The 49th annual meeting of the european society for blood and marrow transplantation: pharmacist committee – poster session (p716-p724)
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

You have full access to this article via your institution. Download PDF 23–26 April, 2023 ● Hybrid Meeting COPYRIGHT: Modified and published with permission from
https://www.ebmt.org/annual-meeting SPONSORSHIP STATEMENT: Publication of this supplement is sponsored by the European Society for Blood and Marrow Transplantation. All content was reviewed
and approved by the EBMT Committee, which held full responsibility for the abstract selections. PHARMACOLOGY POSTER SESSION 36 - PHARMACOLOGY P716 DOSING OF CONDITIONING CHEMOTHERAPY AND
GVHD PROPHYLAXIS IN OBESITY – A SURVEY OF PRACTICE SIOBHAN SMITH 1,2, NICK DUNCAN3,2, HELEN SCARFE4,2, NADJOUA MAOUCHE5,2, RAAKHEE SHAH6,2, ELIZABETH DAVIES7,2 1CARDIFF AND VALE UNIVERSITY
HEALTH BOARD, CARDIFF, UNITED KINGDOM, 2UKBMT PHARMACISTS GROUP, UK, UNITED KINGDOM, 3UNIVERSITY HOSPITALS BIRMINGHAM, BIRMINGHAM, UNITED KINGDOM, 4NOTTINGHAM UNIVERSITY HOSPITALS NHS TRUST,
NOTTINGHAM, UNITED KINGDOM, 5OXFORD UNIVERSITY HOSPITALS NHS FOUNDATION TRUST, OXFORD, UNITED KINGDOM, 6UNIVERSITY COLLEGE LONDON HOSPITAL NHS TRUST, LONDON, UNITED KINGDOM, 7MANCHESTER
UNIVERSITY NHS FOUNDATION TRUST, MANCHESTER, UNITED KINGDOM BACKGROUND: As reflected in a position statement by the American Society for Blood and Marrow Transplantation (ASBMT) (1), there
is a degree of uncertainty as to appropriate practice in relation to dose adjustments of both conditioning chemotherapy and Graft-versus-host disease (GvHD) prophylaxis in obese patients
undergoing haematopoietic stem cell transplantation. This study aimed to evaluate current practice in dosing across UK and European transplant centres. METHODS: Transplant centres were
invited to participate in the survey through the corresponding UK and European BMT pharmacist groups. The survey was completed electronically by haematology pharmacists using the
www.onlinesurveys.ac.uk platform. RESULTS: Responses were received from 30 centres representing 22 UK and 8 European centres. Of these, 28 (93%) were centres undertaking both allogeneic and
autologous transplants. Dose adjustments were made for obesity in 90% (n = 27) of centres (Table 1). Parameters used to define obesity included; BMI categorisation (74%, n = 20), percentage
above ideal body weight (56%, n = 15), BSA threshold (44%, n = 12) and weight above defined threshold (22%, n = 6). Guidelines were used to support dose adjustments in 81.5% (n = 22) of
centres including; Summary product characteristics (73%, n = 16), ASBMT recommendations (73%, n = 16), local guidelines 36%, n = 8), and clinical trial protocols (41%, n = 9). TABLE 1:
FREQUENCY OF DOSE ADJUSTMENT FOR CONDITIONING CHEMOTHERAPY AND GVHD PROPHYLAXIS Conditioning chemotherapy frequency of dose adjustment Number of centres (%) GvHD prophylaxis frequency of
dose adjustment Number of centres (%) BUSULFAN (n = 27) POST-TRANSPLANT CYCLOPHOSPHAMIDE (n = 26) Routinely 24 (88.9) Routinely 16 (61.5) Sometimes 1 (3.7) Sometimes 7 (26.9) Never 2
(7.4) Never 3 (11.5) CYCLOPHOSPHAMIDE (n = 25) ATG – RABBIT (THYMOGLOBULIN) (n = 26) Routinely 16 (64.0) Routinely 9 (34.6) Sometimes 8 (32.0) Sometimes 5 (19.2) Never 1 (4) Never 12
(46.2) THIOTEPA (n = 26) METHOTREXATE (n = 23) Routinely 17 (65.4) Routinely 7 (30.4) Sometimes 6 (23.1) Sometimes 4 (17.4) Never 3 (11.5) Never 12 (52.2) CARMUSTINE (n = 25)
MYCOPHENOLATE MOFETIL (n = 24) Routinely 15 (60.0) Routinely 7 (29.2) Sometimes 8 (32.0) Sometimes 0 Never 2 (8.0) Never 17 (70.8) MELPHALAN (n = 27) TACROLIMUS (n = 25) Routinely 13
