Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Neutrophil extracellular traps (NETs) may play a critical role in smoking-related chronic airway inflammation. However, the mechanism by which NETs induced by cigarette smoke
initiate the adaptive immunity in chronic obstructive pulmonary disease (COPD) is not fully understood. In this study, we explored the effects of NETs induced by cigarette smoke on the
myeloid dendritic cells (mDCs) and Th1 and Th17 cells. Additionally, we observed the inhibitory effect of erythromycin on NETs induced by cigarette smoke. We found that elevated NET levels
in the sputum of COPD patients were correlated with the circulating Th1 response, mDC activation and airflow limitation. NETs induced by cigarette smoke extract (CSE) could activate
monocyte-derived mDCs and promote Th1 and Th17 differentiation in vitro. Erythromycin effectively inhibited NET formation induced by CSE. In vivo, erythromycin decreased NETs in the airway
and ameliorated emphysema with Th1 and Th17 cell down-regulation and CD40+ and CD86+ mDCs suppression in mice chronically exposed to cigarette smoke. These findings provide direct evidence
that NETs promote the differentiation of Th1 and Th17 and play a role in the adaptive immunity of smoking-related chronic lung inflammation. Erythromycin is a potential therapeutic strategy
for NETs inhibition in COPD. SIMILAR CONTENT BEING VIEWED BY OTHERS DNA OF NEUTROPHIL EXTRACELLULAR TRAPS PROMOTE NF-ΚB-DEPENDENT AUTOIMMUNITY VIA CGAS/TLR9 IN CHRONIC OBSTRUCTIVE PULMONARY
DISEASE Article Open access 17 June 2024 THE EFFECTS OF THE MIR-21/SMAD7/TGF-Β PATHWAY ON TH17 CELL DIFFERENTIATION IN COPD Article Open access 18 March 2021 INHALATION OF PATCHOULI
ESSENTIAL OIL ALLEVIATES AIRWAY INFLAMMATION IN CIGARETTE SMOKE-INDUCED COPD MICE Article Open access 30 December 2024 INTRODUCTION Chronic obstructive pulmonary disease (COPD) is a
progressive disease associated with abnormal airway and alveolar inflammatory responses to cigarette smoke or other noxious particles and gases1. Owing to its high mortality and morbidity,
COPD has become a serious global health issue2. Cigarette smoking is the major risk factor for COPD and directly promotes airway neutrophilic inflammation3. Interestingly, cigarette smoke
extract (CSE) or nicotine can trigger neutrophil extracellular trap (NET) formation4,5. NETs were initially described as web-like DNA structures produced by activated neutrophils during the
response to pathogens6. More recently, studies have indicated that NETs are involved in many non-infectious disorders and chronic inflammatory conditions, such as systemic lupus
erythematosus7, autoimmune small-vessel vasculitis8, rheumatoid arthritis9, atherosclerosis10, and others11,12,13,14. Previous studies have revealed that excessive NETs in the sputum are
associated with elevated pro-inflammatory cytokine levels and thus, might contribute to lung damage15,16. However, most studies have focused on the direct pathological effect of NETs17,18,
and relatively little is known about the contribution of NETs to adaptive immunity. The initiation and dysregulation of adaptive immunity are related, in part, to the enhancement and
persistence of airway inflammation in COPD. We recently demonstrated that NETs released by activated polymorphonuclear neutrophils (PMNs) stimulated with CSE promote the activation of
plasmacytoid dendritic cells (DCs) in mice and, subsequently initiate pathologic T cell responses5, suggesting that NETs serve as critical connectors between the innate and adaptive immunity
and could serve as a potential target for COPD treatment. However, whether NETs induced by CSE could activate DCs in human remains to be elucidated. Persistent neutrophilic infiltration in
the airway is a typical feature of smoking-related COPD, which represents an obstacle for conventional therapeutic regimens. The anti-inflammatory and immuno-modulating effects of
macrolides, such as erythromycin, have long been recognised. Emerging evidence has shown that long-term and low-dose erythromycin can inhibit airway neutrophilic inflammation and reduce
acute exacerbations in patients with COPD19. Macrolides are now recommended by a series of clinical guidelines to prevent acute exacerbation of COPD2,20, but the mechanisms underlying their
inhibitory effects on neutrophilic inflammation are still unclear. Several previous studies have reported that erythromycin could attenuate pulmonary inflammation, protect against the
development of emphysema, and alter different types of pulmonary cells in murine models21,22,23,24. However, it is unclear whether erythromycin suppresses NET formation under stimulation
with cigarette smoke and thus, modulates adaptive immunity. Here, we sought to explore the effects of NETs induced by cigarette smoke on the differentiation of Th1 and Th17. We also observed
the effect of erythromycin on NET formation during chronic inflammation induced by cigarette smoke exposure. MATERIALS AND METHODS Detailed methods are provided in the online supplement.
