Genetic trajectory and clonal evolution of multiple primary lung cancer with lymph node metastasis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Multiple primary lung cancer (MPLC) with lymph node metastasis (LNM) is a rare phenomenon of multifocal lung cancer. The genomic landscapes of MPLC and the clonal evolution pattern
between primary lung lesions and lymph node metastasis haven’t been fully illustrated. We performed whole-exome sequencing (WES) on 52 FFPE (Formalin-fixed Paraffin-Embedded) samples from 11
patients diagnosed with MPLC with LNM. Genomic profiling and phylogenetic analysis were conducted to infer the evolutional trajectory within each patient. The top 5 most frequently mutated
genes in our study were TTN (76.74%), MUC16 (62.79%), MUC19 (55.81%), FRG1 (46.51%), and NBPF20 (46.51%). For most patients in our study, a substantial of genetic alterations were mutually
exclusive among the multiple pulmonary tumors of the same patient, suggesting their heterogenous origins. Individually, the genetic profile of lymph node metastatic lesions overlapped with
that of multiple lung cancers in different degrees but are more genetically related to specific pulmonary lesions. SETD2 was a potential metastasis biomarker of MPLC. The mean putative
neo-antigen number of the primary tumor (646.5) is higher than that of lymph node metastases (300, _p_ = 0.2416). Primary lung tumors and lymph node metastases are highly heterogenous in
immune repertoires. Our findings portrayed the comprehensive genomic landscape of MPLC with LNM. We characterized the genomic heterogeneity among different tumors. We offered novel clues to
the clonal evolution between MPLC and their lymphatic metastases, thus advancing the treatment strategies and preventions of MPLC with LNM. SIMILAR CONTENT BEING VIEWED BY OTHERS
EVOLUTIONARY CHARACTERIZATION OF LUNG ADENOCARCINOMA MORPHOLOGY IN TRACERX Article 12 April 2023 GENOMIC CHARACTERISTICS OF INVASIVE MUCINOUS ADENOCARCINOMA OF THE LUNG WITH MULTIPLE
PULMONARY SITES OF INVOLVEMENT Article Open access 21 July 2021 OPTIMIZING THE NGS-BASED DISCRIMINATION OF MULTIPLE LUNG CANCERS FROM THE PERSPECTIVE OF EVOLUTION Article Open access 14
January 2025 INTRODUCTION According to the newest data published in 2022 [1], the lung cancer death rate is 21% worldwide, ranking the first in both genders among all cancer types. Over 350
people die of lung cancer every day. Lung cancer burdens public healthcare annually [2, 3]. In recent decades, multifocal lung cancer has been an increasingly common scenario in clinical
practice [4, 5]. It is reported that up to 15% of lung cancer patients developed two or more lesions [6], among which multiple primary lung cancer (MPLC) is very common [7]. MPLC indicates
patients who developed two or more pulmonary tumors that were originally independent, and the multiple pulmonary lesions can be synchronous or metachronous [8, 9]. In 2016, the International
Association for the Study of Lung Cancer (IALSC) classified multifocal lung cancer into four categories: (1) Second primary cancer, (2) Separate Tumor Nodules (Intrapulmonary metastasis,
IPM), (3) Multifocal Lung Adenocarcinoma with Ground Glass/Lepidic (GG/L) Features and (4) Diffuse Pneumonic Type [10], among which second primary cancer and multifocal GG/L are commonly
acknowledged as MPLC by clinicians. So far, there are no guidelines or widely accepted criteria in the realm of MPC. Identifying the “dominant” lung lesions with the most malignant nature is
crucial for MPLC patients, many of whom are not qualified for radical surgical resection because of operational contraindication, such as advanced age or prolonged anesthesia risk.
Pre-locating the critical tumor before surgery can provide those patients with more treatment options. From the view of evolution, MPLC with lymph node metastasis (LNM) is an ideal material
for studying the genetic trajectory of MPLC, thus identifying the “dominant” lung lesions. However, there are several barriers to the study of MPLC with LNM. Firstly, precise discrimination
between MPLC and lung cancer intrapulmonary metastasis (IPM) has been a clinical dilemma for decades [6, 11]. Both two diseases behave as multiple tumor sites in the lung, but they are of
distinct staging strategies [8], treatment [9,10,11], and prognosis [8, 12, 13]. Secondly, MPLC with LNM is very rare in clinical practice. Thirdly, the difficulties in sample collection of
MPLC with LNM hamper its studies. What we know about MPLC with LNM is far from enough, though some researchers have paid attention to it. In 2016, Gao et al. focused on an MPLC patient with
one lymph node metastasis and applied targeted panel sequencing on him [12]. The lymph node metastasis shared 52 common mutations with one of the pulmonary tumors but had no overlap with
other lung lesions within the individual. A similar conclusion was observed in Omada et al.’s research in 2020 [13], in which five MPLC with LNM patients were included. Our study performed
WES on tumor samples from 11 patients diagnosed as MPLC with LNM. We are the first to characterize the clonal evolution pattern of MPLC with lymph node metastasis using comprehensive genetic
sequencing. Our findings may help grasp the mysterious nature of MPLC and identify the tumor lesion most likely to metastasize before surgery, thus spurring the introduction of MPLC
guidelines. We present the following article by the MDAR reporting checklist. METHODS AND MATERIALS STUDY DESIGN AND PATIENTS According to the 8th edition of the TNM staging system published
in 2017, a total of 1458 patients during 2011 and 2019 who were diagnosed with MPLC receiving no adjuvant treatment before surgery were identified in Cancer Hospital, the Chinese Academy of
Medical Sciences, and Peking Union Medical College. Under the following criteria: (1). Positive lymph node metastasis; (2). Complete access to FFPE specimens of pulmonary tumors, lymph node
metastasis tumors, and matched normal samples, 11 patients were finally included in our study. Two independent certified pathologists confirmed the diagnosis by HE staining and provided the
pathological details of each patient (Table 1). The clinical information was retrieved from the medical record system of our hospital (Table 2). CT (Computerized Tomography) images were
obtained from the department of radiology of our hospital, and two experienced radiological specialists annotated the pulmonary tumor lesions independently. The ethics committee of Cancer
Hospital, Chinese Academy of Medical Science approved the study, and written informed consent was obtained from each involved patient. The analysis was performed in accordance with the local
ethical regulations and the guidelines of the Declaration of Helsinki. WES SEQUENCING We extracted total DNA from archived FFPE samples using the QIAamp DNA FFPE Tissue Kit (Qiagen, cat.