(48.1) Routinely 3 (12.0) Sometimes 6 (22.2) Sometimes 7 (28.0) Never 8 (29.6) Never 15 (60.0) ETOPOSIDE (n = 25) CICLOSPORIN (n = 24) Routinely 12 (48.0) Routinely 2 (8.3) Sometimes 9
(36.0) Sometimes 14 (58.3) Never 4 (16.0) Never 8 (33.3) FLUDARABINE (n = 26) SIROLIMUS (n = 16) Routinely 9 (34.6) Routinely 1 (6.3) Sometimes 7 (26.9) Sometimes 2 (12.5) Never 10
(38.5) Never 13 (81.2) CYTARABINE (n = 23) ALEMTUZUMAB (n = 21) Routinely 8 (34.8) Routinely 1 (4.8) Sometimes 6 (26.1) Sometimes 1 (4.8) Never 9 (39.1) Never 19 (90.5) Data were
collected for 14 conditioning chemotherapy drugs and 8 drugs used for GvHD prophylaxis. Data for drugs most commonly dose adjusted in each category are presented in table 1. The most
commonly adjusted conditioning chemotherapy agent was busulfan with 88.9% (n = 24) of centres making routine dose adjustments. Of these, 83.3% (n = 20) dosed busulfan on an adjusted ideal
body weight. Thiotepa was also commonly dose adjusted with 65% (n = 17) making routine adjustments, of which, 76.5% (n = 13) dosed on an adjusted ideal body weight. Overall, GvHD prophylaxis
was adjusted less frequently than conditioning chemotherapy. The most commonly adjusted GvHD prophylaxis was post-transplant cyclophosphamide with 61.5% (n = 16) routinely dose adjusting.
Of these, 81.3% (n = 13) dosed on an adjusted ideal weight, most commonly in patients at either 20 or 25% above their ideal weight. Rabbit ATG (Thymoglobulin) was dose adjusted in 34.6% (n =
9) of patients with different criteria for dose adjustment applied including; ideal body weight, adjusted ideal body weight and capping at a defined maximum dose. CONCLUSIONS: Dose
adjustments to conditioning chemotherapy and GvHD prophylaxis were considered in the vast majority of centres although there was significant variation in the methods used to categorise
obesity and both the degree and frequency of dose modification. Dose adjustments were applied most commonly to conditioning chemotherapy involving busulfan, cyclophosphamide and thiotepa.
Dose adjustments to GvHD prophylaxis were less commonly observed with the exception of post-transplant cyclophosphamide where adjustments were made in more than half of patients. These
findings present an opportunity for better harmonisation of dose adjustment between transplant centres. 1.Bubalo J, et al. American Society for Blood and Marrow Transplantation Practice
Guideline Committee. Conditioning chemotherapy dose adjustment in obese patients: A review and position statement by the American Society for Blood and Marrow Transplantation practice
guideline committee.Biol Blood Marrow Transplant.2014 May;20(5):600-16.doi:10.1016/j.bbmt.2014.01.019. DISCLOSURE: Nothing to declare 36 - PHARMACOLOGY P717 ROLE OF FLUOROQUINOLONE
PROPHYLAXIS IN ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION IN REGIONS WITH A HIGH PREVALENCE OF FLUOROQUINOLONE RESISTANCE ASHWIN NAIR 1, SHAWETA KAUNDAL1, KRIPA SHANKER KAUSUSDHAN1, MADHU
CHOPRA1, ADITYA JANDIAL1, ARIHANT JAIN1, GAURAV PRAKASH1, ALKA KHADWAL1, PANKAJ MALHOTRA1, DEEPESH LAD1 1PGIMER, CHANDIGARH, INDIA BACKGROUND: The role of fluoroquinolone (FQ) prophylaxis
in preventing gram-negative bacilli (GNB) bacteremia and its impact on graft versus host disease (GVHD) and overall survival (OS) after allogeneic hematopoietic cell transplantation
(allo-HCT) is debatable, and perhaps different in the settings with a low and high prevalence of FQ resistance. In this study, we sought to answer this question in a region with high FQ
resistance. METHODS: In this single-center study, allo-HCT recipients until the year 2016 were given levofloxacin prophylaxis till the need for initiation of IV antibiotics. After the year
