Longitudinal association of homocysteine with depressive and anxiety symptoms among urban adults: healthy aging in neighborhoods of diversity across the life span study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Longitudinal associations of homocysteine (HCY) with depressive symptoms scores among urban adults remain under-studied, especially across sex, race and levels of anxiety. We
examined longitudinal associations of homocysteine (HCY) with depressive symptoms scores among urban adults, before and after stratifying by sex, race and anxiety level, using data from 1460
Healthy Aging in Neighborhoods of Diversity across the Lifespan Study (HANDLS) participants aged 30–64 y at v1 (2004–2009), followed across 3 visits up to 2017. In addition to LnHcyv1, we
used group-based trajectory models predicting z-transformed likelihood of greater LnHcy with age (Hcytraj). Total and domain-specific depression symptoms were scored using Center for
Epidemiologic Studies Depression (CES-D) scale. Mixed-effects linear regression models and Cox proportional hazards models were utilized. A positive association was found between baseline
LnHcyv1 and CES-D total scores in reduced socio-demographic- adjusted Model 1 (β (standard error [SE]) = + 2.337 (0.902), _P_ = 0.010), a relationship slightly attenuated in fully adjusted
Model 2 (Model 1 adjusting for lifestyle and health factors) with a β (SE) = + 1.825 (0.883), _P_ = 0.039. Individuals with lower anxiety levels experienced faster CES-D domain 2 score
annualized increase over time (interpersonal problems) with higher LnHcyv1 (_β_ (SE) = 0.041 (0.018), _P_ = 0.024). Hcytraj was linked to incident elevated depressive symptoms (CES-D total
score ≥16) overall (fully adjusted model: HR = 1.09, 95% CI: 1.03–1.14, _P_ = 0.001), particularly among women and those living in poverty. Baseline and “high trajectory” of LnHcy were
positively associated with depressive symptoms and elevated depressive symptom incidence, in a sex-, race-, poverty status- and anxiety-level specific manner. SIMILAR CONTENT BEING VIEWED BY
OTHERS SUBJECTIVE MENTAL HEALTH, INCIDENCE OF DEPRESSIVE SYMPTOMS IN LATER LIFE, AND THE ROLE OF EPIGENETICS: RESULTS FROM TWO LONGITUDINAL COHORT STUDIES Article Open access 21 September
2020 THE LONGITUDINAL ASSOCIATION BETWEEN MULTIPLE CARDIOMETABOLIC DISEASES, SOCIOECONOMIC STATUS, AND DEPRESSIVE SYMPTOMS IN CHINA Article Open access 23 January 2025 LIFE COURSE BMI
TRAJECTORIES FROM CHILDHOOD TO MID-ADULTHOOD ARE DIFFERENTIALLY ASSOCIATED WITH ANXIETY AND DEPRESSION OUTCOMES IN MIDDLE AGE Article Open access 09 May 2023 INTRODUCTION According to the
World Health Organization and United Nations Development Program projections, the number of those over 60 would increase to 2.1 billion by 2050, from 1.4 billion in 2030 [1, 2]. Declines in
social, cognitive, and physical function associated with aging has been related to worse quality of life [3]. An earlier study discovered a reciprocal association between quality of life in
older age and mental health outcomes, such as anxiety and depression [4]. Depression, while being underdiagnosed frequently, is a major contributor to the worldwide sickness burden and has
been linked to higher rates of morbidity and mortaltiy in the elderly [2, 5]. Hu et al.‘s latest 48-study meta-analysis estimated that 28.4% of older adults had depression, with a 95%
confidence interval (CI) ranging from 24.8% to 32.0% [1]. Personal characteristics associated with geriatric depression include female sex, aging, being single or divorced, having less
education, being unemployed, low income, no health insurance, smoking, having grown up through traumatic experiences, low self-esteem, social deprivation, being alone or lonely, bereavement,
chronic conditions, cognitive impairment, poor health, and history of depression [2]. Studies suggest that stressful life experiences and long-term chronic stress may be linked to geriatric
depression [6,7,8]. Physical symptoms are more common in late life onset depression than in early onset depression, and vascular disease may contribute to, exacerbate, or prolong depressive
symptoms in old age [5]. A meta-analysis of 61 prospective cohort studies found that the presence of late-life depression may raise the risk of cardiovascular disease-related death by
>30% [5]. Investigating connection between stressors and symptoms of depression can help identify strategies for averting late-life depression and its detrimental impact on health.
Homocysteine (HCY), which plays a crucial role in the biochemical balance within the central nervous system, shows strong associations with cardiovascular (i.e., stroke and coronary artery
disease), neurological (i.e., dementia), and psychiatric disease (depression and anxiety) when elevated [9,10,11,12,13,14,15,16,17,18,19,20,21,22]. A meta-analysis of 46-studies revealed
that elevated HCY is associated with depression [23]. Several studies identified positive relationships between elevated HCY levels and anxiety disorders, including PTSD [13, 17, 18, 24].
However, despite such evidence, findings concerning HCY’s associations with depressive symptoms, in the presence or absence of anxiety disorders remains minimal. In this study, urban
middle-aged adults participating in the Health Aging in Neighborhoods of Diversity across the Life Span (HANDLS) investigation had their long-term associations between HCY and depression
examined. We hypothesized that, differently depending on race and sex, higher HCY would be associated with higher symptoms of anxiety and depression. We also investigated whether anxiety
levels affected the relationship over time between HCY and depressive symptoms. Both anxiety and depressive symptoms were compared to the long-term levels and change in HCY with age as a
secondary analysis. METHODS DATABASE Initiated in 2004 by the National Institute on Aging (NIA) Intramural Research Program (IRP), the HANDLS study is a prospective cohort study conducted to
address research topics linked to health disparities in age-associated disorders. Using an area probability sampling strategy, middle-aged African American and White individuals of both
sexes (baseline age: 30-64 years) were chosen from thirteen Baltimore city neighborhoods with widely varying household incomes in order to create the sample of urban adults for the HANDLS
study [25]. Additionally, to increase participation rates and retention among non-traditional research participants, the HANDLS project makes use of mobile medical research vehicles (MRVs)
and innovative research methods. The HANDLS project was authorized by the National Institutes of Health’s Institutional Review Board, and participants gave written informed consent [25].
