The intracellular domain of β-dystroglycan mediates the nucleolar stress response by suppressing ubf transcriptional activity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT β-dystroglycan (β-DG) is a key component of multiprotein complexes in the plasma membrane and nuclear envelope. In addition, β-DG undergoes two successive proteolytic cleavages that
result in the liberation of its intracellular domain (ICD) into the cytosol and nucleus. However, stimuli-inducing ICD cleavage and the physiological relevance of this proteolytic fragment
are largely unknown. In this study we show for the first time that β-DG ICD is targeted to the nucleolus where it interacts with the nuclear proteins B23 and UBF (central factor of Pol
I-mediated rRNA gene transcription) and binds to rDNA promoter regions. Interestingly DG silencing results in reduced B23 and UBF levels and aberrant nucleolar morphology. Furthermore, β-DG
ICD cleavage is induced by different nucleolar stressors, including oxidative stress, acidosis, and UV irradiation, which implies its participation in the response to nucleolar stress.
Consistent with this idea, overexpression of β-DG elicited mislocalization and decreased levels of UBF and suppression of rRNA expression, which in turn provoked altered ribosome profiling
and decreased cell growth. Collectively our data reveal that β-DG ICD acts as negative regulator of rDNA transcription by impeding the transcriptional activity of UBF, as a part of the
protective mechanism activated in response to nucleolar stress. SIMILAR CONTENT BEING VIEWED BY OTHERS THE ASSOCIATION OF UBAP2L AND G3BP1 MEDIATED BY SMALL NUCLEOLAR RNA IS ESSENTIAL FOR
STRESS GRANULE FORMATION Article Open access 14 April 2023 AUTOPHAGY RECEPTOR NDP52 ALTERS DNA CONFORMATION TO MODULATE RNA POLYMERASE II TRANSCRIPTION Article Open access 18 May 2023
DYNAMICS OF DNA DAMAGE-INDUCED NUCLEAR INCLUSIONS ARE REGULATED BY SUMOYLATION OF BTN2 Article Open access 13 April 2024 INTRODUCTION Regulated proteolysis of cell surface receptors that
liberates biologically active proteins/peptides from the plasma membrane (PM) to the cytosol is a critical step in a variety of different signaling pathways that respond to external stimuli.
γ-Secretase is an intramembranous cleaving protease complex consisting of at least four proteins: presenilin-1, nicastrin, anterior pharynx-defective phenotype 1, and presenilin enhancer
21. γ-Secretase is known to be required for the activation of many transmembrane proteins, including the amyloid precursor protein, cadherins, Notch12, and recently, dystroglycan3,4.
Dystroglycan, a key component of the dystrophin-associated protein complex (DAPC), is transcribed from the _DAG1_ gene and translated as a single propeptide, which is proteolytically
processed to generate the extracellular subunit α-dystroglycan (α-DG) and the transmembrane subunit β-dystroglycan (β-DG)5. α-DG binds to different extracellular matrix proteins including
laminin, agrin, or perlecan6, while β-DG connects actin through various cytolinker proteins including dystrophin or utrophin. Thereby, dystroglycan serves as a link between the extracellular
matrix and the actin-based cytoskeleton, acting also as an adhesion and signaling receptor5,7. Besides its structural role in the maintenance of membrane integrity, dystroglycan
localization is not static but dynamic. Phosphorylation of β-DG at Y890 triggers its retrograde trafficking from PM to the nucleus, via the membranous endosome-endoplasmic reticulum (ER)
network, with ezrin activation enhancing the intracellular trafficking and translocon Sec61 facilitating the exit of β-DG from the ER membrane to be accessible for importin-dependent nuclear
import through the nuclear pore8,9,10. In the nucleus, β-DG is assembled with nuclear envelope (NE) components, including emerin, and lamins A/C and B1, to preserve the nuclear
structure/function11,12 and where it can also indirectly regulate gene expression13. This functional diversity of β-DG, acting as a platform for both PM- and NE-associated processes, is
further expanded by proteolytic cleavage of the protein. β-DG is subjected to proteolytic cleavage by MMP-2 and MMP-9 to liberate its extracellular domain14,15, while the remaining fragment,
containing the transmembrane stub and the cytoplasmic portion is thought to be subsequently processed by γ-secretase to deliver an intracellular domain (ICD; 12 kDa in mass but runs
aberrantly on SDS-PAGE at ~26 kDa) into the cytosol3,4. Recent evidence showed that β-DG ICD is targeted to the nucleus in prostate cancer cells3,13,16 nonetheless the biological
significance of such localization is largely unknown. The nucleus is organized into distinct functional compartments containing specific macromolecules that govern nuclear processes;16 for
instance, the nucleolus is a prominent non-membranous nuclear organelle primarily involved in ribosome biogenesis and cellular homeostasis17. Thus, identification of the destination of β-DG
ICD within the nucleus could facilitate further elucidation of its function. In this study we demonstrate for the first time that β-DG ICD is target to the nucleolus where it plays a
negative role in the regulation of ribosomal RNA (rRNA) transcription. We provide evidence that full-length β-DG is proteolytically processed into β-DG ICD in response to nucleolar stress,
via the Notch signaling pathway. Remarkably, β-DG ICD binds to the rDNA promoter to suppress rRNA synthesis by impairing the expression, localization, and ultimately activity of the RNA
polymerase I (Pol I) transcription factor UBF (upstream binding factor), which further results in the downregulation of rRNA expression and cell proliferation. Thus, β-DG ICD appears to be a
key contributor to the nucleolar stress response. RESULTS THE Γ-SECRETASE-GENERATED INTRACELLULAR DOMAIN OF Β-DG IS TARGETED TO THE NUCLEOLUS We previously observed localization of β-DG to
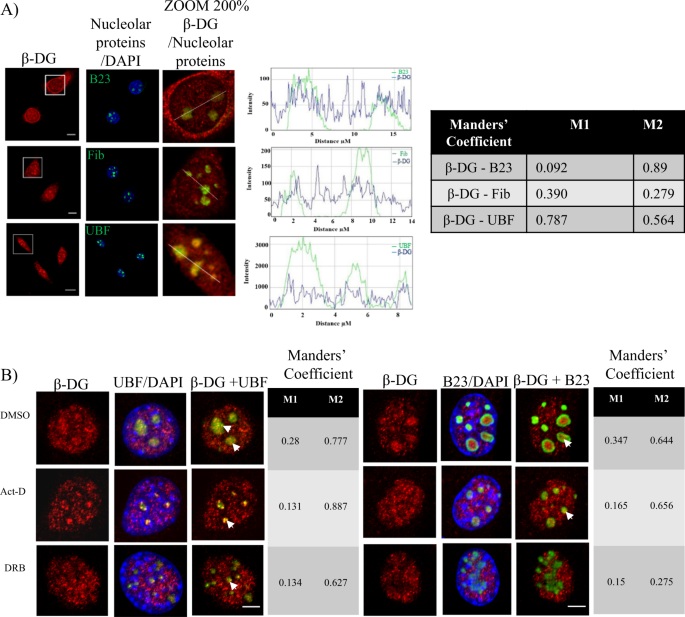
the nucleoli in C2C12 myoblasts11; but no role for β-DG has been described in this nuclear organelle. As a first step, we analyzed whether β-DG colocalizes with proteins that define
functionally distinct compartments of the nucleolus. Cells were double-stained for β-DG (C20 antibody) along with UBF, fibrillarin (markers of the fibrillar center, FC), or B23 (marker of
the granular component, GC) and further analyzed by confocal microscopy. The nucleolar immunostaining of β-DG colocalized at certain extent with all three nucleolar proteins analyzed, as
confirmed by the line intensity scan analysis and Manders’ overlapping coefficients (Fig. 1a). The specificity of C20 antibody was demonstrated using both DG knockout C2C12 cells and primary
fibroblasts from a subject with Walker–Warburg syndrome that do not express _DAG1_ gene all18. Nucleoli were strongly staining by C20 in C2C12 wild-type cells, while a faint nuclear
staining that was excluded from nucleoli was observed in DG knockout C2C12 cells (Supplementary Figure 1A, left panel). Furthermore, the immunoreactive band corresponding to β-DG (~43 kDa),
which was observed in primary fibroblast lysates from a healthy subject, was absent in Walker–Warburg syndrome primary fibroblast lysates (Supplementary Figure 1A, right panel). Finally,
pre-incubation with a blocking peptide virtually eliminated C20 immunostaining in wild-type cells (Supplementary Figure 1B). In addition, nucleolar localization of β-DG was confirmed using
