Subcortical brain volumes in neonatal hypoxic–ischemic encephalopathy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT BACKGROUND Despite treatment with therapeutic hypothermia, hypoxic–ischemic encephalopathy (HIE) is associated with adverse developmental outcomes, suggesting the involvement of
subcortical structures including the thalamus and basal ganglia, which may be vulnerable to perinatal asphyxia, particularly during the acute period. The aims were: (1) to examine
subcortical macrostructure in neonates with HIE compared to age- and sex-matched healthy neonates within the first week of life; (2) to determine whether subcortical brain volumes are
associated with HIE severity. METHODS Neonates (_n_ = 56; HIE: _n_ = 28; Healthy newborns from the Developing Human Connectome Project: _n_ = 28) were scanned with MRI within the first week
of life. Subcortical volumes were automatically extracted from T1-weighted images. General linear models assessed between-group differences in subcortical volumes, adjusting for sex,
gestational age, postmenstrual age, and total cerebral volumes. Within-group analyses evaluated the association between subcortical volumes and HIE severity. RESULTS Neonates with HIE had
smaller bilateral thalamic, basal ganglia and right hippocampal and cerebellar volumes compared to controls (all, _p_ < 0.02). Within the HIE group, mild HIE severity was associated with
smaller volumes of the left and right basal ganglia (both, _p_ < 0.007) and the left hippocampus and thalamus (both, _p_ < 0.04). CONCLUSIONS Findings suggest that, despite advances in
neonatal care, HIE is associated with significant alterations in subcortical brain macrostructure. IMPACT * Compared to their healthy counterparts, infants with HIE demonstrate significant
alterations in subcortical brain macrostructure on MRI acquired as early as 4 days after birth. * Smaller subcortical volumes impacting sensory and motor regions, including the thalamus,
basal ganglia, and cerebellum, were seen in infants with HIE. * Mild and moderate HIE were associated with smaller subcortical volumes. You have full access to this article via your
institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS INSIGHTS FROM SERIAL MAGNETIC RESONANCE IMAGING IN NEONATAL ENCEPHALOPATHY IN TERM INFANTS Article 21 June 2024 HOW WELL DOES
NEONATAL NEUROIMAGING CORRELATE WITH NEURODEVELOPMENTAL OUTCOMES IN INFANTS WITH HYPOXIC-ISCHEMIC ENCEPHALOPATHY? Article Open access 01 March 2023 FUNCTIONAL BRAIN CONNECTIVITY IN EARLY
ADOLESCENCE AFTER HYPOTHERMIA-TREATED NEONATAL HYPOXIC-ISCHEMIC ENCEPHALOPATHY Article Open access 02 March 2025 INTRODUCTION Hypoxic–ischemic encephalopathy (HIE) due to perinatal asphyxia
is a condition that causes a reduction of blood and oxygen to the neonatal brain and can lead to brain damage and adverse developmental outcomes, including cerebral palsy, cognitive
impairment, and epilepsy.1,2,3 Adverse developmental outcomes seen in childhood survivors are often widespread, impacting multiple domains of function, indicating the involvement of
subcortical structures with widespread connectivity to cortical areas. HIE is highly prevalent and affects 1.5 per 1000 live-born term neonates in developed countries.4 The modified Sarnat
examination is the most frequently used tool to assess the severity of HIE.5,6 Staging is based on evidence of the following criteria: history of a perinatal event, prolonged resuscitation,
poor cord gases, low Apgar scores and findings consistent with encephalopathy, such as an abnormal neurological examination or seizures within the first 6 h after birth.5 HIE does not only
cause immediate neuronal cell death (primary phase) but precipitates a complex biochemical cascade leading to delayed neuronal loss (secondary phase).7 This conceptual framework forms the
basis for the timely introduction of therapeutic hypothermia (TH, also known as cooling at 33.5 °C ± 0.5 °C)—to reduce further cell death and neuronal loss. TH started within the first 6 h
after birth, for 72 h, is now the standard of care for moderate to severe HIE in developed countries. TH is currently the only treatment that has shown neuroprotective benefit.8 To optimize
neurodevelopmental outcomes, infants being treated with TH undergo intensive monitoring, including neuromonitoring (seizure detection and treatment, analgesia and sedation),
cardio-respiratory monitoring (respiratory support if needed, blood pressure maintenance), nutrition and correction of metabolic derangements.9 After 72 h, infants are gradually rewarmed,
and magnetic resonance imaging (MRI) is typically performed to characterize brain structure and injury. In the acute period, hypoxic–ischemic injury associated with HIE leads to
characteristic patterns of injury impacting subcortical structures, including the hippocampus, thalamus, and basal ganglia identified on neonatal MRI scans.10 A pilot study of volumetric MRI
for participants with HIE found relatively larger volumes of subcortical white matter in infants treated with TH than in controls.11 Shapiro et al., using deformation-based morphometry in
infants with HIE at 6 months, showed local changes in the perisylvian gray and white matter that were associated with language outcomes at 30 months of age.12 Children and adults treated for
neonatal HIE also demonstrate altered hippocampal volumes.13 Previous studies have also shown reduced total brain and cortical volumes in children with HIE compared with controls.14
Presently, studies examining macrostructure volumes of infants with HIE in the acute period remain an active area of interest due to the challenges faced by their complex care. In turn, less
is known about volumes in newborns with HIE; however, the acute period represents a critical window when brain macrostructure may be most vulnerable to hypoxic–ischemic injury. In the
current study, based on previous research indicating that infants and children diagnosed with HIE at birth suffered long-term neurological consequences, we wished to examine brain
development in neonates with HIE during the acute period. This is the first study to examine subcortical volumes in the first week of life. In this prospective cohort study, we examined the
subcortical macrostructure (thalamus, basal ganglia, hippocampus, amygdala, cerebellum). Our first aim was to examine subcortical macrostructure in infants with HIE (within the first week of
life), compared to healthy term-born babies scanned at a comparable postmenstrual age (PMA), adjusting for demographic variables. The second aim of the study was to examine whether the
severity of HIE, based on Sarnat staging, is associated with changes in subcortical macrostructure, adjusting for clinical factors related to systemic illness and demographic variables.