2016, institutional protocol was revised and fluoroquinolone prophylaxis was omitted. We retrospectively analyzed this data to compare the incidence of GNB bacteremia, duration of IV
antibiotics, hospitalization, acute GVHD, and overall survival between these two cohorts. Acute GVHD was diagnosed and graded as per the MAGIC criteria. OS was defined as the time from HCT
to death from any cause. RESULTS: A total of 135 allo-HCT recipients (43 in the FQ prophylaxis cohort and 92 in the no antibiotic prophylaxis cohort) were analyzed in this study. The two
cohorts were matched for age (median 26 years (IQR 18–36) vs. 24.5 years (IQR 17–38), _p_ = 0.8) and gender. The FQ-cohort had a lower proportion of malignant diagnoses (58% vs. 80%, _p_ =
0.01) and haploidentical transplants (14% vs. 46%, _p_ = 0.004) compared to the ‘no-antibiotic prophylaxis’ cohort. As a result, the no-antibiotic prophylaxis cohort also had a higher
proportion of recipients receiving post-transplant cyclophosphamide (PTCy) (14% vs. 46%, _p_ = 0.003). The conditioning intensity was also matched between the cohorts (Myelobaltive
conditioning 51% vs. 65%, _p_ = 0.12). The neutrophil engraftment was significantly delayed by one day in the ‘no-antibiotic prophylaxis’ cohort (13 vs. 14 days, _p_ = 0.03), probably due to
a higher proportion of haploidentical-HCT and PTCy. The incidence of GNB bacteremia was comparable between the two cohorts (37% vs. 34%, _p_ = 0.690). The median duration of parenteral
antibiotics (16 vs. 12.5, _p_ = 0.05) and hospital stay (20 vs. 22.5, _p_ = 0.2) were also similar between the two cohorts. The incidence of acute GVHD was comparable between the two cohorts
(53% vs. 53%, _p_ = 0.3). The infection (19% vs. 14%, _p_ = 0.7) or GVHD (16% vs 12%, _p_ = 0.7) attributable deaths were comparable between the two cohorts. The median follow-up of the
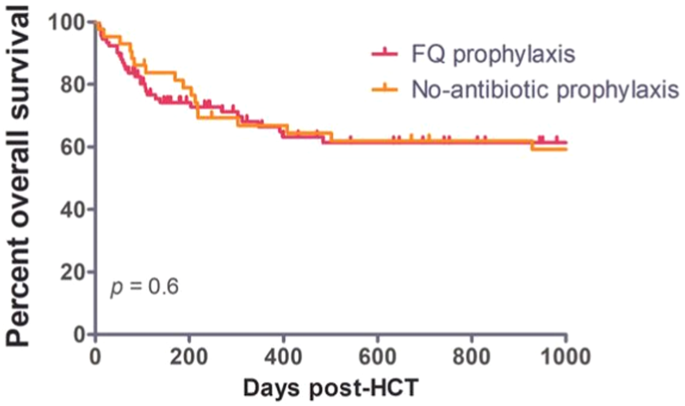
whole cohort was 354 days (107-1319). The median overall survival was not reached in both cohorts. The one-year OS was comparable in the two cohorts (66% vs. 67%, _p_ = 0.6) (Figure 1).
CONCLUSIONS: The role of levofloxacin as bacterial prophylaxis in HCT is controversial, especially in a country like ours where there is a wide prevalence of fluoroquinolone and multi-drug
resistance. With limitations of a retrospective period study, our study shows that fluoroquinolone prophylaxis did not make a difference in the incidence of GNB bacteremia, antibiotic
duration, hospitalization, acute GVHD, and overall survival outcomes post-HCT. DISCLOSURE: Nothing to declare 36 - PHARMACOLOGY P718 TARGETED VS FIXED-DOSE BUSULFAN AS PART OF REDUCED
INTENSITY CONDITIONING PRIOR TO ALLOGENEIC HAEMATOPOIETIC STEM CELL TRANSPLANTATION IN MYELOFIBROSIS CLAUDIA LANGEBRAKE 1,2, ADRIN DADKHAH1,2, DIETLINDE JANSON1, FRANCIS AYUK1, NICOLAUS M.
KRÖGER1 1UNIVERSITY MEDICAL CENTER HAMBURG-EPPENDORF, HAMBURG, GERMANY, 2UNIVERSITY MEDICAL CENTER HAMBURG-EPPENDORF, HOSPITAL PHARMACY, HAMBURG, GERMANY BACKGROUND: In allogeneic
haematopoietic stem cell transplantation (allo-HSCT), busulfan-based conditioning regimens are commonly used for adult and paediatric patients. A high inter-patient variability in drug
exposure, even after intravenous application, makes individual dose selection challenging and therefore using therapeutic drug monitoring (TDM) is advisable in order to ensure that drug
exposure is maintained within the narrow therapeutic range. However, PK data of busulfan from patients with myelofibrosis (MF) receiving reduced intensity conditioning (RIC) are sparse.