Between 2004 and 2009, baseline (Visit 1) data on HANDLS participants were gathered in two stages: (i) an in-home interview including health status, health care utilization, psychosocial
factors, diet, neighborhood characteristics, and demographics comprised the first phase of the study and (ii) the subsequent stage encompassed the following aspects: medical history,
physical examination, dietary recall, cognitive assessment, psychophysiological evaluations (e.g., heart rate variability, arterial thickness, carotid ultrasonography, muscle strength, bone
density assessments), and laboratory assessments (e.g., blood chemistries, hematology, biomarkers of oxidative stress, biomaterials for genetic studies) all carried out in MRVs. Following
the initial visit, HANDLS participants were contacted every five years for Visits 2 and 3, which were conducted between 2009 and 2013 and 2013 and 2017, respectively. HANDLS data
documentation are available at https://handls.nih.gov/06Coll-w00dataDocR.cgi. While some HANDLS study visits involved distinct forms of evaluations, several assessments were repeated
throughout time. This analysis harnessed data from Visits 1, 2, and 3, including data on blood HCY concentrations and depressive symptom scores. MEASURES HOMOCYSTEINE (HCY) Plasma HCY was
measured by the Alinity i analyzer Aeon Technologies, LLC (Frostburg, MD). First, the serum quality was measured by hemolysis, lipemia, and icteria detection. All serum samples passed the
quality check threshold determined by Aeon Technologies. HCY was quantified using the Alinity i Homocysteine assay on the Alinity i analyzer [26]. This assay is a one-step immunoassay that
uses chemiluminescent microparticle immunoassay (CMIA) technology. The analytical measuring range (AMR) of the Alinity i Homocysteine assay is 1.00 to 50.00 mmol/L (0.14 to 6.76 mg/mL).
Samples were run in 12 batches and a serum sample (Cat # 200-0162; Stem Cell Technologies) was run in each batch as a control. The homocysteine values for this serum control ranged from 4.55
to 6.18, with an inter-assay coefficient of variation of 8.38%. Using a STATA plugin (_traj_ and _trajplot_) modified from a well-established SAS approach [27, 28], group-based trajectory
modeling (GBTM) was carried out for Loge transformed HCY, or LnHcy measured at visits 1, 2, and 3. This allowed for the identification of adult groups with comparable developmental
trajectories in terms of level and trends throughout time. This group-based method uses maximum likelihood and a multinomial modeling strategy to estimate model parameters. The quasi-Newton
method was selected as the likelihood optimization technique. The _traj_ command uses maximum likelihood estimation to preserve data and mitigate biases from listwise elimination, assuming
missingness at random. Group-based trajectories with age were displayed with 95% confidence intervals (CI) for each group trajectory. We defined a censored normal distribution for LnHcy,
with intercept (0), linear (1), or quadratic (2) orders for each group trajectory, as appropriate. The most parsimonious model with best the fit was determined using the BIC. If linearity
was established using the _trajplot_, up to three groups were taken into consideration, and the linear model was selected. Group membership probability was the main metric of interest
obtained from the GBTM. If there was an identified trajectory group with less than 10% prevalence in the three-group model, then two groups were selected. Given prior evidence, the
trajectory group with elevated and/or increasing HCY over time was selected as the main exposure of interest [9,10,11,12,13,14,15,16,17,18,19,20,21,22], and the exposure was operationalized
using group membership probability. DEPRESSIVE SYMPTOMS Using self-reported information from the Center for Epidemiological Studies Depression (CES-D) questionnaire, a depressed symptoms
score was computed [29]. Several samples of older adults have demonstrated the adequate psychometric properties of the CES-D questionnaire (e.g. [30]). With 20 items and item scores ranging
from “0” to “3,” the CES-D total score range anywhere from “0” and “60.” The frequency and intensity of depressed symptomatology during the previous week were the main aspects of the CES-D
questions. Participants in the HANDLS study were asked to select whether they experienced an item most or all of the time (score = 3), seldom or a substantial portion of the time (score =
2), rarely or never (score = 0), or some or a little of the time (score = 1). Some questions required reverse coding. Additionally, we looked at domain-specific CES-D scores, such as those
for (1) depressive affect (such as feeling depressed); (2) interpersonal problems (such as experiencing social anxiety); (3) somatic complaints (such as difficulty sleeping or eating); and
(4) positive affect (such as thinking positively) [29]. By summing the scores for depressed symptoms for each item under each domain, we were able to determine the raw CES-D sub-scores for
each domain. Information about the items used to calculate each domain-specific CES-D sub-score is described elsewhere [29]. A binary version of CES-D total score was also computed using a
cutoff of 16, commonly used in other studies [31], for a sensitivity analysis on elevated depressive symptoms (EDS) incidence. ANXIETY SYMPTOMS Self-report information from the Psychiatric
Diagnostic Screening Questionnaire Generalized Anxiety Disorder subscale (PDSQga) was used to compute an anxiety symptom score. There were strong psychometric properties ascribed to the
PDSQga among adults [32, 33]. There are a total of 10 items with a scoring of “No”, “Yes”, “Don’t Know”, “Not Applicable”. A total PDSQga score ≥ 6 indicated substantial anxiety symptoms.
Information about the items used to calculate anxiety symptoms is described in Supplementary Method 1. For a secondary analysis whereby anxiety symptoms were the outcome of interest, GBTM
was used to obtain two groups for anxiety scores between visits 1 and 3 (High vs. Low), using a zero-inflated Poisson (zip) distribution. This binary trajectory outcome was then converted
into a Loge(odds of “High” anxiety) and used as main continuous outcome of interest in a series of multiple linear regression models [27, 28]. Anxiety disorder was also elicited at all 3
visits. This variable was used for validation only by cross-tabulating it with the main anxiety disorder variable (above vs. below median) and examining mean Loge(odds of “High” anxiety)
across baseline anxiety disorder. COVARIATES The 2010 Healthy Eating Index [HEI-2010] and health (body mass index [BMI; weight/height2 in kg.m−2, continuous]) characteristics were among
factors taken into account when examining the hypothesized relationships between HCY and depressive symptoms. Other key confounders included demographics (sex (male, female), age (years),
race (White, African American), poverty status (<125% federal poverty line, ≥125% federal poverty line), education (less than high school, high school, more than high school), lifestyle
(current cigarette smoking [Yes, No]) and use of drugs (Yes, No [using any of marijuana, opiates, and cocaine]). The age at baseline (Visit 1) was examined as a continuous variable, and the
follow-up time durations were computed using the age at Visits 2 and 3. The Department of Health and Human Services’ poverty standards were used to operationalize the state of poverty based
on household income and total household size [34]. The HEI-2010 [34] based its overall diet quality recommendations on food and macronutrient standards for the American population from the
Dietary Guidelines for Americans. We also described the relationship between HCY and several downstream health-related factors including self-rated health and comorbidities without including
those into the main models given their potential mediating effect between HCY and depressive symptoms. Comorbidities were characterized as follows: self-reported history of any of several
cardiovascular diseases (no, yes), including atrial fibrillation, angina, coronary artery disease, congestive heart failure, and myocardial infarction; diabetes (non-diabetic, pre-diabetic,
diabetic); dyslipidemia ([or statin use] no, yes); and hypertension (no, yes). Three categories for self-rated health were established: very good/excellent, good, and poor/average. EFFECT