another anti-β-DG antibody (G5; Supplementary Figure 1C). The nucleolus is a highly dynamic organelle that responds to a variety of cellular stresses with morphological changes19 and
redistribution of proteins implicated in ribosome biogenesis form perinuclear caps upon inhibition of rDNA transcription20. C2C12 cells were therefore treated with actinomycin D (0.08 µg/ml)
or DRB (0.3 µM) to cause nucleolar stress, and the effect of treatments on β-DG distribution was evaluated by confocal microscopy. The formation of nucleolar caps and nucleolar
segregation/nucleolar necklaces was found after treatment with Act D and DRB, respectively, as shown by immunostaining for UBF and B23 (Fig. 1b). Treatment with the vehicle alone dimethyl
sulfoxide (DMSO) produced no change in the immunostaining pattern of nucleolar proteins. Interestingly, nucleolar labeling of β-DG also underwent redistribution in response to Act D and DRB
but remained in colocalization partially with UBF and barely with B23, as shown by Manders’ overlapping coefficient analysis (Fig. 1b), suggesting that β-DG might be involved in nucleolar
organization/plasticity. Next, we investigated whether the nucleolar localization of β-DG is dependent on nucleic acid integrity. C2C12 myoblasts were immunolabeled for β-DG before and after
nuclease treatment, and stained with DAPI to monitor DNA degradation, or immunolabeled for hnRNP C1/C2 antibody (RNA-binding protein) to monitor RNA removal. DNase digestion completely
removed DAPI-labeled DNA, while treatment with RNase caused hnRNP C1/C2 redistribution from the nucleoplasm to the nuclear periphery and cytoplasm, thus confirming the effectiveness of
nucleases treatment. DNase treatment completely abrogated the nucleolar distribution of β-DG, while treatment with RNase apparently increased the intensity of β-DG nucleolar immunostaining,
compared with untreated cells (Supplementary Figure 2A and B). These results suggest that the nucleolar localization of β-DG is dependent on DNA. To demonstrate conclusively that β-DG
resides in the nucleolus, C2C12 were fractionated into total, cytoplasmic, nuclear, and nucleolar fractions to be further analyzed by SDS-PAGE/WB. A prominent ~30 kDa β-DG band was found
enriched, together with fibrillarin, in the nucleolar fraction, as revealed using three different anti-β-DG antibodies: MANDAG (mouse mab that recognizes total β-DG levels), pTyr890 (rabbit
pAb that specifically recognizes β-DG phosphorylated at Tyr890) (Fig. 2a), and C20 (rabbit antibody that recognizes the total β-DG pool Supplementary Figure 1D). Nup62 (nuclear pore protein)
and calnexin (endoplasmic reticulum protein) were recovered in the nuclear and cytoplasmic fractions, respectively, and the two proteins were absent from the nucleolar fraction
demonstrating that no cross-contamination occurred during cellular fractionation. Control experiments using both DG knockout C2C12 cells and Walker–Warburg syndrome primary fibroblasts yield
no immunoreactive bands after incubation with MANDAG antibody (Fig. 2b and Supplementary Figure 1A, right panel), which demonstrated the specificity of this antibody for β-DG detection. We
hypothesized that the ~30 kDa band correspond to the ICD of β-DG generated by γ-secretase cleavage as has been described previously3,13,16. Compatible with this assumption, lysates from
cells treated with DAPT (γ-secretase inhibitor) resulted in a dose-dependent decrease in β-DG ICD levels that paralleled with dose-dependent reduction of the intracellular domain of Notch
(NICD), a well-characterized γ-secretase substrate (Fig. 2c). Collectively these data support the idea that the γ-secretase-derived cleavage fragment of β-DG (~30 kDa), named hereafter as
β-DG ICD, is targeted to the nucleolus where it possibly has a role in regulating nucleoli structure/function. Β-DG ICD INTERACTS WITH UBF AND B23 Colocalization of β-DG with nucleolar
proteins even after induced nucleolar disruption (Fig. 1b), suggests their physical interaction. Consistent with this notion, UBF and traces of B23 but not fibrillarin were recovered in
complex with β-DG after immunoprecipitation (IP) of C2C12 lysates with anti-β-DG antibody (Fig. 3a). None of the nucleolar proteins were immunoprecipitated using nonspecific IgG antibodies.
Because anti-β-DG antibody immunoprecipitated both β-DG full-length and β-DG ICD, we wondered what form is bound to nucleolar proteins. To approach this, C2C12 cells were transiently
transfected to express GFP-β-DG full-length or GFP-β-DG ICD and subjected to GFP-based IP assays. Both UBF and B23 were found preferentially bound to GFP-β-DG ICD, with UBF interaction
predominant over that with B23 (Fig. 3b). GFP alone interacted with neither B23 nor UBF, while lamin B1 (β-DG nuclear partner) was bound to GFP-β-DG full-length but not GFP-β-DG ICD,
demonstrating the specificity of GFP-Trap assays. SILENCING OF DG DISRUPTS NUCLEOLAR MORPHOLOGY AND DECREASES B23 AND UBF LEVELS To elucidate whether nucleolar localization of β-DG is
physiologically relevant, the impact of knocking down of β-DG expression on nucleolar structure and expression of nucleolar proteins was examined. Significant reduction of dystroglycan mRNA
levels and the consequent decrease in β-DG full-length and β-DG ICD levels were observed upon stably transfection of C2C12 cells with a vector expressing a short hairpin RNA against _DAG1_
gene (DG shRNA), compared to cells expressing an unspecific shRNA (control shRNA) (Fig. 4a, b). Remarkably, the depletion of β-DG resulted in decreased proteins levels of B23 (80%),
fibrillarin (30%), and UBF (60%) (Fig. 4b). Such effects were accompanied by a clear alteration in nucleolar morphology; while control shRNA cells contain numerous relatively small nucleoli,
β-DG-depleted cells exhibited fewer larger and more amorphous nucleoli, as shown by immunostaining for UBF and B23 (Fig. 4c, d) and quantification of nucleolar area (right panels). Β-DG
BINDS TO THE RDNA PROMOTER TO POSSIBLY REGULATE RRNA EXPRESSION Because knockdown of β-DG resulted in decreased levels of UBF, a key component of the RNA polymerase I preinitiation complex
in rRNA transcription, we sought to determine the expression of 28 S and 18 S pre-rRNAs in β-DG knockdown cells by quantitative RT-PCR (Figure 4a). β-DG knockdown cells exhibited an ~40%
decrease in 18 S levels, while 28 S expression showed a slight but significant increase, compared with control shRNA (Fig. 5a). To provide insight into the mechanism by which β-DG depletion
influences rDNA gene expression, we analyzed whether β-DG is physically associated with the rDNA promoter. Chromatin was immunoprecipitated from wild-type and DG knockout (negative control)
C2C12 cells, using β-DG or nonspecific IgG antibodies; moreover, ChIP assays using RNA polymerase I (Pol I) antibodies were performed in parallel (positive control). Then, rDNA was amplified
using primers specific for the rDNA promoter, 5.8 S or intergenic spacer (IGS) regions. ChIP assays revealed the presence of β-DG in the 5.8 S and IGS regions, while Pol I was found to
occupy all three regions of the rDNA (Fig. 5b). As expected, no PCR amplification was detected upon ChIP assays on DG knockout cells (right panel), which demonstrated the specificity of
Mandag antibody for immunoprecipitating β-DG from chromatin. Overall these data suggest that β-DG may modulate rRNA transcript expression through its binding to the rDNA regulatory region.
