inexpensive imaging modality that uses minimal radiation to quantify regional fat distribution (e.g., total abdominal or
truncal fat)16,17,18. In recent years, advanced software has enabled visceral fat cross-sectional area to be ascertained in a predominantly automated manner from a standard DXA scan
(DXA-VAT)19. Multiple studies among the general population have demonstrated that DXA-VAT approximates VAT as measured by other imaging modalities including CT15,19,20,21. However, the
accuracy of DXA-VAT has not been evaluated among individuals with HIV, who may exhibit substantial expansion of this depot. Moreover, the use of DXA to quantify changes in visceral fat over
time (i.e., in response to an intervention) has never before been assessed among any patient population. In the current analysis, we leveraged data from previously conducted clinical studies
to compare DXA-VAT with CT-VAT among a large sample of men and women with and without HIV. Specifically, we sought to assess the accuracy of DXA in the measurement of (1) visceral fat at a
single time point, (2) changes in visceral fat over time that occur spontaneously, and (3) changes in visceral fat over time that occur in response to an intervention. Before DXA-VAT can be
readily applied in clinical and research settings, it is pivotal to validate its accuracy in diverse populations and to delineate potential limitations to its use. SUBJECTS AND METHODS
SUBJECTS We combined all available data from five studies of individuals with HIV, which were conducted from 2006 to 2014 (Supplementary Table 1)22,23,24,25,26. In each study, participants
recruited from the Boston area underwent abdominal CT and DXA concurrently per protocol. Controls without HIV were co-enrolled in three of the studies and their data were included for
comparison22,23,25. In four of the studies, participants were followed longitudinally for at least 6 months with CT and DXA repeated at fixed intervals23,24,25,26. In our cross-sectional
analysis, we compared DXA with CT in the measurement of visceral fat among individuals with and without HIV at a single time point. Each participant contributed one set of paired CT and DXA
data to this analysis (Supplementary Fig. 1). For subjects who took part in more than one study, only baseline data from their first longitudinal study was included for analysis. If no
longitudinal data was available, data from the cross-sectional study was used. We prioritized inclusion of baseline longitudinal data in the cross-sectional analysis to facilitate
consistency between the cross-sectional and longitudinal sub-studies. In our longitudinal analysis, we sought to determine the accuracy of DXA compared with CT with respect to repeated
measurements of visceral fat over time. This analysis consisted of two arms (Supplementary Fig. 1). First, we devised a natural history group, which comprised participants with or without
HIV, who had been assigned to receive either no treatment or placebo for at least 12 months23,25,26. Changes in visceral fat from baseline to 12 months as measured by DXA and CT were
compared. As with our cross-sectional analysis, for subjects who had participated in more than one longitudinal study, only data from their first study was eligible for inclusion. Second, we
assembled a group of individuals with HIV, who had been randomized to receive tesamorelin or placebo for 6 months24. Tesamorelin is a growth hormone-releasing hormone (GHRH) analog that
selectively reduces visceral fat without substantially altering subcutaneous fat content or BMI27,28. As such, this randomized trial enabled us to assess the agreement between DXA and CT
with regard to an intervention known to reduce visceral fat. We included all participants of this study, who had DXA and CT data available at baseline and 6 months. Subjects gave informed
consent before their participation in study procedures. The Institutional Review Boards at Massachusetts General Hospital and/or Massachusetts Institute of Technology approved each original
study and the current combined analysis. STUDY PROCEDURES In each of the five studies, all participants underwent a detailed history and physical exam. HIV viral load and T-cell subsets were
performed using standard assays. At baseline, whole-body DXA (Hologic QDR 4500 A, Hologic Discovery A, and Hologic Horizon A) was performed and analyzed by licensed research dieticians
certified as bone densitometry technologists by the International Society for Clinical Densitometry. In addition, at baseline, each subject underwent a single-slice CT scan of the abdomen at
L4 level (General Electric, Waukesha, WI) for assessment of VAT and subcutaneous adipose tissue area (SAT). In four of the five studies, repeated DXA and CT were performed at set intervals