METHODS: In our institution, busulfan dosing in MF-patients was changed from fixed-dose (10 x 0.8 mg/kg dosing weight - total body weight (TBW) or in obese patients adjusted ideal body
weight (AIBW)) to targeted-dose (AUC 60 h*mg/L) with once-daily busulfan, starting with 3.2 mg/kg dosing weight and performing therapeutic drug monitoring (TDM) using a model-based precision
dosing approach after the first dose. Here we report data of the first 15 patients with targeted busulfan and compare them to patients receiving fixed-dose busulfan, in whom busulfan levels
were taken as part of a pharmacometric study. RESULTS: Altogether, 15 targeted-dose and 30 fixed-dose patients could be included. All patients received busulfan/fludarabine/ATG and
peripheral blood stem cells (7.2 vs 7.6 CD34+ cells x 106/kg), mainly from unrelated donors (12/15 vs. 28/30 patients). Patient characteristics were comparable between groups (age: 63 vs. 61
years, TBW: 78.2 vs. 73.5 kg, BMI: 26.4 vs. 24.4 kg/m2). In the targeted-dose group, initial dosing was calculated on TBW in 6/15 (40%), while in the fixed-dose group this was true for
24/30 (80%) patients. Median dose adjustment in the targeted-dose group was an increase of 15.3% (-4.3 to +91,2%), resulting in a cumulative dose of 11.0 (9,0-18.4) mg/kg dosing weight,
while fixed-dose patients received 8,0 mg/kg dosing weight (p < 0.001). The resulting median cumulative busulfan AUC were 56.3 (40.0-61.9) and 36.45 (18.7-52,6) h*mg/L, respectively (p
< 0.001). Leucocyte engraftment took place in median at d + 12 in both groups. Transient increases in bilirubin occurred in both groups with a maximum of 3.1 and 3.0 mg/dl at d + 2 and d
+ 1, respectively. Transient and mild elevations of liver transaminases were also similar in both groups. Acute GvHD occurred in 8/15 (53%) patients (I°: n = 4, II°: n = 4) vs 17/30 (57 %)
patients (I°: n = 10, II°: n = 4, III°: n = 3) with a possibly earlier, but not statistically significant, onset in the fixed-dose group (median day +28 vs 57). CONCLUSIONS: Patients with
targeted-dose busulfan received significantly higher doses and resulting cumulative AUC as compared to the fixed-dose patients. There were no differences in engraftment or toxicity,
especially the transient elevations in liver parameters were most likely be caused by the use of ATG. At the current time, with a median follow-up of 5 months (min 30 days) in the
targeted-dose group, it is too early to perform analyses regarding relapse incidence or survival. At the present time, there are indications of a less severe and later occurrence of aGvHD in
the targeted-dose group, which needs to be further analysed during longer follow-up. Furthermore, effects on chimerism and molecular markers will be evaluated. CLINICAL TRIAL REGISTRY: not
applicable DISCLOSURE: nothing to disclose 36 - PHARMACOLOGY P719 EARLY PREDICTION OF CYTOKINE RELEASE SYNDROME BY MEASURING PHOSPHATE AND MAGNESIUM LEVELS FOLLOWING CHIMERIC ANTIGEN
RECEPTOR T CELL THERAPY MASAHIRO YOSHIDA 1, SATOSHI MITSUYUKI1, YOSHINORI MATSUOKA1, NOBORU YONETANI1, JUNICHI KAWAI1, TADAKAZU KONDO1, TAKAYUKI ISHIKAWA1 1KOBE CITY MEDICAL CENTER GENERAL
HOSPITAL, KOBE, JAPAN BACKGROUND: Cytokine release syndrome (CRS) is the most common adverse event of anti-CD19 chimeric antigen receptor T (CAR-T) cell therapy. Early diagnosis and
therapeutic intervention with tocilizumab are important because untreated CRS can progress to a serious, life-threatening condition. Several factors associated with CRS have been reported,
but predictive markers for CRS onset have not yet been elucidated. Hypophosphatemia and hypomagnesemia have been reported as adverse events after CAR-T administration. However, at present,
little is known about how iP or Mg levels change throughout the clinical course of CRS. Therefore, we measured serum iP and Mg levels over time in CAR-T treated patients and analyzed whether
they are useful predictive markers of CRS onset. METHODS: A total of 14 patients who were treated at our hospital for cases of relapsed/refractory diffuse large B-cell lymphoma with CAR-T
cell therapy using Tisa-Cel were enrolled in the study, between January 2021 and September 2022. The day of Tisa-Cel administration was defined as "Day 0." Pre-treatment iP and Mg
levels were used as a baseline, and changes relative to this baseline were calculated at seven time points (days 1, 2, 3, 5, 7, 10, and 14) after Tisa-Cel administration. Then, we compared
changes in iP/Mg levels between the CRS and non-CRS groups using the Mann-Whitney U test. RESULTS: The median age of the 14 patients (6 male, 8 female) was 66 years (range: 48-72 years). CRS
was observed in nine patients (CRS grade 1, 6 patients; grade 3, 2 patients; grade 4, 1 patient), eight of whom required tocilizumab. The median time to onset of CRS was day 3 (range: 1-5)
after Tisa-Cel administration. In the CRS group, iP levels decreased on day 2 after Tisa-Cel administration, reaching the lowest levels around day 7, and recovered with remission of CRS. Mg
levels decreased just after Tisa-Cel administration, were lowest on day 3, and recovered to the pre-treatment levels by day 7. Phosphorus was not administered during this period, except in
one severely affected patient. On day 2 after Tisa-Cel administration, the change in the iP level in the CRS group was significantly lower than that in the non-CRS group (0.78 [IQR,
0.71-0.82] vs. 0.96 [IQR, 0.93-0.99] P = 0.049). Mg was not significantly different on day 2, but tended to be lower in the CRS group than in the non-CRS group (0.88 [IQR, 0.79-0.96] vs.