METHODS USE OF HUMAN SUBJECTS This study was conducted as part of an ongoing prospective study of infants at risk of neurological injury. All participants were recruited from the Neonatal
Intensive Care Unit (NICU) at the Children’s Hospital of South Western Ontario, London, Canada. The study was approved by the Health Sciences Research Ethics Board at Western University.
Informed consent was provided by the parents/caregivers of the infants enrolled in the study. The study was performed in accordance with the Declaration of Helsinki. PARTICIPANTS Infants
with HIE were eligible for recruitment in this prospective study between January 2020 and June 2022. The following criteria were required for inclusion in the sub-study: gestational age (GA)
≥ 36 weeks, with a birth weight ≥2000 g and admitted with a diagnosis of HIE.5 HIE diagnosis was based on the following criteria: cord gas pH of ≤7.0 and/or base deficit of ≥16 mmol per L;
if pH was between 7.01 and 7.15 or a base deficit was between 10 and 15.9 mmol per L, additional history of an acute perinatal event and an APGAR score at 10 min of ≤5, or need for assisted
ventilation/resuscitation at birth; the presence of seizures or moderate or severe encephalopathy by standardized neurological examination.15 We included infants regardless of whether they
were born at our NICU or transferred from an outside hospital for tertiary care. Infants were excluded if they had evidence of major anomalies of the brain or other organs, congenital
infections (e.g., TORCH), intrauterine growth restriction (IUGR), identifiable metabolic disorder or ultrasound evidence of a large parenchymal hemorrhagic infarction. TYPICALLY DEVELOPING
NEWBORNS In addition to the participants with HIE, the study included 28 healthy newborns with no reported brain injury in the analysis, who were compared to age- and sex-matched neonates
with HIE within the first week of life. The participants were infants born at term from the publicly available Developing Human Brain Connectome Project (DHCP).16 DHCP is the first
open-access data release of brain images in healthy neonates born at term who had an MRI scan within the first few days of life (37–44 weeks GA), comparable with the HIE participants. With
these data, we had access to the T1-weighted images. Additional MRI data-acquisition-related information is included in Supplementary File (DHCP MRI Protocol). The images from the healthy
newborns were selected based on participants’ GA and age at the MRI scan to ensure minimal variability. CLINICAL AND DEMOGRAPHIC VARIABLES Maternal and infant data were abstracted from
electronic medical records. Demographic data included GA, birth weight, sex, HIE stage, mode of delivery, place of birth, resuscitation details, Apgar scores and cord pH. Details of 72 h
treatment with TH (33–34 °C) and the presence of brain injury on MRI were collected. As part of the unit protocol, infants with HIE treated with TH underwent at least one MRI scan during
hospitalization (after rewarming, within the first week of life). If infants underwent multiple scans, imaging obtained immediately after rewarming was prioritized for scoring. Days on
ventilation (invasive and non-invasive) were recorded. Invasive ventilation: a sum of the days on conventional, jet or high-frequency oscillatory ventilation. Non-invasive ventilation:
continuous positive airway pressure (CPAP), high- or low-flow oxygen. Additional variables of interest included seizures, postnatal infections (clinical sepsis or positive culture infection,
confirmed necrotizing enterocolitis) and brain injury identified on MRI. MRI IMAGE ACQUISITION All HIE participants underwent an MRI scan as part of clinical care during the first week of
life (median day of life [DOL] at scan=4, interquartile range [IQR] = 4.4). All scans were obtained while participants were resting or asleep. None of the infants was sedated for the MRI
scan. Structural MRI scans were acquired on a 1.5T 450 W GE scanner. Each infant underwent a clinical MRI scan consisting at a minimum of a whole-brain T1-weighted structural image (TR =
8.4–11.5 ms, depending on clinical requirements, TE = 4.2 ms, flip angle=12/25°, matrix size 512 × 512, 99–268 slices, voxel size typically 0.39 × 0.39 × 0.5 mm (0.31 × 31 × 5 to 0.43 × 0.43
× 0.6 for some infants), and a T2-weighted structural image (TR = 3517–9832 ms, TE = 7.3–8.4 ms, flip angle=90/160°, matrix size 256 × 256, 19–60 slices, 0.7 × 0.7 × 2–5 mm voxel
resolution). Alternative imaging sequences such as DWI (Diffusion Weighted Imaging), EPI (echo planar imaging, resting-state functional MRI), FLAIR (fluid-attenuated inversion recovery), PD
(proton density), and SWAN (susceptibility-weighted angiography) sequences were added if requested by the attending physician. BRAIN INJURY CHARACTERIZATION A Pediatric Neuroradiology Fellow
(L.T.) scored the anatomical images for brain injury severity using a combination of T1- and T2-weighted images along with other relative sequences if required including the DW images (see
Supplementary Fig. 1). These were verified by a Pediatric Neuroradiologist (M.J.). White matter injury (WMI) was defined as foci exhibiting T1 hyperintensity without T2 hypointensity or by
low-intensity T1 foci and was scored on a 3-point scale (none=0, minimal=1, moderate–severe=2–3 combined) using the methods of de Vries.10 Intraventricular hemorrhage (IVH) was graded