of 6 or more months among participants receiving either treatment, placebo, or no intervention. CT scan was performed with participants in the supine position. Lateral and frontal scout
images were obtained to identify the L4 vertebral level, which served as a landmark for the single-slice scan. Scan parameters were standardized: 144 table height, 80 kV, 70 mA, 2 seconds
scan time, 1 cm slice thickness, 48 cm field of view. A single expert (M.T.) supervised the reading of all scans to ensure uniformity. Quantification of abdominal fat depots was performed
using image analysis software (ViTrak, Merge/eFilm, Inc., Chicago, IL). Briefly, thresholding methods were applied to identify adipose tissue using a threshold set to −50 to −250 Hounsfield
units. Then, manual delineation with tools provided by the software was used to separate VAT from SAT. Pixels within the threshold that were not anatomically one of the two adipose tissue
depots were removed. Using this technique, the coefficient of variation for repeated measurements of the same scan on consecutive days by the same analyst has been reported as 2.3% for VAT
and 1.7% for SAT20. For the purposes of the current analysis, DXA was re-analyzed by a single research dietician (J.H.) in 2018 using Hologic Horizon A, APEX 6.6.0.5 software (Hologic, Inc.,
Bedford, MA, USA) for measurement of visceral fat cross-sectional area. Standard procedures per the Horizon Bone Densitometry Systems User Guide were employed29. Briefly, for each scan, the
software automatically located the outer and inner margins of the abdominal wall in a 5 cm region of the abdomen. The lower border of this region coincided with the L4 vertebral level and
marked the plane through which DXA-VAT cross-sectional area was reported. The software measured total abdominal fat and subcutaneous fat directly, and reported visceral fat content as the
difference between these measurements20,30. To assess the reproducibility of our DXA-VAT readings, 98 DXA scans were re-analyzed by a second research dietician (T.H.). Owing to the high
level of automaticity of the APEX software, there was a strong correlation (_r_ > 0.99) of DXA-VAT measurements between readers. Both dieticians were kept blind to the CT data.
STATISTICAL ANALYSIS In our comparisons of DXA vs. CT, individuals with and without HIV were analyzed separately. For the cross-sectional analysis, the correlation between CT-VAT and DXA-VAT
was determined using Pearson’s correlation coefficient. Measurement bias was calculated as the mean difference between the two techniques (DXA–CT) ± SD. Bland–Altman plots were subsequently
used to display the relationship of average VAT between modalities vs. measurement bias31. Visual inspection followed by linear regression was used to assess for systematic bias.
Multivariable models also were constructed to adjust for age, sex, race, and ethnicity, and to test for interactions of VAT with age (≥ vs. <50 years), sex, race, BMI (≥ vs. <30
kg/m2), CD4 count (≥ vs. <250 cells/mm3), HIV viral load (≥ vs. <50 copies/mL), and antiretroviral therapy (ART) duration (≥ vs. <5 years), and class (among individuals receiving
ART). For interactions that were significant, subgroup analyses were performed. A comparable statistical approach was used to compare DXA with CT with respect to SAT. For the natural history
longitudinal analysis, a linear regression was performed to relate change in CT-VAT to change in DXA-VAT over 12 months. Multivariable models were constructed to compare the changes in VAT
between these two modalities, while controlling for age, sex, race, and ethnicity, and to test for an interaction between sex and change in VAT. Changes in VAT as measured by DXA and CT also
were compared using a Bland–Atlman plot. An analogous statistical approach was taken for the tesamorelin longitudinal analysis, which compared changes in visceral fat as measured by CT and
DXA over 6 months among individuals with HIV only. All two-group comparisons used Student’s two-tailed _t_-test assuming unequal variances for continuous outcomes and _χ_2-test for
categorical outcomes. Continuous variables were reported as mean ± SD or mean (95% confidence interval). A critical value of _P_ < 0.05 was used to designate statistical significance. All
statistical analyses were performed using JMP Pro 12.0.1 (SAS Institute, Inc., Cary, NC). RESULTS CLINICAL CHARACTERISTICS A total of 313 individuals with HIV and 144 controls were included
in these analyses (Table 1). Participants with and without HIV were similar in age (48 ± 7 vs. 47 ± 7 years) and racial distribution. Individuals with HIV tended to have long-standing
infection (15 ± 7 years) that was well controlled (viral load < 50 copies/mL in 76%) on ART (88%). Although BMI was comparable between HIV and non-HIV groups (27 ± 5 vs. 28 ± 5 kg/m2),