no. 56404). To enhance the tumor purity, two independent pulmonary pathologists marked tumor areas on FFPE slides, and the non-tumor components were scraped off before DNA extraction. DNA
from paired normal lung tissue samples was used to eliminate the influence of germline mutation. Isolated genomic DNA quality was verified using three combined methods: (1) DNA degradation,
and contamination was monitored on 1% agarose gels. (2) DNA purity was checked using the NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). (3) DNA concentration was measured by Qubit® DNA
Assay Kit in Qubit® 2.0 Fluorometer (Invitrogen, USA). WES libraries were prepared using Agilent SureSelect Human All Exon V6 kit (Agilent Technologies, CA, USA). Following the
manufacturer’s recommendations, index codes were added to each sample, and the clustering of the index-coded samples was performed on a cBot Cluster Generation System using Hiseq PE Cluster
Kit (Illumina). DNA libraries were sequenced on the Illumina Hiseq platform with a paired-end 2 × 150 protocol. The WES was conducted at CapitalBio Technology Inc. Beijing, China, from
January 2020.01 to October 2020. DATA PROCESSING AND BIOINFORMATICS SEQUENCE ALIGNMENT, AND VARIANT CALLING We trimmed and filtered raw data using Trimmomatic 0.33 [14]. Paired-end clean
reads were aligned to the human reference sequence hg19 using the BWA-MEM algorithm (BWA version 0.7.10-r789) with default parameters [15]. To guarantee meaningful downstream analysis,
duplicated sequencing reads were excluded by Picard v.2.13. In contrast, low-confidence reads (reads containing adapter contamination, low-quality nucleotides, and unrecognizable nucleotide
(N)) were removed by the criteria of TLOD < 10. All high-confident mutations were then annotated into the MAF format using the tool vcf2maf. We called somatic single-nucleotide variants
(SNVs) and small indels using MuTect (version 3.1-0-g72492bb) and Strelka (version 1.0.14) [16, 17]. All mutations in coding regions were manually checked using the Integrative Genomics
Viewer (version2.3.34) [18]. DEFINITIONS OF PUTATIVE DRIVER MUTATIONS We compared all non-silent mutations with lists of lung cancer/pan-cancer potential driver genes in the COSMIC cancer
gene census (September 2021). We identified the driver mutations in our data if they are in the lists. MUTATION SPECTRA AND SIGNATURE ANALYSES We extracted each mutation’s 5′ and 3′ sequence
context from the hg19 reference genome (BSgenome.Hsapiens.UCSC.hg19). We categorized the SNVs into C > A, C > G, C > T, T > A, T > C, and T > G bins according to the type
of substitution and then subcategorized them into 96 sub-bins according to the nucleotides preceding (5′) and succeeding (3′) the mutated base. The deconstructSigs package (v1.8.0) was used
to infer the contributions of 30 published signatures from the Catalog of Somatic Mutations in Cancer (COSMIC) (https://cancer.sanger.ac.uk/cosmic/signatures_v2) in each sample [19]. The
contribution of each signature for each tumor was statistically quantified. PHYLOGENETIC TREE CONSTRUCTION AND LABELING All nonsynonymous somatic mutations were considered for determining
phylogenetic trees. We built trees using binary presence/absence matrices generated from the distribution of variants within different tumors within each patient. R Bioconductor package
phangorn [20] was used to perform the parsimony ratchet method, generating unrooted trees. Branch and trunk lengths were proportional to the number of nonsynonymous mutations. To label the
clonal phylogenetic trees for each patient, we defined 5 categories of mutations according to the driver genes lists in the COSMIC cancer gene census (September 2021): (1) Tier 1 NSCLC/lung
cancer driver genes. (2) Tier 2 NSCLC/lung cancer driver genes. (3) Tier 1 pan-cancer driver genes. (4) Tier 2 pan-cancer driver genes. (5) Genes not on the list. UNSUPERVISED CLUSTERING The
hclust function (the agglomeration method is “ward.D2”) in R software (Version 4.0.2) was utilized to perform unsupervised clustering. According to the driver gene list from the Catalog of
Somatic Mutations in Cancer (COSMIC) (https://cancer.sanger.ac.uk/cosmic/signatures_v2), a totally of 40 genes were utilized, including 20 genes with the highest mutation frequencies in our
data, 10 pan-cancer driver genes with top mutation frequencies except for the top 20 genes, and 10 lung cancer driver genes with top mutation frequencies except for the top 20 genes.
FUNCTIONAL ENRICHMENT ANALYSIS We applied the 468 genes from the MSK-IMPACT assay to our cohort and conducted GO functional enrichment analysis, and showed the essential genes. VENN DIAGRAM
AND UPSET GRAPH Venn diagrams and Upset charts were used to illustrate the mutational overlaps among multiple samples within individuals. For patients with no more than 5 tumor samples, we
drew the Venn diagrams using Omicshare (an online tool, https://www.omicshare.com/tools/Home/Soft/venn). For Patient 1 and Patient 11, each of whom has 6 tumor samples, the R software
package UpSetR was applied to draw Upset graphs. PUTATIVE NEOANTIGENS IDENTIFICATION AND BINDING AFFINITY PREDICTION Polysolver algorithm [21] was applied to conduct HLA typing. Non-silent
mutations were used to generate a list of mutant peptides of approximately 9–11 amino acids in length, with the mutated residues represented in each position. NetMHCpan (v3.0) was used to
predict the binding affinity of each mutant peptide and its corresponding wild-type peptide to the patient’s germline HLA alleles [22]. Candidate neoantigens were identified with a predicted
mutant peptide binding affinity of <500 nmol/L and rank<2. STATISTICAL ANALYSIS Statistical analysis was performed using Fisher’s exact test for categorical variables and the Wilcoxon
or Mann–Whitney U test for continuous variables. We estimated the mutual exclusivity of mutations by Monte Carlo simulation. We calculated the relevance between constant variables using
Pearson’s correlation or Spearman’s correlation. Kaplan–Meier curves and the log-rank tests were used for the survival analysis. Statistical tests were performed in R (v3.2.0) and GraphPad
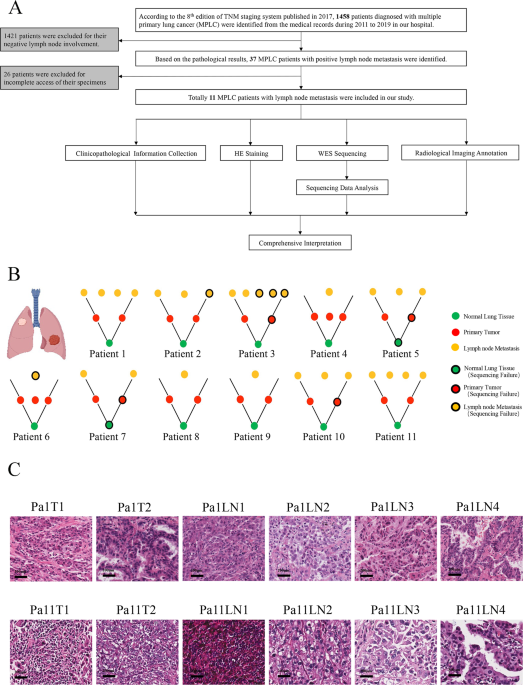
(v8). We regarded a two-tailed _P_ value < 0.05 as statistically significant. RESULTS PATIENT COHORT The workflow was presented in Fig. 1A. Initially, 1458 patients were selected, based
on whom 1421 patients were excluded for they lacked lymph node metastasis, and 26 patients were excluded for their FFPE samples were not available. Finally,11 MPLC with LNM patients were
recruited. We explored these patients from clinicopathological information, hematoxylin-eosin (HE) staining, WES sequencing, and radiological images. The ideograph of all 11 patients was
displayed in Fig. 1B. Representative HE images of Patients 1 and 11 were shown in Fig. 1C (Supplementary Fig 1). CLINICOPATHOLOGICAL AND DEMOGRAPHIC INFORMATION The clinicopathological