1.13 [IQR, 1.06-1.19] P = 0.085). CRS occurred in all cases when the relative change in the iP level on day 2 was less than 0.85, even when the iP level was within the normal range.
CONCLUSIONS: We found considerable decreases in iP and Mg levels prior to the onset of mild-to-severe CRS. Changes in iP at day 2 may be a useful predictive marker for CRS onset in patients
treated with Tisa-Cel. DISCLOSURE: Nothing to declare. 36 - PHARMACOLOGY P720 INTERIM ANALYSIS OF AN AUDIT ON VARIANCE IN TARGET CICLOSPORIN CONCENTRATION FOLLOWING LOADING DOSE IN
ALLOGENEIC STEM CELL TRANSPLANT RECIPIENTS DOUGLAS SEMPLE 1 1QUEEN ELIZABETH UNIVERSITY HOSPITAL, GLASGOW, UNITED KINGDOM BACKGROUND: Ciclosporin is a calcineurin inhibitor which
downregulates the expression of Interleukin 2, reversibly inhibiting the growth and differentiation of T cells involved in the adaptive immune response. Ciclosporin is used in the post
allogeneic stem cell transplant setting as prophylaxis against graft versus host disease (GVHD) and it is essential that patients reach therapeutic levels quickly following commencement of
therapy. We audited how many patients became therapeutic following their initial ciclosporin loading dose - our standard is that 100% of patients should be therapeutic after a loading dose.
For those who measured sub-therapeutic levels, we will try to elucidate any common or patient factors which may have contributed to this. To ensure a balance between GVHD risk and the risk
of infection and relapse, serum concentrations of ciclosporin are monitored three times weekly in all inpatients. Within our unit, the agreed therapeutic range for ciclosporin concentrations
is 150-200 micrograms/L. In non-haploidentical allogeneic patients, dosing commences on day minus 1 at 2.5mg/kg TWICE daily as a loading dose followed by 1.5mg/kg TWICE daily thereafter
starting on day 0. In haploidentical transplants, ciclosporin dosing starts on day 5 with the same dosing schedule. METHODS: We audited 30 random allogeneic transplant recipients, both
retrospectively and prospectively. The first level post loading dose was recorded, along with the following two levels. The variance was noted and the intervention to correct this was
recorded. The data was collected both retrospectively and prospectively (data collection ongoing: so far 20/30 patients have been captured). Concurrent medicines and renal function was noted
throughout. RESULTS: At interim analysis (20 patients), 30% of patients had a therapeutic first serum level. None of these patients had low levels over the next two assays. 70% (14
patients) had a sub-therapeutic first level. The average deficit was -54.5 micrograms/L from the above concentration range. Three of these patients achieved an acceptable second and third
level, one was reloaded at 2.5mg/kg BD for one day (61% dose increase), one had a 10% dose increase and one had a 7% dose increase. At interim analysis, 10 of the remaining 11 patients (one
had a level outstanding) were sub-therapeutic at second serum level with an average dose increase of 21%. The average deficit at second level in those 10 patients was -43.5 micrograms/L. The
average dose increase at second level was 10.8% with 7 patients (70%) achieving a therapeutic third level. Three patients (15% of the total cohort at interim analysis) failed to achieve a
therapeutic ciclosporin concentration after three serum assays. CONCLUSIONS: Data from interim analysis perhaps shows a need to adjust the dosing guidelines of ciclosporin in our transplant
recipients. I plan to collect data on another 10 patients prior to final analysis. We will also look at confounding factors such as drug interactions and renal function however in the twenty
patients analysed so far, I have not found evidence of confounding factors which would lead to the results shown. We aim to review the data collected and change our local ciclosporin dosing
guideline to improve practice. DISCLOSURE: Nothing to declare 36 - PHARMACOLOGY P721 PENICILLIN ALLERGY DE-LABELLING IN THE SCOTTISH NATIONAL ADULT ALLOGENEIC STEM CELL TRANSPLANT UNIT
FRANCESCA MCARTHUR 1, SINÉAD CONNOLLY1 1NHS GLASGOW AND GREATER CLYDE, GLASGOW, UNITED KINGDOM BACKGROUND: Unverified penicillin allergies can lead to a number of clinical complications
including sub-optimal anti-microbial cover, increased risk of infections such as _Clostridium difficile_, antimicrobial resistance, and increased costs to healthcare institutions. One study
suggests that a true penicillin allergy is confirmed in less than 10% of patients; the Scottish Antimicrobial Prescribing Group (SAPG) reports that penicillin allergies are recorded in up to