MODIFIERS The main effect modifiers in this study were sex (male vs. female) and race (African American vs. White). In addition, baseline anxiety symptoms (above vs. below median of total
score) was also considered as an important effect modifier in the association between HCY and depressive symptoms over time. STATISTICAL METHODS Stata version 18 (StataCorp, College Station,
TX) was used to conduct descriptive, bivariate, and multivariable analyses. While counts and percentages were employed to describe categorical data, measures of central tendency (mean,
median) and dispersion (standard deviation, standard error, interquartile range) were used to characterize continuous variables. Testing for multicollinearity among the variables included in
mixed-effects models using correlation matrices was one of the model-building procedures. The _mixed_ command in Stata removes missing data from analysis by removing observations with
missing outcomes, but can cause data loss and biases if not random. Nevertheless, the model includes observations with at least one outcome available, assuming missingness at random. Given
that each covariate had less than 5% missing data on average, we ensured sample sizes were constant between different adjusted models by performing multiple imputations (5 imputations, 10
iterations) using the chained equations methodology in order to reduce missing data caused by the addition of covariates into different models. Only the potentially confounding covariates
were imputed, while outcome and exposures were not. Stata commands to this end included _mi impute_, _mi passive_ and _mi estimate_ among others. During this estimation method, all
covariates were entered simultaneously, and continuous covariates were centered on their means, just like in earlier research [35, 36]. First, using the largest sample after excluding HANDLS
subjects with missing CES-D data, baseline sociodemographic, lifestyle, and health characteristics, CES-D test scores (at baseline and change over time), as well as LnHcyv1 and
z-transformed probability of higher LnHcy with age, were described before and after stratifying according to Hcyv1 tertiles. Differences across tertiles were tested using a series of
bivariate linear regression and multinomial logistic regression models to compare means and odds of belonging to specific categories across these tertiles. Specifically, the uppermost two
tertiles of LnHcyv1 were compared to the lowest tertile. Second, different sets of covariates were accounted for when building a series of mixed-effects linear regression models for baseline
HCY as a predictor of CES-D test scores (at baseline and change over time) and z-transformed probability of higher HCY trajectory as a predictor of CES-D test scores (at baseline and change
over time), (Supplementary method 2). Time spent on study, measured in years, between visits 1 and 3 was the time variable employed. Age, sex, race, poverty status, inverse mills ratio
(IMR), time spent on studies, and its interaction with variables including LnHcyv1 and Hcytraj alternative exposures were all taken into account while adjusting _Model 1_. Model 2 included
time on study and its interaction with LnHcyv1 or Hcytraj and other covariates such as age, sex, race, poverty status, education, literacy, smoking, drug use, and the 2010-HEI, BMI, and IMR.
For Models 1 and 2, the interaction effects of LnHcyv1 or Hcytraj with sex and race were assessed as a sensitivity analysis. Moreover, stratified analyses were carried out independently for
White and African American HANDLS participants, males, and women, as was done in previous studies examining blood biomarkers in relation to depressive symptoms [31, 37,38,39]. Therefore, we
applied Models 1-2 to two exposures (LnHcyv1 and Hcytraj), five CES-D test scores (one total score and four domain-specific scores), two stratifying variables (sex, race), and up to two
repeats (impact on baseline CES-D test scores and effect on change in CES-D test scores). Using a two-stage Heckman selection technique, we corrected for sample selectivity resulting from
missing data in all models [26]. We estimated those mixed-effects linear regression models adjusted for the IMR in addition to the aforementioned covariates after using a probit regression
model to predict an indicator of selection with sex, age at Visit 1, race, and poverty status. This model produced an IMR, or a function of the probability of being selected given these
characteristics [40]. Predictive margins from the main mixed-effects linear regression models (Model 1, CES-D total score) were obtained and plotted across time for illustration purposes. As
a secondary outcome, the global anxiety score was treated similarly, but with a maximum of two visits (visits 1 and 3). In contrast to the CES-D score outcome, Loge odds(“high anxiety
trajectory”) was the main outcome of interest which was entered into a linear regression model. Prior to multiple testing correction, the type I error rate for the main and interaction
effects was predetermined to be 0.05 and 0.10, respectively [41]. We used the familywise Bonferroni correction approach [42] to adjust for outcome multiplicity (i.e., five CES-D test scores
and one global anxiety test score), especially for Model 1. Following that, Model 2 was regarded as a sensitivity model that incorporated variables that could be mediating or confounding. In
line with earlier research [43], we therefore changed the significance thresholds for the main effects to _p_ < 0.010 (0.05/5) and the two-way interaction terms to 0.10/5 = 0.020. Four
sensitivity analyses were also performed. First, Ln(odds of elevated anxiety) was the outcome in a linear regression model with primary predictor being baseline Loge(Hcy) [LnHcy]. A reduced
and a fully adjusted model was conducted. Second, mixed-effects linear regression models were carried out whereby the main outcomes were CES-D total and domain-specific scores and the
predictor was annualized change in LnHcy between visits 1 and 2. The models were incrementally adjusted as in earlier models. Moreover, a third sensitivity analysis was conducted whereby a
mixed-effects linear regression model with time-dependent LnHcy as the outcome was conducted in the overall sample examining its association with various exposures, including baseline CES-D
total score, ordinal anxiety score, probability of higher anxiety score and anxiety disorder at baseline. Finally, Cox proportional hazards models were conducted on the time-dependent binary
version of the CES-D total score with a cutoff of 16. Time on study was used as the time variable. Analyses were also conducted on multiple imputed data. Specifically, the fully adjusted
models were presented, examining associations of baseline and trajectory exposures of Hcy with incidence of EDS, after adjustment for the same vector of covariates included in the
mixed-effects linear regression models. Stratification by sex, race, poverty status and anxiety score level was carried out, while also testing for interaction between each exposure and
effect modifier separately in the fully adjusted models. The full Stata script will be made available on github at: https://github.com/baydounm/HANDLS_Hcy_Depression_Anxiety. RESULTS As
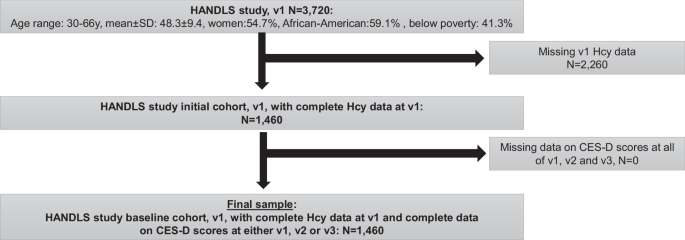
shown in Fig. 1, of 3,720 participants recruited initially in the HANDLS study, 1,460 participants had complete data on Hcy at visit 1, all of whom had complete data on CES-D scores at any
of visits 1, 2 or 3, which made the final analytic sample for the main outcome of depressive symptoms over time. Based on Fig. 2, three separate groups were identified using group-based
trajectory modeling for LnHcy levels, indicating a positive linear trend with age and an initial level that can be labelled as “Low”, “Medium” and “High”. The probability of belonging to the
“High” group was on average 0.12. Importantly, 1 SD in Hcytraj in the selected sample was equivalent to a 0.28 increased probability of membership in the “High” group (data shown on
https://github.com/baydounm/HANDLS_Hcy_Depression_Anxiety). Figure 3 shows the findings for the anxiety score using GBTM, which yielded two groups, namely “High” (46.5%) vs. “Low” (53.5%).
