Lncrna bcyrn1 inhibits glioma tumorigenesis by competitively binding with mir-619-5p to regulate cuedc2 expression and the pten/akt/p21 pathway
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Glioma is the most common malignant tumor in the central nervous system. Altered long noncoding RNAs (lncRNAs) are playing regulatory roles in physiological and pathogenic processes
in cancer. Here, we uncovered a differentially expressed lncRNA called brain cytoplasmic RNA 1 (BCYRN1), and elucidated its function and molecular mechanism in the progression and
development of glioma. Three fresh tumor tissues from glioma patients and three normal brain tissues from craniocerebral trauma patients were prepared for high-throughput RNA sequencing.
Differential RNA transcripts and BCYRN1 were identified by RT-qPCR in glioma samples and controls. CCK-8, colony formation assays, flow cytometry, TUNEL assays, cell migration assays,
wound-healing assays, and xenograft model were established to investigate the biological function of BCYRN1 both in vitro and in vivo. Various bioinformatics analysis, dual-luciferase
reporter assays, biotinylated RNA pulldown assays, and rescue experiments were conducted to reveal the underlying mechanisms of competitive endogenous RNAs (ceRNAs). 183 lncRNAs were
identified with significant dysregulation in glioma and randomly selected differential RNAs were further confirmed by RT-qPCR. Among them, BCYRN1 was the most downregulated lncRNA, and its
low expression positively correlated with glioma progression. Functionally, BCYRN1 overexpression inhibited cell proliferation, migration in glioma cell lines, whereas BCYRN1 depletion
resulted in the opposite way. MiR-619-5p was further confirmed as the direct target of BCYRN1. Mechanistically, miR-619-5p specifically targeted the CUE domain containing protein 2 (CUEDC2),
and BCYRN1/miR-619-5p suppressed glioma tumorigenesis by inactivating PTEN/AKT/p21 pathway in a CUEDC2-dependent manner. Overall, our data presented that the reduced expression of BCYRN1
was associated with poor patient outcome in glioma. BCYRN1 functioned as a ceRNA to inhibit glioma progression by sponging miR-619-5p to regulate CUEDC2 expression and PTEN/AKT/p21 pathway.
Our results indicated that BCYRN1 exerted tumor suppressor potential and might be a candidate in the diagnosis and treatment of glioma. SIMILAR CONTENT BEING VIEWED BY OTHERS LNCRNA
LINC00998 INHIBITS THE MALIGNANT GLIOMA PHENOTYPE VIA THE CBX3-MEDIATED C-MET/AKT/MTOR AXIS Article Open access 02 December 2020 LNCRNA ANCR PROMOTES GLIOMA CELLS INVASION, MIGRATION,
PROLIFERATION AND INHIBITS APOPTOSIS VIA INTERACTING WITH EZH2 AND REPRESSING PTEN EXPRESSION Article 08 December 2020 LNCRNA HOXA11-AS PROMOTES GLIOMA MALIGNANT PHENOTYPES AND REDUCES ITS
SENSITIVITY TO ROS VIA TPL2-MEK1/2-ERK1/2 PATHWAY Article Open access 09 November 2022 INTRODUCTION Glioma is the most prevalent and malignant primary intracranial tumor. Glioblastoma
multiforme (GBM) is one of the most lethal forms of human cancers with average survival rate of 12–15 months [1, 2]. With the aggravation of population aging, glioma is becoming a more and
more serious threat to human health. Therefore, early diagnosis and treatment is a crucial medical problem. Due to the invasiveness nature of glioma, it is very difficult to be completely
removed in the surgery [3]. What’s worse is this tumor resistant to radiation therapy and chemotherapy and easy to recurrence [1, 2]. Thus, molecular diagnosis, especially various biomarkers
are necessary to be investigated during glioma occurrence and progression. Dysregulated transcripts including mRNAs, microRNAs (miRNAs), lncRNAs, and circular RNAs (circRNAs) can be a
primary feature in human cancers [4,5,6,7,8,9,10,11,12,13]. With the rapid development of high-throughput RNA sequencing and the wide application of bioinformatics, lncRNAs, as one subset of
ncRNAs with the length >200 nucleotides, were identified to participate in diverse biological processes in cancer [7,8,9]. There are multiple kinds of mechanisms in cell development and
disease for lncRNAs. For instance, Evx1as, a nuclear antisense lncRNA, was reported to regulate gene transcription _in cis_ [14]. Xist is one of the most famous lncRNAs, which recruits
blocking factors leading to X inactivation [15]. Metastasis associated lung adenocarcinoma transcript 1 (Malat1) is thought to form molecular scaffolds for ribonucleoprotein complexes, which
was also reported to serve as a transcriptional regulator for numerous genes, including some genes involved in cancer metastasis, cell migration, and cell cycle [16, 17]. 5S-OT is a lncRNA
overlapped with 5 S rRNA, which interacts with U2AF65 to modulate alternative splicing through RNA: RNA pairing [10]. Some lncRNAs act as the miRNA precursors to give rise to miRNAs to
target genes [18]. Growing evidences have implicated that a number of lncRNAs functioned as ceRNAs to affect the occurrence and development of cancers. Taken as an example, H19, a well-known
lncRNA, was highly regulated in glioma. H19 induced endothelial cell proliferation, migration, and tube formation in vitro, which were proved to exert an H19-mir-29a-VASH2 axis to regulate
the tumorigenesis [19]. In glioma, a number of lncRNAs have been found to be significantly dysregulated, such as HOTAIR, TUG1, and ECONEXIN [20,21,22,23,24]. Nevertheless, the various
functions and multiple mechanisms of lncRNAs in glioma have not been explored comprehensively. MiRNAs are endogenously expressed small noncoding RNAs with a length of ~20–22 nucleotides,