fat distribution varied as would be expected8,32. In particular, individuals with HIV had higher CT-VAT (141 ± 101 vs. 115 ± 84 cm2, _P_ = 0.004) and lower CT-SAT (236 ± 137 vs. 289 ± 152
cm2, _P_ = 0.0005) than controls. Total abdominal adiposity did not differ between groups. CROSS-SECTIONAL COMPARISON OF DXA WITH CT IN HIV Among individuals with HIV, CT-VAT and DXA-VAT
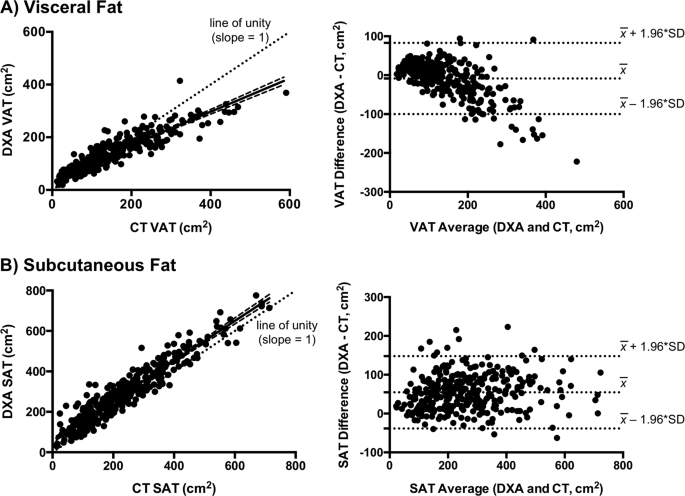
were strongly correlated (_r_ = 0.91, _P_ < 0.0001) (Fig. 1a). Moreover, DXA-VAT only mildly underestimated CT-VAT on average (−9 ± 47 cm2, _P_ = 0.001) (Table 2). However, the difference
between these modalities (DXA–CT) became progressively more negative with greater visceral fat content (_P_ < 0.0001) (Fig. 1a). As such, the measurement bias was 7 ± 29 cm2 among
individuals with VAT < 200 cm2, compared with −61 ± 58 cm2 among those with VAT ≥ 200 cm2 (_P_ < 0.0001). Sex-stratified analyses revealed contrasting relationships of DXA-VAT with
CT-VAT in men vs. women. Clinical characteristics between men and women with HIV were compared in Supplementary Table 2. On average, DXA-VAT underestimated CT-VAT among men, whereas it
overestimated CT-VAT among women (−20 ± 50 vs. 14 ± 27 cm2, _P_ < 0.0001) (Table 2). The relationship between visceral adiposity and measurement bias (DXA–CT) also differed between sexes
as evidenced by a significant sex × VAT interaction (_P_ < 0.0001) in models that adjusted for age, race, and ethnicity. When each sex was analyzed separately, the line relating VAT
average (DXA and CT) to VAT difference (DXA–CT) had a slope of −0.41 (−0.47 to −0.36) in men and −0.09 (−0.17 to −0.003) in women (Fig. 2). Thus, DXA progressively underestimated VAT as a
function of visceral fat content in men, whereas this relationship was less pronounced in women. In addition, in HIV, obesity did not modify the inverse relationship between visceral
adiposity and measurement bias (DXA–CT). However, at any given visceral fat content, the measurement bias was modestly smaller in obese compared with non-obese individuals (Supplementary
Fig. 2). There was no effect or interaction with VAT for age, race, CD4 count, HIV viral load, or ART duration or class (among participants receiving ART) in relation to measurement bias. In
HIV, CT-SAT and DXA-SAT were strongly correlated (_r_ = 0.94, _P_ < 0.0001) (Fig. 1b) with an overall measurement bias (DXA–CT) of 55 ± 48 cm2 (_P_ < 0.0001). Unlike visceral fat, the
bias did not substantially vary across the subcutaneous fat spectrum (Fig. 1b). However, among individuals with high visceral fat in whom DXA routinely underestimated VAT, DXA conversely
overestimated SAT (Fig. 3). Thus, SAT bias (DXA–CT) became increasingly more positive as a function of visceral adiposity (_P_ < 0.0001), although it did not systematically relate to
subcutaneous fat content per se. LONGITUDINAL COMPARISON OF DXA WITH CT IN HIV A total of 106 individuals with HIV who were followed for at least 12 months on no active treatment were
included in the natural history analysis. The linear relationship between change in CT-VAT and change in DXA-VAT over 12 months had a slope of 0.55 (0.45–0.65), indicating that DXA
systematically underestimated CT with respect to change in VAT over time (Fig. 4a). As such, among the subset of participants who had a decline in CT-VAT at 12 months compared with baseline
(_n_ = 46), change in CT-VAT was −27 ± 28 cm2, whereas change in DXA-VAT was −15 ± 24 cm2. Similarly, among the subset of participants who had a gain in CT-VAT over 12 months (_n_ = 60),
change in CT-VAT was 23 ± 21 cm2, compared with change in DXA-VAT of 13 ± 20 cm2. Unlike the sex differences seen in the cross-sectional analysis, sex did not modify the relationship between
change in CT-VAT and change in DXA-VAT over 12 months in a model that adjusted for age, race, and ethnicity. The tendency for DXA to underestimate changes in VAT also was evident in a
Bland–Altman plot, in which the measurement bias was largest at the most extreme gains and losses of VAT (Fig. 4a). In this regard, measurement bias (DXA–CT) was negative among individuals
who had a gain in VAT and positive among individuals who had a loss of VAT (_P_ < 0.0001). We next examined subjects with HIV, who had been assigned to receive tesamorelin (_n_ = 23) or
placebo (_n_ = 20) for 6 months in a randomized-controlled trial. Analogous to the natural history analysis, among the tesamorelin-treated participants, the linear relationship between
change in CT-VAT and change in DXA-VAT had a slope that was significantly <1 (0.29, 0.01–0.57). In this group, over 6 months, the change in CT-VAT was −33 ± 46 cm2, whereas the change in
DXA-VAT was −12 ± 31 cm2. When the placebo-treated participants from the original randomized-controlled trial were included in the model, treatment assignment did not modify the relationship