(none=0, mild=1–2, and moderate–severe=3–4) using Papile’s method.17 Only supratentorial injuries were scored. PROCESSING AND SEGMENTATION The raw DICOM data were converted to NifTI files
through MRIcroGL. The resulting T1-weighted imaging scans converted to 3D images were segmented automatically using infant FreeSurfer.18 Infant FreeSurfer is an automatic processing stream
for T1-weighted MRI scans in infants.18 Automatic processing steps include intensity normalization, skull stripping, and segmentation of the cortex, white matter and subcortical
structures.18 Intensity normalization involves standardizing MRI signal intensity which may differ across participants, scanners, or visits.19 Skull-stripping is the process of removing all
non-brain tissue from MRI images. Segmentation involves segmenting the brain into its anatomical features, including cortical/subcortical brain structures and white/gray matter. The
Freesurfer method involves a multi-atlas approach, in which multiple atlases of newborns are first registered to native space and structure labels are transferred. The atlases were developed
from infant MRI scans.20 To initially create the atlases, manually segmented labels were developed using MRI scans from a representative sample of infants (0–2 years of age). In the current
study, developmentally appropriate atlases for newborns were employed. Labels are fused into a single segmentation result, providing higher accuracy than single-atlas approaches.20,21
Volumetric measurements for anatomical features can then be extracted.18 Infant brain subcortical brain structure volumes were extracted as well as cerebral white matter and cerebral cortex
volumes, to compute total cerebral volumes (TCV). Subcortical brain structures included the left and right: cerebellum cortex, thalamus, caudate, putamen, pallidum, hippocampus, amygdala,
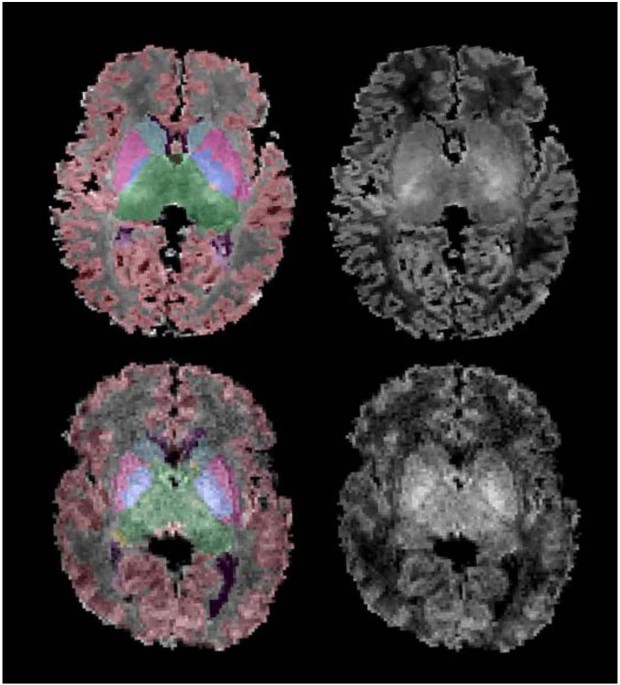
accumbens area, and ventral diencephalon. The ventricles (lateral, third, and fourth) were also automatically labeled. An example of automatic segmentation of a newborn with HIE and a
healthy newborn with T1-weighted MRI scans is provided in Fig. 1. Each segmented T1-weighted image was visually inspected by one of the authors (B.K.) using the Freeview software, available
within the FreeSurfer suite of tools. Seven (25%) T1-weighted images underwent additional manual relabeling. Further manual segmentation was employed to correct any segmentation errors
(i.e., partial volume effects) in the subcortical gray matter using ITK-SNAP (http://www.itksnap.org/). STATISTICAL ANALYSIS All statistical analyses were performed using Statistical Package
for the Social Sciences (v.27 [SPSS], Chicago, IL). The main aim of the study was to assess volumetric differences in subcortical structures between newborns with HIE compared to healthy
newborns. A multivariate model was run to address the first aim of the study. In the model, the volumes of the left and right basal ganglia (summed values of the caudate, putamen, and
pallidum), thalamus, hippocampus, amygdala, and cerebellum were the dependent variables and the independent variable was group (HIE, healthy newborns), adjusting for sex, GA, PMA at the scan
and TCV. As one model was implemented to assess a single hypothesis, with subcortical volumes being smaller in the HIE group compared to healthy newborns, the alpha level was set at _p_ =
0.05. The second aim of the study was to examine whether the severity of HIE was associated with subcortical development. A basic model including the dependent variables (left and right
basal ganglia, thalamus, hippocampus, amygdala, cerebellum) and HIE severity was entered as a continuous variable to examine the heterogeneity of the disorder in relation to brain
macrostructure. The model was adjusted for TH (yes/no), sex, GA, TCV and the total number of days on mechanical ventilation. We had a single hypothesis that HIE severity would be associated
with subcortical volume size, and in turn, we set the alpha level at _p_ = 0.05. RESULTS PARTICIPANTS We identified 33 infants with HIE. Five were excluded due to unavailable MRI (early
death or discharge home) or poor image quality resulting from significant motion artifacts. Hence, our study comprised 28 newborns (19 male, 9 female) with HIE. Of the 28 participants, three
were diagnosed with mild HIE, 21 with moderate HIE, and four with severe HIE in accordance with Sarnat staging.5 THERAPEUTIC HYPOTHERMIA If the neonate met the TH criteria, the decision was
made within the first 6 h of life, as per the unit policy. Of the 28 infants, 23 (82%) underwent whole body TH treatment for 72 hours at 33.5°C, followed by gradual re-warming over 6 h (at
0.5°C per hour) until the core temperature reached 36.5 °C. Five neonates did not undergo 72 h TH due to contraindications, specifically intracranial hemorrhage and hypotension, refractory
to multiple vasopressors. Based on Sarnat staging, of the 23 neonates who underwent TH, 1 had mild HIE, 19 had moderate, and 3 had severe HIE. CLINICAL CHARACTERISTICS The HIE and healthy
newborn groups were matched for sex, GA, PMA at scan and birth weight (Table 1). The HIE participants included in the study had a variety of clinical criteria assessed (Supplementary Table