which play central roles in the ceRNA hypothesis [25]. MiRNAs are highly conserved across species, and regulate gene expression by binding to the 3′-untranslated region (3′-UTR) of target
mRNAs. More and more miRNAs were demonstrated to play important roles in glioma [26,27,28]. In this study, we investigated the expression profiling of lncRNAs and identified a most
downregulated lncRNA called BCYRN1 in glioma from RNA-seq. Lower BCYRN1 expression was related to poor prognosis of glioma patients. Furthermore, we found that BCYRN1 affected the
proliferation, apoptosis, migration, invasion abilities in vitro and inhibited the tumor formation in vivo. In addition, our study revealed a mechanism that BCYRN1 competitively bind
miR-619-5p to regulate glioma progression through inactivating the CUEDC2/PTEN/AKT/p21 pathway. Our results indicate that BCYRN1 exerts as a tumor suppressor in glioma and has the potential
to be the promising therapeutic target for glioma diagnosis, therapy, and prognosis. RESULTS TRANSCRIPTOME PROFILING IN GLIOMA AND CHARACTERIZATION OF LNCRNA BCYRN1 In order to obtain a
comprehensive and profound view of lncRNA transcripts in glioma, we performed high-throughput RNA sequencing using three tissue samples from glioma patients (diagnosed by enhanced MRI and
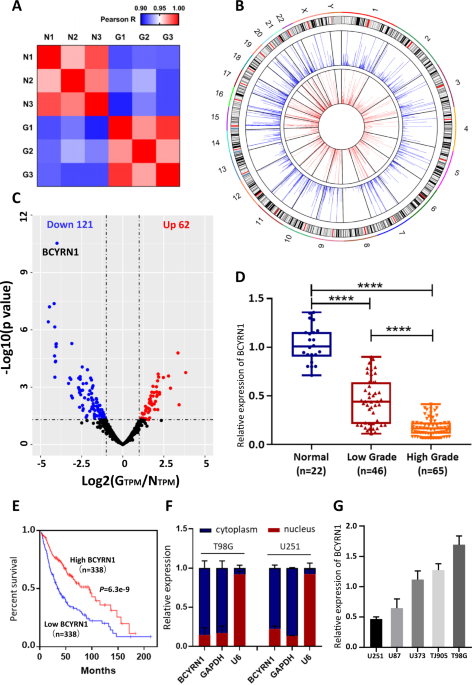
pathology) and three normal brain tissues from craniocerebral trauma patients (Fig. s1a, b). To ensure the authenticity and validity of RNA-seq data, we performed the Pearson’s correlation
coefficient analysis of all transcripts between each sample (Fig. 1a). Tumor tissues or control tissues themselves demonstrated the higher correlation compared to each other (Fig. 1a).
Notably, we found the expression levels of some previously reported mRNAs and lncRNAs were significantly dysregulated in our data (Table S1). Circos plots globally and genome-widely
displayed 1344 kinds of detected lncRNAs in glioma tissues and controls (Fig. 1b). Volcano plots were performed for all expressed lncRNAs (Fig. 1c). There were 183 lncRNAs showed
significantly dysregulated in glioma, in which 121 were downregulated while 62 were upregulated (Fig. 1c, Fig. s1c). Hierarchical cluster analysis was also constructed to reveal the
differential expression of lncRNAs in glioma tissues and controls (Fig. s1c). To validate the results, 6 lncRNAs were randomly selected for RT-qPCR analysis. THBS3, ATP6VOE, and LINC01235
were significantly upregulated while USP32P2, RTN3, and LINC00294 were markedly downregulated in glioma compared to controls (Fig. s1d). All these validations were consistent with the
RNA-seq data. BCYRN1, as the most downregulated lncRNA in glioma, was chosen for further studies. Next, we confirmed the downregulated expression of BCYRN1 in 22 normal tissues and 111
glioma tissues (46 Grade I and II, 65 Grade III and IV) by RT-qPCR, and found that the expression of BCYRN1 was significantly lower in glioma tissue, especially reduced with advanced glioma
grade (Fig. 1d, Table 1). In addition, we assessed the correlation between BCYRN1 expression and prognosis of glioma patients. Kaplan–Meier analysis of TCGA database demonstrated that
patients with lower BCYRN1 expression were more likely to be poor overall survivals (Fig. 1e). Taken together, these data suggested that BCYRN1 downregulation was common in glioma tissues
and was correlated with poor prognosis. BCYRN1 INHIBITS GLIOMA PROGRESSION IN VITRO AND IN VIVO To identify cellular localization of BCYRN1, RT-qPCR analysis of nuclear and cytoplasmic RNAs
was carried out to show that BCYRN1 was preferentially located in the cytoplasm (Fig. 1f), consistent with the subcellular fractionation in other cancer types [29,30,31]. Furthermore, five
kinds of glioma cell lines were tested the endogenous expression level of BCYRN1. Based on the results, T98G and U251 were selected for the loss-of-function and gain-of-function assays in
vitro (Fig. 1g). To explore the functional roles of BCYRN1, we overexpressed the full length in U251 and T98G cells (Fig. 2a, Fig. s2a). Two short interfering RNAs against different region
of BCYRN1 were performed, which effectively knockdown BCYRN1 expression level in T98G and U251 cells (Fig. 2b, Fig. s2b). CCK-8 and colony formation assay revealed that overexpression of
BCYRN1 inhibited cell growth and colony formation (Fig. 2c, e, Fig. s2c, e). On the other hand, flow cytometry and TUNEL assay demonstrated overexpression of BCYRN1 induced cell apoptosis
(Fig. 2g, i, Fig. s2g, i). In addition, transwell and wound-healing assay displayed that upregulation of BCYRN1 significantly reduced cell migratory capacity (Fig. 2k, m, Fig. s2k, m). In
contrast to overexpression, ablation of BCYRN1 promoted cell growth, colony formation, migration, and repressed cell apoptosis in U251 and T98G cells (Fig. 2d, f, h, j, l, n, Fig. s2d, f, h,
j, l, n). In order to investigate regulatory roles of BCYRN1 on tumor growth in vivo, we established U251 stable cell line with lentivirus-BCYRN1 to overexpress BCYRN1. The procedure of in
vivo tumor xenograft assay was showed (Fig. 3a). Overexpression efficiency of BCYRN1 was revealed by RT-qPCR assays (Fig. 3b). We observed that tumor size and weights were reduced in the
LV-BCYRN1 group compared with those in the LV-vector group (Fig. 3c–h). IHC staining revealed that Ki-67 expression was downregulation upon BCYRN1 overexpression in dissected tumors (Fig.