between change in CT-VAT and change in DXA-VAT over time (Fig. 4b). The Bland–Altman plot of the data from the tesamorelin trial was comparable to that seen in the natural history analysis
(Fig. 4b). COMPARISON OF DXA TO CT IN CONTROLS WITHOUT HIV The comparison between DXA and CT in controls without HIV yielded similar findings to those seen in HIV. There was a strong
correlation between CT-VAT and DXA-VAT (_r_ _=_ 0.89, _P_ < 0.0001) (Supplementary Fig. 3) and only a modest bias of DXA compared with CT overall (1 ± 40 cm2) (Table 2). However, as in
HIV, the measurement difference between modalities (DXA–CT) became progressively more negative with greater visceral adiposity (_P_ < 0.0001) (Supplementary Fig. 3). In addition, once
again, men predominantly drove this relationship with a significant sex × VAT interaction in a model that adjusted for age, race, and ethnicity (_P_ < 0.0001) (Supplementary Fig. 4).
Lastly, although there was no obesity × VAT interaction, the magnitude of the measurement bias was slightly smaller in obese compared with non-obese individuals across the spectrum of
visceral adiposity (Supplementary Fig. 2). A total of 80 controls without HIV were included in the longitudinal analysis. As in HIV, the linear regression relating change in CT-VAT to change
in DXA-VAT over 12 months had a slope that was <1 (0.51, 0.39–0.62), suggesting that DXA underestimated longitudinal changes in VAT compared with CT (Supplementary Figure 5). This
relationship was not modified by sex in a model that adjusted for age, race, and ethnicity. DISCUSSION In the current work, we used data from five previously conducted studies of
participants with and without HIV, to assess the accuracy of DXA in the automated measurement of VAT. In our cross-sectional analysis of individuals with HIV, we showed that DXA
underestimated VAT relative to CT among those with high visceral fat content. Sex-stratified comparisons revealed this measurement bias was largely driven by men rather than women, even upon
controlling for VAT. In our longitudinal assessment of participants with HIV, we found that DXA underestimated changes in VAT over time, irrespective of sex. Analogous findings were
demonstrated in cross-sectional and longitudinal comparisons among controls without HIV. To our knowledge, this is the first study to examine the accuracy of DXA-VAT specifically among
individuals with HIV. People living with HIV as a group are prone to visceral fat accumulation due to an interplay of medication toxicities, viral effects on host metabolism, and
contemporary lifestyle trends33,34. Moreover, HIV lipodystrophy is an extreme form of visceral fat accumulation that remains common among long-term survivors35 or those with drug resistance,
who require older-generation therapies36. Visceral fat accumulation is a key mediator of cardiometabolic comorbidities in HIV such as glucose intolerance12,37, nonalcoholic fatty liver
disease38, and coronary artery disease39. Accordingly, our group developed the GHRH analog tesamorelin as a novel strategy to selectively reduce visceral fat in HIV without substantially
altering subcutaneous fat or BMI27,28. As anticipated, a reduction in visceral fat with tesamorelin has been associated with metabolic benefits including decreased triglycerides40 and liver
enzymes41 among this patient population. Tesamorelin is the only medication that has been approved by the Food and Drug Administration to treat excess abdominal fat in HIV. Given the pivotal
contribution of visceral fat accumulation to cardiometabolic risk in HIV, a technique that allows for the safe, affordable, and convenient measurement of visceral fat may substantially
improve the quality of care among the HIV population. Despite the initial promise of DXA in this regard, we found that this modality systematically underestimated VAT among participants with
HIV and visceral fat accumulation compared with CT. Of note, sex-specific comparisons revealed that men rather than women were predominantly responsible for this relationship. In contrast
to VAT, the measurement difference for SAT between DXA and CT did not substantially vary across the subcutaneous fat spectrum. However, for the individuals in whom DXA underestimated VAT,
DXA conversely overestimated SAT. Our findings were analogous for controls without HIV, although the range of visceral fat content examined was narrower among this group. Multiple studies
from the general population have posited that visceral fat quantification by DXA is accurate compared with CT and magnetic resonance imaging (MRI)15,19,20,21,42. However, in several of these