1). Twelve (42.9%) of the 28 newborns were outborn (born at another facility or home) and then transferred to our NICU. The mean Apgar at 5 min was 4.35 ± 1.05, and the mean first pH was
6.97 ± 0.05. Half of the study population required intubation at birth. Seizures were recorded in 9 neonates: all 4 in the severe HIE group and 5 in the moderate HIE group. We also recorded
the total number of days on ventilation (invasive or non-invasive) as the mean number of days on invasive ventilation, non-invasive ventilation, or a combination of the 2 (4.54 ± 1.89). In
the severe HIE group, 1 neonate had fetal opioid exposure, another was a home birth whose mother lacked prenatal care, yet another had an ischemic event in utero, while the fourth neonate
had glucose variability during the first 72 h. Lastly, as per the unit protocol, most newborns had their scans on the fourth day following rewarming. BRAIN INJURY Of the 28 infants with HIE,
27 (96%) had at least mild injury on MRI at a median age of 5 (IQR 4–6) days. Grade 1 (mild) white matter injury was commonly seen in the neonates, regardless of HIE severity (_n_ = 22,
79%). Basal ganglia injury was evident in 2 (7%) neonates with severe HIE, while IVH was seen in 2 (7%) participants, bilateral grade IV in 1 (3.6%) participant with mild HIE, and bilateral
grade II in 1 participant (3.6%) with moderate HIE. More than one injury pattern was seen in 4 (14%) neonates. No evidence of watershed injury was evident in any of the neonates with HIE
(Table 2). BETWEEN-GROUP ANALYSIS: RELATIONSHIP BETWEEN SUBCORTICAL MACROSTRUCTURE AND HIE DIAGNOSIS A multivariate model revealed a main effect of the group (_F_ = [6,42]9.84, _p_ <
0.001). Smaller subcortical volumes were seen in 6 (60%) of the 10 regions assessed between groups. Newborns with HIE had significantly smaller right (_F_ = 16.2, df=1, _p_ < 0.001) and
left thalamic (_F_ = 10.8, df=1, _p_ = 0.002) volumes compared to typically developing newborns (Fig. 2 and Table 3). The right (_F_ = 34.4, df=1, _p_ < 0.001) and left basal ganglia (_F_
= 12.6, df=1, _p_ < 0.001) as well as the right hippocampus (_F_ = 41.3, df=1, _p_ < 0.001) and right cerebellar (_F_ = 5.7, df=1, _p_ = 0.02) volumes were also smaller in the
newborns with HIE compared to healthy newborns. Volumetric differences in amygdala volumes were not evident (_p_ > 0.05). The SD of brain volumes in HIE and healthy neonates is also shown
in Supplementary Table 2. WITHIN-GROUP ANALYSIS: RELATIONSHIP BETWEEN SUBCORTICAL MACROSTRUCTURE AND HIE SEVERITY To address our second aim, subcortical volumes were examined in relation to
HIE severity based on Sarnat staging while adjusting for sex, GA, TH, days of mechanical ventilation, and TCV. A multivariate model revealed a main effect of HIE severity (_F_ = [10,7]4.34,
_p_ = 0.032). Smaller volumes were seen in 4 (40%) of the 10 subcortical regions that were assessed. The left (_F_ = 9.68, _p_ = 0.007) and right (_F_ = 15.11, _p_ = 0.001) basal ganglia,
the left hippocampus (_F_ = 5.78, _p_ = 0.029), and the left thalamus (_F_ = 4.8, _p_ = 0.044) were associated with HIE severity (Table 4). As part of an exploratory analysis, the data from
the two neonates with basal ganglia injury were removed and the results were comparable, whereby an association between HIE severity and volumetric changes in bilateral basal ganglia (both,
_p_ < 0.001) and thalamus (both, _p_ < 0.048), and the left hippocampus (_p_ = 0.03) was evident. Post hoc univariate general linear models for the main analysis revealed that milder
HIE severity was associated with smaller volumes in all structures, including the left (_B_ = 1409.3, df=1, _p_ = 0.008) and right (_B_ = 1268.2, df=1, _p_ < 0.001) basal ganglia, left
hippocampus (_B_ = 291.3, df=1, _p_ = 0.008), and the left (_B_ = 1084.9, df=1, _p_ = 0.047) thalamus, adjusting for the same clinical and demographic covariates. The SD of brain volumes in
neonates based on HIE severity is also shown in Supplementary Table 3. DISCUSSION HIE remains prevalent and is associated with myriad adverse neurodevelopmental outcomes. Despite advances in
neonatal care, including therapeutic hypothermia, HIE is associated with significant alterations in brain macrostructure. We compared the subcortical brain macrostructure between neonates
with HIE and their healthy counterparts. The initial volumetric comparisons demonstrated reductions in the basal ganglia, thalami, and right hippocampal and cerebellar volumes in patients
with HIE compared to typically developing children, consistent with other reports in children with HIE. Further, we found that HIE severity was associated with regionally specific volume
changes particularly in the basal ganglia, even when removing the data from participants with injury to the basal ganglia. Mild HIE was associated with smaller basal ganglia, hippocampal and
thalamic volumes, a finding that may be reflective of the etiologies of the disorder in the participants with severe HIE, hospital-related care that the participants received or a
combination of these factors. In this sample, the newborns with severe HIE were a heterogeneous group. The generalizability of the findings to other populations of infants with severe HIE
could be enhanced through larger cohort studies. Assessment of brain injury in the acute period is vital to determine the severity of the disease and prognosis. Hence, the findings of this