3i, j). Collectively, these results in vitro and in vivo strongly suggested that BCYRN1 was a potential tumor suppressor in glioma. BCYRN1 ACTS AS A CERNA FOR MIR-619-5P IN GLIOMA As shown
in Fig. 1f, BCYRN1 was preferentially located in the cytoplasm. It has been shown that cytoplasmic lncRNAs can act as miRNAs sponge to regulate downstream targets [32,33,34]. In order to
explore whether BCYRN1 might also function as the ceRNA mechanism, we searched candidate miRNAs by miRanda prediction. We observed that multiple miRNAs were able to align to lncRNA BCYRN1
and blast score of miR-619-5p was the highest (Fig. 4a). MiR-619-5p showed a severe overexpression in 30 glioma samples compare to control samples (Fig. s3a, Table S2). MiR-619-5p expression
was the highest in U251 and was the lowest in T98G, which was inverse to BCYRN1 in the two cell lines (Fig. s3b). Furthermore, the miR-619-5p level was negatively correlated with the lncRNA
BCYRN1 level in the glioma tissues (Fig. 4b). Overexpression of BCYRN1 led to the significant downregulation of miR-619-5p, whereas its silencing resulted in the upregulation of miR-619-5p
(Fig. 4c, d). AGO2 immunoprecipitation (IP) was performed to determine whether BCYRN1 served as a component for AGO2 and miR-619-5p complex. It turned out BCYRN1 was enriched in AGO2 IP of
miR-619-5p transfected cells (Fig. 4e, f). To verify that BCYRN1 could bind to miR-619-5p, luciferase reporters containing wild-type (BCYRN1-WT) and mutated miR-619-5p binding sites
(BCYRN1-MUT) were constructed. We observed that luciferase activity of BCYRN1-WT was significantly reduced after co-transfection of miR-619-5p mimic, but luciferase activity of BCYRN1-MUT
did not change, which suggested that miR-619-5p was a target of BCYRN1 in a sequence-specific manner (Fig. 4g, h). In addition, biotin-coupled BCYRN1 successfully pulled down the competitive
binding of miR-619-5p, while biotin-labeled miR-619-5p also pulled down the binding of BCYRN1 (Fig. 4i, Fig. s3c). All these findings suggested BCYRN1 acted as a ceRNA for miR-619-5p in
glioma. We further tested the fundamental role of miR-619-5p itself in glioma. MiR-619-5p expression was increased or decreased after T98G and U251 cells were transfected with miR-619-5p
mimic or inhibitor, respectively (Fig. s3d, e). Overexpression of miR-619-5p induced cell growth and migration, inhibited cell apoptosis (Fig. s3f, h, j). On the other hand, ablation of
miR-619-5p suppressed cell growth and migration, promoted cell apoptosis (Fig. s3g, i, k). More importantly, upregulation of BCYRN1 could partially rescue the promotive effects of miR-619-5p
on cell growth and migration, and the reductive effect of miR-619-5p on cell apoptosis in glioma (Fig. 4j–o). Meanwhile, ablation of BCYRN1 could partially rescue the reductive effects of
miR-619-5p on cell growth and migration, and the inductive effect of miR-619-5p on cell apoptosis in glioma (Fig. s4). Taken together, our results indicated BCYRN1 served as a sponge for
miR-619-5p in the regulation of glioma progression. BCYRN1 INHIBITS GLIOMA PROGRESSION BY TARGETING MIR-619-5P/CUEDC2 MiRNAs exert various biological functions by targeting mRNAs. To explore
the mechanism of BCYRN1/miR-619-5p in glioma progression, we performed comprehensive bioinformatic analysis using four datasets and found that 44 mRNAs might be the potential targets of
miR-619-5p including CUEDC2 (Fig. 5a), which was reported to be a tumor suppressor in glioma [35]. CUEDC2 showed an obvious downregulation in 30 glioma samples compared to control samples
(Fig. s5a, Table S2). Kaplan–Meier analysis of TCGA database demonstrated that patients with lower CUEDC2 expression were more likely to be poor overall survivals (Fig. s5b). Importantly,
the miR-619-5p level was inversely correlated with the CUEDC2 level in the glioma tissues (Fig. 5b). Furthermore, both the mRNA and protein levels of CUEDC2 were decreased or increased after
treated with miR-619-5p mimic or inhibitor, respectively (Fig. 5c). Then we used TargetScan database to predict the binding site of miR-619-5p on CUEDC2 3′-UTR region (Fig. 5d). Luciferase
assays were performed to verify a direct interaction between miR-619-5p and CUEDC2. CUEDC2 3′-UTR sequence containing the seed region of miR-619-5p (CUEDC2-WT) and mutated miR-619-5p binding
sites (CUEDC2-MUT) were constructed and transfected into 293 T cell line. The results showed that luciferase activity of CUEDC2-WT was significantly reduced after co-transfection of
miR-619-5p mimic, but luciferase activity of CUEDC2-MUT did not change (Fig. 5d). Upregulation of CUEDC2 could partially rescue the promotive effects of miR-619-5p on cell growth and
migration (Fig. 5e–g), while inhibition of CUEDC2 could partially rescue the reductive effects of miR-619-5p on cell growth and migration in glioma (Fig. s5c-e). These findings indicated
that CUEDC2 was the target of miR-619-5p. We found that the CUEDC2 level was positively correlated with the BCYRN1 level in the glioma tissues (Fig. 5h). To verify whether the CUEDC2 gene