Table 1 describes socio-demographic, lifestyle and health characteristics at baseline as well as HCY and CES-D total scores (at baseline and change over time), overall, and across tertiles
of baseline HCY. The means ( ± standard error [19]) for baseline LnHcy and CES-D total scores were estimated at 2.15 ( ± 0.01) and 14.01 ( ± 0.29), respectively. Similarly, the means ( ±
SEM) for probability of high LnHcy and between-visit change in CES-D total score (annualized empirical bayes estimator) were estimated at 0.12 ± 0.01 and −0.12 ± 0.00, respectively. Means
and proportions of several characteristics differed across baseline HCY tertiles. Mean age increased across the tertiles of Hcy as did the prevalence proportions of hypertension, diabetes,
and elevated cholesterol. In contrast, we observed lower HEI-2010 scores, proportions of female subjects, and proportions of cigarette smokers across higher tertiles of HCY. Non-linear
J-shaped associations between Hcy tertiles and CES-D total and sub-domain scores were detected. Similarly, ordered baseline anxiety score exhibited a non-linear inverted U-shaped association
with HCY tertiles and an inverse relationship with the Log-odds of “high anxiety” probability. The associations of LnHcyv1 with depressive symptoms over time (total and domain-specific
scores), both baseline and annualized change are presented in Table 2, overall, and across sex and race. Overall, and after adjusting for multiple testing, a positive cross-sectional
relationship was observed baseline LnHcy and CES-D total scores in Model 1 (β (standard error [SE]) = + 2.337 (0.902), _P_ = 0.010), a relationship that was slightly attenuated in Model 2
but remained statistically significant [Model 2 (β (SE) = 1.825 (0.883), _P_ = 0.039). The finding from Model 1 is illustrated in Fig. 4 as predictive margins of CES-D total scores across
time based on this model. Among women, LnHcyv1 was only associated with reduced scores on the positive affect sub-domain (Domain #4) in both the reduced (Model 1: _β_ (SE) = −0.86 (0.30),
_P_ = 0.004) and the fully adjusted models (Model 2: _β_ (SE) = −0.68 (0.30), _P_ = 0.025). Importantly, when we stratified the analyses by levels of anxiety (below vs. above median),
heterogeneity across these groups were detected. Most notably, among individuals below median anxiety, LnHcy was associated with a faster increase in CES-D domain 2 score over time
(“interpersonal problems”: _β_ (SE) = 0.043 (0.018), _P_ = 0.018) for Model 1; and _β_ (SE) = 0.041 (0.018), _P_ = 0.024) for Model 2). In contrast, among individuals above median anxiety,
LnHcy showed a strong positive cross-sectional association with CES-D total score (_β_ (SE) = 4.02 (1.46), _P_ = 0.006) for Model 1; and _β_ (SE) = 3.59 (1.45), _P_ = 0.013) for Model 2).
The probability of belonging to the “High” LnHcy trajectory was _z_-scored (Hcytraj) and entered into another set of mixed-effects linear regression models with outcome being CES-D total and
sub-domain scores (baseline and between-visit change), overall, and by stratifying variables (Table 3). Overall, a “High” LnHcy trajectory probability (_z_-scored), or Hcytraj, was directly
associated with baseline CES-D total score for Model 1 (β (SE) = 0.74 (0.29), _P_ = 0.009), an association that was slightly attenuated in Model 2 (β (SE) = 0.64 (0.28), _P_ = 0.021). This
association was mainly driven by the relationship between Hcytraj and the interpersonal problems (Domain 2 score) and somatic complaints (Domain 3 score) sub-domains. Hcytraj was also linked
to a higher score on the depressed affect sub-domain (Domain 1 score) among men at baseline, an association that remained statistically significant in Model 2. Among individuals with an
above median anxiety score, Hcytraj was inversely related to the annualized rate of increase in the “interpersonal problems” sub-domain (Domain 2 score) in the reduced and fully adjusted
models. Four sensitivity analyses were also conducted. In the first sensitivity analysis, LnHcy at visit 1 was studied in relation to the Loge odds of high anxiety (Table S1). No association
was detected in both the reduced and fully adjusted models. In a second sensitivity analysis (Table S2), annualized rate of change in Hcy between visits 1 and 2 was studied in relation to
CES-D total and sub-scores in the overall sample. After adjustment for multiple testing, annualized change in LnHcy between the first two visits was not associated with neither baseline nor
annualized change in CES-D scores, in both reduced and fully adjusted models. Third, a mixed-effects linear regression model with LnHcy as the outcome was conducted in the overall sample
examining its association with various exposures, including baseline CES-D total score, ordinal anxiety score, probability of higher anxiety score and anxiety disorder at baseline (Table
S3). Most notably, baseline CES-D total score was associated with LnHcyv1 in both the reduced and fully adjusted models. In contrast, a higher probability of elevated anxiety over time was
associated with faster increase in LnHcy over time, a finding only detected in the reduced model. In the fourth sensitivity analysis (Table S4), the two main Hcy exposures of interest were
tested as predictors for incidence of EDS over time, using a series of fully adjusted Cox proportional hazards models, while stratifying and testing for potential effect modification by sex,
race, poverty status and anxiety score levels. Baseline Hcy was not associated with incident EDS overall. In fact, there was strong heterogeneity in this association across race, poverty
status and anxiety levels. Most notably, baseline Hcy was inversely associated with incident EDS among White participants and participants living above poverty while being positively
associated with this outcome among African American adults, adults with low level of anxiety at baseline, and adults living below poverty (_P_ < 0.05 for 2-way interaction terms between
baseline Hcy exposure and those 3 effect modifiers). In contrast, Hcytraj was positively associated with incident EDS overall (HR = 1.09, 95% CI: 1.03–1.14, _P_ = 0.001), more strongly so
among women and individuals living below poverty (_P_ < 0.05 for 2-way interaction between Hcytraj and those 2 effect modifiers). For Hcytraj, the strongest association with incident EDS
was noted in the “Below poverty” stratum: HR = 1.22, 95% CI: 1.13–1.18, _P_ < 0.001. DISCUSSION In this prospective cohort study of 1460 urban adults, 30 to 64 years of age at baseline,
mean LnHcyv1 and CES-D total scores were estimated at 2.15 and 14.01, respectively. Overall, a positive cross-sectional relationship was observed LnHcyv1 and CES-D total scores in Model 1 (β
([SE]) = + 2.337 (0.902), _P_ = 0.010), a relationship that was slightly attenuated in Model 2 [(β (SE) = + 1.825 (0.883), _P_ = 0.039). However, heterogeneity was detected across anxiety
levels, with individuals below median anxiety experiencing faster increases in CES-D domain 2 scores with increased LnHcyv1 (interpersonal problems: _β_ [SE] = 0.041 [0.018], _P_ = 0.024). A
“High” LnHcy trajectory probability (_z_-scored), or Hcytraj, was directly associated with baseline CES-D total score for Model 1 (β (SE) = + 0.74 (0.29), _P_ = 0.009), an association that
was slightly attenuated in Model 2 (β (SE) = + 0.64 (0.28), _P_ = 0.021). This association was mainly driven by the relationship between Hcytraj and the interpersonal problems and somatic
complaints sub-domains. Among individuals with an above median anxiety score, Hcytraj was inversely related to the annualized rate of increase in the “interpersonal problems” sub-domain
(Domain 2 score) in the reduced and fully adjusted models. Hcytraj was positively associated with incident elevated depressive symptoms (CES-D total score ≥ 16) overall (fully adjusted
model: HR = 1.09, 95% CI: 1.03–1.14, _P_ = 0.001), though more strongly so among women and individuals living below poverty. Though research in this area of work is limited, prior studies