reports, the sample was limited to women20,21. Concordant with these studies, our data suggest that DXA is relatively accurate in women with and without HIV, even among those with increased
visceral adiposity. Moreover, consonant with our findings in men with and without HIV, several previous studies have shown that DXA loses accuracy with greater visceral adiposity15,42,43.
In addition, multiple prior reports have found a tendency for DXA to underestimate visceral fat compared with other modalities43,44. In one such study of older males, visceral fat volume as
measured by DXA was consistently 30% less than that measured by MRI44. Of note, the authors of this previous work attributed this bias to a difference in compartment sizes between modalities
rather than to a true measurement inaccuracy44. Similarly, a study among Korean men and women found that DXA underestimated visceral fat volume relative to CT as a function of visceral
adiposity43. Sex-stratified analyses were not performed in this previous study43. A higher proportion of visceral fat is common to men45 and Asians46 relative to women and other races,
respectively, which may account for the more pronounced measurement bias seen among these groups. Strategies to reduce visceral fat have been developed for the clinical care of individuals
with HIV as a means toward improving metabolic health. However, to our knowledge, the accuracy of DXA with respect to detecting changes in visceral fat over time has never before been
assessed. In the current analysis, among individuals with and without HIV receiving no intervention for 12 months, we found that DXA systematically underestimated changes in VAT as measured
by CT, irrespective of sex. We additionally showed that DXA underestimated changes in VAT over 6 months among individuals with HIV that were randomized to receive tesamorelin or placebo.
Among the tesamorelin-treated participants, the average VAT reduction as measured by DXA was <50% of that measured by CT. Strengths of this analysis include its large sample of
individuals with and without HIV. Furthermore, both men and women were included in our analysis, which allowed for us to draw important sex-specific distinctions between groups. In addition,
we assessed longitudinal changes in DXA-VAT over time both in the presence and the absence of a metabolic intervention. An important limitation to our analysis is that DXA-VAT was
exclusively determined using a Hologic scanner and APEX software. Although this lends consistency to our comparisons, we were not able to assess the accuracy of DXA-VAT as ascertained by
other systems. Similarly, our analyses compared DXA and CT with respect to visceral fat cross-sectional area, but did not evaluate visceral fat volume or mass. Measurement of visceral fat
volume by CT was not performed in our original studies to minimize the dose of radiation to which participants were exposed. Nonetheless, as visceral fat cross-sectional area is highly
correlated with visceral fat volume on CT (_r_ > 0.95)47, we would expect a comparison of this parameter between modalities to yield similar findings. However, further research is needed
to confirm this. In the current study, we showed that DXA underestimated visceral fat content among men with and without HIV, who had increased visceral adiposity. We also demonstrated that
DXA underestimated changes in visceral fat content over time among both men and women, irrespective of HIV status. These findings represent important caveats to the use of DXA for the
automated determination of visceral fat. DXA-VAT should be interpreted with caution in HIV, particularly among men. REFERENCES * Fox, C. S. et al. Abdominal visceral and subcutaneous adipose
tissue compartments: association with metabolic risk factors in the Framingham Heart Study. _Circulation_ 116, 39–48 (2007). Article Google Scholar * Hayashi, T. et al. Visceral adiposity
and the prevalence of hypertension in Japanese Americans. _Circulation_ 108, 1718–1723 (2003). Article Google Scholar * Kwon, H., Kim, D. & Kim, J. S. Body fat distribution and the
risk of incident metabolic syndrome: a longitudinal cohort study. _Sci. Rep._ 7, 10955 (2017). Article Google Scholar * Neeland, I. J. et al. Associations of visceral and abdominal
subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. _Obesity (Silver Spring)_ 21, E439–E447 (2013). CAS Google Scholar * Ohashi, N. et al. Visceral fat
accumulation as a predictor of coronary artery calcium as assessed by multislice computed tomography in Japanese patients. _Atherosclerosis_ 202, 192–199 (2009). Article CAS Google Scholar
* Marques, M. D. et al. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. _Atherosclerosis_ 209, 481–486 (2010). Article CAS
Google Scholar * McLaughlin, T., Lamendola, C., Liu, A. & Abbasi, F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. _J. Clin.