details and WES depth were in Table 1. We defined the pathological subtype with the highest proportion as the major pathological component. In 72.73% (8/11) patients, the multiple lung
tumors were of heterogenous major pathological components, while in Patient1, 5, and 11, all their pulmonary tumors were solid subtypes. In 81.2% (9/11) patients, we captured pathological
consistency between primary tumors and metastases, but in Patient7 and 10, their lymph node metastasis sites were heterogenous to primary tumors pathologically. Stage annotations were based
on the proposed TNM classification criteria of lung cancers with multiple pulmonary sites by IASLC in 2016 [6]. Most lung tumors were staged as T1 and T2, and most lymph node metastatic
lesions were staged as N1 or N2. The average sequencing depth of tumor and normal samples were 275x (range 69x-435x) and 332x (range 63x-487x), respectively. Table 2 exhibits demographic
information. According to the proposed differentiation criteria of lung cancer with multiple pulmonary sites of involvement by IASCL in 2016 [10], all patients in our study were MPLC. The
average age of our cohort was 59.6 years old (range 42-76). 27.27% (3/11) patients had a smoking history, and 27.27% (3/11) patients were alcohol users. Over one-third of patients (36.36%,
4/11) had a positive family history, especially Patient 9, who had a familial history of both lung and prostate cancer. All patients were synchronous MPLC, except Patient6 (metachronous
MPLC). The post-surgery recurrence/metastasis rate was 55.56% (5/9) patients. The average follow-up time of all patients is 61.1 months. In this study, both the primary lung/pulmonary tumor
samples and the lymph node metastases tumor samples of each patient are identified as the tumor samples of this patient. We emphasize the different locations of tumor samples when the
analysis requires us to separate the tumor samples into primary lung/pulmonary tumor samples and lymph node metastases tumor samples, during which the location-based integration logic is
identical for all patients. GENOMIC ALTERATIONS We successfully conducted WES on 52 DNA samples from 11 patients to assess genomic alterations globally. Sequencing quality information of all
the samples was in Supplementary Data 1, and the list of somatic nonsynonymous mutations was in Supplementary Data 2. The alteration spectrum is Fig. 2A (Supplementary Data 3). According to
the driver gene list from COSMIC Cancer Gene Census (Sep. 2021, https://cancer.sanger.ac.uk/census, Supplementary Data 4), we marked the pan-cancer driver genes (light brown) and lung
cancer/NSCLC driver genes (green). The mean tumor mutation burden (TMB) for pulmonary tumors and lymph node metastases was 12.78 and 8.94 mutations per megabase, respectively. Among the top
20 genes with the highest alteration rates, 30% (6/20) were pan-cancer driver genes. TTN (Titin, 76.74%), MUC16 (Mucin16, 62.79%), and MUC19 (Mucin19, 55.81%) were the 3 genes with the
highest alteration rates in our cohort. Pan-cancer driver genes with high alteration rates include FLNA (Filamin A, 41.86%), MUC4 (Mucin 4, 41.86%), and FAT3 (FAT Atypical Cadherin 3,
39.53%). Lung cancer/NSCLC driver genes with high alteration rates include BIRC6 (Baculoviral IAP Repeat Containing 6, 30.23%), EGFR (Epidermal Growth Factor Receptor, 30.23%), and TP53
(Tumor Protein P53, 20.93%). SETD2 (SET Domain Containing 2, Histone Lysine Methyltransferase) is the histone H3 lysine 36 histone (H3K36) methyltransferase [23] and has been reported in
various solid tumors and blood malignancies [24,25,26]. In our cohort, SETD2 alternated in all 6 tumor samples (2 primary tumors and 4 lymph node metastasis) of the Patient1, including 5
splice site mutations and 1 missense mutation. Other genes alternating in both primary tumors and lymph node metastasis tumors within individuals include CALR (Calreticulin), FRG1(FSHD
Region Gene 1), and ACTG1 (Actin Gamma 1). The single-nucleotide variations (SNVs) displayed considerable variations across and within patients, indicating intratumor heterogeneity (Fig. 2B,
Supplementary Data 5). For Patient 4, 9, and 10 with smoking history, most of their sequenced samples displayed a preponderance of C > A transitions, which is associated with tobacco
exposure [27]. In the Patient 9, the SNV pattern of Patient 9LN1 is highly similar to that of Patient 9T1 rather than Patient 9T2, implying the metastasis dominance of Patient 9T1. The
contributions of various known signatures to each sample are demonstrated in Fig. 2C (Supplementary Data 6). Signature 4 was prevalent in Patient4, 9, and 10, consistent with the fact that
they were smokers. However, signature 4 was also observed in non-smoker patients, and signature 29 (representing tobacco chewing) was prevalent in a large proportion of samples, suggesting
that tobacco exposure may play a critical role in the pathogenesis of MPLC with LNM. Signature 3 was identified in 93.20% (40/43) of tumor samples, indicating that DNA mismatch repair was
highly involved in the etiology of MPLC with LNM. The signatures echoes between primary tumors and lymph node metastasis tumors offer clues for identifying the dominant primary tumor. For
example, in the Patient 1, signature 22 only appeared in T1 and LN1, suggesting their unique connection. In the Patient 9, the signatures contribution of LN1 was almost identical to that of
T1, implying that T1 might be the source of LN1. Based on the MSK-IMPACT 468 panel genes (Supplementary Data 7), we acquired 315 genes from our data and conducted functional enrichment
analysis (Fig. 2D, Supplementary Data 8). Several signaling pathways were involved, including histone modification, chromatin remolding, MAPK, and cell cycle. Overall, the mutation spectrum
of MPLC with lymph node metastasis was distinct from that of typical lung adenocarcinoma, indicating the unique genomic profile of this disease. Meanwhile, primary tumor and lymph node
metastasis echo in both SNVs and COSMIC signature levels, showing the potentiality of clarifying metastasis trajectory and identifying the dominant primary tumor. GENOMIC HETEROGENEITY The
Upset map and Venn diagram show the mutation repertoire overlap within representative individuals (Fig. 3A, Supplementary Data 9, Supplementary Fig 2, 3.). For the 9 patients with matched
normal samples of our study, the mean number of genes shared by all tumor samples within the individual patient (regardless of the tumor locations) was 3.67 (range 1–10). FRG1B
(LOC102724813) mutation was shared by all tumor samples of Patient 1, 2, 6, and 9. Within Patient1, both SETD2 p.X1529_splice and STAG2 (Stromal Antigen 2) p.D1014E were identified by
Patient 1T2, Patient 1LN2, and Patient 1LN4. SETD2 is a histone lysine methyltransferase playing a significant role in renal malignancies [28], prostate cancer [24], and NSCLC [29]. STAG2 is
