Melanoma recurrence patterns and management after adjuvant targeted therapy: a multicentre analysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Adjuvant targeted therapy (TT) improves relapse free survival in patients with resected BRAF mutant stage III melanoma. The outcomes and optimal management of patients who relapse after
adjuvant TT is unknown.
Patients from twenty-one centres with recurrent melanoma after adjuvant TT were included. Disease characteristics, adjuvant therapy, recurrence, treatment at relapse and outcomes were
examined.
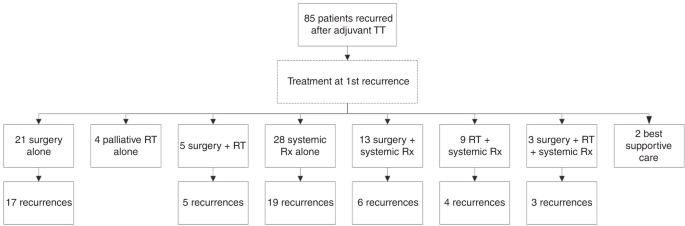
Eighty-five patients developed recurrent melanoma; nineteen (22%) during adjuvant TT. Median time to first recurrence was 18 months and median follow-up from first recurrence was 31 months.
Fifty-eight (68%) patients received immunotherapy (IT) or TT as 1st line systemic therapy at either first or subsequent recurrence and had disease that was assessable for response. Response
to anti-PD-1 (±trial agent), combination ipilimumab-nivolumab, TT rechallenge and ipilimumab monotherapy was 63%, 62% 25% and 10% respectively. Twenty-eight (33%) patients had died at
census, all from melanoma. Two-year OS was 84% for anti-PD-1 therapy (±trial agent), 92% for combination ipilimumab and nivolumab, 49% for TT and 45% for ipilimumab monotherapy (p = 0.028).
Patients who relapse after adjuvant TT respond well to subsequent anti-PD-1 based therapy and have outcomes similar to those seen when first line anti-PD-1 therapy is used in stage IV
melanoma.
The management of cutaneous melanoma has been revolutionised in the last decade. Targeted therapies (TT) inhibiting the MAPK pathway and immunotherapy (IT) with T-cell checkpoint inhibitors
have each been demonstrated to prolong survival of patients with metastatic melanoma.1,2,3,4,5 As a result, these therapies are now mainstays of treatment for patients with unresectable
stage III or stage IV disease.
Recent studies have tested adjuvant systemic TT and IT for resected stage III/IV melanoma, with the aims of reducing melanoma recurrence and prolonging patient survival.6,7 Whilst TT is
reserved for patients with BRAF V600 mutant melanoma, IT may be used in patients irrespective of their BRAF status.
A number of randomised, Phase 3 trials have investigated IT as adjuvant treatment in melanoma. The EORTC-18071 trial compared adjuvant ipilimumab 10 mg/kg to placebo in patients with
resected stage III disease and demonstrated an improvement in both relapse free survival (RFS, hazard ratio (HR) 0.76, p = 0.0008) and overall survival (OS) (HR 0.72, p = 0.001). However,
toxicity rates were high, with 45% of patients having grade 3–4 adverse events (AEs), resulting in a third of patients discontinuing treatment.8,9,10 The Phase 3 E1609 trial examined the use
of adjuvant ipilimumab at two doses (3 mg/kg and 10 mg/kg) and interferon alfa-2b. The lower ipilimumab dose improved OS compared to interferon, however, rates of grade 3–4 AEs were not
negligible, and therefore single agent ipilimumab is rarely used in clinical practice.11
The Checkmate-238 trial randomised patients with resected stage IIIB/C or IV disease to nivolumab 3 mg/kg or ipilimumab 10 mg/kg for 1 year, finding an improvement in RFS (HR 0.66, p