10% of all patients1, although many cannot recall what the reaction was. METHODS: Using the SAPG Penicillin Allergy Assessment Tool2, patients admitted to the Scottish National Adult
Allogeneic Stem Cell Transplant Unit with a reported penicillin allergy were assessed for suitability for penicillin re-challenge. Only patients stratified as low risk were re-challenged and
all were given a single dose of amoxicillin. Their observations were monitored regularly by nursing staff after the dose. Patient’s reactions or lack thereof were then interpreted, and if
patients were found not to have a true penicillin allergy this was communicated to the patient and any penicillin allergy notes were removed from hospital records. This information was also
communicated to the patient’s GP in their discharge letter, where the GP was also asked to remove the allergy status from the patient’s records. RESULTS: Between July 2021 and October 2022
ten patients between the ages of 19 and 77 were selected and underwent a penicillin allergy re-challenge. Of these ten patients none had a reaction to the dose and therefore had their
allergy de-labelled. All of these patients met the low risk criteria where the type of reaction was unknown and/or the allergy label was added over ten years ago. CONCLUSIONS: We expect to
increase our patient numbers to present for EBMT but current data suggest that this will produce encouraging results. Although this is a small patient group, this work has clearly shown the
benefit of re-challenging penicillin allergies in patients who are at low risk of a repeat reaction. In all cases we were able to remove the patient’s allergy status meaning that they were
able to receive standard of care antibiotics. These antibiotics have been chosen by the local antimicrobial team based on local sensitivities to stop the spread of drug-resistant pathogens
and decrease the risk of secondary infections such as _Clostridium difficile_, as well as reduce antimicrobial spending by the health board. It was found that although all patient’s GPs were
alerted to this change in allergy status, 33% did not change the allergy status on their own system, suggesting that further communication with primary care about this protocol may be
needed. References: 1 – SAPG Procotol for Implementation of Penicillin Allergy De-labelling. Visited 30/11/22
https://www.sapg.scot/media/5586/protocol-for-implementing-pencillin-allergy-delabelling-process.pdf 2 – SAPG Penicillin Allergy Risk Algorithm. Visited 30/11/22
https://www.sapg.scot/media/5585/penicillin-allergy-risk-algorithm.pdf DISCLOSURE: Nothing to declare 36 - PHARMACOLOGY P722 PRIMIDONE IN ALLOGENEIC HAEMATOPOIETIC STEM CELL TRANSPLANTATION
– A CASE REPORT WITH FOCUS ON DRUG-DRUG INTERACTIONS WITH TACROLIMUS CLAUDIA LANGEBRAKE 1,2, ADRIN DADKHAH1,2, DIETLINDE JANSON1, FRANCIS AYUK1, NICOLAUS M. KRÖGER1 1UNIVERSITY MEDICAL
CENTER HAMBURG-EPPENDORF, HAMBURG, GERMANY, 2UNIVERSITY MEDICAL CENTER HAMBURG-EPPENDORF, HOSPITAL PHARMACY, HAMBURG, GERMANY BACKGROUND: Primidone and its active metabolite phenobarbital
are potent inducers of cytochrome P450 and P-glycoprotein (PgP). Many drugs (e.g. calcineurin inhibitors (CNI), cytostatics) are substrates of these enzymes and transporters, therefore
clinically relevant drug-drug interactions need to be considered. METHODS: We report on a 63-year old female patient (total body weight (TBW): 121kg, length: 165cm, ideal body weight (IBW):
57kg, BMI: 44kg/m2) receiving an allogeneic haematopoietic stem cell transplantation (allo-HSCT) from a matched related donor because of a chronic myelomonocytic leukaemia transformed to a
secondary acute myeloid leukaemia with blast persistence. She was diagnosed with epilepsy since she was seven years old and she has received valproate and primidone for many years, during
which she had no or very few seizures. RESULTS: Although a change of the antiepileptic treatment regimen was desirable regarding the expected drug-drug interactions and toxicities, primidone
was maintained under blood level control due to the short time until allo-HSCT and the high risk of withdrawal convulsions. Valproate was successfully tapered over three weeks starting at
day -11. In parallel, levetiracetam was started and gradually increased to the target dose of 2x1000mg orally. For salvage and conditioning therapy, cytostatic drugs that are not supposed to
be affected by CYP, UGT and PgP were chosen: azacitidine/cytarabine and treosulfan/fludarabine/ATG. Since it was to be expected that sufficient blood levels could not be achieved with CNI,
we decided to additionally apply methotrexate (MTX) and mycophenolate mofetil (MMF) and to start with tacrolimus (0.03mg/kg TBW/d as continuous infusion [0,064mg/kg IBW/d]) earlier than