suggested that higher levels of HCY were related to depressive episodes, symptoms, and risk for depression and anxiety disorders, though it remains unclear if anxiety partially explains
these relationships [13, 17,18,19,20,21,22]. Differences in sample compositions across age, sex, nationality, and prior medical history of psychiatric disorders may be reasons for these
discrepancies. Furthermore, studies have varied on their assessments of depression, depressive symptoms, and anxiety, as well as that of other conditions such as PTSD. The growing body of
work in this area warrants further inquiry into how these relationships develop over time and to what extent the heterogeneity across anxiety levels observed in the present study highlight
more nuanced influences per depression- or anxiety-related disorders. The cross-sectional and longitudinal evidence of the positive association of HCY and symptoms of depression are
consistent with the findings from other studies [15, 16]. Most notably, a systematic review and meta-analysis comparing homocysteine levels in healthy subjects and those with depression
found that individuals with higher homocysteine levels had a higher risk of depression [15]. This was found in studies using various depression diagnostic tools, including DSM-IV, GDS, ZDRS,
and BDI-II [15]. The review and meta-analysis also found that participants with hyperhomocysteinaemia had a higher chance of depression [15]. The findings suggest that future research
should focus on the tools used for depression assessment to better understand the relationship between homocysteine levels and depression [15]. A cross-sectional study along with a
systematic review, and meta-analysis were conducted on 3752 men aged 70 years or older [16]. The results showed that the odds ratio of prevalent depression increased 4% with every unit
increase of total homocysteine (tHcy) [16]. The methylenetetrahydrofolate reductase (MTHFR) C677T TT genotype was 0.19 mg/L higher among participants with the MTHFR C677T TT genotype
compared to the CC genotype [16]. The meta-analysis showed that older adults with high tHcy had an increased risk of depression, and TT carriers were 22% more likely than CC carriers to have
current depression or a history of depression [16]. The triangular association between the MTHFR genotype, tHcy, and depression implies that higher concentrations of tHcy increase the risk
of depression [16]. Confirmatory data from sufficiently powered randomized trials of homocysteine-lowering therapy are now required to test the causal relationship between tHcy and
depression [16]. Nevertheless, a study conducted in the Longitudinal Aging Study Amsterdam (LASA) involving 1352 men and women aged ⩾65 years, assessed depressive symptoms six times from
1995/1996 to 2011/2012 [14]. Multiple linear regression and mixed models were used to assess the associations of vitamin B-12 as well as Hcy with depressive symptoms over 16 years [14]. The
results showed that vitamin B-12 was not cross-sectionally or prospectively associated with depressive symptoms, and no association was found with incident depression [14]. For Hcy, no
associations were found, except for a lower risk of depression in younger participants [14]. The study concluded that further research is needed to understand the influence of Hcy metabolism
on mental health [14]. Our present study detected a similar inverse association between baseline Hcy and incident EDS among White adults and individuals living over poverty with no
detectable association overall. In contrast, there was a positive association detected among African American adults and individuals living below poverty. Therefore, the inverse association
detected in the previous study may be specific to middle-aged adults of higher SES, independently of other socio-demographic, lifestyle and health-related factors. Although the relationship
of HCY to depression was first noted in 1970 [9], the exact mechanisms explaining the association remain unclear. High levels of homocysteine can result from a variety of reasons including a
dietary deficiency of vitamins B6, B12, and folate, genetic variation of the enzymes essential for the metabolism of homocysteine such as MTHFR and cystathionine beta-synthase, enzymes,
gastric atrophy, inflammatory bowel disease and methionine loading [9, 16]. Elevated HCY may result in deficiency of methyl transfer reactions, resulting in deficits to neurotransmitter
synthesis, and is associated with reduced cortical and hippocampal volumes in the brain. These changes may explain how HCY levels mediate symptoms of depression [44]. The effects of HCY on
depression have been reported to be independent of dementia [45]. However, the interaction of HCY and depression can influence cognitive performance [46]. Evaluated HCY and depression appear
to be associated with white matter damage, gray matter atrophy, β-amyloid deposition, and cardiovascular disease risk [46, 47]. Yet not all studies have found the association between HCY
and depression which could reflect the many causes for elevated HCY, including genetic predisposition and brain disease, and the several types of depression in samples of different
ethnicities and ages [9, 48]. A growing interest in Plasma HCY and its sequelae has spurred a set of evidence linking HCY to adverse outcomes for brain health. Much of this interest has
grown out of observational studies linking high plasma HCY and allelic variants of the MTHFR gene to psychiatric abnormalities [9, 49]. HCY production is controlled through one-carbon
metabolism [50, 51]. Methionine synthase, a B12-dependent enzyme, is a key enzyme that governs the utilization of HCY [50, 51]. Vitamin B12 deficiency or a mutation in the enzyme slow the
conversion of HCY to methionine, leading to the accumulation of HCY [50, 51]. An inborn error of metabolism in the MTHFR gene, which is responsible for the production of the other substrate
of the methionine synthase reaction, 5-methyl THF, can also cause HCY accumulation [52]. Animal studies have shown that loss-of-function mutations in MTHFR decrease levels of monoamine
neurotransmitters in key regions of the brain, such as glutamate in the amygdala and γ-aminobutyric acid in the thalamus [53]. Hyperhomocysteinemia has also been linked to the production of
homocysteic acid, a compound that is known to be neurotoxic to dopaminergic regions of the brain and that functions as an N-methyl-D-aspartate (NMDA) receptor agonist [54]. Indeed, it
appears that the downstream neurotoxic effects of HCY on dopaminergic circuits and the changes in monoamine neurotransmitter concentrations may help explain the observed associations from
our study, which we find are also consistent across several other epidemiological studies [9, 55]. Our study has several strengths including its being the first study conducted among
ethnically and socio-economically diverse urban middle-aged adults to examine these intricate research questions. Second, the study was well-powered to examine associations across sex and
race, as well as across anxiety levels. Furthermore, analyses included advanced techniques such as multiple imputation coupled with mixed-effects linear regression models and 2-stage Heckman
selection. There was also a wealth of data available to control for potential confounders in all our models. A few limitations are worth considering when interpreting study results. First,
subsamples of the initial HANDLS participants were used to assess potential associations between HCY and depressive symptoms, which may have resulted in selection bias. Second, as a large
number of exposure, outcome, and covariate assessment factors were self-reported, measurement errors are probably present and could result in skewed measures of association. Third, our
ability to compare our findings with published literature may have been hampered by various criteria used to categorize HCY as well as different means of measuring it in serum. Fourth,
there’s a chance that the amount of time spent in follow-up between HANDLS Visits 1, 2, and 3 was insufficient to see any clinically significant improvements in depression symptoms.