HUMAN BLOOD AND SPUTUM SPECIMEN COLLECTION AND PREPARATION Human blood and sputum samples were collected from 32 patients with COPD and 16 healthy controls. The diagnosis of COPD was based
on forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) detected by post-bronchodilator spirometry (FEV1 < 80% predicted and FEV1/FVC < 70%) established in the Global
Initiative for Chronic Obstructive Lung Disease (GOLD)24. The grading of severity of airflow limitation in COPD was defined as: GOLD stage 1: FEV1 ≥ 80% predicted; GOLD stage 2: 50% ≤ FEV1
< 80% predicted; GOLD stage 3: 30% ≤ FEV1 < 50% predicted; GOLD stage 4: FEV1 < 30% predicted24. Peripheral blood mononuclear cells (PBMCs) were separated by Lymphoprep (Stemcell
Technologies, Canada) centrifugation. Th1 (CD4+ IFN-γ+ T) and Th17 (CD4+ IL-17+ T) cells as well as CD40+ and CD86+ myeloid dendritic cells (mDCs) in PBMCs were analysed by flow cytometry.
In some experiments, PBMCs of healthy donors were used to generate monocyte-derived mDCs or to isolate naïve CD4+ T lymphocytes. PMNs in the blood were isolated as described previously25.
Sputum induction was performed as previously described26. Cell-free extracellular DNA in sputum was detected by using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA)
following the manufacturer’s instructions. All the subjects provided informed consents, and the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University granted
ethical approval. ANIMALS Thirty male C57BL/6J mice (8 weeks old) were randomly divided into three groups: Air group, cigarette smoke (CS) group, and erythromycin (EM) group (_n_ =
10/group). The CS group and EM group were exposed to cigarette smoke for 24 weeks as previously described27. The Air group was exposed to room air for 24 weeks. In the EM group, erythromycin
(Sigma-Aldrich, St. Louis, MO, USA) was orally administered at 100 mg/kg/d from the 12th week of cigarette exposure. In the CS and Air group, an identical volume of vehicle was administered
orally. At the end of the 24th week, mice were anaesthetised and sacrificed. PMNs were isolated using peripheral blood neutrophil separation medium for mouse (TBD, Tianjin, China).
Bronchoalveolar lavage fluid (BALF) was collected. Extracellular DNA in BALF was detected by PicoGreen. The total cell count in the BALF was determined in a Bürcker chamber, and the
neutrophil cell count was obtained after Wright’s Giemsa staining. The severity of emphysema was assessed by the mean linear intercept (MLI). Single-cell suspensions from the lungs were
prepared for flow cytometry as described previously28. All mice were purchased from the Guangxi Medical University Laboratory of Animal Centre and all animal experiments in this study were
approved by the Laboratory Animal Ethics Committee of Guangxi Medical University (Nanning, China). IMMUNOFLUORESCENCE OF NETS NETs were stained for DNA with propidium iodide (PI;
Sigma-Aldrich) or 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich). In some experiment, neutrophil elastase (NE), myeloperoxidase (MPO), and citrullinated histone H3
(CITH3) on NETs were observed by immunofluorescence staining. CSE-INDUCED NETS ASSAY CSE was prepared as described previously29. Freshly isolated PMNs from the blood of COPD patients or mice
with emphysema were seeded in 24-well culture plates (1.0 × 106 cells) with serum-free RPMI 1640. PMNs were stimulated with 0.3% CSE for 4 h, with or without pre-treatment with erythromycin
(10 μg/mL for human samples; 2 μg/mL for mice) for 30 min. Phorbol-12-myristate-13-acetate (PMA, 100 nmol/L = 61.68 μg/mL; Sigma-Aldrich) and diphenyl iodine (DPI; Sigma-Aldrich) were used
in some of the experiments as positive controls for stimulating and inhibiting NETs, respectively. Cell-free extracellular DNA in supernatants was detected using PicoGreen. For NE and MPO
concentration assay, PMNs (2.0 × 106 cells) were seeded in 24-well culture plates and stimulated with 0.3% CSE for 4 h with or without pre-treatment of erythromycin for 30 min.