Endocrinol. Metab._ 96, E1756–E1760 (2011). Article CAS Google Scholar * Joy, T. et al. Relationship of body composition to body mass index in HIV-infected patients with metabolic
abnormalities. _J. Acquir. Immune Defic. Syndr._ 47, 174–184 (2008). Article Google Scholar * McComsey, G. A. et al. Body composition changes after initiation of raltegravir or protease
inhibitors: ACTG A5260s. _Clin. Infect. Dis._ 62, 853–862 (2016). Article CAS Google Scholar * Hadigan, C. et al. Prediction of coronary heart disease risk in HIV-infected patients with
fat redistribution. _Clin. Infect. Dis._ 36, 909–916 (2003). Article Google Scholar * Lake, J. E. et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study.
_AIDS Care_ 23, 929–938 (2011). Article Google Scholar * Glesby, M. J. et al. Abdominal fat depots and subclinical carotid artery atherosclerosis in women with and without HIV infection.
_J. Acquir. Immune Defic. Syndr._ 77, 308–316 (2018). Article Google Scholar * Wajchenberg, B. L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome.
_Endocr. Rev._ 21, 697–738 (2000). Article CAS Google Scholar * Thaete, F. L., Colberg, S. R., Burke, T. & Kelley, D. E. Reproducibility of computed tomography measurement of visceral
adipose tissue area. _Int. J. Obes. Relat. Metab. Disord._ 19, 464–467 (1995). CAS PubMed Google Scholar * Neeland, I. J., Grundy, S. M., Li, X., Adams-Huet, B. & Vega, G. L.
Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. _Nutr. & Diabetes_ 6, e221
(2016). Article CAS Google Scholar * Svendsen, O. L., Haarbo, J., Hassager, C. & Christiansen, C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in
vivo. _Am. J. Clin. Nutr._ 57, 605–608 (1993). Article CAS Google Scholar * Mazess, R. B., Barden, H. S., Bisek, J. P. & Hanson, J. Dual-energy x-ray absorptiometry for total-body and
regional bone-mineral and soft-tissue composition. _Am. J. Clin. Nutr._ 51, 1106–1112 (1990). Article CAS Google Scholar * Glickman, S. G., Marn, C. S., Supiano, M. A. & Dengel, D.
R. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. _J. Appl. Physiol. (1985)_ 97, 509–514 (2004). Article Google Scholar * Kaul, S.
et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. _Obes. (Silver Spring)._ 20, 1313–1318 (2012). Article Google Scholar * Micklesfield, L. K., Goedecke, J. H.,
Punyanitya, M., Wilson, K. E. & Kelly, T. L. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. _Obesity (Silver Spring)_ 20,
1109–1114 (2012). Article Google Scholar * Bredella, M. A. et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity.
_Obesity (Silver Spring)_ 21, 2458–2464 (2013). Article CAS Google Scholar * Lo, J. et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed
tomography angiography in HIV-infected men. _AIDS_ 24, 243–253 (2010). Article Google Scholar * Fitch, K. V. et al. Effects of aging and smoking on carotid intima-media thickness in
HIV-infection. _AIDS_ 27, 49–57 (2013). Article CAS Google Scholar * Stanley, T. L. et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat
accumulation: a randomized clinical trial. _JAMA_ 312, 380–389 (2014). Article Google Scholar * Looby, S. E. et al. Increased hot flash severity and related interference in perimenopausal
human immunodeficiency virus-infected women. _Menopause_ 21, 403–409 (2014). PubMed PubMed Central Google Scholar * Lo, J. et al. Effects of statin therapy on coronary artery plaque
volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. _Lancet Hiv._ 2, e52–e63 (2015).