was modulated by BCYRN1, overexpression and knockdown of BCYRN1 were performed. The results showed that both the mRNA and protein levels of CUEDC2 were decreased or increased upon BCYRN1
overexpression or knockdown, respectively (Fig. 5i–k). Upregulation of BCYRN1 could rescue the inhibitory effect of miR-619-5p mimic on CUEDC2 expression, while knockdown of BCYRN1 could
partially rescue the induction of miR-619-5p inhibitor on CUEDC2 expression both in mRNA and protein level (Fig. 5l–n). Taken together, these data provided evidences that inhibition glioma
progression of BCYRN1 was primarily dependent on the miR-619-5p/ CUEDC2 axis. BCYRN1/MIR-619-5P/CUEDC2 AXIS NEGATIVELY REGULATES GLIOMA VIA PTEN/AKT/P21 PATHWAY Gene Ontology (GO) biological
process enrichment analyses of the 44 mRNAs in the network demonstrated the functional associations with several important pathways, including biological processes such as response to
stimulus, cell communication, signal transduction, especially PI3K/Akt signaling pathway (Fig. 6a). It is well established that PI3K/Akt signaling played important regulatory role in glioma,
and we examined the pathway related proteins by western blot. Knockdown BCYRN1 showed activation of AKT signaling, increased expression of Phosphorylated-AKT (P-AKT) protein, decreased
expression of PTEN and P21 (Fig. 6b). In addition, overexpression of BCYRN1 in vivo led to decrease of P-AKT and increase of CUEDC2, PTEN, and P21(Fig. 3i, j). As expected, overexpression of
miR-619-5p, as well as knockdown CUEDC2, also activated AKT signaling, increased expression of P-AKT protein, decreased expression of PTEN and P21(Fig. 6c, d). Therefore, downregulation of
BCYRN1 could rescue the promotive effect of miR-619-5p inhibitor on PI3K/Akt signaling protein expression level (Fig. 6e). In addition, CUEDC2 induced abnormal expression level of PI3K/Akt
signaling related proteins reversed with miR-619-5p (Fig. s5f-m). In conclusion, our results indicated that PTEN/AKT signaling participated in BCYRN1-mediated inhibition of glioma
progression via miR-619-5p/CUEDC2 axis. DISCUSSION Transcriptome profiling is an effective approach for the global view of cancers [36]. More importantly, it is crucial to identify tumor
suppressors and oncogenic lncRNAs then elucidate their functions and mechanisms. In this study, we identified hundreds of markedly altered transcripts including 183 lncRNAs through RNA-seq
in glioma. Interestingly, altered transcripts were downregulated in glioma compared to controls in our present study as well as other reports [37]. Among them, BCYRN1 was the most
significantly downregulated lncRNA in glioma. Gain-of-function and loss-of-function experiments demonstrated that BCYRN1 inhibited the occurrence and development of glioma in vitro and in
vivo. BCYRN1 exhibited its function as a ceRNA that competitively bind to miR-619-5p, then regulated target gene CUEDC2 expression and the PTEN/AKT/p21 pathway (Fig. 6f). LncRNAs are
emerging as important regulators in different disease processes including cancer [34, 38,39,40,41,42]. Recently, growing numbers of lncRNAs have been found to be dysregulated in glioma. HOX
transcript antisense RNA (HOTAIR) was overexpressed in GBM, crucial to sustain tumor cell proliferation, and regulated by BET bromodomain protein [43]. ECONEXIN is a potential oncogene that
regulates TOP2A by sponging miR-411-5p in glioma [23]. In this study, we found that lncRNA BCYRN1 was significantly lower in glioma than normal brain tissues and it was negatively correlated
with glioma grade. BCYRN1, termed as BC200, has been found in a variety of cancers as previous studies. Either as an oncogenic lncRNA or a tumor suppressor, BCYRN1 was reported to exert
significant biological function in cancers such as NSCLC, ovarian cancer, and gastric cancer [31, 44,45,46,47,48]. However, as a lncRNA derived from brain cytoplasm, the expression and
function of BCYRN1 in glioma remain unknown. We believe that it is the first comprehensive characterization of BCYRN1 function and mechanism in glioma. Our results revealed the clinical
significance of BCYRN1 and indicated that BCYRN1 might be a candidate in diagnosis and treatment of glioma. It has been extensively reported that cytoplasmic lncRNAs could function as miRNA
sponge to modulate mRNA stability or translation and affect related signaling pathways [49,50,51,52,53]. Bioinformatic analyses and luciferase reporter assays verified that miR-619-5p was
the binding target of BCYRN1. MiR-619-5p has been reported to play important roles in several diseases [54, 55]. For example, miR-619-5p was markedly downregulated in colorectal carcinoma
and predicted to be a prognostic indicator of CRC patients [55]. However, the regulatory roles of miR-619-5p in glioma remain unclear. In this study, we found that miR-619-5p could promote
cell proliferation and migration in glioma and sponged by BCYRN1. Of course, the ceRNA network has its own limitation, and additional studies of lncRNAs as ceRNA in human cancers are needed.