NET-associated NE and MPO were detected using the NETosis Assay kit (Cayman Chemical, Ann Arbor, MI, USA) and MPO ELISA kit (Cusabio, Wuhan, China), respectively. For the intracellular
reactive oxygen species (ROS) production assay, PMNs (2.0 × 106 cells) of patients with COPD were seeded in 24-well culture plates and stimulated with 0.3% CSE for 1 h in a CO2 incubator at
37 °C with or without pre-treatment of erythromycin or DPI for 30 min. After stimulation, PMNs were harvested and further incubated with 1 μmol/L ROS probe 2′,7′-dichlorofluorescein
diacetate (DCFH-DA; Sigma-Aldrich) at 37 °C for 30 min. The DCFH-DA stained PMNs were detected by flow cytometry immediately. PREPARATION OF MONOCYTE-DERIVED MDCS Monocytes isolated by
adherence from PBMCs of healthy donors were cultured with RPMI 1640 containing 10% FBS, 1000 IU/mL granulocyte-macrophage colony stimulating factor (GM-CSF; Peprotech, Rocky Hill, USA) and
500 IU/mL IL-4 (Peprotech) for 6 days as previously described30. At day 6, immature mDCs were harvested and isolated by positive selection (CD209 (DC-SIGN) MicroBead Kit, human, Miltenyi
Biotec, Aubum, CA). PREPARATION OF CSE-INDUCED NETS AND STIMULATION OF MDCS PMNs from patients with COPD were stimulated with 0.3% CSE for 4 h. CSE-induced NETs were collected as previously
described5. To determine the effect of CSE-induced NETs on mDCs maturation, the purified mDCs were plated in 6-well plates at a density of 1.0 × 106 cells/mL in the presence or absence of
CSE-induced NETs (20 ng/mL) for 15 h. Subsequently, CD11c, CD40, CD86, and HLA-DR on the surface of mDCs were analysed by flow cytometry. The supernatants of mDCs were collected and detected
IL-1β, IL-12, and TNF-α by ELISA. In some experiments, mDCs were generated for assessing the capacity to promote the differentiation of naïve CD4+ T lymphocytes. The purified immature mDCs
of day 6 were primed with IFN-γ (10 ng/mL; Peprotech) until day 7. Then the primed mDCs were stimulated with or without NETs (20 ng/mL) for 24 h. CD4+ naïve T cells isolated by negative
selection (Naive CD4+ T Cell Isolation Kit II human, Miltenyi) from PBMCs isolated from blood of healthy donors were cultured with mDCs (pre-treated with or without NETs) at a DC/T cell
ratio of 1:5 for 4 days in 24-well plates. Cells were stimulated with 50 ng/mL PMA and 1 µg/mL ionomycin in the presence of GolgiStop (BD Pharmingen, San Diego, CA, USA) for 5 h. Cells were
then collected and stained for CD4, IFN-γ and IL-17A which were detected by flow cytometry. FLOW CYTOMETRY The detection of surface molecule, intracellular cytokine, and intracellular ROS
were performed by flow cytometry. Detailed methods are provided in the online supplementary file. ELISA The IL-1β, IL-12, and TNF-α concentration in supernatants of mDCs and the soluble NE,
MPO, and CITH3 levels in BALF of mice were measured by ELISA (IL-1β, IL-12, TNF-α, and MPO: Cusabio; NE: USCN Life Sciences, Wuhan, China; CITH3: Cayman Chemical) following the
manufacturer’s instructions. STATISTICAL ANALYSIS Results were expressed as medians. Comparisons between two groups were evaluated using Mann–Whitney test. Comparisons between three or more
groups were evaluated using Kruskal–Wallis one-way ANOVA on ranks. The correlation analyses were performed using Spearman’s rank correlation coefficient. Analyses were implemented in SPSS
version 17.0 and _P_ _<_ 0.05 was considered significant. RESULTS CHARACTERISTICS OF PARTICIPANTS The characteristics of the 32 patients with COPD and the 16 healthy controls are shown in
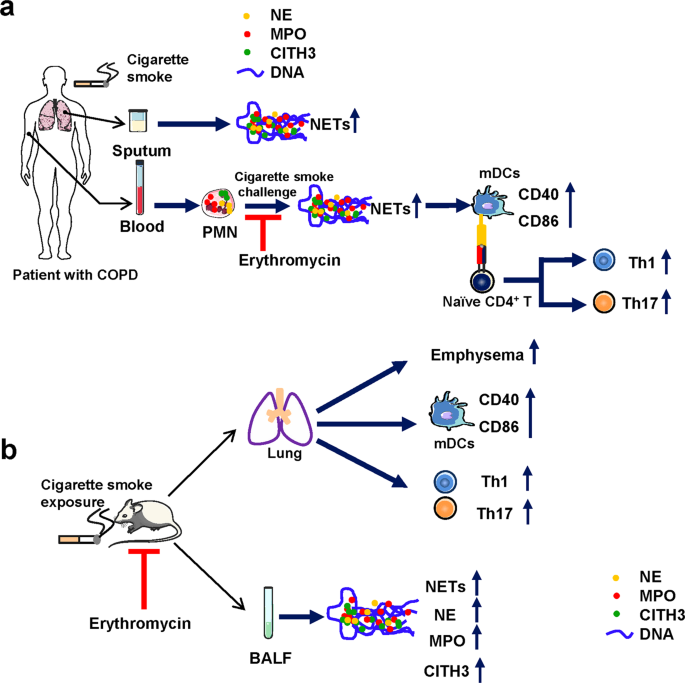
Table 1. NETS ARE ABUNDANTLY PRODUCED IN THE SPUTUM OF PATIENTS WITH COPD A flow diagram of the experimental design of this study is shown in Fig. 1. We first observed NETs in the sputum of