NUCLEOLAR STRESS-INDUCED Β-DG ICD CLEAVAGE CORRELATES WITH IMPAIRED RIBOSOME BIOGENESIS In an effort to understand the potential role of β-DG ICD in the nucleolus, we investigated whether
β-DG ICD cleavage is induced by nucleolar stress. C2C12 cells were subjected to different kind of stresses, namely oxidative stress, acidosis, and UV irradiation, and effectiveness of
treatments was monitored by p53 activation. Interestingly, the ratio of β-DG ICD/full-length β-DG was augmented in response to all treatments with concomitant decrease in UBF content, with
H2O2 and UV being the most efficient inductors (Fig. 6a, b). As expected, H2O2-induced nucleolar stress resulted in nucleolar segregations, with a formation of UBF-stained nucleolar caps at
the periphery of B23-stained nucleoli (Supplementary Figure 3), which elicited ultimately downregulation of 45 S rRNA expression (Fig. 6c) and abnormal ribosomal profiling characterized by
decreased monosomes and polysome absorbance peaks (Fig. 6d). We hypothesized that augmented β-DG ICD levels could be reflected in a higher binding activity to the rDNA promoter; consistent
with this idea, occupancy of the three rDNA regulatory regions by β-DG markedly increased after H2O2 treatment, as revealed by ChIP-qPCR assays (Fig. 6e). OVEREXPRESSION OF Β-DG ICD IMPAIRS
RIBOSOMAL BIOGENESIS BY SUPPRESSING UBF FUNCTION To analyze directly the implication of β-DG ICD in ribosome biogenesis, we stably transfected C2C12 cells with a GFP-β-DG ICD vector, to
mimic high levels of the cleavage fragment. GFP-β-DG ICD localization was restricted to the nucleus showing homogenous distribution throughout the nucleoplasm, with less intense staining in
nucleoli (Fig. 7a). Remarkably, the significant number of GFP-β-DG ICD-transfected cells exhibited mislocalization of UBF foci to the cytoplasm, compared to GFP alone (50% and 20%,
respectively), while the nucleolar distribution of B23 remained unaltered (Fig. 7a, bottom panel and right panel). Impaired nucleolar localization of UBF due to exogenous β-DG ICD expression
was confirmed by transfecting FLAG-tagged β-DG ICD (Supplementary Figure 4, top rows). Furthermore, overexpression of GFP-β-DG ICD resulted in decreased levels of UBF with no effect on B23,
compared to GFP alone (Fig. 7b and right panel). We have demonstrated previously that the phosphorylation of β-DG at Tyr890 is not only a signal for the proteolysis and internalization of
dystroglycan3,16,22 but also acts to target dystroglycan to the nucleus10. We considered therefore whether phosphorylation of β-DG ICD at the PPxY motif (Tyr890) plays a role in UBF
mislocalization. Thus, FLAG-β-DG ICD variants that either mimic (Y890E) or block (Y890A) the Tyr890 phosphorylation were analyzed for UBF localization. Similar percentage of transfected
cells showing UBF outside of the nucleus were observed between β-DG ICD FLAGY890A and β-DG ICD-FLAGY890E; thus, overexpression of β -DG ICD resulted in UBF mislocalization irrespective of a
charged residue at residue 890 that mimics phosphorylation (Supplementary Figure 4). We hypothesized that decreased expression/mislocalization of UBF through β-DG ICD overexpression would
ultimately impact rRNA expression. Consistent with this idea, an ~50% decrease in 45 S rRNA and 28 S but not 18 S was found in cells stably overexpressing β-DG ICD, compared with those
expressing GFP alone (Fig. 8a). To ascertain whether β-DG ICD overexpression influences Pol I transcription, functional rRNA promoter reporter assays were carried out in β-DG ICD stably
transfected cells, using a vector that expresses Firefly luciferase under the control of the mouse rRNA promoter and a vector that expresses Renilla luciferase to normalize transfection
efficiency. Pol I promoter activity was found to decrease by 40% in GFP-β-DG ICD-transfected, compared with those with GFP alone (Fig. 8b). Because the rRNA content is a crucial point of
regulation for ribosome biogenesis, we hypothesized that decreased 40 S and 60 S levels would impact the ribosome profile. Consistent with this notion, altered ribosome profiles devoid of
the peaks corresponding to free 40 S and 60 S ribosomal subunits were observed in cells expressing β-DG ICD (Fig. 8c). Such impairment in rRNA expression and polysome profiling ultimately
leads to defective proliferative capacity of β-DG ICD overexpressing cells, as shown by MTT-based proliferation assays (Fig. 8d). Collectively these data indicate that β-DG ICD acts as
negative regulator of rRNA expression by affecting both the levels and transcriptional activity of UBF. DISCUSSION β-DG is a key component of both PM and NE, acting as a platform for the
proper anchorage of organelle-specific protein assemblies. At the PM β-DG modulates adhesion/signaling by connecting extracellular matrix proteins with the actin-based cytoskeleton5,7, while
in the nucleus β-DG interacts with the NE proteins emerin and lamins A/C and B1 to regulate nuclear structure and function11,12. This functional diversity could be wider than originally
thought, due to the potential function of β-DG proteolytic fragments. Recent in vitro and in vivo evidence showed that β-DG undergoes two successive and possibly coordinate proteolytic
cleavages that result in the liberation of the intracellular domain (ICD) into the cytosol; first matrix metalloproteinases, MMP-2 and MMP-9 cleave the extracellular domain of β-DG23 and
create a membrane-tethered intermediate that is subsequently processed by γ-secretase, a PM-embedded protease complex, to render a final cleavage product that approximates the entire ICD21.