BCYRN1 shared common miR-619-5p binding sites with CUEDC2. CUEDC2 played critical roles in many biological processes, such as cell cycle, inflammation, and tumorigenesis
[56,57,58,59,60,61,62]. As a multifunctional protein, the function of CUEDC2 in cancers is debated. Li et al. demonstrated that CUEDC2 acted as a tumor suppressor and inhibited the
tumorigenicity of glioma by inactivating STAT3 and NF-κB signaling pathways [35]. While Wang et al. found that CUEDC2 contributed to cisplatin-based chemotherapy resistance by regulating p38
MAPK signaling and was a promising biomarker and therapeutic target of cisplatin resistance in ovarian serous carcinoma [62]. In this study, we showed that CUEDC2 was downregulated in
glioma and the lower expression was more likely to be poor overall survivals, which supported that reduced level of BCYRN1 was correlated with poor prognosis. Finally, we explored downstream
targets of CUEDC2 essential for BCYRN1-mediated tumor suppressor function. Knockdown BCYRN1, as well as overexpression of miR-619-5p, activated AKT signaling, increased expression of P-AKT
protein, decreased expression of PTEN and P21 through downregulating CUEDC2. In fact, PTEN/AKT signaling has been widely reported to play vital regulatory roles in cancers including glioma
[63,64,65]. Our findings demonstrated that the role of the regulatory network between BCYRN1/miR-619-5p/CUEDC2 in glioma was achieved by affecting PTEN/AKT signaling pathway activity. Based
on the fact that inactivation of the PTEN signaling pathway, frequently found to be disrupted in human glioma [66], we suspected that there were some other miRNAs or other mRNA targets of
BCYRN1, contributing to the effect of BCYRN1, when PTEN was lost. However, other mechanisms underlying the downregulation of BCYRN1 in glioma need to be further investigated in our future
studies. In conclusion, we performed high-throughput RNA sequencing to uncover a downregulated lncRNA BCYRN1 in glioma and verified with clinical glioma samples. BCYRN1 inhibited glioma
progression in vitro and in vivo. Our study revealed a mechanism for BCYRN1 that competitively bind miR-619-5p, in turn, suppressed glioma proliferation, migration through inactivating the
CUEDC2/PTEN/AKT/p21 pathway. Overall, our study provided a new perspective to identify the potential biomarkers and therapeutic targets for glioma. MATERIALS AND METHODS CLINICAL SAMPLES
PREPARATION All fresh glioma patient tumor samples and normal tissues were collected from Department of Neurosurgery of The First Affiliated Hospital of University of Science and Technology
of China, which was approved by the Human Research Ethics Committee of the hospital. In this study, we obtained the written informed consent from each patient. A total of 111 resected brain
tumors were obtained from May 2015 to May 2019, and all tumor tissues were clinically and histopathological diagnosed as glioma (WHO I/II 46, WHO III/IV 65). Normal brain tissues obtained
from 22 patients with brain tissue resection due to craniocerebral injury during the period from May 2015 to May 2019. All samples were rinsed with PBS after operation, and then were cut
into small pieces with RNAhold (TransGen) immersed. All samples were stored in −80 °C for the following experiments. CELL CULTURE Glioma cell lines U251, T98G, U87, TJ905 and U373 in the
experiments were purchased from the ATCC. All cell lines were cultured in DMEM medium (HyClone) containing 10% FBS (Clark Bioscience) and stored in a humidified incubator at 37 °C with 5%
CO2. PLASMIDS CONSTRUCTION AND CELL TRANSFECTION Our plasmids were constructed with restriction-enzyme digestion and ligation or with recombinant methods (Vazyme). Full length of BCYRN1 was
inserted into Plko.1 vector and CDS of CUEDC2 was inserted into PEGFP-C1 vector, respectively. SiRNAs, miRNA mimic and inhibitors were purchased from RiboBio. Plasmid and siRNAs transfection
were conducted with Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol. The sequences are showed in Table S3. CHANGE HISTORY * _ 16 AUGUST 2021 A Correction to this
paper has been published: https://doi.org/10.1038/s41388-021-01990-4 _ REFERENCES * Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. Article CAS PubMed
Google Scholar * Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin.