Article Google Scholar * Falutz, J. et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. _N. Engl. J. Med._ 357, 2359–2370 (2007). Article CAS Google
Scholar * Falutz, J. et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled
analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. _J. Clin. Endocrinol. Metab._ 95, 4291–4304 (2010). Article CAS Google Scholar *
_Horizon Bone Densitometry Systems User Guide MAN-04871 Revision 004_. p. 51–58 (Heron House, Wythenshawe, Manchester M23 9HZ, UK, 2016). * Katzmarzyk, P. T., Greenway, F. L., Heymsfield, S.
B. & Bouchard, C. Clinical utility and reproducibility of visceral adipose tissue measurements derived from dual-energy X-ray absorptiometry in White and African American adults.
_Obesity (Silver Spring)._ 21, 2221–2224 (2013). Article Google Scholar * Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods for clinical
measurement. _Lancet_ 1, 307–310 (1986). Article CAS Google Scholar * Brown, T. T. et al. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without
clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. _AIDS Res. Ther._ 6, 8 (2009). Article Google Scholar * Erlandson, K. M.
& Lake, J. E. Fat matters: understanding the role of adipose tissue in health in HIV infection. _Curr. HIV/AIDS Rep._ 13, 20–30 (2016). Article Google Scholar * Koethe, J. R. Adipose
tissue in HIV infection. _Compr. Physiol._ 7, 1339–1357 (2017). Article Google Scholar * Grenha, I. et al. HIV-infected patients with and without lipodystrophy under combined
antiretroviral therapy: evaluation of body composition. _J. Clin. Densitom._ 21, 75–82 (2018). Article Google Scholar * Martinez, E. Disorders of fat partitioning in treated HIV-infection.
_Best. Pract. Res. Clin. Endocrinol. Metab._ 25, 415–427 (2011). Article Google Scholar * Grunfeld, C. et al. Association of upper trunk and visceral adipose tissue volume with insulin
resistance in control and HIV-infected subjects in the FRAM study. _J. Acquir. Immune Defic. Syndr._ 46, 283–290 (2007). Article Google Scholar * Hadigan, C., Liebau, J., Andersen, R.,
Holalkere, N. S. & Sahani, D. V. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. _J. Acquir. Immune Defic. Syndr._ 46, 312–317
(2007). Article Google Scholar * Guaraldi, G. et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected
subjects. _Atherosclerosis_ 208, 222–227 (2010). Article CAS Google Scholar * Stanley, T. L. et al. Reduction in visceral adiposity is associated with an improved metabolic profile in
HIV-infected patients receiving tesamorelin. _Clin. Infect. Dis._ 54, 1642–1651 (2012). Article CAS Google Scholar * Fourman, L. T. et al. Visceral fat reduction with tesamorelin is
associated with improved liver enzymes inHIV. _AIDS_ 31, 2253–2259 (2017). Article CAS Google Scholar * Mohammad, A. et al. Validity of visceral adiposity estimates from DXA against MRI
in Kuwaiti men and women. _Nutr. Diabetes_ 7, e238 (2017). Article CAS Google Scholar * Choi, Y. J., Seo, Y. K., Lee, E. J. & Chung, Y. S. Quantification of visceral fat using
dual-energy x-ray absorptiometry and its reliability according to the amount of visceral fat in Korean adults. _J. Clin. Densitom._ 18, 192–197 (2015). Article Google Scholar * Cheung, A.