Except for cell density21, other cellular stimuli inducing β-DG ICD release as well as the physiological consequences of such processing event are largely unknown. In this study we showed
that β-DG ICD associates to nucleoli and its cleavage is promoted by the induction of a different kind of nucleolar stresses. We also ascribed for the first time a role for β-DG ICD in
regulating rRNA transcription through negative regulation of the expression and transcriptional activity of UBF, in the context of the response to nucleolar stress. We show herein that β-DG
colocalizes and interacts with the key nucleolar proteins B23 and UBF, and, based on the converging redistribution of β-DG with B23, fibrillarin, or UBF in response to both actinomycin- and
DRB-induced nucleoli disorganization, we hypothesized that β-DG participates in the functional plasticity of nucleoli. Interestingly, cell fractionation experiments revealed the presence of
β-DG ICD but not β-DG full-length in the nucleolar fraction of C2C12 cells; however, GFP-based IP experiments revealed that both β-DG ICD and β-DG full-length are able to bind UBF. Because
full-length β-DG is an NE protein associated with nuclear lamin and because the interaction of intranuclear filaments of lamins with nuclear compartments (including nucleoli) has been well
established24,25,26 it is possibly that IP of β-DG full-length pulled down a nuclear macromolecular complex containing NE (lamin B1) and nucleolar (UBF) proteins. The trafficking pathway
underlying nucleolar targeting of β-DG ICD remains to be deciphered. Full-length β-DG is translocated to the nucleus through recognition of its NLS by importins α2/β19, therefore it is
likely that β-DG ICD enters the nucleus by virtue of the NLS that is still present in the cleaved fragment, or alternatively by passive diffusion through the nuclear pore complex (NPC),
because its molecular mass (<30 kDa) is clearly below the NPC permissive size27. No obvious nucleolar localization signal is found in β-DG ICD, thus, its nucleolar accumulation might be
mediated by a retention mechanism via interaction with nucleolar components. Supporting this idea, β-DG ICD was found to interact with B23, a shuttle protein that transports cargos between
the cytoplasm and the nucleolus and mediates nucleolar retention of other proteins28. Furthermore, binding of β-DG to the rDNA gene regulatory region (see below) could serve to anchor β-DG
ICD to nucleoli too. Owing to the main contribution of nucleoli in ribosome biogenesis16, we wondered whether β-DG is involved in this process. We provide here solid evidence supporting this
hypothesis: first, β-DG interacts with UBF, an HMG box-containing protein that binds directly with rDNA and plays an important role in the recruitment of SL1 (selectivity factor 1) and Pol
I to the rRNA promoter to constitute the Pol I preinitiation machinery complex29; second, ChIP assays with anti-β-DG antibody demonstrated β-DG recruitment to the rDNA 5.8 S and IGS
regulatory regions; and third, DG silencing resulted in reduced UBF levels, decreased 18 S pre-rRNA expression but not 28 S pre-rRNA expression, and aberrant nucleolar morphology. Discordant
expression between 28 S and 18 S rRNAs is intriguingly. Even though both 28 S rRNA and 18 S rRNA are originated from 45 S rRNA precursor, their processing/maturation pathways differ from
each other, and each one is assisted by specific exo and endoribonucleasas30. Therefore, we argue that aside from its effect on rDNA basal transcription machinery (as shown here), β-DG ICD
might modulate processing pathways of 18 S rRNA and 28 S rRNA differentially by indirect mechanisms. Additional experiments are needed to clear this issue. The nucleolus is a central hub for
orchestrating stress response, with downregulation of rRNA synthesis taking place as a part of ribosome biogenesis surveillance31, furthermore, numerous proteins that functions are not
generally ascribed to ribosome biogenesis are recruited to the nucleolus in response to environmental stimuli32. Therefore, we ascertained whether β-DG ICD cleavage is coupled to the
response to nucleolar stress. Remarkably, triggers of β-DG ICD cleavage by Notch signaling activation occurred in response to different nucleolar stressor treatments, including oxidative
stress, acidosis, and UV irradiation. Supporting a role for β-DG ICD in the response to nucleolar stress, β-DG ICD overexpression was sufficient to elicit several features of nucleolar
stress, including mislocalization and decreased levels of UBF, repression of rRNA transcription, altered ribosome profile, and decreased cell proliferation. The results raise the question of
how β-DG ICD exerts a negative regulation on rDNA expression. β-DG lacks DNA-binding motifs or enzymatic activity and is considered as a scaffold protein, due to the presence of protein
modules supporting interactions with diverse proteins (e.g. SH3, SH2, and WW domains)5. Therefore, we postulate that binding of β-DG ICD to UBF may lead to mislocalization and decreased
levels of the latter protein, possibly by altering its stability, thereby interfering with the assembly of the Pol I initiation complex and the subsequent UBF-dependent activation of rDNA
transcription (Fig. 9). Because the occupancy of rDNA regulatory regions by β-DG increased in response to nucleolar stress, it is also possible that β-DG ICD exerts its suppressive effect by
displacing UBF from the Pol I transcription complex, which is consistent with mislocalization of UBF to the nucleolar periphery and cytoplasm that was observed in H202-treated cells.
Further ChIP assays to ascertain whether β-DG and UBF coexist or not in the rDNA promoter region under nucleolar stress conditions are needed to solve this question. Furthermore, since β-DG
has multiple partners in the nucleus, the existence of an indirect mechanism by which β-DG ICD represses UBF activity cannot be ruled out. The implication of β-DG ICD in rDNA expression
could be envisioned as an extension of the homeostatic role of β-DG, where induction of ICD cleavage and its further targeting to the nucleolus in response to environmental stress, like
oxidative stress, acts as a protective response to inhibit rDNA transcription and further proliferation of damaged cells. In summary, our data are compatible with the paradigm that β-DG ICD
functions as a negative regulator of rRNA transcription by impeding the transcriptional activity of UBF, as a part of the nucleolar stress response mechanisms that are activated under
physiologically relevant stress conditions. Thus, in addition to its roles as PM and NE signaling scaffold, β-DG should be regarded as a regulator of Pol I transcription in the nucleolus.