2010;60:166–93. Article PubMed PubMed Central Google Scholar * D’Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma-a review of the cutting edge. World Neurosurg.
2017;103:538–49. Article PubMed Google Scholar * Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. Article CAS PubMed Google Scholar * Esteller
M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. Article CAS PubMed Google Scholar * Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the
management of cancer and other diseases. Nat Rev Drug Disco. 2017;16:203–22. Article CAS Google Scholar * Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape
of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. Article CAS PubMed PubMed Central Google Scholar * Kung JT, Colognori D, Lee JT. Long noncoding RNAs:
past, present, and future. Genetics. 2013;193:651–69. Article CAS PubMed PubMed Central Google Scholar * Hu S, Shan G. LncRNAs in stem cells. Stem Cells Int. 2016;2016:2681925. Article
PubMed PubMed Central CAS Google Scholar * Hu S, Wang X, Shan G. Insertion of an Alu element in a lncRNA leads to primate-specific modulation of alternative splicing. Nat Struct Mol
Biol. 2016;23:1011–9. Article CAS PubMed Google Scholar * Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct
Mol Biol. 2015;22:256–64. Article PubMed CAS Google Scholar * Shao M, Liu W, Wang Y. Differentially expressed LncRNAs as potential prognostic biomarkers for glioblastoma. Cancer Genet.
2018;226-227:23–29. Article CAS PubMed Google Scholar * Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell 2019;179:1033–55. Article CAS PubMed PubMed Central
Google Scholar * Luo S, Lu JY, Liu L, Yin Y, Chen C, Han X, et al. Divergent lncRNAs regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell.
2016;18:637–52. Article CAS PubMed Google Scholar * Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–23. Article CAS
PubMed Google Scholar * Sun Q, Hao Q, Prasanth KV. Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genet. 2018;34:142–57. Article CAS PubMed PubMed Central
Google Scholar * Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor
phosphorylation. Mol Cell. 2010;39:925–38. Article CAS PubMed PubMed Central Google Scholar * Dhir A, Dhir S, Proudfoot NJ, Jopling CL. Microprocessor mediates transcriptional
termination of long noncoding RNA transcripts hosting microRNAs. Nat Struct Mol Biol. 2015;22:319–27. Article CAS PubMed PubMed Central Google Scholar * Jia P, Cai H, Liu X, Chen J, Ma
J, Wang P, et al. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett.
2016;381:359–69. Article CAS PubMed Google Scholar * Zhang JX, Han L, Bao ZS, Wang YY, Chen LY, Yan W, et al. HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of
survival, is preferentially expressed in classical and mesenchymal glioma. Neuro Oncol. 2013;15:1595–603. Article CAS PubMed PubMed Central Google Scholar * Katsushima K, Natsume A,
Ohka F, Shinjo K, Hatanaka A, Ichimura N, et al. Targeting the Notch-regulated non-coding RNA TUG1 for glioma treatment. Nat Commun. 2016;7:13616. Article PubMed PubMed Central Google
Scholar * Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. m6A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell
Proliferation Program. Cancer Cell. 2017;31:591–606.e6. Article CAS PubMed PubMed Central Google Scholar * Deguchi S, Katsushima K, Hatanaka A, Shinjo K, Ohka F, Wakabayashi T, et al.
Oncogenic effects of evolutionarily conserved noncoding RNA ECONEXIN on gliomagenesis. Oncogene. 2017;36:4629–40. Article CAS PubMed Google Scholar * Han M, Wang S, Fritah S, Wang X,
Zhou W, Yang N, et al. Interfering with long non-coding RNA MIR22HG processing inhibits glioblastoma progression through suppression of Wnt/β-catenin signalling. Brain. 2020;143:512–30.
Article PubMed Google Scholar * Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20–51. Article CAS PubMed PubMed Central Google Scholar * Huang T, Alvarez AA, Pangeni RP, Horbinski CM,
Lu S, Kim SH, et al. A regulatory circuit of miR-125b/miR-20b and Wnt signalling controls glioblastoma phenotypes through FZD6-modulated pathways. Nat Commun. 2016;7:12885. Article CAS
PubMed PubMed Central Google Scholar * Sun B, Zhao X, Ming J, Liu X, Liu D, Jiang C. Stepwise detection and evaluation reveal miR-10b and miR-222 as a remarkable prognostic pair for
glioblastoma. Oncogene. 2019;38:6142–57. Article CAS PubMed PubMed Central Google Scholar * Ahir BK, Lakka SS. Elucidating the microRNA-203 specific biological processes in glioblastoma
cells from comprehensive RNA-sequencing transcriptome profiling. Cell Signal. 2019;53:22–38. Article CAS PubMed Google Scholar * Wu DI, Wang T, Ren C, Liu L, Kong D, Jin X, et al.
Downregulation of BC200 in ovarian cancer contributes to cancer cell proliferation and chemoresistance to carboplatin. Oncol Lett. 2016;11:1189–94. Article CAS PubMed Google Scholar *
Singh R, Gupta SC, Peng WX, Zhou N, Pochampally R, Atfi A, et al. Regulation of alternative splicing of Bcl-x by BC200 contributes to breast cancer pathogenesis. Cell Death Dis.