proven to be involved with viral infection via STING signaling [30]. CEP192 p.L1701F, a gene involved with PLK1 activity regulation at G2/M Transition, mitotic centrosome maturation, and
bipolar assembly [31, 32], was the only mutation common to all 6 tumor samples of Patient 11. Mutation distribution analysis reveals that MPLC with lymph node metastasis has high intratumor
heterogeneity. The genomic complexity of pulmonary and lymph node metastasis tumors is hard to gauge (Fig. 3B, Supplementary Data 10). For all patients, the mutations private to only one
tumor sample within a patient account for the most significant proportion (ratio range 66.40–99.72%, median 96.08%; number range 518 to 6721, median 1596), indicating enormous heterogeneity
among tumors. For Patient 5 and Patient 7, the percentages of mutations shared by all tumor samples within individual patients are higher than that of other patients (Patient 5, 19.74%,
Patient 7, 3.74%), we believe it results from that the two patients lack matched normal samples. Generally, pulmonary tumor samples take larger percentages of private mutations than lymph
node metastasis tumor samples (pulmonary, range 37.92–77.86%, mean 55.35%; lymph node metastasis, range 2.51–59.48%, mean 36.71%. _p_ = 0.0245), emphasizing the significance of primary tumor
management. However, in Patient 2, 7, 10, and 11, lymph node metastasis tumors claim more private mutations than pulmonary tumors, suggesting that lymph node metastasis tumors may be more
complex in the genomic background. We visualized the intratumor heterogeneity by global unsupervised clustering analysis (Fig. 3C, Supplementary Data 11). Patient 11 was the only patient
whose tumors were closely clustered, except Patient 5 and 7, who lacked paired normal samples. Pulmonary tumors and lymph node metastasis tumors from distinct individuals tended to be
clustered together, respectively, implying genomic differences between primary lung tumors and lymph node metastasis sites. CLONAL ARCHITECTURE AND EVOLUTIONAL TRAJECTORY Filtered
nonsynonymous variances from each tumor sample were used to construct phylogenetic trees using the parsimony ratchet method [33]. The branch lengths were proportional to the number of
nonsilent alterations within individuals (the scales were different among patients for better visual effect), and the driver genes were annotated next to the branches. Individual CT
(computerized tomography) images before surgery were provided to validate the multiple pulmonary tumors. Heatmaps were used to show mutation distribution (Fig. 4, Supplementary Data 9,
Supplementary Fig 4.). Based on the driver gene list from COSMIC Cancer Gene Census (Sep. 2021, https://cancer.sanger.ac.uk/census), we annotated the driver genes to the branches. We
observed diverse evolutional patterns between primary pulmonary tumors and lymph node metastases. Firstly, in Patient 1, there were 2 lymph node tumors (Pa1LN3 and Pa1LN4) that were earlier
in evolution than the two pulmonary tumors. The same phenomenon was found in Patient 11LN1, indicating that lymph node metastasis could be an early event in the pathogenesis of MPLC.
Secondly, for Patient 4, 8, and 9, lymph node metastasis lesions were more closely related to the specific individual pulmonary tumor, suggesting these pulmonary tumors were more aggressive
in nature. Thirdly, in Patient 2 and 11, lymph node metastases were associated with more than one pulmonary tumor, indicating that multiple pulmonary lesions of MPLC could contribute to
metastasis within individuals, for whom radical resection is necessary. Fourthly, we observed that the multiple lymph node metastases tumor samples within the same patient could be very
close in evolution. For example, Pa1LN1 and Pa1LN2 in Patient1, Pa5LN1 and Pa5LN2 in Patient 5, Pa10LN1 and Pa10LN2 in Patient 10, and Pa11LN2 and Pa11LN3 in Patient 11. These findings imply
the metastasis potentiality of lymph node tumors, and there might be the dominant lesions among multiple lymph node metastases. Moreover, for Patient 3, 5, 7, and 10, their results were
consistent with Patient 4, 8, and9, but needed further exploration since each of them had only one valid pulmonary tumor. The three pulmonary tumors of the Patient6 were highly heterogeneous
in genetic background, suggesting the intricate nature of MPLC. Generally, the diverse evolution patterns suggested the complex nature of MPLC with LNM. IMMUNE REPERTOIRES OF MPLC WITH LNM
Currently, the major management for MPLC is surgical resection, and the clues of chemotherapy or immunotherapy are rare. To offer new insights into the immunotherapy administration in MPLC,
we performed neoantigen number prediction, neoantigen binding affinity prediction, and clone cluster calculation in Fig. 5. Overall, multiple tumors in MPLC showed extremely high
heterogeneity in the immune background, which is more complicated than the mutational spectrum. Both primary pulmonary tumors and lymph node metastasis tumors could have diverse clone
clusters. In Fig. 5A (Supplementary Data 12), we showed the predicted neoantigen number of each sample. For key patients (Patient 1, 2, 4, 8, 9, and 11), the mean predicted neoantigen number
of primary lung cancers (mean 646.5, range 59–5186) was higher than that of lymph node metastases (mean 300, range 45–4562). Still, this discrepancy was not significant statistically (_p_ =
0.2416). For all 11 patients, neoantigens private to only one tumor sample possessed absolute advantage (range 71.40–100%, mean 99.36%). In Patient 3 and 6, all their predicted neoantigens
were private to one tumor (Fig. 5B Left, Supplementary Data 13). Neoantigens of primary lung tumors (range 35.07–76.02%, mean 52.72%) hold similar proportions to that of lymph node
metastases (range 2.38–64.70%, mean 46.80%), while the two parts shared few neoantigens (range 0–28.60%, mean 0.27%), suggesting the two parts might response to immunotherapy in different
manners (Fig. 5B Right, Supplementary Data 13). Binding affinity analysis shows that primary lung tumors and lymph node metastases harbor distinct neoantigens repertoires (Fig. 5C,
Supplementary Data 14), implying heterogeneous tumor microenvironments and potentially various reactions to immune checkpoint blockers. Most sequenced samples were oligoclonal, and the
clonal structures of the primary tumor and metastases were in diverse patterns (Fig. 5D, Supplementary Data 15). DISCUSSION In our study, the mutation landscape of MPLC with LNM is quite
different from that of east Asian LUAD patients [34] in our study, suggesting there might be unique features of this disease and the necessity of further research. Our study’s most
frequently mutated oncogenes were MUC16, FLNA, MUC4, FAT3, SETD2, and CALR, all of which were pan-cancer driver genes, and no highly mutated lung cancer driver gene ranked in the top 20
mutations, such as EGFR, KRAS, and TP53. This deviation might result from the insufficient sequencing depth of a few samples in our study. Still, we have reason to infer that such