study bear implications, given the exponential growth of the neonatal brain during the first year of life, and may be amenable to early therapeutic interventions.22 Volumetric analysis
showed reduced volumes in the basal ganglia, thalami, right cerebellum and right hippocampus in the HIE group compared to typically developing newborns. Our findings are aligned with
previous studies. A prospective study with preterm infants (with and without perinatal HIE) found decreased cerebellum and brainstem volumes at term-equivalent age compared to healthy
term-born infants, a finding that persisted at 2 years.23 Although the HIE population was preterm (GA 23–32 weeks), a similar inclusion criterion was used in addition to neurological
deficits and abnormal radiological findings that were consistent with HIE. Additionally, none of the babies received TH. The application of TH in preterm neonates (<36 weeks GA) remains
unclear and is currently being studied.24 Nonetheless, their findings support the results of our study. Spencer et al. reported reduced thalamic, hippocampal, and gray matter volumes in
children aged 6–8 (31 patients and 32 controls) treated with TH for neonatal HIE. Although their study was done outside infancy, it further shows that subcortical brain volume loss in HIE
survivors persists even at school-going age and is associated with decreased functional outcomes.25 Other childhood studies have consistently reported similar findings.13,14 Our findings of
reduced basal ganglia and thalami (BGT) volumes in the participants with HIE compared to typically developing newborns support well-known animal and clinical evidence showing BGT injury as
one of the predominant patterns of hypoxic–ischemic injury.2,10,26,27 Deep gray matter regions, including BGT, are particularly vulnerable to hypoxic–ischemic injury due to their high
metabolic rate, ongoing myelination and high concentration of glutamate receptors, making them prone to volume loss following hypoxic–ischemic injury.26,28 Of note, we report bilateral
reductions in volumes in BGT in the newborns with HIE, but other subcortical structures including the hippocampus and cerebellum demonstrated unilateral reductions in volumes in the HIE
cohort compared to healthy newborns. Evidence from post-mortem and imaging studies has reported bilateral structural changes in the basal ganglia in HIE.29 Bilateral and unilateral changes
in volumes seen in the current study may be reflective of alterations in glutamate receptor density in the subcortical structures. Adult studies following traumatic brain injury show
subcortical volume loss in the BGT, further reinforcing that these regions are likely to suffer volume loss.30 Information regarding decreased BGT volumes is important, given the importance
of these regions in sensorimotor functions. The thalamus is the conduit of all sensory and motor information from the peripheral nervous system to the cerebral cortex.31 Previous studies
have consistently reported that neonates with reduced basal ganglia volumes experience more developmental delays and deficits than their healthy counterparts.32,33,34,35,36 Smaller
subcortical structures are associated with poor motor and higher-order executive functions, as found in the 7-year follow-up of neonates born with HIE.37 We also found that HIE severity was
associated with subcortical volumes, particularly impacting the basal ganglia, adjusting for clinical and demographic factors, including TH. Within the HIE group, newborns with mild and
moderate HIE had small subcortical volumes in regionally specific areas, while the newborns with severe HIE had large volumes. Existing animal evidence shows that increasing hypoxic–ischemic
injury severity is associated with a shorter latent phase, worse secondary energy failure and more severe cortical damage.38 Further, existing human evidence demonstrates more severe acute
neurological injury, with HIE severity, TH notwithstanding.38,39 Clinical care has been shown to have neuroprotective effects in HIE.40 In turn, the newborns in the current sample with
severe HIE who had larger brain volumes within the HIE group may have been exposed to additional neuroprotective agents as part of clinical care that were not captured by our analyses. An
additional consideration is the babies with severe HIE were a heterogeneous group and the resulting encephalopathy may have resulted from antenatal or postnatal events that were not captured
in our analyses that impacted neuronal and/glial processes, which resulted in larger volumes. For newborns with mild and moderate HIE who demonstrated smaller volumes of the basal ganglia,
these structures are particularly vulnerable to hypoxic–ischemic injury. Glutamate receptors are highly prevalent in the basal ganglia, and glutamate-induced toxicity plays an important role
in ischemic injury in term infants, which can ultimately result in neuronal cell death. Another plausible explanation for the subcortical volume changes we saw could be cerebral edema,
which usually peaks 72 h after the insult.41 Second, the neonatal brain, particularly the BGT, may be sensitive to oxidative injury following perinatal asphyxia. All these factors, combined
with mitochondrial dysfunction and the release of inflammatory processes, contribute to cellular alterations, including synaptic pruning that could impact subcortical macrostructure.