MATERIALS AND METHODS CELL CULTURING AND TREATMENTS Mouse C2C12 myoblasts (ATCC® CRL-1772™) were grown as previously (Vásquez-Limeta, et al 2014). Generation of C2C12-derivative DG knockout
cell lines with the CRISP/Cas9 system will be published elsewhere. For nucleoli disorganization treatments, cells were treated for 3 h with 0.01 μg/ml Act D (Sigma-Aldrich, St Louis,
Missouri) or 25 µg/ml DRB (5,6 dichloro-1–d-ribofuranosylbenzimidazole; Sigma-Aldrich, St Louis, Missouri) or vehicle alone (DMSO), prior to confocal laser scanning microscopy (CLSM)
analysis. The ɤ-secretase inhibitor N-(N-(3,5-difluorophenacetyl)-L-alanyl). S-phenylglycine t-butyl ester (DAPT; Sigma-Aldrich, St Louis, Missouri) was used at 10 µM for 24 h. For induction
of the nucleolar stresses response, cells were cultured in DMEM supplemented with serum adjusted to pH 6, treated with 1.4 mM H2O2 (7722-84-1,Sigma-Aldrich) for 4 h or subjected to UV
radiation using a pulse of 20 J/cm2 each 24 h for 2 days. For nuclease digestion assays, cells grown on coverslips were treated with either 100 μg/ml protease-free RNase A in CSK buffer (10
mM Pipes pH 6.8, 100 mM NaCl, 30 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 0.5% (v/v) Triton X-100) for 40 min at 37 °C or with 200 μg/ml protease-free DNase I in PBS with 5 mM MgCl2 for 1 h at 37
°C, then the cells were fixed with PFA 4% and immunolabeled using primary antibodies and the corresponding fluorochrome_-_conjugated secondary antibodies. Negative control experiments were
carried out by incubating cells only with CSK buffer (RNase) or PBS with 5 mM MgCl2 (DNase) prior to fixation. PLASMIDS AND TRANSFECTION The expression vectors Flag-β-DG ICD WT, Flag-β-DG
ICDY890E, and Flag-β-DG ICDY890A were generated by PCR amplification using DGWT-DG, DGY890E-GFP, and DGY890A-GFP plasmids as template, respectively10. The cDNA sequence encoding the
intracellular domain of β-DG (ICD, amino acids 774–895) was amplified using an M-MLV reverse transcriptase coupled to a high-fidelity polymerase (_Pfu_ turbo; Strategene) and primers
containing Hind III and EcoRI restriction sites. Forward primer was 5′-CCTAAGCTTTATCGCAAGAAGAGGAAGGGC-3′ and the respective reverse primers were 5′-CTGAATTCTTAAGGGGGAACATACGGAGGG-3′ (ICD
WT), 5′-CTGGAATCCTTAAGGGGGAACCTCCGGAGGG-3′ (ICD Y890E mutation), and 5′-CTGAATTCTTAAGGGGGAACTGCCGGAGGG-3′ (ICD Y890A mutation). PCR products were double digested with Hind III and EcoRI and
cloned into the Hind III-EcoRI digested pFlag-CMV-10 vector. The integrity of the constructs was confirmed by DNA sequencing. Transfection was performed following supplier’s recommendations.
Briefly, cells seeded onto glass coverslips were incubated overnight and then transfected with 3 μg of the corresponding vector premixed with 3 μl of Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA). Transiently transfected cells were analyzed at 24 h post-transfection. When indicated, cells were stably transfected by culturing them for 12 days in the presence of 2
µg/ml puromycin (Invitrogen) or for 5 days in the presence of 800 µg/ml gentamicin (G418), prior to being used for further experiments. For knockdown experiments, cells were stably
transfected with psi-mH1 vector expressing a small interfering RNA (RNAi) specific for mouse DAG1 gene or a scrambled RNAi, as negative control (GeneCopoeia, Inc., Rockville, MD). For
overexpression analysis, cells were stably transfected with GFP constructions as previously reported9. ANTIBODIES The following anti-β-DG primary antibodies were used: rabbit polyclonal
antibodies G5 (Royuela et al, 2001) and Dystroglycan pTyr892 (Ilsley et al, 2002); goat polyclonal antibody C20 (Santa Cruz Biotechnology, CA), and mouse monoclonal antibodies MANDAG233 and
7D11 (Santa Cruz). Rabbit polyclonal antibodies anti-B23 (C19-R), anti-Nup62 (H-122), anti-calnexin (H70), anti-fibrillarin (Ab5821), anti-GFP (sc8334) and mouse monoclonal antibodies
anti-UBF (F9), anti-B23 (NPM1-FC-61991) (anti-RNA polymerase I (sc-46699), anti-lamin B1 (Ab16048), anti-and anti-RPA(sc-46699), and α-Tubulin (sc-32293) were purchased from Santa Cruz
Biotechnology, CA, USA. Rabbit polyclonal anti-Flag (2368) and rabbit monoclonal anti-Notch1 (4147) antibodies were acquired from cell signaling, while mouse monoclonal anti-actin antibody
was a gift from Dr. Manuel Hernández (CINVESTAV, Mexico City). IMMUNOFLUORESCENCE AND CONFOCAL MICROSCOPY ANALYSIS Cells grown on coverslips were fixed with 4% paraformaldehyde for 10 min in
PBS, permeabilized with 0.2% Triton X-100-PBS, blocked with 0.5% fetal bovine serum and 3% bovine serum albumin (BSA) in PBS and incubated overnight at 4 °C with the appropriate primary
antibodies. The following day, cells were washed with 0.2% Triton X-100-PBS for 5 min and then with PBS alone three times, prior to be incubated for 1 h at room temperature with the
appropriate fluorochrome_-_conjugated secondary antibody. For double immunolabeled samples, this was followed by overnight incubation at 4 °C with corresponding primary antibodies and the
next day, cells were incubated with secondary fluorochrome_-_conjugated antibodies. Where indicated F-actin was labeled using TRITC-conjugated Phalloidin (Sigma-Aldrich St. Louis, Mo. USA)
diluted 1:500 in PBS for 10 min at room temperature. For nuclease digestion assays, cells grown on coverslips were treated with either 100 μg/ml protease-free RNase A in CSK buffer (10 mM
Pipes pH 6.8, 100 mM NaCl, 30 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 0.5% (v/v) Triton X-100) for 40 min at 37 °C or with 200 μg/ml protease-free DNase I in PBS with 5 mM MgCl2 for 1 h at 37 °C.
Cells were then immunolabeled using primary antibodies and the corresponding fluorochrome_-_conjugated secondary antibodies. Finally, coverslip preparations were incubated for 20 min at
room temperature with 0.2 µg/ml diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for nuclei visualization, mounted on microscope slides with VectaShield (Vector Laboratories Inc. Burlingame,
CA, USA) and further analyzed by CLSM (TCP-SP5, Leica Microsystems, Heidelberg, Germany) using a Plan Neo Fluor 63 × (NA = 1.4) oil-immersion objective. Analyses of digitized images were
carried out using ImageJ 1.62 software. The nucleolar area (μm2) of 600 nucleoli was analyzed in maxima projection using 3D objects counter and the data were analyzed using Prism6 software.