inconsistencies indicate that MPLC with LNM may have a unique mutation spectrum, which needs further validation in larger cohorts. SETD2 (SET Domain Containing 2) mutated in all 6 tumors of
the Patient 1 (five of them were splice-site mutations), suggesting its potentiality to be a driving force of lymph node metastasis in MPLC. It is a histone lysine methyltransferase, closely
involved with cancer behavior [35, 36], and recurrently mutated in several cancer types [24, 37]. Moreover, SETD2 mutated in one pulmonary tumor of an MPLC with an LNM patient in a previous
study [13], which was partly consistent with our results. TTN (Titin), the gene with the highest alteration rate in our cohort, codes a sarcomere protein which is a significant player in
cardiomyocyte stiffness and cardiac train sensing [38]. In lung cancer, several recent studies reported that TTN mutations enhanced anti-tumor immunity and were associated with favorable
responses to ICI administration [39,40,41]. Therefore, we believe that MPLC and MPLC with LNM might be ideal substrates for ICI. Our study imposed further deliberation on the current MPLC
diagnosis standard. In the clinical practice of multifocal lung cancer, precise differentiation between MPLC and IPM has been a dilemma for decades [42], rendering treatment selection
challenging [9,10,11]. Many previous studies have paid attention to this field [6, 11,12,13, 43,44,45,46,47,48]. They believed that at most 1 mutation could be shared by any two pulmonary
tumors of MPLC. And the patients whose pulmonary tumors share two or more than two (≥2) mutations should be defined as IPM [11]. However, in our study, the shared mutations by any two
pulmonary tumor samples within an individual patient ranged from 1 to 78 (median: 20, Patient 3, 5, 7, and 10 were excluded for they only had one valid lung tumor, thus could not perform
this analysis), inconsistent with previous studies, and very few overlap genes were oncogenes. This inconsistency could result from the sequencing strategies used by previous studies, most
of them chose targeted sequencing, which only detected cancer-related genes [11, 49, 50], while WES/WGS was applied to only a few patients [12, 43]. Our research indicates that multiple
pulmonary tumors of MPLC could be more like each other in the genomic profile than we know before, and it might be arbitrary to distinguish MPLC and IPM under the current “1 mutation”
criteria. Moreover, consistent with Qu et al.’s work [51], our study suggests that the absence of LNM may not be necessary for diagnosing MPLC with similar tumor pathology. MPLC with LNM
should be carefully diagnosed to guarantee that the patients do not miss the best treatment opportunity. We offered novel clues for identifying the “dominant” lung tumors in MPLC and
challenged the current recognition of MPLC. At present, the clonal evolution relationships among different tumor lesions of MPLC with lymph node metastasis are unclear but of great
significance, since we may catch the driving force dictating the metastasis progression and identify the tumor site that has the most potential for metastasis so that doctors can react in
advance, and get a thorough understanding of the biological behavior of MPLC. However, these works are hampered by the enormous genetic heterogeneity of MPLC. While in MPLC with LNM, the
genetic trajectory between primary pulmonary tumor and lymph node metastasis is an ideal material to illustrate this dilemma. In 2016, Liu et al. [12] firstly sequenced one MPLC with an LNM
patient who was adenocarcinoma. In 2020, Higuchi et al. [13] performed targeted sequencing on 5 MPLC with LNM patients whose pulmonary tumors were all LUSC in histology. These two studies
indicated that lymph node metastasis was genetically related to one specific lung lesion, suggesting metastasis dominance. In our research, we drew phylogenetic trees for each patient and
inferred the evolutional process of multiple tumors based on the theory of clonal heterogeneity and tumor evolution [52]. We found that the lymph node metastases could originate from both
one and multiple pulmonary tumors within the individual, indicating that the “dominant” tumors could be more than one. In addition, our results challenge the current understanding that MPLC
is early in staging since some lymph node metastases are evolutionally earlier than pulmonary tumors. For clinical doctors, we provided new choices for MPLC management by advancing dominant
tumor identification. Currently, the most prevalent treatment for MPLC is surgical resection [53, 54]. However, many therapies are feasible in MPLC, including photodynamic therapy [55],
stereotactic ablative radiotherapy [56], and a combination of local radiofrequency ablation and melatonin [57]. When the multiple tumors of MPLC are in different lobes or lungs, multiple
operations become necessary, which is riskier than one-time resection. However, many MPLC patients cannot endure highly radical resections because of surgical contraindications. Therefore,
if we can pre-locate and remove the dominant tumor, more patients will benefit from surgery more safely. In addition, recurrence is widespread for multiple lung cancer in clinical practice,
the underlying reasons for which are unclear. At the same time, dominant tumor resection can be a critical indicator for recurrence prevention. We offered novel insights for the
immunotherapy administration in MPLC and MPLC with LNM. In the recent decade, immune checkpoint inhibitors achieved considerable success in multiple malignancies [58], including NSCLC [59]
(Non-Small Cell Lung Cancer). However, MPLC and MPLC with LNM are seldom involved. In Fig. 5, we observed that the multiple tumors within individuals were highly heterogeneous in neoantigen,
while disparities exist between primary tumors and metastases. Our findings shed light on the application of ICIs (immune checkpoint inhibitors) on MPLC and MPLC with LNM and suggested that
different strategies should be applied to primary and metastases samples. There are mainly three obstacles in unveiling the nature of MPLC and MPLC with LNM: Firstly, the lack of a golden
standard or generally accepted definitions of MPLC hindered the integration of data generated by different researchers who might adopt other criteria. Secondly, it is not easy to collect
MPLC samples in surgery since some tumor nodules are too small to resect, and the multiple nodules might locate on different sides of the lungs. In contrast, only one side of the lung will
be touched in one surgery. Thirdly, WES or WGS (whole-genome sequencing) still cannot be afforded by most scientific institutes, so most researchers used target sequencing, which could not
give a profound coverage of the genomic profile of MPLC. In our study, the incidence of MPLC with LNM was 2.54% (37/1458), and we estimate that the incidence is higher in the real world.