S. et al. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. _Int. J. Obes. (Lond.)._ 40, 1325–1328 (2016). Article CAS
Google Scholar * Schreiner, P. J. et al. Sex-specific associations of magnetic resonance imaging-derived intra-abdominal and subcutaneous fat areas with conventional anthropometric
indices. The Atherosclerosis Risk in Communities Study. _Am. J. Epidemiol._ 144, 335–345 (1996). Article CAS Google Scholar * Lear, S. A. et al. Visceral adipose tissue accumulation
differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). _Am. J. Clin. Nutr._ 86, 353–359 (2007). Article CAS Google Scholar *
Kvist, H., Chowdhury, B., Grangard, U., Tylen, U. & Sjostrom, L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women:
predictive equations. _Am. J. Clin. Nutr._ 48, 1351–1361 (1988). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank the participants of our studies and the Nursing
and Bionutrition Staff of the MGH and MIT Clinical Research Centers. Funding was provided by NIH 5T32HL076136-14 (L.T.F.), NIH K23NR011833-01A1 (S.E.L.), NIH K23HL092792 (J.L.), NIH
F32HL088991 (J.L.), NIH K23DK089910 (T.L.S.), NIH R01HL095123 (S.K.G.), NIH R01DK049302 (S.K.G.), NIH R01DK063639 (S.K.G.), Bristol Myers Squibb, Inc. (S.K.G.), and NIH 1UL1TR002541-01, NIH
1UL1RR025758, NIH M01-RR-01066, and NIH P30DK040561. S.E.L. also received developmental awards from the Harvard University Center for AIDS Research (CFAR), an NIH funded program
(P30AI060354), supported by NIAID, NCI, NICHD, NIDCR, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, NIMHS, FIC, and OAR. The content is solely the responsibility of the authors and does not
necessarily represent the official views of the National Institutes of Health. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Program in Nutritional Metabolism, Department of Medicine,
Massachusetts General Hospital, Boston, MA, USA Lindsay T. Fourman, Emma M. Kileel, Sara E. Looby, Kathleen V. Fitch, Meghan N. Feldpausch, Janet Lo, Takara L. Stanley & Steven K.
Grinspoon * Translational and Clinical Research Center, Massachusetts General Hospital, Boston, MA, USA Jane Hubbard, Tara Holmes & Ellen J. Anderson * Yvonne L. Munn Center for Nursing
Research, Massachusetts General Hospital, Boston, MA, USA Sara E. Looby * MGH Metabolic Imaging Core, Department of Radiology, Massachusetts General Hospital, Boston, MA, USA Martin Torriani
Authors * Lindsay T. Fourman View author publications You can also search for this author inPubMed Google Scholar * Emma M. Kileel View author publications You can also search for this
author inPubMed Google Scholar * Jane Hubbard View author publications You can also search for this author inPubMed Google Scholar * Tara Holmes View author publications You can also search
for this author inPubMed Google Scholar * Ellen J. Anderson View author publications You can also search for this author inPubMed Google Scholar * Sara E. Looby View author publications You
can also search for this author inPubMed Google Scholar * Kathleen V. Fitch View author publications You can also search for this author inPubMed Google Scholar * Meghan N. Feldpausch View
author publications You can also search for this author inPubMed Google Scholar * Martin Torriani View author publications You can also search for this author inPubMed Google Scholar * Janet
Lo View author publications You can also search for this author inPubMed Google Scholar * Takara L. Stanley View author publications You can also search for this author inPubMed Google
Scholar * Steven K. Grinspoon View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Steven K. Grinspoon. ETHICS
DECLARATIONS CONFLICT OF INTEREST The authors have no disclosures relevant to the design of this study or the preparation of this manuscript. Unrelated to this work, S.E.L. is a non-paid
Board member of the community non-profit organization Healing Our Community Collaborative, and received one-time compensation for CME educational offerings sponsored by the Association of
Nursing in AIDS Care (Conference, Atlanta, GA, 2017) and New England AIDS Education and Training Center, (Boston, MA, 2018). J.L. has provided consulting services to Gilead Sciences and Viiv
Healthcare. T.L.S. has received investigator-initiated funding from Novo Nordisk and Kowa Pharmaceuticals. S.K.G. has received research funding from Gilead Sciences, Kowa Pharmaceuticals,
and Theratechnologies, and served as a consultant for Navidea and Theratechnologies. All other authors declare no competing financial interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURE LEGENDS SUPPLEMENTAL
TABLE 1 SUPPLEMENTAL TABLE 2 SUPPLEMENTAL FIGURE 1 SUPPLEMENTAL FIGURE 2 SUPPLEMENTAL FIGURE 3 SUPPLEMENTAL FIGURE 4 SUPPLEMENTAL FIGURE 5 RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fourman, L.T., Kileel, E.M., Hubbard, J. _et al._
Comparison of visceral fat measurement by dual-energy X-ray absorptiometry to computed tomography in HIV and non-HIV. _Nutr. Diabetes_ 9, 6 (2019). https://doi.org/10.1038/s41387-019-0073-1
Download citation * Received: 15 October 2018 * Revised: 07 December 2018 * Accepted: 31 January 2019 * Published: 25 February 2019 * DOI: https://doi.org/10.1038/s41387-019-0073-1 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