healthy controls and patients with COPD. Under the fluorescence microscopy, the patients with COPD presented a considerable amount of spontaneous NETs in sputum (Fig. 2a). As determined by
PicoGreen, the extracellular DNA concentration in the sputum was markedly higher in individuals with COPD than in healthy controls (_P_ _<_ 0.001; Fig. 2b). In addition, we observed that
NE, MPO, and CITH3 were embedded in the network structure of extracellular DNA in the sputum of COPD patients (Fig. 2c). PMNS ISOLATED FROM COPD PATIENTS ARE MORE SENSITIVE TO CSE We
previously observed that PMNs of mice with emphysema seem to be highly sensitive to NET production16. To investigate whether PMNs of COPD were more susceptible to NET formation than those of
healthy controls, we used 0.3% CSE to trigger NET formation in the blood PMNs of healthy controls and COPD patients and observed NET formation (Fig. 3a). As determined by PicoGreen, the
PMNs from both COPD patients and healthy controls produced more extracellular DNA under the stimulation of CSE than their un-stimulated controls (Both _P_ _<_ 0.05; Fig. 3b). Notably, The
PMNs from COPD patients produced more extracellular DNA than those from healthy controls under the stimulation of CSE (_P_ _<_ 0.001; Fig. 3b). We observed NE, MPO, and CITH3 in the
network structure of CSE-induced NETs in the blood of COPD patients by immunofluorescence (Fig. 3c). We also quantitatively analysed the extracellular DNA, and NET-associated NE and MPO
released by CSE-stimulated PMNs from COPD patients. The levels of extracellular DNA, as well as NET-associated NE and MPO were significantly increased by CSE stimulation (All _P_ _<_
0.001; Fig. 3d). CORRELATIONS BETWEEN NETS IN SPUTUM, TH1/TH17 RESPONSES, DCS ACTIVATION, AND THE SEVERITY OF COPD We observed Th1 and Th17 cells as well as the costimulatory molecules CD40
and CD86 on mDCs in PBMCs by flow cytometry (Fig. 4a, b). Th1 and Th17 cells were more prominent in COPD patients than in healthy controls (both _P_ _<_ 0.01; Fig. 4c). Moreover,
circulating mDCs in patients showed a more activated phenotype with elevated expression of CD40 and CD86 (_P_ _<_ 0.05 and _P_ _<_ 0.001, respectively; Fig. 4c). Next, we assessed the
correlations between NETs in the sputum, and circulating Th1 and Th17 responses, and the activation of mDCs in COPD patients. Th1 cells and CD40 on mDCs were positively correlated with
extracellular DNA in the sputum (_r_ = 0.723, _P_ _<_ 0.001; _r_ = 0.391, _P_ = 0.027, respectively; Fig. 4d). However, there was no correlation between the frequencies of circulating
Th17 cells or CD86 and the levels of extracellular DNA in sputum (_r_ = 0.045, _P_ = 0.805; _r_ = 0.250, _P_ = 0.168, respectively; Fig. 4d). Moreover, we found that extracellular DNA in the
sputum was negatively correlated with percent predicted FEV1 (_r_ = −0.437, _P_ _=_ 0.013), and positively correlated with GOLD stage (_r_ = 0.360, _P_ _=_ 0.043; Fig. 4e). However,
extracellular DNA in sputum did not correlate with age, smoking pack years or body mass index in patients with COPD (Table 2). NETS INDUCED BY CSE PROMOTE THE ACTIVATION OF MDCS AND THE
GENERATION OF TH1 AND TH17 CELLS IN VITRO To test if NETs induced by CSE could directly contribute to the activation of mDCs and promote the generation of Th1 and Th17, we isolated and used
CSE-induced NETs to simulate mDCs, and then co-cultured CD4+ naïve T cells with mDCs pre-treated with or without NETs. As expected, mDCs stimulated with CSE-induced NETs for 15 h expressed
higher levels of CD40, CD86 and HLA-DR than un-stimulated mDCs (_P_ _<_ 0.01, _P_ _<_ 0.01 and _P_ _<_ 0.05, respectively; Fig. 5a, b). Meanwhile, the levels of IL-1β, IL-12, and
TNF-α were higher in the supernatant of mDCs stimulated with CSE-induced NETs than in those without NETs stimulation (_P_ _<_ 0.05, _P_ _<_ 0.01 and _P_ _<_ 0.05, respectively; Fig.