Therefore, it is significant to study this malignancy regardless of several obstacles. Several limitations existed in our study. Firstly, due to the limited number of patients (only 11 cases
were included) and the lack of large-scale validation cohorts in this study, most conclusions we reached should be interpreted with caution. Secondly, a few samples failed in the WES
quality control, causing imperfection in some patients. Thirdly, the sequencing depth of many samples is insufficient for producing highly valid results, many key mutations were missing.
Fourthly, we only performed WES on the patients in our study. Therefore, we missed the data in the non-coding area of the genome, which contains significant biological information. RNA-seq
and profiling of other omics, such as methylation, were not performed in our study, limiting our perception of this disease. In conclusion, by characterizing the molecular features of MPLC
with LNM, we identified potential driver genes of this disease. We revealed various evolution patterns among primary pulmonary and lymph node metastasis tumors. We found that lymph node
metastasis might be an early event in the etiology of MPLC. Our findings push the boundaries of MPLC and MPLC with LNM by providing new information for dominant tumor identification. Further
solid evidence of MPLC and MPLC with LNM is warranted. DATA AVAILABILITY We deposited our data in the Genome Sequence Archive-Human in BIG Data Center, Beijing Institute of Genomics (BIG),
under accession number HRA001717 (https://bigd.big.ac.cn/gsa-human/browse/HRA001717). REFERENCES * Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin.
2022;72:7–33. Article PubMed Google Scholar * Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments.
Lancet. 2017;389:299–311. Article CAS PubMed Google Scholar * Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54.
Article CAS PubMed Google Scholar * Leventakos K, Peikert T, Midthun DE, Molina JR, Blackmon S, Nichols FC, et al. Management of multifocal lung cancer: results of a survey. J Thorac
Oncol. 2017;12:1398–402. Article PubMed PubMed Central Google Scholar * Zheng R, Shen Q, Mardekian S, Solomides C, Wang ZX, Evans NR 3rd. Molecular profiling of key driver genes improves
staging accuracy in multifocal non-small cell lung cancer. J Thorac Cardiovasc Surg. 2020;160:e71–e9. Article PubMed Google Scholar * Murphy SJ, Harris FR, Kosari F, Barreto Siqueira
Parrilha Terra S, Nasir A, Johnson SH, et al. Using genomics to differentiate multiple primaries from metastatic lung cancer. J Thorac Oncol. 2019;14:1567–82. Article CAS PubMed Google
Scholar * Jiang L, He J, Shi X, Shen J, Liang W, Yang C, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer.
2015;87:303–10. Article PubMed Google Scholar * Zhao L, Liu C, Xie G, Wu F, Hu C. Multiple primary lung cancers: a new challenge in the era of precision medicine. Cancer Manag Res.
2020;12:10361–74. Article CAS PubMed PubMed Central Google Scholar * Wang Y, Yeung JC, Hanna WC, Allison F, Paul NS, Waddell TK, et al. Metachronous or synchronous primary lung cancer
in the era of computed tomography surveillance. J Thorac Cardiovasc Surg. 2019;157:1196–202. Article PubMed Google Scholar * Detterbeck FC, Nicholson AG, Franklin WA, Marom EM, Travis WD,
Girard N, et al. The IASLC Lung Cancer Staging Project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the
forthcoming eighth edition of the TNM classification. J Thorac Oncol. 2016;11:639–50. Article PubMed Google Scholar * Chang JC, Alex D, Bott M, Tan KS, Seshan V, Golden A, et al.
Comprehensive next-generation sequencing unambiguously distinguishes separate primary lung carcinomas from intrapulmonary metastases: comparison with standard histopathologic approach. Clin
Cancer Res. 2019;25:7113–25. Article CAS PubMed PubMed Central Google Scholar * Liu Y, Zhang J, Li L, Yin G, Zhang J, Zheng S, et al. Genomic heterogeneity of multiple synchronous lung
cancer. Nat Commun. 2016;7:13200. Article CAS PubMed PubMed Central Google Scholar * Higuchi R, Nakagomi T, Goto T, Hirotsu Y, Shikata D, Yokoyama Y, et al. Identification of clonality
through genomic profile analysis in multiple lung cancers. J Clin Med. 2020;9:573. Article CAS PubMed PubMed Central Google Scholar * Bolger AM, Lohse M, Usadel B. Trimmomatic: a
flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20. Article CAS PubMed PubMed Central Google Scholar * Li H, Durbin R. Fast and accurate long-read alignment
with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. Article PubMed PubMed Central Google Scholar * Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et
al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–9. Article CAS PubMed PubMed Central Google Scholar * Saunders
CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28:1811–7. Article CAS
PubMed Google Scholar * Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform.
2013;14:178–92. Article PubMed Google Scholar * Rosenthal R, McGranahan N, Herrero J, Taylor BS, Swanton C. DeconstructSigs: delineating mutational processes in single tumors
distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 2016;17:31. Article PubMed PubMed Central Google Scholar * Schliep KP. phangorn: phylogenetic
analysis in R. Bioinformatics. 2011;27:592–3. Article CAS PubMed Google Scholar * Shukla SA, Rooney MS, Rajasagi M, Tiao G, Dixon PM, Lawrence MS, et al. Comprehensive analysis of
cancer-associated somatic mutations in class I HLA genes. Nat Biotechnol. 2015;33:1152–8. Article CAS PubMed PubMed Central Google Scholar * Nielsen M, Andreatta M. NetMHCpan-3.0;
improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med. 2016;8:33. Article PubMed PubMed Central
Google Scholar * Chen R, Zhao WQ, Fang C, Yang X, Ji M. Histone methyltransferase SETD2: a potential tumor suppressor in solid cancers. J Cancer. 2020;11:3349–56. Article CAS PubMed
PubMed Central Google Scholar * Yuan H, Han Y, Wang X, Li N, Liu Q, Yin Y, et al. SETD2 restricts prostate cancer metastasis by integrating EZH2 and AMPK signaling pathways. Cancer Cell.