Similarly, fluctuation of depressive symptoms over time cannot be fully captured by the total CES-D score. Future research should therefore investigate proposed correlations over extended
follow-up periods. Fifth, even after adjusting for a number of factors, residual confounding is likely because HANDLS is an observational study, which means we were unable to demonstrate
causal associations. Sixth, while analyzing HCY in connection to depressive symptoms, the impact of interaction effects by sex and race might have been understated. Lastly, the HANDLS
study’s sampling design limits the generalizability of the study’s findings to middle-aged and older persons living in metropolitan areas across the United States. CONCLUSION Among urban
middle-aged adults, baseline and “high trajectory” of LnHcy were positively associated with depressive symptoms and elevated depressive symptom incidence, in a sex-, race-, poverty status-
and anxiety-level specific manner. More research is needed using longitudinal designs with larger sample sizes and longer follow-up times, in order to replicate and confirm our present study
findings. DATA AVAILABILITY The study protocol (09-AG-N248) received approval from the National Institute on Environmental Health Sciences’ Institutional Review Board (IRB) of the National
Institutes of Health (NIH). Upon request, data can be made available to researchers with approved proposals, after they have agreed to confidentiality as required by our IRB. Policies are
publicized on: https://handls.nih.gov. Data access request can be sent to principal investigators (PI) or the study manager, Jennifer Norbeck at [email protected]. These data are owned
by the National Institute on Aging at the NIH. The PIs have made those data restricted to the public for two main reasons: “(1) The study collects medical, psychological, cognitive, and
psychosocial information on racial and poverty differences that could be misconstrued or willfully manipulated to promote racial discrimination; and (2) Although the sample is fairly large,
there are sufficient identifiers that the PIs cannot guarantee absolute confidentiality for every participant as we have stated in acquiring our confidentiality certificate.” [56] REFERENCES
* Hu T, Zhao X, Wu M, Li Z, Luo L, Yang C, et al. Prevalence of depression in older adults: a systematic review and meta-analysis. Psychiatry Res. 2022;311:114511. Article PubMed Google
Scholar * Zenebe Y, Akele B, Selassie MW, Necho M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry. 2021;20:55. Article
PubMed PubMed Central Google Scholar * Obuobi-Donkor G, Nkire N, Agyapong VIO. Prevalence of major depressive disorder and correlates of thoughts of death, suicidal behaviour, and death
by suicide in the geriatric population—a general review of literature. Behav Sci. 2021;11:142. Article PubMed PubMed Central Google Scholar * Hohls JK, Konig HH, Quirke E, Hajek A.
Anxiety, depression and quality of life—a systematic review of evidence from longitudinal observational studies. Int J Environ Res Public Health. 2021;18:12022. Article PubMed PubMed
Central CAS Google Scholar * Wei J, Hou R, Zhang X, Xu H, Xie L, Chandrasekar EK, et al. The association of late-life depression with all-cause and cardiovascular mortality among
community-dwelling older adults: systematic review and meta-analysis. Br J Psychiatry. 2019;215:449–55. Article PubMed Google Scholar * Krause N. Life stress as a correlate of depression
among older adults. Psychiatry Res. 1986;18:227–37. Article PubMed CAS Google Scholar * Rauch SA, Morales KH, Zubritsky C, Knott K, Oslin D. Posttraumatic stress, depression, and health
among older adults in primary care. Am J Geriatr Psychiatry. 2006;14:316–24. Article PubMed Google Scholar * Zannas AS, McQuoid DR, Payne ME, Steffens DC, MacFall JR, Ashley-Koch A, et
al. Negative life stress and longitudinal hippocampal volume changes in older adults with and without depression. J Psychiatr Res. 2013;47:829–34. Article PubMed PubMed Central Google
Scholar * Folstein M, Liu T, Peter I, Buel J, Arsenault L, Scott T, et al. The homocysteine hypothesis of depression. Am J Psychiatry. 2007;164:861–7. Article PubMed Google Scholar *
Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry.
2000;69:228–32. Article PubMed PubMed Central CAS Google Scholar * Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:1–10. Article
Google Scholar * Marinou K, Antoniades C, Tousoulis D, Pitsavos C, Goumas G, Stefanadis C. Homocysteine: a risk factor for coronary artery. Hellenic J Cardiol. 2005;46:59–67. PubMed Google
Scholar * Levine J, Timinsky I, Vishne T, Dwolatzky T, Roitman S, Kaplan Z, et al. Elevated serum homocysteine levels in male patients with PTSD. Depress Anxiety. 2008;25:E154–E157.
Article PubMed Google Scholar * Elstgeest LE, Brouwer IA, Penninx BW, van Schoor NM, Visser M. Vitamin B(12), homocysteine and depressive symptoms: a longitudinal study among older
adults. Eur J Clin Nutr. 2017;71:468–75. Article PubMed CAS Google Scholar * Moradi F, Lotfi K, Armin M, Clark CCT, Askari G, Rouhani MH. The association between serum homocysteine and
depression: a systematic review and meta-analysis of observational studies. Eur J Clin Invest. 2021;51. https://doi.org/10.1111/eci.13486. * Almeida OP, McCaul K, Hankey GJ, Norman P,
Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–94. Article PubMed CAS Google Scholar * de Vries G-J, Lok A, Mocking R, Assies J,
Schene A, Olff M. Altered one-carbon metabolism in posttraumatic stress disorder. J Affect Disord. 2015;184:277–85. Article PubMed Google Scholar * Jendricko T, Vidovic A, Grubisic-Ilic
M, Romic Z, Kovacic Z, Kozaric-Kovacic D. Homocysteine and serum lipids concentration in male war veterans with posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry.