Manders overlap coefficients were calculated for double labeling inmunofluorescences, using red (Alexa 594 nm) and green (FITC 488 nm) channels and the ImageJ plugin JACoB. A line intensity
scan analysis was performed by ROI manager Multiplot. WESTERN BLOTTING Cell lysates were electrophoresed on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes
(Hybond-Nb, Amersham Pharmacia, GE Healthcare, Bukinghamshire, UK). Membranes were blocked in TBST (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5 % (v/v) Tween-20) with low fat-dried milk and
then incubated overnight at 4 °C with the appropriate primary antibodies. The specific protein signal was developed using the corresponding secondary antibodies and enhanced
chemiluminescence western blotting detection system (ECLTM; Amersham Pharmacia, GE Healthcare), according to the manufacturer's instructions. CELL FRACTIONATION Total cell extracts were
obtained with lysis Buffer (10 mM Tris-HCl pH 8, 30 mM NaCl, 0.2% Triton X-100, 4 mM Na3O4V, 50 mM NaF, 20 mM Na2MoO4, 1 mM PMSF, 1X complete protease inhibitor) sonicated three times at
3.5 μm with 15 s bursts and analyzed by western blotting. Purification of the cytosolic, nuclear, and nucleolar fractions was performed as previously reported34. A total amount of fifteen
100 mm dishes grown to 90% confluence were used; cells were washed twice with 1 ml of ice-cold PBS and collected by centrifugation at 15,000 rpm for 15 min at 4 °C. The pellet was
resuspended in 1 ml of buffer TM (10 mM Tris-HCl pH 8, 2 mM MgCl2, 25 mM NaF, 10 mM Na2MoO4, 2 mM Na3VO4, 1 mM PMSF), supplemented with 1× complete protease inhibitor mixture (Roche Applied
Science, Indianapolis, USA), and incubated on ice for 10 min. Then, 2% Triton X_-_100_-_PBS was added, and the homogenate incubated for 10 min on ice, transferred to a glass Dounce
homogenizer, stroked 30 times with B pestle, and centrifuged at 5,000 rpm for 15 min at 4 °C. The supernatant was recovered as the cytosolic fraction and the nuclear pellet was resuspended
in 1 ml of buffer I (0.32 M Sucrose, 3 mM CaCl2, 2 mM Mg(CH3COO)2, 0.1 mM EDTA, 10 mM Tris-HCl pH 8, 1 mM DTT, 0.5 mM PMSF, 0.5% (v/v) NP40) and 1 ml of Sucrose buffer II (2 M Sucrose, 5 mM
Mg (CH3COO)2, 0.1 mM EDTA, 10 mM Tris-HCl pH 8.0, 1 mM DTT, 0.5 mM PMSF), and further purified by sucrose gradient centrifugation at 16,000 rpm for 1 h at 4 °C. The pellet was resuspended in
600 µl of buffer III (0.34 M Sucrose, 1 mM MgCl2, 0.1 mM EDTA, 10 mM Tris-HCl pH 8.0, 1 mM DTT, 0.5 mM PMSF) and aliquots collected for extraction of nuclear and nucleolar proteins. For
nuclear proteins, the aliquot was centrifuged at 1,000 rpm for 5 min at 4 °C and the pellet resuspended in 200 µl of lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM PMSF, 1% (v/v)
Triton X-100), supplemented with protease inhibitor cocktail and phosphatase inhibitors, sonicated 3 times at 4μm with 15 s bursts and 4 min on ice between intervals, and then pre-cleared at
13,000 rpm for 2 min at 4 °C. For purification of nucleoli, the remaining 350 µl aliquot was sonicated 5 times at 5 μm with 30 s bursts and analyzed under a light microscope to ensure
nucleoli integrity, prior to purification by centrifugation at 3,000 g for 20 min at 4 °C through a sucrose gradient, using buffer III and four volumes of Sucrose buffer IV (0.88 M Sucrose,
0.1 mM EDTA, 10 mM Tris-HCl pH 8.0, 1 mM DTT, 0.5 mM PMSF). Finally, the pellet was washed in 500 µl of buffer III and centrifuged at 2,000 g for 2 min at 4 °C, to save the supernatant as
the nucleolar fraction34. IMMUNOPRECIPITATION Recombinant protein G-agarose beads (10 μl per sample; Invitrogen, Carlsbab, CA, USA) were equilibrated by gently agitation in lysis buffer (50
mM Tris-HCl pH 8, 150 mM NaCl, 1% Triton X-100, 2 mM Na3VO4, 25 mM NaF, 10 mM Na2MoO4, 1 mM PMSF, and 1× complete protease inhibitor mixture). We quantified 500 ug of protein and the lysates
were pre-cleared with equilibrated beads for 2 h at 4 °C, the beads were removed by centrifugation at 3,500 rpm for 5 min and the pre-cleared extracts incubated overnight at 4 °C with the
appropriate antibody. Incubations with an irrelevant IgG antibody were carried out in parallel. Thereafter, equilibrated protein G-agarose beads blocked previously with 4% BSA were added to
the lysates and incubated overnight at 4 °C. Immune complexes were collected by centrifugation at 3,500 rpm for 5 min, washed twice for 10 min with 500 µl of washing buffer (50 mM Tris-HCl,
150 mM NaCl, 5 mM EDTA, 1% Tritón X-100, 1X complete protease inhibitor, 1 mM PMSF). Bound proteins were eluted from beads by boiling in sample buffer (50 mM Tris-HCl pH 6.8, 2% (w/v) SDS,
10% (v/v) glycerol, 0.1% (v/v) 2-mercaptoethanol, 0.001% bromophenol blue), prior to being subjected to western blot analysis. C2C12 cell lysates expressing GFP-tagged proteins were
subjected to IP using the GFP-Trap® system (Chromotek, Germany), according to the manufacturer’s instructions. QUANTITATIVE REVERSE PCR (RT-QPCR) For analysis of rRNA transcripts, total RNA
was extracted using Direct-zol TM RNA Miniprep Kit (Zymo Research, Irvine, CA, USA), according to the manufacturer's instructions. And further analyzed by qRT-PCR with specific primers
amplifying 45 S pre-rRNA (forward, 5′-GTGTCCAAGTGTTCATGCCA; reverse, 5′-CGATCTAAGAGTGAGCAACGAC), 18 S rRNA (forward, 5′-TTCCGACCATAAACGATGCC; reverse, 5′-GCTCCACCAACTAAGAACGG), 28 S rRNA
(forward, 5′-GATGGTGAACTATGCTTGGG; reverse, 5′-GAATAGGTTGAGATCGTTTCGG), and GAPDH mRNA (forward, 5′-CTTGGGCTACACTGAGGACC; reverse, 5′-CTGTTGCTGTAGCCGTATTC). For analysis of _DAG1_ mRNA
expression, the following primers were used: forward, 5′-GAGATCATCAAGGTGTCTGCA and reverse, 5′-GTGGCTCATTGTGGTCTTCAG. RT-qPCR reactions were carried out in the StepOneplus System (Life
technologies), using KAPA SYBR® FAST One-Step qRT-PCR Master Mix (2 × ) Kit, and further analyzed with the StepOne Software v2.3. The data were analyzed by comparative method 2−ΔΔ_CT_. RNA
POL I REPORTER ASSAYS C2C12 cells stably expressing GFP-ICD-βDG or GFP alone were co-transfected with both the Renilla Luciferase vector (transcribed by RNA polymerase II) as a control to
normalize for differences in transfection efficiency and with the firefly luciferase RNA polymerase I reporter vector (pMrTsp-9-T10). The two vectors were kindly donated by Gernot Längst
Lab., Regensburg University, Germany. After 48 h, the luciferase activity was measured using Dual Luciferase Assay kit (Promega). The firefly luciferase counts of the RNA polymerase I
reporter were divided by the Renilla luciferase counts and compared to GFP control transfections. CELL PROLIFERATION ASSAYS C2C12 cells stably expressing GFP-ICD-βDG or GFP alone were