2020;38:350–65. Article CAS PubMed Google Scholar * Mar BG, Chu SH, Kahn JD, Krivtsov AV, Koche R, Castellano CA, et al. SETD2 alterations impair DNA damage recognition and lead to
resistance to chemotherapy in leukemia. Blood. 2017;130:2631–41. Article CAS PubMed PubMed Central Google Scholar * Huang KK, McPherson JR, Tay ST, Das K, Tan IB, Ng CC, et al. SETD2
histone modifier loss in aggressive GI stromal tumours. Gut. 2016;65:1960–72. Article CAS PubMed Google Scholar * Swanton C, Govindan R. Clinical implications of genomic discoveries in
lung cancer. N. Engl J Med. 2016;374:1864–73. Article CAS PubMed Google Scholar * Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and
branched evolution revealed by multiregion sequencing. N. Engl J Med. 2012;366:883–92. Article CAS PubMed PubMed Central Google Scholar * Kim IK, McCutcheon JN, Rao G, Liu SV, Pommier
Y, Skrzypski M, et al. Acquired SETD2 mutation and impaired CREB1 activation confer cisplatin resistance in metastatic non-small cell lung cancer. Oncogene. 2019;38:180–93. Article CAS
PubMed Google Scholar * Ding S, Diep J, Feng N, Ren L, Li B, Ooi YS, et al. STAG2 deficiency induces interferon responses via cGAS-STING pathway and restricts virus infection. Nat Commun.
2018;9:1485. Article PubMed PubMed Central Google Scholar * Joukov V, Walter JC, De Nicolo A. The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar
spindle assembly. Mol Cell. 2014;55:578–91. Article CAS PubMed PubMed Central Google Scholar * Chinen T, Yamazaki K, Hashimoto K, Fujii K, Watanabe K, Takeda Y, et al. Centriole and PCM
cooperatively recruit CEP192 to spindle poles to promote bipolar spindle assembly. J Cell Biol. 2021;220:e202006085. Article CAS PubMed PubMed Central Google Scholar * Murugaesu N,
Wilson GA, Birkbak NJ, Watkins T, McGranahan N, Kumar S, et al. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Disco. 2015;5:821–31.
Article CAS Google Scholar * Chen J, Yang H, Teo ASM, Amer LB, Sherbaf FG, Tan CQ, et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat Genet. 2020;52:177–86. Article CAS
PubMed Google Scholar * Niu N, Lu P, Yang Y, He R, Zhang L, Shi J, et al. Loss of Setd2 promotes Kras-induced acinar-to-ductal metaplasia and epithelia-mesenchymal transition during
pancreatic carcinogenesis. Gut. 2020;69:715–26. Article CAS PubMed Google Scholar * Chen BY, Song J, Hu CL, Chen SB, Zhang Q, Xu CH, et al. SETD2 deficiency accelerates MDS-associated
leukemogenesis via S100a9 in NHD13 mice and predicts poor prognosis in MDS. Blood. 2020;135:2271–85. Article CAS PubMed PubMed Central Google Scholar * Cancer Genome Atlas Research
Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9. * Loescher CM, Hobbach AJ, Linke WA. Titin (TTN): from molecule to modifications,
mechanics and medical significance. Cardiovasc Res. 2022;118:2903–18. Article PubMed Google Scholar * Su C, Wang X, Zhou J, Zhao J, Zhou F, Zhao G, et al. Titin mutation in circulatory
tumor DNA is associated with efficacy to immune checkpoint blockade in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2021;10:1256–65. Article CAS PubMed PubMed Central
Google Scholar * Wang Z, Wang C, Lin S, Yu X. Effect of TTN mutations on immune microenvironment and efficacy of immunotherapy in lung adenocarcinoma patients. Front Oncol. 2021;11:725292.
Article PubMed PubMed Central Google Scholar * Xie X, Tang Y, Sheng J, Shu P, Zhu X, Cai X, et al. Titin mutation is associated with tumor mutation burden and promotes antitumor immunity
in lung squamous cell carcinoma. Front Cell Dev Biol. 2021;9:761758. Article PubMed PubMed Central Google Scholar * Mitsudomi T, Yatabe Y, Koshikawa T, Hatooka S, Shinoda M, Suyama M,
et al. Mutations of the P53 tumor suppressor gene as clonal marker for multiple primary lung cancers. J Thorac Cardiovasc Surg. 1997;114:354–60. Article CAS PubMed Google Scholar * Ma P,
Fu Y, Cai MC, Yan Y, Jing Y, Zhang S, et al. Simultaneous evolutionary expansion and constraint of genomic heterogeneity in multifocal lung cancer. Nat Commun. 2017;8:823. Article PubMed
PubMed Central Google Scholar * Roepman P, Ten Heuvel A, Scheidel KC, Sprong T, Heideman DAM, Seldenrijk KA, et al. Added value of 50-gene panel sequencing to distinguish multiple primary
lung cancers from pulmonary metastases: a systematic investigation. J Mol Diagn. 2018;20:436–45. Article CAS PubMed Google Scholar * Takahashi Y, Shien K, Tomida S, Oda S, Matsubara T,
Sato H, et al. Comparative mutational evaluation of multiple lung cancers by multiplex oncogene mutation analysis. Cancer Sci. 2018;109:3634–42. Article CAS PubMed PubMed Central Google
Scholar * Murphy SJ, Aubry MC, Harris FR, Halling GC, Johnson SH, Terra S, et al. Identification of independent primary tumors and intrapulmonary metastases using DNA rearrangements in
non-small-cell lung cancer. J Clin Oncol. 2014;32:4050–8. Article CAS PubMed PubMed Central Google Scholar * Sozzi G, Miozzo M, Pastorino U, Pilotti S, Donghi R, Giarola M, et al.