5c). Regarding antigen-specific T-cell response, CD4+ naïve T cells cultured with mDCs pre-treated with NETs generated more Th1 and Th17 cells than those cultured with un-stimulated mDCs
(_P_ _<_ 0.01 and _P_ _<_ 0.05, respectively; Fig. 5d, e). ERYTHROMYCIN EFFECTIVELY INHIBITS NET FORMATION AND ROS PRODUCTION INDUCED BY CSE IN VITRO To evaluate the effects of
erythromycin on NET formation, we pre-treated human and mice neutrophils with erythromycin before stimulating NET formation with CSE in vitro. The immunofluorescence results showed that
erythromycin- or DPI-pretreated PMNs from COPD patients had fewer filaments (Fig. 6a). Using PicoGreen, we found a lower extracellular DNA concentration in the supernatants of PMNs
pre-treated with erythromycin before stimulation, than in PMNs stimulated with CSE alone (_P_ _<_ 0.001; Fig. 6b). In addition, NET-associated NE and MPO were also decreased in PMNs
pre-treated with erythromycin when compared to PMNs stimulated by CSE alone (_P_ _<_ 0.001 and _P_ _<_ 0.01; Fig. 6b). Moreover, we observed an increased level of ROS production in
PMNs stimulated with CSE (_P_ _<_ 0.05; Fig. 6c), which could be suppressed by DPI, the inhibitor of phagocyte NADPH oxidase (_P_ _<_ 0.05; Fig. 6c). Intracellular ROS production was
also decreased in PMNs pre-treated with erythromycin when compared to PMNs stimulated by CSE alone (_P_ _<_ 0.05; Fig. 6c). For PMNs of mice with emphysema, erythromycin-pretreated PMNs
had fewer NETs than those without erythromycin pre-treatment (Fig. 7a). NE, MPO, and CITH3 were observed on CSE-induced NETs by immunofluorescence (Fig. 7b). We found that extracellular DNA
as well as NET-associated NE and MPO were lower in the erythromycin-pretreated PMNs than in PMNs stimulated by CSE alone (All _P_ _<_ 0.05; Fig. 7c). ERYTHROMYCIN INHIBITS NETS, WITH
AMELIORATION OF EMPHYSEMA, THE DOWN-REGULATION OF TH1/TH17 RESPONSES AND MDCS ACTIVATION IN MICE EXPOSED TO CIGARETTE SMOKE The flow diagram of the experimental design of the mice
experiments is shown in Fig. 1b. The mice in the EM group had significantly lower levels of extracellular DNA and soluble NE, MPO, and CITH3 in their BALF than those in the CS group (All _P_
_<_ 0.05; Fig. 8a). In addition, neutrophils count and the proportions of CD11b+Ly-6G+ neutrophils in BALF were also decreased in the erythromycin-treated group compared with CS group
(Fig. 8b–d). The MLI was lower in the EM group than in the CS group (_P_ _<_ 0.05; Fig. 8e, f) suggesting a reduction of emphysema in mice treated with erythromycin. Moreover, the MLI of
mice with emphysema positively correlated with the levels of extracellular DNA in the BALF (r = 0.845, _P_ _=_ 0.002; Fig. 8g). Furthermore, we observed that mice that treated with
erythromycin expressed lower levels of CD40 and CD86 on pulmonary mDCs, along with decreases in Th1 cells and Th17 cells in the lungs than untreated mice (All _P_ _<_ 0.05; Fig. 9a–d).