harvested and plated in triplicate onto 12 wells microplates at 1 × 103 cells/mL confluence. Proliferation was measured for 13 days using the MTT (3-(4,5-dimethylthiazole)− 2–5-diphenyl
tetrazolium bromide) commercial kit (Sigma-Aldrich), following the manufacturer’s instructions. CHROMATIN IMMUNOPRECIPITATION ASSAYS C2C12 cells were incubated with 1% formaldehyde in PBS
for 10 min at room temperature. Cross-link reaction was stopped by adding glycine to a final concentration of 125 mM. Cells were washed and harvested with cold PBS. After centrifugation at
1500 g, the pellet was resuspended in sonication buffer (50 mM Hepes pH 7.9, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS and protease inhibitors). Soluble and
sheared chromatin were obtained by applying five cycles of sonication on ice at 10% of amplitude for 20 s (Q55 sonicator, Qsonica) and recovered by centrifugation. Soluble chromatin was
pre-clarified by mixing with 50 µl of recombinant protein G-agarose beads (rPGA Invitrogen) and incubated with shaking for 1 h at 4 °C. The mix was centrifuged at 3,000 × g for 1 min to
remove rPGA. Twenty five micrograms of pre-clarified chromatin diluted 1:10 in sonication buffer were mixed with 7.8 µg of mouse purified serum anti-β-dystroglycan or 7.8 µg of mouse IgG
(irrelevant control). Finally 25 µl of rPGA were added to each sample and incubate with shaking overnight at 4 °C. Immunoprecipitated DNA-protein complexes were extensively and sequentially
washed with sonication buffer, wash buffer A (50 mM Hepes pH 7.9, 500 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS), wash buffer B (20 mM Tris-Cl pH 8, 1 mM EDTA,
250 mM LiCl, 0.5% NP40, 0.5% deoxycholate), and TE buffer. Finally, the pellet was incubated with shaking for 10 min at 65 °C in elution buffer (50 mM Tris-Cl pH 8, 1 mM EDTA, 1% SDS, 50 mM
NaHCO3). Supernatant was recovered by centrifugation and the cross-link reversion was carried out by adding RNAase A and Proteinase K to each sample and incubating with shaking for 4 h at 65
°C. DNA was recovered by phenol–chloroform extraction and ethanol precipitation. Twenty nanograms of DNA was used to perform quantitative PCR (qPCR), using SYBR green and rDNA-specific
primers as previously reported35,36. RIBOSOMAL PROFILE ANALYSIS Cells grown at 80–90% confluency were harvested, washed with PBS with 100 μg/mL cycloheximide and centrifuged at 5,000 rpm for
5 min at 4 °C, prior to suspension in 200 μl of lysis buffer containing 100 μg/mL cycloheximide and 1× complete protease inhibitor mixture. Thereafter cells were centrifuged for 10 min at
10,000 rpm and the supernatant was layered on top of a 15–50% sucrose gradient to be centrifuged at 25,000 rpm for 5:30 h at 4 °C in a Beckman SW28Ti rotor. All gradients were scanned at 260
nm from the top with an ISCO gradient collector. Collected fractions were precipitated with ethanol and centrifuged at 15,000 rpm for 30 min to obtain ribosomal particles. REFERENCES *
Vieceli Dalla Sega, F. et al. Context-dependent function of ROS in the vascular endothelium: The role of the Notch pathway and shear stress. _Biofactors_ 43, 475–485 (2017). Article CAS
Google Scholar * Walter, J., Kemmerling, N., Wunderlich, P. & Glebov, K. γ-Secretase in microglia - implications for neurodegeneration and neuroinflammation. _J. Neurochem._ 143,
445–454 (2017). Article CAS Google Scholar * Leocadio, D., Mitchell, A. & Winder, S. J. γ-Secretase dependent nuclear targeting of dystroglycan. _J. Cell. Biochem_. 117, 2436 (2016).
* Leiton, C. V. et al. Laminin promotes metalloproteinase-mediated dystroglycan processing to regulate oligodendrocyte progenitor cell proliferation. _J. Neurochem._ 135, 522–538 (2015).
Article CAS Google Scholar * Moore, C. J. & Winder, S. J. Dystroglycan versatility in cell adhesion: a tale of multiple motifs. _Cell. Commun. Signal._ 8, 3 (2010). Article Google
Scholar * Moore, C. J. & Winder, S. J. The inside and out of dystroglycan post-translational modification. _Neuromuscul. Disord._ 22, 959–965 (2012). Article Google Scholar * Bozzi,
M., Morlacchi, S., Bigotti, M. G., Sciandra, F. & Brancaccio, A. Functional diversity of dystroglycan. _Matrix Biol._ 28, 179–187 (2009). Article CAS Google Scholar * Vásquez-Limeta,
A. et al. Nuclear import of β-dystroglycan is facilitated by ezrin-mediated cytoskeleton reorganization. _PLoS ONE_ 9, e90629 (2014). Article Google Scholar * Lara-Chacón, B. et al.
Characterization of an importin alpha/beta-recognized nuclear localization signal in beta-dystroglycan. _J. Cell. Biochem._ 110, 706–717 (2010). Article Google Scholar * Gracida-Jiménez,
V. et al. Retrograde trafficking of β-dystroglycan from the plasma membrane to the nucleus. _Sci. Rep._ 7, 9906 (2017). Article Google Scholar * Martínez-Vieyra, I. A. et al. A role for
β-dystroglycan in the organization and structure of the nucleus in myoblasts. _Biochim. Biophys. Acta_ 1833, 698–711 (2013). Article Google Scholar * Vélez-Aguilera, G. et al. Control of
nuclear β-dystroglycan content is crucial for the maintenance of nuclear envelope integrity and function. _Biochim. Biophys. Acta_ 1865, 406–420 (2018). Article Google Scholar * Mathew, G.
et al. Nuclear targeting of dystroglycan promotes the expression of androgen regulated transcription factors in prostate cancer. _Sci. Rep._ 3, 2792 (2013). Article CAS Google Scholar *
Sbardella, D. et al. Enzymatic processing by MMP-2 and MMP-9 of wild-type and mutated mouse β-dystroglycan. _IUBMB Life_ 64, 988–994 (2012). Article CAS Google Scholar * Sbardella, D. et
al. α-dystroglycan is a potential target of matrix metalloproteinase MMP-2. _Matrix Biol._ 41, 2–7 (2015). Article CAS Google Scholar * Mitchell, A. et al. Dystroglycan function is a
novel determinant of tumor growth and behavior in prostate cancer. _Prostate_ 73, 398–408 (2013). Article CAS Google Scholar * Sleeman, J. E. & Trinkle-Mulcahy, L. Nuclear bodies: new
insights into assembly/dynamics and disease relevance. _Curr. Opin. Cell Biol._ 28, 76–83 (2014). Article CAS Google Scholar * Sharifi, S. & Bierhoff, H. Regulation of RNA polymerase
I transcription in development, disease, and aging. _Annu. Rev. Biochem._ https://doi.org/10.1146/annurev-biochem-062917-012612 (2018). * Riemersma, M. et al. Absence of α- and
β-dystroglycan is associated with Walker-Warburg syndrome. _Neurology_ 84, 2177–2182 (2015). Article CAS Google Scholar * Tsekrekou, M., Stratigi, K. & Chatzinikolaou, G. The
Nucleolus: In Genome Maintenance and Repair. _Int. J. Mol. Sci_. 18, https://doi.org/10.3390/ijms18071411 (2017). * Shav-Tal, Y. et al. Dynamic sorting of nuclear components into distinct
nucleolar caps during transcriptional inhibition. _Mol. Biol. Cell._ 16, 2395–2413 (2005). Article CAS Google Scholar * Lipscomb, L., Piggott, R. W., Emmerson, T. & Winder, S. J.