Genetic evidence for an independent origin of multiple preneoplastic and neoplastic lung lesions. Cancer Res. 1995;55:135–40. CAS PubMed Google Scholar * Takamochi K, Oh S, Matsuoka J,
Suzuki K. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer. 2012;75:313–20. Article PubMed Google Scholar * Pei G, Li M,
Min X, Liu Q, Li D, Yang Y, et al. Molecular identification and genetic characterization of early-stage multiple primary lung cancer by large-panel next-generation sequencing analysis. Front
Oncol. 2021;11:653988. Article PubMed PubMed Central Google Scholar * Park E, Ahn S, Kim H, Park SY, Lim J, Kwon HJ, et al. Targeted sequencing analysis of pulmonary adenocarcinoma with
multiple synchronous ground-glass/lepidic nodules. J Thorac Oncol. 2018;13:1776–83. Article PubMed Google Scholar * Qu R, Tu D, Ping W, Zhang N, Fu X. Synchronous multiple lung cancers
with lymph node metastasis and different EGFR mutations: intrapulmonary metastasis or multiple primary lung cancers? Onco Targets Ther. 2021;14:1093–9. Article PubMed PubMed Central
Google Scholar * McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–28. Article CAS PubMed Google Scholar * Zhang Y,
Wang Y, Lv C, Shu X, Wang J, Yang Q. Clinical analysis of 56 cases of simultaneous bilateral video-assisted thoracoscopic surgery for bilateral synchronous multiple primary lung
adenocarcinoma. J Thorac Dis. 2018;10:6452–7. Article PubMed PubMed Central Google Scholar * Liu M, He W, Yang J, Jiang G. Surgical treatment of synchronous multiple primary lung
cancers: a retrospective analysis of 122 patients. J Thorac Dis. 2016;8:1197–204. Article PubMed PubMed Central Google Scholar * Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K,
Maehara S, et al. Management of multiple primary lung cancer in patients with centrally located early cancer lesions. J Thorac Oncol. 2010;5:62–8. Article PubMed Google Scholar * Chang
JY, Liu YH, Zhu Z, Welsh JW, Gomez DR, Komaki R, et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer.
2013;119:3402–10. Article PubMed Google Scholar * Li M, Hao B, Zhang M, Reiter RJ, Lin S, Zheng T, et al. Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer
metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct Target Ther. 2021;6:330. Article CAS PubMed PubMed Central Google Scholar * Sanmamed
MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–26. Article CAS PubMed PubMed Central Google Scholar * Uprety D, Mandrekar
SJ, Wigle D, Roden AC, Adjei AA. Neoadjuvant immunotherapy for NSCLC: current concepts and future approaches. J Thorac Oncol. 2020;15:1281–97. Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS We would like to express our sincere appreciation to the Department of Pathology of Cancer Hospital, Chinese Academy of Medical Sciences, for providing technical
support in the process of IHC. FUNDING Supported by the Special Research Fund for Central Universities, Peking Union Medical College (3332022132); the Special Research Fund for Central
Universities, Peking Union Medical College (3332021028, 3332021029); the National Natural Sciences Foundation (82203827, 82102886); the Beijing Nova Program (Z211100002121055); the Beijing
Natural Science Foundation (7222146); the Beijing Hope Run Special Fund of Cancer Foundation of China (LC2021B22, LC2020B09, LC2020A33); the Guangdong Association of Clinical Trials (GACT)
/Chinese Thoracic Oncology Group (CTONG) and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (Grant No. 2017B030314120); the Beijing Natural Science Foundation(No.
7224342); the National Key R&D Program of China (2020AAA0109505, YS2021YFF120009), the National Natural Science Foundation of China (81972196), the CAMS Innovation Fund for Medical
Sciences (CIFMS) (2021-1-I2M-012), and the R&D Program of Beijing Municipal Education Commission (KJZD20191002302). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Thoracic
Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, 100021, China He
Tian, Zhenlin Yang, Tao Fan, Chu Xiao, Guangyu Bai, Chunxiang Li & Jie He * Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer
Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, 100021, China Yalong Wang * Department of Medical Oncology, Yancheng No. 1 People’s Hospital, Yancheng,
Jiangsu, 224000, China Ping Chen * Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences
and Peking Union Medical College, Beijing, China Jiachen Xu * Guangdong Provincial People’s Hospital/Guangdong Provincial Academy of Medical Sciences, Guangdong Provincial Key Lab of
Translational Medicine in Lung Cancer, Guangzhou, China Jiachen Xu * Department of Thoracic Surgery/Head & Neck Medical Oncology, UT MD Anderson Cancer Center, Houston, TX, 77030, USA
Yanhua Tian * Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College,
Beijing, 100021, China Lin Li & Bo Zheng Authors * He Tian View author publications You can also search for this author inPubMed Google Scholar * Yalong Wang View author publications
You can also search for this author inPubMed Google Scholar * Zhenlin Yang View author publications You can also search for this author inPubMed Google Scholar * Ping Chen View author
publications You can also search for this author inPubMed Google Scholar * Jiachen Xu View author publications You can also search for this author inPubMed Google Scholar * Yanhua Tian View
author publications You can also search for this author inPubMed Google Scholar * Tao Fan View author publications You can also search for this author inPubMed Google Scholar * Chu Xiao View
author publications You can also search for this author inPubMed Google Scholar * Guangyu Bai View author publications You can also search for this author inPubMed Google Scholar * Lin Li
View author publications You can also search for this author inPubMed Google Scholar * Bo Zheng View author publications You can also search for this author inPubMed Google Scholar *
Chunxiang Li View author publications You can also search for this author inPubMed Google Scholar * Jie He View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS HT, CL, and JH are responsible for the study design. JH, CL, BZ, and LL are responsible for conception identification. HT, YW, and ZY are responsible for the
acquisition of data. HT, ZY, and PC are responsible for the analysis and interpretation of data. HT, CL, GB, and TF, CX are responsible for drafting the manuscript. HT, TF, YT, and JX are
responsible for revising the article critically for important intellectual content. HT and CX provide the final approval of the version to be published. All authors read and approved the
final manuscript. CORRESPONDING AUTHORS Correspondence to Chunxiang Li or Jie He. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL Written
informed consent was signed by each participant of this study. The Ethics Committee approved the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union
Medical College approved this study (NCC3231). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SUPPLEMENTARY DATA 5 SUPPLEMENTARY DATA 6
SUPPLEMENTARY DATA 7 SUPPLEMENTARY DATA 8 SUPPLEMENTARY DATA 9 SUPPLEMENTARY DATA 10 SUPPLEMENTARY DATA 11 SUPPLEMENTARY DATA 12 SUPPLEMENTARY DATA 13 SUPPLEMENTARY DATA 14 SUPPLEMENTARY
DATA 15 SUPPLEMENTARY FIGURE 1 SUPPLEMENTARY FIGURE 2 SUPPLEMENTARY FIGURE 3 SUPPLEMENTARY FIGURE 4 SUPPLEMENTARY FIGURE 5 SUPPLEMENTARY FIGURE 6 SUPPLEMENTARY MATERIALS LEGEND (FOR
SUPPLEMENTARY FIGURES) RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Tian, H., Wang, Y., Yang, Z. _et al._ Genetic trajectory and clonal evolution of multiple primary lung cancer with lymph node metastasis. _Cancer Gene Ther_ 30, 507–520
(2023). https://doi.org/10.1038/s41417-022-00572-0 Download citation * Received: 20 June 2022 * Revised: 10 November 2022 * Accepted: 25 November 2022 * Published: 19 January 2023 * Issue
Date: March 2023 * DOI: https://doi.org/10.1038/s41417-022-00572-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