Microglia play an important role in controlling synaptic pruning in a healthy developing brain. In HIE, however, this process could be impaired, leading to inappropriate synaptic
connections.42 Besides the known association of HIE targeting the basal ganglia resulting in overt motor deficits43, there is also an increased risk for neuropsychiatric disorders in the
long term. Basal ganglia injury is associated with up to a sevenfold increased risk of developing schizophrenia in HIE survivors.44 Neuropsychiatric disorders are associated with larger
subcortical volumes45; however, the association between BGT injury and later development of neuropsychiatric disorders remains an active area of investigation. An additional interpretation
may be that increased lactate in the basal ganglia contributes to the macrostructural changes seen on MRI. In fact, a preliminary report imaged asphyxiated newborns who were encephalopathic,
within the first 24 h of life, using multimodal neuroimaging methods, and reported increased lactate levels in the basal ganglia.46 Further, while volumes were not examined in the cohort of
asphyxiated newborns, evidence for atrophy in the basal ganglia was evident in some participants with others showing alterations in the thalamus. Improved information concerning subcortical
volumetric development within the first week of life in newborns impacted by HIE provides an opportunity to identify neonates who will benefit from close interdisciplinary follow-up or
additional neuroimaging. In the current study, all newborns had MRI scans completed within the first week of life. We included a comparable control group in terms of baseline
characteristics. This permitted the investigation of subcortical macrostructure in infants with HIE. Although our study offers new insights into brain subcortical volumes in newborns with
HIE, the findings should be considered with respect to some limitations. First, a major limitation was that imaging data were acquired at different field strengths (1.5 T for the HIE group
and 3 T for the control group). However, the HIE infants were scanned on a clinical scanner and it would not be possible to include healthy babies, nor did we have access to a
research-dedicated 1.5 T scanner. Of note, all the segmentations were visually inspected for the HIE and typically developing cohorts, and volumetric differences were only evident in
regionally specific locations. Second, with a relatively small sample size of 28 newborns with HIE, the conclusions drawn require more research to support them. Particularly in relation to
HIE severity, fewer newborns in our sample were diagnosed with mild or severe HIE compared to newborns who were diagnosed with moderate HIE. Clinical care practices differed for newborns
with HIE in that not all newborns received TH—five newborns did not undergo TH due to contraindications that led to the redirection of care. Additionally, with a relatively small sample
size, adjusting for all clinical care factors was not possible. However, HIE at our center is seen in relatively low incidence, and to recruit newborns for an extensive period, during which
time changes in clinical care may occur, could unduly influence results in relation to changes in brain macrostructure. Future prospective studies with larger sample sizes are needed.
Lastly, our study only reports imaging findings in the acute period with no linking to neurodevelopmental outcomes. CONCLUSIONS Consistent with findings from childhood survivors of HIE,
newborns with HIE, scanned with MRI within the first days of life, had smaller subcortical volumes impacting sensory and motor regions, including the thalamus, basal ganglia and cerebellum,
compared to healthy newborns. The results may be reflective of the initial insult associated with hypoxic ischemia; however, the results may also be influenced by the clinical care,
particularly TH, received by the newborns with HIE. Additionally, HIE severity was associated with subcortical volumes, particularly impacting the basal ganglia, suggesting these regions may
be important brain-based biomarkers in newborns impacted by hypoxic–ischemic injury. These findings suggest that despite advances in neonatal care, HIE is associated with significant
alterations in brain macrostructure. DATA AVAILABILITY The data supporting this study’s findings are available from the corresponding author upon reasonable request. REFERENCES * Lee, A. C.
et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. _Pediatr. Res._ 74(Suppl 1), 50–72 (2013). Article
PubMed PubMed Central Google Scholar * Gunn, A. J. & Bennet, L. Fetal hypoxia insults and patterns of brain injury: insights from animal models. _Clin. Perinatol._ 36, 579–593 (2009).
Article PubMed PubMed Central Google Scholar * Lee, B. L. & Glass, H. C. Cognitive outcomes in late childhood and adolescence of neonatal hypoxic-ischemic encephalopathy. _Clin.
Exp. Pediatr._ 64, 608–618 (2021). Article PubMed PubMed Central Google Scholar * Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and
hypoxic-ischaemic encephalopathy. _Early Hum. Dev._ 86, 329–338 (2010). Article PubMed Google Scholar * Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress.
A clinical and electroencephalographic study. _Arch. Neurol._ 33, 696–705 (1976). Article CAS PubMed Google Scholar * Mrelashvili, A., Russ, J. B., Ferriero, D. M. & Wusthoff, C. J.
The Sarnat score for neonatal encephalopathy: looking back and moving forward. _Pediatr. Res._ 88, 824–825 (2020). Article PubMed PubMed Central Google Scholar * de Vries, L. S. &
Cowan, F. M. Evolving understanding of hypoxic-ischemic encephalopathy in the term infant. _Semin. Pediatr. Neurol._ 16, 216–225 (2009). Article PubMed Google Scholar * Jacobs, S. E. et
al. Cooling for newborns with hypoxic ischaemic encephalopathy. _Cochrane Database Syst. Rev_. 2013, CD003311 (2013). * Wintermark, P., Mohammad, K. & Bonifacio, S. L. & Newborn
Brain Society Guidelines and Publications Committee. Proposing a care practice bundle for neonatal encephalopathy during therapeutic hypothermia. _Semin. Fetal Neonatal Med._ 26, 101303
(2021). Article PubMed Google Scholar * de Vries, L. S. & Groenendaal, F. Patterns of neonatal hypoxic-ischaemic brain injury. _Neuroradiology_ 52, 555–566 (2010). Article PubMed
PubMed Central Google Scholar * Parikh, N. A. et al. Volumetric and anatomical MRI for hypoxic-ischemic encephalopathy: relationship to hypothermia therapy and neurosensory impairments.