Dasatinib as a treatment for Duchenne muscular dystrophy. _Hum. Mol. Genet._ 25, 266–274 (2016). Article CAS Google Scholar * Bozzi, M. et al. Enzymatic processing of beta-dystroglycan
recombinant ectodomain by MMP-9: identification of the main cleavage site. _IUBMB Life_ 61, 1143–1152 (2009). Article CAS Google Scholar * Martin, C. et al. Lamin B1 maintains the
functional plasticity of nucleoli. _J. Cell. Sci._ 122, 1551–1562 (2009). Article CAS Google Scholar * Legartová, S. et al. Nuclear structures surrounding internal lamin invaginations.
_J. Cell. Biochem._ 115, 476–487 (2014). Article Google Scholar * Sen Gupta, A. & Sengupta, K. Lamin B2 modulates nucleolar morphology, dynamics, and function. _Mol. Cell. Biol._ 37,
https://doi.org/10.1128/MCB.00274-17 (2017). * Christie, M. et al. Structural biology and regulation of protein import into the nucleus. _J. Mol. Biol._ 428, 2060–2090 (2016). Article CAS
Google Scholar * Lindström, M. S. NPM1/B23: a multifunctional chaperone in ribosome biogenesis and chromatin remodeling. _Biochem. Res. Int._ 2011, 195209 (2011). Article Google Scholar *
Panov, K. I., Friedrich, J. K., Russell, J. & Zomerdijk, J. C. UBF activates RNA polymerase I transcription by stimulating promoter escape. _EMBO J._ 25, 3310–3322 (2006). Article CAS
Google Scholar * Aubert, M., O’Donohue, M. F., Lebaron, S. & Gleizes, P. E. Pre-ribosomal RNA processing in human cells: from mechanisms to congenital diseases. _Biomolecules_ 8,
https://doi.org/10.3390/biom8040123 (2018). * Yang Kai, Y. J. & Jing, Ji Nucleolar Stress: hallmarks, sensing mechanism and diseases. _Cell Stress_ 2, 125–140 (2018). Article Google
Scholar * Németh, A. & Grummt, I. Dynamic regulation of nucleolar architecture. _Curr. Opin. Cell Biol._ 52, 105–111 (2018). Article Google Scholar * Pereboev, A. V., Ahmed, N., thi
Man, N. & Morris, G. E. Epitopes in the interacting regions of beta-dystroglycan (PPxY motif) and dystrophin (WW domain). _Biochim. Biophys. Acta_ 1527, 54–60 (2001). Article CAS
Google Scholar * Hernández-Ibarra, J. A., Laredo-Cisneros, M. S., Mondragón-González, R., Santamaría-Guayasamín, N. & Cisneros, B. Localization of α-dystrobrevin in cajal bodies and
nucleoli: a new role for α-dystrobrevin in the structure/stability of the nucleolus. _J. Cell. Biochem._ 116, 2755–2765 (2015). Article Google Scholar * Todd, M. A., Huh, M. S. &
Picketts, D. J. The sub-nucleolar localization of PHF6 defines its role in rDNA transcription and early processing events. _Eur. J. Hum. Genet._ 24, 1453–1459 (2016). Article CAS Google
Scholar * Hisaoka, M., Ueshima, S., Murano, K., Nagata, K. & Okuwaki, M. Regulation of nucleolar chromatin by B23/nucleophosmin jointly depends upon its RNA binding activity and
transcription factor UBF. _Mol. Cell. Biol._ 30, 4952–4964 (2010). Article CAS Google Scholar Download references ACKNOWLEDGMENTS This work was funded by CONACYT Mexico, a grant to BC
(237123) and MRC PhD studentship to LAJ (G1000405-1/1 MR//J500513/1. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departamento de Genética y Biología Molecular, Centro de Investigación y de
Estudios Avanzados Del Instituto Politécnico Nacional, 07360, Ciudad de México, Mexico Paulina Margarita Azuara-Medina, Ariana María Sandoval-Duarte, Ricardo Modragón-González, Griselda
Vélez-Aguilera, Juan de Dios Gómez-López, Guadalupe Elizabeth Jiménez-Gutiérrez, Reynaldo Tiburcio-Félix & Bulmaro Cisneros * Departamento de Neurociencia Cognitiva, Instituto de
Fisiología Celular, Universidad Nacional Autónoma de México, 04510, Ciudad de México, Mexico Sara L. Morales-Lázaro * Laboratorio de Hematobiología, Escuela Nacional de Medicina y
Homeopatía, Instituto Politécnico Nacional, 07320, Ciudad de México, Mexico Ivette Martínez-Vieyra * Laboratorio de Medicina Genómica, Instituto Nacional de Rehabilitación, 14389, Ciudad de
México, Mexico Rocío Suárez-Sánchez & Jonathan Javier Magaña * Biochemistry Centre Regensburg (BCR), Universität Regensburg, 93053, Regensburg, Germany Gernot Längst * Department of
Biomedical Science, University of Sheffield, Sheffield, S10 2TN, UK Steve J. Winder & Laura A. Jacobs * Departamento de Toxicología, Centro de Investigación y de Estudios Avanzados Del
Instituto Politécnico Nacional, 07000, Ciudad de México, Mexico Arturo Ortega * Department of Medicine, Lillehei Heart Institute, University of Minnesota, Minneapolis, MN, USA Rita de Cassia
Ramos Perlingeiro Authors * Paulina Margarita Azuara-Medina View author publications You can also search for this author inPubMed Google Scholar * Ariana María Sandoval-Duarte View author
publications You can also search for this author inPubMed Google Scholar * Sara L. Morales-Lázaro View author publications You can also search for this author inPubMed Google Scholar *
Ricardo Modragón-González View author publications You can also search for this author inPubMed Google Scholar * Griselda Vélez-Aguilera View author publications You can also search for this
author inPubMed Google Scholar * Juan de Dios Gómez-López View author publications You can also search for this author inPubMed Google Scholar * Guadalupe Elizabeth Jiménez-Gutiérrez View
author publications You can also search for this author inPubMed Google Scholar * Reynaldo Tiburcio-Félix View author publications You can also search for this author inPubMed Google Scholar
* Ivette Martínez-Vieyra View author publications You can also search for this author inPubMed Google Scholar * Rocío Suárez-Sánchez View author publications You can also search for this
author inPubMed Google Scholar * Gernot Längst View author publications You can also search for this author inPubMed Google Scholar * Jonathan Javier Magaña View author publications You can
also search for this author inPubMed Google Scholar * Steve J. Winder View author publications You can also search for this author inPubMed Google Scholar * Arturo Ortega View author
publications You can also search for this author inPubMed Google Scholar * Rita de Cassia Ramos Perlingeiro View author publications You can also search for this author inPubMed Google
Scholar * Laura A. Jacobs View author publications You can also search for this author inPubMed Google Scholar * Bulmaro Cisneros View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Bulmaro Cisneros. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest.
ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by S. Lavandero
SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURES SUPPLEMENTAL FIGURE LEGENDS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Azuara-Medina, P.M., Sandoval-Duarte, A.M., Morales-Lázaro, S.L. _et al._ The
intracellular domain of β-dystroglycan mediates the nucleolar stress response by suppressing UBF transcriptional activity. _Cell Death Dis_ 10, 196 (2019).
https://doi.org/10.1038/s41419-019-1454-z Download citation * Received: 26 September 2018 * Revised: 21 January 2019 * Accepted: 11 February 2019 * Published: 27 February 2019 * DOI:
https://doi.org/10.1038/s41419-019-1454-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