usual at day -4. Tacrolimus level at day -3 was 7,5µg/L and remained relatively stable with a median of 9.4µg/L at median doses of 0.03mg/kg TBW and 0.06mg/kg IBW, respectively. Therefore,
we decided to stop MTX after two doses and MMF at day +6. Leucocyte engraftment took place at day +13 and tacrolimus was switched to oral (extended release capsules) at a conversion rate of
1:2, starting with 2x3mg. Tacrolimus levels fell constantly to below the detection limit within three days despite steady dose increases to 2x8mg, therefore MMF was restarted. Only when
tacrolimus was further increased to 2x10mg (0.17mg/kg TBW, 0.35mg/kg IBW) target tacrolimus levels (6-7µg/L) were reached, so that MMF could be stopped again. At day +36 she was discharged
and tacrolimus levels remained stable during the following two weeks of current follow-up. Phenobarbital levels were in the lower therapeutic range on admission and remained almost unchanged
during the course of the inpatient stay and the patient did not show any signs of seizures. CONCLUSIONS: It is possible to maintain primidone during allo-HSCT as long as interacting drugs
are avoided or close blood level monitoring is performed, especially when changing the mode of administration. Tacrolimus is a substrate of CYP3A4 and PgP, and therefore the high oral doses
with co-medication with primodone are plausible and in line with some case reports. As the necessary IV tacrolimus-doses were not unusually high, the influence on intestinal
absorption/metabolism by primidone seems to play a greater role than the induction of the hepatic metabolising enzymes. CLINICAL TRIAL REGISTRY: not applicable DISCLOSURE: no conflicts of
interest 36 - PHARMACOLOGY P723 IMPACT OF HYDROXYUREA ON SOCIAL HEALTH PROBLEMS OF BETA THALASSEMIA PATIENTS WITH A MODERATING ROLE OF HEALTHCARE PROFESSIONALS’ PERFORMANCE AFFAF SHEIKH 1,2,
MUNAZA BIBI1, SAIMA SIDDIQUI2, KOUSAR PERVEEN2, TAHIR SHAMSI2 1BAHRIA UNIVERSITY KARACHI CAMPUS, KARACHI, PAKISTAN, 2NATIONAL INSTITUTE OF BLOOD DISEASES AND BONE MARROW TRANSPLANTATION,
KARACHI, PAKISTAN BACKGROUND: In hemoglobinopathies β-thalassemia (BT) is the commonest monogenic disorder around the globe. Transfusion - dependent thalassemia (TDT) [imbalance ratio of
β-globin chains] patients have low health related quality of life (HR-QoL) as compared to healthy individuals. The objective of the study, along with treatment of BT patients, was to improve
the life expectancy and social health problems (family and school-functioning) with involvement of healthcare professionals. METHODS: In this research, a deductive approach with explanatory
nature was adopted, based on primary quantitative data. The data was collected through the TranQoL questionnaire using Likert scale, with the range of 1-5 and 1-7. The cross-sectional,
non-probability, purposive judgment sampling was conducted after getting approval from Institutional Review Board (IRB-NIBD) of National Institute of Blood Diseases and Bone Marrow
Transplantation (NIBD & BMT) Hospital, Gulshan campus. WHO sample size calculator 2.0 was used to estimate, a sample of 290 TDT patients who visited an out- patient and daycare
department of the hospital. To analyze the data, regression and correlation tests were applied using IBM SPSS (Statistical Package for Social Sciences) Statistics 23.0 and moderation was
done by Process Hayes model 3.5.2. RESULTS: After taking Hydroxyurea, responders were classified into three categories: complete responders (15.9%) who were independent of blood transfusion
dependency; partial responders (27.6%) who remained on transfusion but with increased interval; and non-responders (56.6%) who remained on the same frequency of blood transfusion, after six
months of treatment. After data analysis, it was revealed that there was no significant impact of Hydroxyurea therapy in improving social health which includes family interaction and
school-functioning of the respondents (r = 0.081, p = 0.171). Similarly, a weak positive non-significant correlation exists between Hydroxyurea therapy and the performance of healthcare
professionals in the hospital (r = 0.016; p = 0.782). Moreover, no significant improvement was observed in social health problems of BT patients by inducing moderating the role of healthcare
professional performance (p = 0.0807 and 0.3367, respectively) as shown in Table 1. Model Summary Model R R – Square MSE F df1 df2 p 1 0.1525 0.0232 1.9985 2.2688 3.0000 286.0000 0.0807
MODEL COEFF SE T P LLCI ULCI Constant 1.5000 1.0260 1.4620 0.1448 -0.5194 3.5195 Hydro -0.0861 0.2507 -0.3436 0.7314 -0.5795 0.4073 HEPH 0.1277 0.0688 1.8570 0.0643 -0.0077 0.2631 Int_1