Moreover, Th1 and Th17 cells, and CD40 and CD86 in the lungs of mice with emphysema positively correlated with the levels of extracellular DNA in BALF (r = 0.758, _P_ _=_ 0.011; r = 0.830,
_P_ _=_ 0.003; r = 0.855, _P_ _=_ 0.002; r = 0.673, _P_ _=_ 0.033; respectively; Fig. 9e). DISCUSSION NET formation can be considered a protective strategy by the host in response to
invading pathogens or, in response to tissue damage caused by other harmful environmental irritants. However, an excessive NETs generation may aggravate airway/lung inflammation by eliciting
a pathological immune response under conditions of chronic cigarette smoke exposure16. Here, our results demonstrated that PMNs from patients with COPD are hypersensitive to cigarette smoke
and showed a substantial accumulation of NETs in the airways. NETs induced by CSE promoted mDCs activation, and elicited antigen-specific T-cell responses leading to Th1 and Th17 generation
in vitro. We also showed that erythromycin could suppress cigarette smoke-induced NET formation, thus attenuating the pulmonary inflammation in experimental emphysema murine models.
Additionally, NETs in mice exposed to cigarette smoke were increased and correlated with severity of emphysema and accompanied with the activation of mDCs and Th1 and Th17 cell responses,
which could be suppressed by erythromycin treatment. Previous studies have reported that NETs in airways were elevated and were positively correlated with airway limitation26,31. In
agreement with previously published data, neutrophils from smoking COPD patients are hypersensitive to stimulation with CSE with respect to NET formation32. However, healthy controls (all
non-smokers) did not show increased NETs in the airway. Meanwhile, we found that NETs in sputum did not correlate with smoking pack years. COPD is a highly heterogeneous disorder and there
may be differences in the sensitivity in response to cigarette smoke stimulation among individuals. Our finding indicated that NETs in the airway might represent a biomarker of COPD
patients. Chronic neutrophilic inflammation is prominent in the airways of COPD patients33,34. However, the development of an effective medication targeting neutrophilic inflammation remains
a major challenge. Corticosteroids are commonly used in many chronic airway diseases, but they are not effective for neutrophilic inflammation in COPD. NETs have been proposed as a novel
therapeutic target for neutrophilic inflammation in airways including cystic fibrosis, severe asthma and COPD5,11,35,36. In this study, erythromycin showed a good inhibitory effect on
CSE-induced NETs and NET-associated NE and MPO in vitro, but the mechanism was not so clear. DPI, an inhibitor of the phagocyte NADPH oxidase (NOX2) and NOX2-dependent ROS production, could
inhibit CSE-induced NETs with decreased ROS production, which indicated that NOX2-dependent mechanism was probably responsible for NET formation induced by CSE. It has been established that
the neutrophil NADPH oxidase activity could be suppressed by erythromycin37. Given that erythromycin has a similar effect as DPI, it is very likely that the suppressive capacity of
erythromycin on NET formation attributes to suppressing the NOX2. In addition, we found a significant decrease in the neutrophil count in the BALF of erythromycin-treated mice. We also
cannot rule out the possibility that erythromycin causes a reduction in extracellular DNA in BALF by decreasing the number of neutrophils. A previous study established that macrolides
ameliorate emphysema and reduce airway macrophages, lymphocytes, neutrophils, and pro-inflammatory cytokines in experimental smoking murine models21. We extended these previous findings to a
mechanistic analysis and found that erythromycin negatively regulated airway neutrophilic inflammation and the release of NETs in the BALF of smoke-exposed mice. Thus, our findings provided
direct evidence that erythromycin serves as a potential therapeutic strategy for COPD/emphysema in which neutrophilic inflammation may drive disease progression. Another perhaps more
important consideration is how NETs interact with other components of the immune system and how this affects the processes involved in COPD. This is further emphasised by the observation
that inflammation continues in COPD patients after the cessation of smoking. Indeed, high levels of NETs reduce the activation threshold of T cells and DCs.We recently demonstrated that
CSE-induced NETs are able to activate plasmacytoid DCs, and then induce CD4+T cell polarisation toward Th1 and Th17 phenotypes5. In this study, we translated the murine findings to human
disease and found that excessive NET formation in sputum is accompanied with over-activated mDCs and aggravated Th1/Th17 cell responses in the blood stream of COPD patients. In vitro,
CSE-induced NETs could activate mDCs, and promoted the generation of Th1 and Th17 cells, which showed direct evidence. Further, it was noteworthy that IL-1β, IL-12, and TNF-α released by
mDCs were increased with NETs stimulation. TNF-α could promote the maturation of DCs, and IL-12 and IL-1β were involved in CD4+ T cell-derived IFN-γ and IL-17 production, respectively38,39.