_J. Perinatol._ 29, 143–149 (2009). Article CAS PubMed Google Scholar * Shapiro, K. A. et al. Early changes in brain structure correlate with language outcomes in children with neonatal
encephalopathy. _Neuroimage Clin._ 15, 572–580 (2017). Article PubMed PubMed Central Google Scholar * Annink, K. V. et al. The long-term effect of perinatal asphyxia on hippocampal
volumes. _Pediatr. Res._ 85, 43–49 (2019). Article PubMed Google Scholar * Bregant, T. et al. Region-specific reduction in brain volume in young adults with perinatal hypoxic-ischaemic
encephalopathy. _Eur. J. Paediatr. Neurol._ 17, 608–614 (2013). Article PubMed Google Scholar * Lemyre, B. & Chau, V. Hypothermia for newborns with hypoxic-ischemic encephalopathy.
_Paediatr. Child Health_ 23, 285–291 (2018). Article PubMed PubMed Central Google Scholar * Hughes, E. J. et al. A dedicated neonatal brain imaging system. _Magn. Reson. Med._ 78,
794–804 (2017). Article CAS PubMed Google Scholar * Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a
study of infants with birth weights less than 1,500 gm. _J. Pediatr._ 92, 529–534 (1978). Article CAS PubMed Google Scholar * Zollei, L., Iglesias, J. E., Ou, Y., Grant, P. E. &
Fischl, B. Infant FreeSurfer: an automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0-2 years. _Neuroimage_ 218, 116946 (2020). Article
PubMed Google Scholar * Sun, X. et al. Histogram-based normalization technique on human brain magnetic resonance images from different acquisitions. _Biomed. Eng. Online_ 14, 73 (2015).
Article PubMed PubMed Central Google Scholar * de Macedo Rodrigues, K. et al. A FreeSurfer-compliant consistent manual segmentation of infant brains spanning the 0-2 year age range.
_Front. Hum. Neurosci._ 9, 21 (2015). Article PubMed PubMed Central Google Scholar * Iglesias, J. E. & Sabuncu, M. R. Multi-atlas segmentation of biomedical images: a survey. _Med.
Image Anal._ 24, 205–219 (2015). Article PubMed PubMed Central Google Scholar * Knickmeyer, R. C. et al. A structural MRI study of human brain development from birth to 2 years. _J.
Neurosci._ 28, 12176–12182 (2008). Article CAS PubMed PubMed Central Google Scholar * Raguz, M. et al. Structural changes in the cortico-ponto-cerebellar axis at birth are associated
with abnormal neurological outcomes in childhood. _Clin. Neuroradiol._ 31, 1005–1020 (2021). Article PubMed Google Scholar * Sabir, H. et al. Unanswered questions regarding therapeutic
hypothermia for neonates with neonatal encephalopathy. _Semin. Fetal Neonatal Med._ 26, 101257 (2021). Article PubMed Google Scholar * Spencer, A. P. C. et al. Brain volumes and
functional outcomes in children without cerebral palsy after therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy. _Dev. Med. Child Neurol_. 65, 367–375 (2023). * Miller, S.
P. et al. Patterns of brain injury in term neonatal encephalopathy. _J. Pediatr._ 146, 453–460 (2005). Article PubMed Google Scholar * Misser, S. K. et al. A proposed magnetic resonance
imaging grading system for the spectrum of central neonatal parasagittal hypoxic-ischaemic brain injury. _Insights Imaging_ 13, 11 (2022). Article PubMed PubMed Central Google Scholar *
Roland, E. H., Poskitt, K., Rodriguez, E., Lupton, B. A. & Hill, A. Perinatal hypoxic-ischemic thalamic injury: clinical features and neuroimaging. _Ann. Neurol._ 44, 161–166 (1998).
Article CAS PubMed Google Scholar * Mota-Rojas, D. et al. Pathophysiology of perinatal asphyxia in humans and animal models. _Biomedicines_ 10, 347 (2022). * Gooijers, J. et al.
Subcortical volume loss in the thalamus, putamen, and pallidum, induced by traumatic brain injury, is associated with motor performance deficits. _Neurorehabil. Neural Repair_ 30, 603–614
(2016). Article PubMed Google Scholar * Torrico, T. J. & Munakomi, S. _Neuroanatomy, Thalamus_ (StatPearls, 2022). * Schneider, J. et al. Procedural pain and oral glucose in preterm
neonates: brain development and sex-specific effects. _Pain_ 159, 515–525 (2018). Article CAS PubMed Google Scholar * Ilves, N. et al. Ipsilesional volume loss of basal ganglia and
thalamus is associated with poor hand function after ischemic perinatal stroke. _BMC Neurol._ 22, 23 (2022). Article PubMed PubMed Central Google Scholar * Nivins, S. et al. Associations
between neonatal hypoglycaemia and brain volumes, cortical thickness and white matter microstructure in mid-childhood: an MRI study. _Neuroimage Clin._ 33, 102943 (2022). Article PubMed
PubMed Central Google Scholar * Geva, S. et al. Volume reduction of caudate nucleus is associated with movement coordination deficits in patients with hippocampal atrophy due to perinatal
hypoxia-ischaemia. _Neuroimage Clin._ 28, 102429 (2020). Article PubMed PubMed Central Google Scholar * Martinez-Biarge, M. et al. Predicting motor outcome and death in term
hypoxic-ischemic encephalopathy. _Neurology_ 76, 2055–2061 (2011). Article CAS PubMed PubMed Central Google Scholar * Loh, W. Y. et al. Neonatal basal ganglia and thalamic volumes: very
preterm birth and 7-year neurodevelopmental outcomes. _Pediatr. Res._ 82, 970–978 (2017). Article PubMed PubMed Central Google Scholar * Iwata, O. et al. “Therapeutic time window”
duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. _Brain Res._ 1154, 173–180 (2007). Article CAS
PubMed Google Scholar * Morales, M. M. et al. Association of Total Sarnat Score with brain injury and neurodevelopmental outcomes after neonatal encephalopathy. _Arch. Dis. Child. Fetal
Neonatal Ed._ 106, 669–72. (2021). Article PubMed Google Scholar * Allen, K. A. & Brandon, D. H. Hypoxic ischemic encephalopathy: pathophysiology and experimental treatments. _Newborn
Infant Nurs. Rev._ 11, 125–133 (2011). Article PubMed PubMed Central Google Scholar * Sorokan, S. T., Jefferies, A. L. & Miller, S. P. Imaging the term neonatal brain. _Paediatr.