-0.0097 0.0101 -0.9623 0.3367 -0.0295 0.0101 Product term keys: Int_1 : Hydr × HEPH Test(s) of highest order unconditional interaction(s) R2-CHNG F DF1 DF2 P X*W 0.0032 0.9261 1.0000
286.0000 0.3367 MODEL : 1 Y: SocHea X: Hydr W: HEPH TABLE 1: MODERATION ANALYSIS BY PROCESS HAYES MODEL 3.5.2 Coeff -Coefficient, SE-Standard error, LLCI-Lower level confidence interval,
ULCI-Upper-level confidence interval, HEPH- Hydroxyurea Effect on Social Health. CONCLUSIONS: It is concluded that social health problems of BT patients are not dependent on Hydroxyurea
therapy to have better quality of life. With the introduction of healthcare professionals’ performance as a moderator it does not improve the link between Hydroxyurea and social domain of
QoL. DISCLOSURE: Nothing to declare 36 - PHARMACOLOGY P724 CAR-T CELLS THERAPY: HIGH PAYBACK, HIGH RISK? SAFETY PROFILE IN SINGLE CENTER EXPERIENCE VERA PIRES1, MARIA TEIXEIRA1, VERA
DOMINGOS 1, RUTE VARELA1 1IPO, LISBON, PORTUGAL BACKGROUND: Genetically modified chimeric antigen receptor CAR-T cell targeting CD19 induce high rates of remission in the treatment of highly
refractory and relapsing hematological malignancies.[1] Yescarta® (Y), Kymriah® (K) e Tecartus® (T) have been approved by EMA in 2017, 2018 and 2020 respectively. Since then, we have been
using these three therapies in our hospital, however, this therapy can lead to serious and potentially fatal complications. Cytokine release syndrome (CRS) and immune effector
cell-associated neurotoxicity syndrome (ICANS) are the most common and feared toxicities.[2] Severe CRS may be treated with tocilizumab and corticosteroids. [1] The aim of this study is to
describe the incidence and management of toxicities in patients who underwent car-t cells therapy in our hospital. METHODS: This retrospective database review included all adult patients who
undergo CAR-T cell infusion between January 2020 and October of 2022 and follow them till day +30. We reviewed the clinical files and retrieved the following information: age, sex,
diagnosis, prophylaxis, number of previous lines of treatment and adverse effects (AE). The management of the AEs was conducted according to the protocol of the Institution’s CAR-T team and
were scored as in EBMT/EA CAR-T Cell Handbook. All underwent prophylaxis with acyclovir, posaconazole, levetiracetam and antibiotics (cotrimoxazole or ciprofloxacin). RESULTS: Of the 17 who
infused the CAR-T cells (2 K, 1 T and 14 Y), 64,7% were men and the median age was 60 years (36-73). The most common diagnoses were: DLBCL 82.4% (14), HGBCL 11.8 % (2), MCL 5.9% (1) and 3
(2-5) was the median previews lines of treatment. There was no record of infusion reactions and the median hospitalization time was 22 (11-32) days (K:15, T:22 and Y:22 days). 76.5% (13)
experienced CRS any grade and the most frequent symptom was fever. 29.4% (5) Grade 1, 29.4% (5) grade 2, 17.6% (3) grade 3 and 0 patients had grade 4. Time for the begging of symptoms were 5
days (1-8) (median). Patients who had CRS 84.6% (n = 11/13) required tocilizumab, dose of 8 mg/kg and the median of doses administered was 1 (1–3). Regarding to ICANS 28.6% (4/17) had any
grade of neurotoxicity, all with Y; n = 1 grade 1 and grade 2 and n = 2 grade 3. Time for the begging of symptoms were 7 (4-13) days (median). 30.8% (4/13) of patients who had CLS also had
neurological toxicity. We recorded n = 1 treatment-related death in the context of an infection. CONCLUSIONS: CAR-T cell therapy is a revolutionary new pillar in cancer treatment. However,
the benefit of this treatment is accompanied by potentially significant toxicity, that we are still learning to manage. Although we still have few patients infused (we stopped the program
for 6 months during the pandemic) we think this study is important because allow us to understand how our patients behave with this new therapy and learn as a multidisciplinary team to
improve the efficacy and toxicity outcomes. DISCLOSURE: No conflict of interest CHANGE HISTORY * _ 16 JULY 2024 A Correction to this paper has been published:
https://doi.org/10.1038/s41409-024-02372-6 _ ADDITIONAL INFORMATION The original online version of this article was revised: The Results section of abstract P723 has been corrected. RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE The 49th Annual Meeting of the European Society for Blood and Marrow Transplantation: Pharmacist Committee –
Poster Session (P716-P724). _Bone Marrow Transplant_ 58 (Suppl 1), 668–673 (2023). https://doi.org/10.1038/s41409-023-02061-w Download citation * Published: 09 November 2023 * Issue Date:
November 2023 * DOI: https://doi.org/10.1038/s41409-023-02061-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