2016;7:e2262. Article CAS PubMed PubMed Central Google Scholar * Booy EP, McRae EK, Koul A, Lin F, McKenna SA. The long non-coding RNA BC200 (BCYRN1) is critical for cancer cell
survival and proliferation. Mol Cancer. 2017;16:109. Article PubMed PubMed Central CAS Google Scholar * Bossi L, Figueroa-Bossi N. Competing endogenous RNAs: a target-centric view of
small RNA regulation in bacteria. Nat Rev Microbiol. 2016;14:775–84. Article CAS PubMed Google Scholar * Wang R, Zhang S, Chen X, Li N, Li J, Jia R, et al. CircNT5E Acts as a Sponge of
miR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res. 2018;78:4812–25. Article CAS PubMed Google Scholar * Wu P, Cai J, Chen Q, Han B, Meng X, Li Y, et al. Lnc-TALC promotes
O6-methylguanine-DNA methyltransferase expression via regulating the c-Met pathway by competitively binding with miR-20b-3p. Nat Commun. 2019;10:2045. Article PubMed PubMed Central CAS
Google Scholar * Li F, Tang C, Jin D, Guan L, Wu Y, Liu X, et al. CUEDC2 suppresses glioma tumorigenicity by inhibiting the activation of STAT3 and NF-κB signaling pathway. Int J Oncol.
2017;51:115–27. Article CAS PubMed PubMed Central Google Scholar * Casamassimi A, Federico A, Rienzo M, Esposito S, Ciccodicola A. Transcriptome profiling in human diseases: new
advances and perspectives. Int J Mol Sci. 2017;18:1652. Article PubMed Central CAS Google Scholar * Balci T, Yilmaz Susluer S, Kayabasi C, Ozmen Yelken B, Biray Avci C, Gunduz C.
Analysis of dysregulated long non-coding RNA expressions in glioblastoma cells. Gene. 2016;590:120–2. Article CAS PubMed Google Scholar * Hua JT, Chen S, He HH. Landscape of noncoding
RNA in prostate cancer. Trends Genet. 2019;35:840–51. Article CAS PubMed Google Scholar * McDonel P, Guttman M. Approaches for understanding the mechanisms of long noncoding RNA
regulation of gene expression. Cold Spring Harb Perspect Biol. 2019;11:a032151. Article CAS PubMed PubMed Central Google Scholar * Liu K, Wang BJ, Han W, Chi CH, Gu C, Wang Y, et al.
CFIm25-regulated lncRNA acv3UTR promotes gastric tumorigenesis via miR-590-5p/YAP1 axis. Oncogene. 2020. https://doi.org/10.1038/s41388-020-1213-8. * Kopp F, Mendell JT. Functional
classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. Article CAS PubMed PubMed Central Google Scholar * Li L, Wu P, Wang Z, Meng X, Zha C, Li Z, et
al. NoncoRNA: a database of experimentally supported non-coding RNAs and drug targets in cancer. J Hematol Oncol. 2020;13:15. Article CAS PubMed PubMed Central Google Scholar * Pastori
C, Kapranov P, Penas C, Peschansky V, Volmar CH, Sarkaria JN, et al. The Bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proc Natl
Acad Sci USA. 2015;112:8326–31. Article CAS PubMed PubMed Central Google Scholar * Hu T, Lu YR. BCYRN1, a c-MYC-activated long non-coding RNA, regulates cell metastasis of
non-small-cell lung cancer. Cancer Cell Int. 2015;15:36. Article PubMed PubMed Central CAS Google Scholar * Zhang XY, Zhang LX, Tian CJ, Tang XY, Zhao LM, Guo YL, et al. LncRNAs BCYRN1
promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am J Transl Res. 2016;8:3409–18. CAS
PubMed PubMed Central Google Scholar * Peng J, Hou F, Feng J, Xu SX, Meng XY. Long non-coding RNA BCYRN1 promotes the proliferation and metastasis of cervical cancer via targeting
microRNA-138 in vitro and in vivo. Oncol Lett. 2018;15:5809–18. PubMed PubMed Central Google Scholar * Zhai H, Li Y. BCYRN1 is correlated with progression and prognosis in gastric cancer.
Biosci Rep. 2019;39:BSR20190505. Article CAS PubMed PubMed Central Google Scholar * Yu W, Xiang D, Jia H, He X, Sheng J, Long Y, et al. The lncRNA BCYRN1 Functions as an Oncogene in
Human Glioma by Downregulating miR-125a-5p in vitro. Cancer Manag Res. 2020;12:1151–61. Article CAS PubMed PubMed Central Google Scholar * Hansen TB, Jensen TI, Clausen BH, Bramsen JB,
Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. Article CAS PubMed Google Scholar * Karreth FA, Pandolfi PP. ceRNA
cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3:1113–21. Article CAS PubMed PubMed Central Google Scholar * Tay Y, Rinn J, Pandolfi PP. The multilayered
complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52. Article CAS PubMed PubMed Central Google Scholar * Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence
and controversy. Nat Rev Genet. 2016;17:272–83. Article CAS PubMed Google Scholar * Ge X, Li GY, Jiang L, Jia L, Zhang Z, Li X, et al. Long noncoding RNA CAR10 promotes lung
adenocarcinoma metastasis via miR-203/30/SNAI axis. Oncogene. 2019;38:3061–76. Article CAS PubMed PubMed Central Google Scholar * Chen G, Gu Y, Han P, Li Z, Zhao JL, Gao MZ. Long
noncoding RNA SBF2-AS1 promotes colorectal cancer proliferation and invasion by inhibiting miR-619-5p activity and facilitating HDAC3 expression. J Cell Physiol. 2019;234:18688–96. Article
CAS PubMed Google Scholar * Qiu G, Zhang XB, Zhang SQ, Liu PL, Wu W, Zhang JY, et al. Dysregulation of MALAT1 and miR-619-5p as a prognostic indicator in advanced colorectal carcinoma.