2009;33:134–40. Article PubMed CAS Google Scholar * Kuebler U, Linnebank M, Semmler A, Stoffel-Wagner B, La Marca R, Ehlert U, et al. Plasma homocysteine levels increase following stress
in older but not younger men. Psychoneuroendocrinology. 2013;38:1381–7. Article PubMed CAS Google Scholar * Chung KH, Chiou HY, Chen YH. Associations between serum homocysteine levels
and anxiety and depression among children and adolescents in Taiwan. Sci Rep.2017;7:8330. Article PubMed PubMed Central Google Scholar * Fraguas R Jr, Papakostas GI, Mischoulon D,
Bottiglieri T, Alpert J, Fava M. Anger attacks in major depressive disorder and serum levels of homocysteine. Biol Psychiatry. 2006;60:270–4. Article PubMed CAS Google Scholar *
Saraswathy KN, Ansari SN, Kaur G, Joshi PC, Chandel S. Association of vitamin B12 mediated hyperhomocysteinemia with depression and anxiety disorder: a cross-sectional study among Bhil
indigenous population of India. Clin Nutr Espen. 2019;30:199–203. Article PubMed Google Scholar * Moradi F, Lotfi K, Armin M, Clark CC, Askari G, Rouhani MH. The association between serum
homocysteine and depression: a systematic review and meta‐analysis of observational studies. Eur J Clin Investig. 2021;51:e13486. Article CAS Google Scholar * Chung K-H, Chiou H-Y, Chen
Y-H. Associations between serum homocysteine levels and anxiety and depression among children and adolescents in Taiwan. Sci Rep. 2017;7:8330. Article PubMed PubMed Central Google Scholar
* Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a
longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–75. PubMed Google Scholar * Suen KFK, Lee GR, Finnegan M, Halton K, Borovickova I,
Trench C, et al. Total plasma homocysteine measurement: evaluation of the Abbott immunoassay, comparison with the JEOL ion exchange chromatography and investigation of its clinical utility.
Pr Lab Med. 2022;32:e00295. CAS Google Scholar * Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res.
2001;29:374–93. Article Google Scholar * Jones B, Nagin D. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Method Res. 2007;35:542–71. Article
Google Scholar * Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally
representative sample. Psychiatry Res. 2004;126:177–87. Article PubMed Google Scholar * Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the
Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–5. Article PubMed CAS
Google Scholar * Beydoun MA, Obhi HK, Weiss J, Canas JA, Beydoun HA, Evans MK, et al. Systemic inflammation is associated with depressive symptoms differentially by sex and race: a
longitudinal study of urban adults. Mol Psychiatry. 2020;25:1286–1300. Article PubMed Google Scholar * Zimmerman M, Chelminski I. Screening for anxiety disorders in depressed patients. J
Psychiatr Res. 2006;40:267–72. Article PubMed Google Scholar * Gibbons RD, Rush AJ, Immekus JC. On the psychometric validity of the domains of the PDSQ: an illustration of the bi-factor
item response theory model. J Psychiatr Res. 2009;43:401–10. Article PubMed Google Scholar * Annual Update of the HHS Poverty Guidelines; Notice.
https://aspe.hhs.gov/sites/default/files/documents/ff1ac67af87462a031279a1a462bcd13/HHS-Poverty-Guidelines-Fed-Register-2004.pdf, Date Accessed 2004 Accessed. * Beydoun HA, Huang S, Beydoun
MA, Hossain S, Zonderman AB. Mediating-moderating effect of allostatic load on the association between dietary approaches to stop hypertension diet and all-cause and cause-specific
mortality: 2001-2010 National Health and Nutrition Examination Surveys. Nutrients. 2019;11:503. Article Google Scholar * Beydoun MA, Beydoun HA, Mode N, Dore GA, Canas JA, Eid SM, et al.
Racial disparities in adult all-cause and cause-specific mortality among us adults: mediating and moderating factors. BMC Public Health. 2016;16:1113. Article PubMed PubMed Central CAS
Google Scholar * Beydoun MA, Beydoun HA, Dore GA, Canas JA, Fanelli-Kuczmarski MT, Evans MK, et al. White blood cell inflammatory markers are associated with depressive symptoms in a
longitudinal study of urban adults. Transl Psychiatry. 2016;6:e895. Article PubMed PubMed Central CAS Google Scholar * Beydoun MA, Beydoun HA, Dore GA, Fanelli-Kuczmarski MT, Evans MK,
Zonderman AB. Total serum cholesterol, atherogenic indices and their longitudinal association with depressive symptoms among US adults. Transl Psychiatry. 2015;5:e518. Article PubMed
PubMed Central CAS Google Scholar * Beydoun MA, Hossain S, Chitrala KN, Tajuddin SM, Beydoun HA, Evans MK, et al. Association between epigenetic age acceleration and depressive symptoms
in a prospective cohort study of urban-dwelling adults. J Affect Disord. 2019;257:64–73. Article PubMed PubMed Central Google Scholar * Beydoun MA, Beydoun HA, Kitner-Triolo MH, Kaufman
JS, Evans MK, Zonderman AB. Thyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. J Clin Endocrinol Metab. 2013;98:3470–81. Article
PubMed PubMed Central CAS Google Scholar * Selvin S. Statistical Analysis of Epidemiologic Data. 3rd edn. Oxford University Press, 2004. * Hochberg Y, Tamhane, AC. Multiple comparison
procedures. Wiley: New York, 1987. * Beydoun MA, Canas JA, Dore GA, Beydoun HA, Rostant OS, Fanelli-Kuczmarski MT, et al. Serum uric acid and its association with longitudinal cognitive
change among urban adults. J Alzheimers Dis. 2016;52:1415–30. Article PubMed PubMed Central Google Scholar * Bremner JD, Goldberg J, Vaccarino V. Plasma homocysteine concentrations and
depression: a twin study. J Affect Disord Rep. 2021;4:100093. Google Scholar * Castro F, Melgarejo J, Chavez CA, de Erausquin GA, Terwilliger JD, Lee JH, et al. Total plasma homocysteine
and depressive symptoms in older hispanics. J Alzheimers Dis. 2021;82:S263–9. Article PubMed PubMed Central CAS Google Scholar * Zhou H, Zhong X, Chen B, Wu Z, Zhang M, Mai N, et al.