Child Health_ 23, 322–328 (2018). Article PubMed PubMed Central Google Scholar * Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development.
_Science_ 333, 1456–1458 (2011). Article CAS PubMed Google Scholar * Perlman, J. M. Intrapartum hypoxic-ischemic cerebral injury and subsequent cerebral palsy: medicolegal issues.
_Pediatrics_ 99, 851–859 (1997). Article CAS PubMed Google Scholar * Ursini, G. et al. Convergence of placenta biology and genetic risk for schizophrenia. _Nat. Med._ 24, 792–801 (2018).
Article CAS PubMed Google Scholar * Hagberg, H., Gressens, P. & Mallard, C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in
children and adults. _Ann. Neurol._ 71, 444–457 (2012). Article PubMed Google Scholar * Barkovich, A. J. et al. Proton spectroscopy and diffusion imaging on the first day of life after
perinatal asphyxia: preliminary report. _AJNR Am. J. Neuroradiol._ 22, 1786–1794 (2001). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the NICU
families who participated in this study. FUNDING This study was financially supported by the Whaley and Harding Fellowship from the Children’s Health Foundation, Canada First Research
Excellence Fund (BrainsCAN), and the Molly Towell Perinatal Research Foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Neuroscience program, Western University, London, ON, Canada
Lilian M. N. Kebaya, Sandrine de Ribaupierre, Michael T. Jurkiewicz & Emma G. Duerden * Division of Neonatal-Perinatal Medicine, Department of Paediatrics, London Health Sciences Centre,
London, ON, Canada Lilian M. N. Kebaya, Paula Camila Mayorga, Paige Meyerink, Kathryn Foglton, Talal Altamimi & Soume Bhattacharya * Applied Psychology, Faculty of Education, Western
University, London, ON, Canada Bhavya Kapoor, Emily S. Nichols & Emma G. Duerden * Western Institute for Neuroscience, Western University, London, ON, Canada Bhavya Kapoor, Emily S.
Nichols, Sandrine de Ribaupierre, Michael T. Jurkiewicz & Emma G. Duerden * Division of Neonatal Intensive Care, Department of Pediatrics, College of Medicine, Imam Abdulrahman Bin
Faisal University, Dammam, Saudi Arabia Talal Altamimi * Department of Clinical Neurological Sciences, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
Sandrine de Ribaupierre & Michael T. Jurkiewicz * Children’s Health Research Institute, London, ON, Canada Sandrine de Ribaupierre & Emma G. Duerden * Department of Medical Imaging,
London Health Sciences Centre, London, ON, Canada Leandro Tristao & Michael T. Jurkiewicz Authors * Lilian M. N. Kebaya View author publications You can also search for this author
inPubMed Google Scholar * Bhavya Kapoor View author publications You can also search for this author inPubMed Google Scholar * Paula Camila Mayorga View author publications You can also
search for this author inPubMed Google Scholar * Paige Meyerink View author publications You can also search for this author inPubMed Google Scholar * Kathryn Foglton View author
publications You can also search for this author inPubMed Google Scholar * Talal Altamimi View author publications You can also search for this author inPubMed Google Scholar * Emily S.
Nichols View author publications You can also search for this author inPubMed Google Scholar * Sandrine de Ribaupierre View author publications You can also search for this author inPubMed
Google Scholar * Soume Bhattacharya View author publications You can also search for this author inPubMed Google Scholar * Leandro Tristao View author publications You can also search for
this author inPubMed Google Scholar * Michael T. Jurkiewicz View author publications You can also search for this author inPubMed Google Scholar * Emma G. Duerden View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.M.N.K., B.K., P.C.M., P.M., K.F., T.A., E.S.N., S.B., S.d.R., L.T., M.J., and E.G.D. were involved in the study
design, database variable creation, and data acquisition design and execution of the data analytic strategy and reviewed and/or revised the final version of the manuscript. L.M.N.K., B.K.,
and E.G.D. contributed to the execution of the data analytic strategy, analyzed the data, and wrote the initial draft of the manuscript. All authors approved the final manuscript as
submitted and agree to be accountable for all aspects of the work. CORRESPONDING AUTHOR Correspondence to Lilian M. N. Kebaya. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ETHICS APPROVAL AND CONSENT TO PARTICIPATE Consent was obtained from guardians. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a
society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript
version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kebaya, L.M.N., Kapoor,
B., Mayorga, P.C. _et al._ Subcortical brain volumes in neonatal hypoxic–ischemic encephalopathy. _Pediatr Res_ 94, 1797–1803 (2023). https://doi.org/10.1038/s41390-023-02695-y Download
citation * Received: 20 February 2023 * Revised: 07 May 2023 * Accepted: 21 May 2023 * Published: 23 June 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41390-023-02695-y
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