Oncol Lett. 2016;12:5036–42. Article CAS PubMed PubMed Central Google Scholar * Zhang PJ, Zhao J, Li HY, Man JH, He K, Zhou T, et al. CUE domain containing 2 regulates degradation of
progesterone receptor by ubiquitin-proteasome. EMBO J. 2007;26:1831–42. Article CAS PubMed PubMed Central Google Scholar * Chen Y, Wang SX, Mu R, Luo X, Liu ZS, Liang B, et al.
Dysregulation of the miR-324-5p-CUEDC2 axis leads to macrophage dysfunction and is associated with colon cancer. Cell Rep. 2014;7:1982–93. Article CAS PubMed Google Scholar * Wu QY, Zhu
YY, Liu Y, Wei F, Tong YX, Cao J, et al. CUEDC2, a novel interacting partner of the SOCS1 protein, plays important roles in the leukaemogenesis of acute myeloid leukaemia. Cell Death Dis.
2018;9:774. Article PubMed PubMed Central CAS Google Scholar * Li HY, Liu H, Wang CH, Zhang JY, Man JH, Gao YF, et al. Deactivation of the kinase IKK by CUEDC2 through recruitment of
the phosphatase PP1. Nat Immunol. 2008;9:533–41. Article CAS PubMed Google Scholar * Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao Y, et al. Elevated expression of CUEDC2 protein confers
endocrine resistance in breast cancer. Nat Med. 2011;17:708–14. Article CAS PubMed Google Scholar * Thomas C, Gustafsson JAA. CUE hints at tumor resistance. Nat Med. 2011;17:658–60.
Article CAS PubMed Google Scholar * Wang A, Li J, Zhou T, Li T, Cai H, Shi H, et al. CUEDC2 contributes to cisplatin-based chemotherapy resistance in ovarian serious carcinoma by
regulating p38 MAPK signaling. J Cancer. 2019;10:1800–7. Article CAS PubMed PubMed Central Google Scholar * Lauria A, Peirone S, Giudice MD, Priante F, Rajan P, Caselle M, et al.
Identification of altered biological processes in heterogeneous RNA-sequencing data by discretization of expression profiles. Nucleic Acids Res. 2020;48:1730–47. Article CAS PubMed Google
Scholar * Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71. Article CAS PubMed PubMed Central Google Scholar * Hu L, Li X, Liu Q,
Xu J, Ge H, Wang Z, et al. UBE2S, a novel substrate of Akt1, associates with Ku70 and regulates DNA repair and glioblastoma multiforme resistance to chemotherapy. Oncogene. 2017;36:1145–56.
Article CAS PubMed Google Scholar * Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12:495–508.
Article CAS PubMed Google Scholar Download references FUNDING This study was support by the National Natural Science Foundation of China (No:81902525), Natural Science Foundation of
Anhui Province (No: 1908085QH336), USTC Research Funds of the Double First-Class Initiative (No: YD9100002002), the Fundamental Research Funds for the Central Universities (No: WK9110000033)
and Special Fund Project for Guiding Local Science and Technology Development by the Central Government (No: 2017070802D144). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Neurosurgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230001, Anhui, P.R. China Maolin Mu,
Wanxiang Niu, Xiaoming Zhang, Shanshan Hu & Chaoshi Niu * Anhui Key Laboratory of Brain Function and Diseases, Hefei, 230001, Anhui, P.R. China Maolin Mu, Wanxiang Niu, Xiaoming Zhang,
Shanshan Hu & Chaoshi Niu * Anhui Provincial Stereotactic Neurosurgical Institute, Hefei, 230001, Anhui, P.R. China Shanshan Hu & Chaoshi Niu Authors * Maolin Mu View author
publications You can also search for this author inPubMed Google Scholar * Wanxiang Niu View author publications You can also search for this author inPubMed Google Scholar * Xiaoming Zhang
View author publications You can also search for this author inPubMed Google Scholar * Shanshan Hu View author publications You can also search for this author inPubMed Google Scholar *
Chaoshi Niu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS CSN and SSH designed this project. MLM and WXN performed the experiments. XMZ
collected specimen. SSH analyzed the data and wrote the paper. All authors have discussed the results and made comments on the experiment. CORRESPONDING AUTHORS Correspondence to Shanshan Hu
or Chaoshi Niu. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION FIGURE S1 FIGURE S2 FIGURE S3 FIGURE S4 FIGURE S5
TABLE S1 TABLE S2 TABLE S3 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Mu, M., Niu, W., Zhang, X. _et al._ LncRNA BCYRN1 inhibits glioma tumorigenesis by competitively binding with miR-619-5p to regulate CUEDC2 expression and the PTEN/AKT/p21
pathway. _Oncogene_ 39, 6879–6892 (2020). https://doi.org/10.1038/s41388-020-01466-x Download citation * Received: 20 March 2020 * Revised: 05 August 2020 * Accepted: 10 September 2020 *
Published: 25 September 2020 * Issue Date: 05 November 2020 * DOI: https://doi.org/10.1038/s41388-020-01466-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