Interactive effects of elevated homocysteine and late-life depression on cognitive impairment. J Affect Disord. 2020;277:212–7. Article PubMed CAS Google Scholar * Ford AH, Flicker L,
Singh U, Hirani V, Almeida OP. Homocysteine, depression and cognitive function in older adults. J Affect Disord. 2013;151:646–51. Article PubMed CAS Google Scholar * Forti P, Rietti E,
Pisacane N, Olivelli V, Dalmonte E, Mecocci P, et al. Blood homocysteine and risk of depression in the elderly. Arch Gerontol Geriatr. 2010;51:21–25. Article PubMed CAS Google Scholar *
Beydoun MA, Tajuddin SM, Shaked D, Beydoun HA, Evans MK, Zonderman AB. One-carbon metabolism gene polymorphisms are associated with cognitive trajectory among African-American adults.
Neurobiol Aging. 2019;84:70–82. Article Google Scholar * Watkins D, Ru M, Hwang HY, Kim CD, Murray A, Philip NS, et al. Hyperhomocysteinemia due to methionine synthase deficiency, cblG::
structure of the gene, genotype diversity, and recognition of a common mutation, P1173L. Am J Hum Genet. 2002;71:143–53. Article PubMed PubMed Central CAS Google Scholar * Pawlak R. Is
vitamin B12 deficiency a risk factor for cardiovascular disease in vegetarians? Am J Prev Med. 2015;48:e11–26. Article PubMed Google Scholar * Zaghloul A, Iorgoveanu C, Desai A,
Balakumaran K, Chen K. Methylenetetrahydrofolate reductase polymorphism and premature coronary artery disease. Cureus. 2019;11:e5014. PubMed PubMed Central Google Scholar * Jadavji NM,
Wieske F, Dirnagl U, Winter C. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Mol Genet Metab Rep. 2015;3:1–4. Article
PubMed PubMed Central CAS Google Scholar * Bhatia P, Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam Clin Pharm. 2015;29:522–8.
Article CAS Google Scholar * Lee ES, Chen H, Soliman KF, Charlton CG. Effects of homocysteine on the dopaminergic system and behavior in rodents. Neurotoxicology. 2005;26:361–71. Article
PubMed CAS Google Scholar * Beydoun MA, Weiss J, Obhi HK, Beydoun HA, Dore GA, Liang H, et al. Cytokines are associated with longitudinal changes in cognitive performance among urban
adults. Brain Behav Immun. 2019;80:474–487. Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to thank all HANDLS
participants, staff and investigators, as well as internal reviewers of the manuscript at NIA/NIH/IRP. The authors would like to thank Nicolle Mode for her help with sample selection for the
homocysteine assay. Dr. Hind A. Beydoun worked on this manuscript outside her tour of duty at the U.S. Department of Veterans Affairs. FUNDING This research was supported entirely by the
Intramural Research Program of the NIH, National Institute on Aging (Z01-AG000513). Open access funding provided by the National Institutes of Health. AUTHOR INFORMATION Author notes * These
authors contributed equally: Michael F. Georgescu, May A. Beydoun. * These authors jointly supervised this work: Michele K. Evans; Alan B. Zonderman. AUTHORS AND AFFILIATIONS * Laboratory
of Epidemiology and Population Sciences, National Institute on Aging Intramural Research Program, Baltimore, MD, 21224, USA Michael F. Georgescu, May A. Beydoun, Christian A. Maino Vieytes,
Marie T. Fanelli-Kuczmarski, Jason Ashe, Nicole Noren Hooten, Michele K. Evans & Alan B. Zonderman * VA National Center on Homelessness Among Veterans, U.S. Department of Veterans
Affairs, Washington, DC, 20420, USA Hind A. Beydoun * Department of Management, Policy, and Community Health, School of Public Health, University of Texas Health Science Center at Houston,
Houston, TX, 77030, USA Hind A. Beydoun * Department of Human Services, State of Maryland, Baltimore, MD, 21202, USA Sharmin Hossain Authors * Michael F. Georgescu View author publications
You can also search for this author inPubMed Google Scholar * May A. Beydoun View author publications You can also search for this author inPubMed Google Scholar * Christian A. Maino Vieytes
View author publications You can also search for this author inPubMed Google Scholar * Marie T. Fanelli-Kuczmarski View author publications You can also search for this author inPubMed
Google Scholar * Jason Ashe View author publications You can also search for this author inPubMed Google Scholar * Hind A. Beydoun View author publications You can also search for this
author inPubMed Google Scholar * Sharmin Hossain View author publications You can also search for this author inPubMed Google Scholar * Nicole Noren Hooten View author publications You can
also search for this author inPubMed Google Scholar * Michele K. Evans View author publications You can also search for this author inPubMed Google Scholar * Alan B. Zonderman View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Michael F. Georgescu: study concept, plan of analysis, assistance with data management and statistical
analysis, literature search and review, write-up of parts of the manuscript, revision of the manuscript. May A. Beydoun: study concept, plan of analysis, data management and statistical
analysis, literature search and review, write-up of parts of the manuscript, revision of the manuscript. Christian A. Maino Vieytes: study concept, plan of analysis, literature search and
review, write-up of parts of the manuscript, revision of the manuscript. Marie T. Fanelli-Kuczmarski: study concept, plan of analysis, literature search and review, write-up of parts of the
manuscript, revision of the manuscript. Jason Ashe: plan of analysis, literature search and review, write-up of parts of the manuscript, revision of the manuscript. Hind A. Beydoun: plan of
analysis, literature search and review, write-up of parts of the manuscript, revision of the manuscript. Sharmin Hossain: plan of analysis, literature search and review, write-up of parts of
the manuscript, revision of the manuscript. Nicole Noren Hooten: study concept, literature search and review, write-up of parts of the manuscript, review of the manuscript. Michele K.
Evans: data acquisition, plan of analysis, write-up of parts of the manuscript, revision of the manuscript. Alan B. Zonderman: data acquisition, plan of analysis, write-up of parts of the
manuscript, revision of the manuscript. CORRESPONDING AUTHOR Correspondence to May A. Beydoun. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS
APPROVAL AND CONSENT The HANDLS project was authorized by the National Institutes of Health’s Institutional Review Board, and participants gave written informed consent [25]. All methods
were performed in accordance with the relevant guidelines and regulations. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION ONLINE SUPPLEMENTARY MATERIALS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Georgescu, M.F., Beydoun, M.A., Maino Vieytes, C.A. _et al._ Longitudinal
association of homocysteine with depressive and anxiety symptoms among urban adults: healthy aging in neighborhoods of diversity across the life span study. _Transl Psychiatry_ 14, 444
(2024). https://doi.org/10.1038/s41398-024-03111-7 Download citation * Received: 27 February 2024 * Revised: 19 September 2024 * Accepted: 23 September 2024 * Published: 19 October 2024 *
DOI: https://doi.org/10.1038/s41398-024-03111-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