CSE-induced NETs is not the only harmful stimulation to the adaptive immune in COPD. For example, CSE could stimulate DCs directly and promote Th17 response40. Nonetheless, our findings
suggested that NETs may contribute to the activation of the adaptive immune response in COPD, illustrating a unique way in which neutrophils play a synergetic role in this disorder. The
down-regulation of NETs under conditions of chronic inflammation induced by cigarette smoke exposure might decrease the activation of the adaptive immune response. In conclusion, we
demonstrated that NETs promote the differentiation of Th1 and Th17 cells and play a role in the adaptive immunity of smoking-related chronic lung inflammation, which indicates that NETs
could serve as an important target in COPD. Additionally, cigarette smoke-induced NET formation is inhibited by erythromycin treatment in vitro and in vivo. These findings offer a potential
therapeutic strategy for smoking-related COPD/emphysema. REFERENCES * Brusselle, G. G., Joos, G. F. & Bracke, K. R. New insights into the immunology of chronic obstructive pulmonary
disease. _Lancet (Lond., Engl.)_ 378, 1015–1026 (2011). Article CAS Google Scholar * Criner, G. J. et al. Executive summary: prevention of acute exacerbation of COPD: American College of
Chest Physicians and Canadian Thoracic Society Guideline. _Chest_ 147, 883–893 (2015). Article Google Scholar * Heijink, I. H. et al. Cigarette smoke-induced damage-associated molecular
pattern release from necrotic neutrophils triggers proinflammatory mediator release. _Am. J. respiratory cell Mol. Biol._ 52, 554–562 (2015). Article CAS Google Scholar * Hosseinzadeh,
A., Thompson, P. R., Segal, B. H. & Urban, C. F. Nicotine induces neutrophil extracellular traps. _J. Leukoc. Biol._ 100, 1105–1112 (2016). Article CAS Google Scholar * Qiu, S. L. et
al. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. _Thorax_ 72, 1084–1093 (2017). Article Google Scholar * Brinkmann, V. et al. Neutrophil
extracellular traps kill bacteria. _Sci. (New Y., N. Y.)_ 303, 1532–1535 (2004). Article CAS Google Scholar * Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I
IFN production in pediatric systemic lupus erythematosus. _Sci. Transl. Med._ 3, 73ra20 (2011). Article Google Scholar * Kessenbrock, K. et al. Netting neutrophils in autoimmune
small-vessel vasculitis. _Nat. Med._ 15, 623–625 (2009). Article CAS Google Scholar * Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory
responses in rheumatoid arthritis. _Sci. Transl. Med._ 5, 178ra140 (2013). Article Google Scholar * Warnatsch, A., Ioannou, M., Wang, Q. & Papayannopoulos, V. Inflammation. Neutrophil
extracellular traps license macrophages for cytokine production in atherosclerosis. _Sci. (New York., N. Y.)_ 349, 316–320 (2015). Article CAS Google Scholar * Gray, R. D. et al. Delayed
neutrophil apoptosis enhances NET formation in cystic fibrosis. _Thorax_ 73, 134–144 (2018). Article Google Scholar * Laridan, E. et al. Neutrophil extracellular traps in ischemic stroke
thrombi. _Ann. Neurol._ 82, 223–232 (2017). Article CAS Google Scholar * Stakos, D. A. et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of
acute myocardial infarction. _Eur. heart J._ 36, 1405–1414 (2015). Article CAS Google Scholar * Huang, H. et al. Damage-associated molecular pattern-activated neutrophil extracellular
trap exacerbates sterile inflammatory liver injury. _Hepatol. (Baltim., Md.)_ 62, 600–614 (2015). Article CAS Google Scholar * Wright, T. K. et al. Neutrophil extracellular traps are
associated with inflammation in chronic airway disease. _Respirology. (Carlton, Vic. )_ 21, 467–475 (2016). Article Google Scholar * Porto, B. N. & Stein, R. T. Neutrophil
extracellular traps in pulmonary diseases: too much of a good thing? _Front. Immunol._ 7, 311 (2016). Article Google Scholar * Schreiber, A. et al. Necroptosis controls NET generation and
mediates complement activation, endothelial damage, and autoimmune vasculitis. _Proc. Natl Acad. Sci. USA_ 114, E9618–e9625 (2017). Article CAS Google Scholar * Saffarzadeh, M. et al.
Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. _PloS one_ 7, e32366 (2012). Article CAS Google Scholar * He, Z.-Y.
et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. _Respiration_ 80, 445–452 (2010).
Article CAS Google Scholar * Vogelmeier, C. F. et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive
Summary. _The European Respir. J._ 49, https://doi.org/10.1183/13993003.00214-2017 (2017). Article Google Scholar * Bai, J. et al. Erythromycin enhances CD4+Foxp3+ regulatory T-cell
responses in a rat model of smoke-induced lung inflammation. _Mediators Inflamm._ 2012, 410232 (2012). Article Google Scholar * Shimizu, T., Shimizu, S., Hattori, R., Gabazza, E. C. &
Majima, Y. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. _Am. J. Respir. Crit. Care Med_ 168, 581–587 (2003). Article Google Scholar
* Li, M. et al. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-kappaB activity in human macrophages in vitro. _Int. Immunopharmacol._
12, 643–650 (2012). Article CAS Google Scholar * Vestbo, J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive
summary. _Am. J. Respir. Crit. Care Med_ 187, 347–365 (2013). Article CAS Google Scholar * Brinkmann, V., Laube, B., Abu Abed, U., Goosmann, C. & Zychlinsky, A. Neutrophil
extracellular traps: how to generate and visualize them. _J Vis. Exp.: JoVE_, https://doi.org/10.3791/1724 (2010). * Grabcanovic-Musija, F. et al. Neutrophil extracellular trap (NET)
formation characterises stable and exacerbated COPD and correlates with airflow limitation. _Respir. Res._ 16, 59 (2015). Article Google Scholar * D’Hulst, A. I., Vermaelen, K. Y.,
Brusselle, G. G., Joos, G. F. & Pauwels, R. A. Time course of cigarette smoke-induced pulmonary inflammation in mice. _Eur. respiratory J._ 26, 204–213 (2005). Article Google Scholar *
Qiu, S. L. et al. Cigarette smoke induction of Interleukin-27/WSX-1 regulates the differentiation of Th1 and Th17 cells in a smoking mouse model of emphysema. _Front. Immunol._ 7, 553
(2016). Article Google Scholar * Moodie, F. M. et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory
cytokine release in alveolar epithelial cells. _FASEB J.: Off. Publ. Federation Am. Societies Exp. Biol._ 18, 1897–1899 (2004). Article CAS Google Scholar * Hautefort, A. et al. T-helper
17 cell polarization in pulmonary arterial hypertension. _Chest_ 147, 1610–1620 (2015). Article Google Scholar * Pedersen, F. et al. Neutrophil extracellular trap formation and
extracellular DNA in sputum of stable COPD patients. _Respiratory Med._ 109, 1360–1362 (2015). Article Google Scholar * Lee, J. et al. Nicotine drives neutrophil extracellular traps
formation and accelerates collagen-induced arthritis. _Rheumatol. (Oxf., Engl.)_ 56, 644–653 (2017). CAS Google Scholar * Singh, D., Edwards, L., Tal-Singer, R. & Rennard, S. Sputum
neutrophils as a biomarker in COPD: findings from the ECLIPSE study. _Respiratory Res._ 11, 77 (2010). Article Google Scholar * Baraldo, S. et al. Neutrophilic infiltration within the
airway smooth muscle in patients with COPD. _Thorax_ 59, 308–312 (2004). Article CAS Google Scholar * Dicker, A. J. et al. Neutrophil extracellular traps are associated with disease
severity and microbiota diversity in patients with chronic obstructive pulmonary disease. _J. allergy Clin. Immunol._ 141, 117–127 (2018). Article Google Scholar * Krishnamoorthy, N. et
al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. _Science immunology_ 3, https://doi.org/10.1126/sciimmunol.aao4747 (2018).
Article Google Scholar * Umeki, S. Anti-inflammatory action of erythromycin. Its inhibitory effect on neutrophil NADPH oxidase activity. _Chest_ 104, 1191–1193 (1993). Article CAS Google
Scholar * DuPage, M. & Bluestone, J. A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. _Nat. Rev. Immunol._ 16, 149–163 (2016). Article CAS Google
Scholar * Agalioti, T., Villablanca, E. J., Huber, S. & Gagliani, N. TH17cell plasticity: the role of dendritic cells and molecular mechanisms. _J. Autoimmun._ 87, 50–60 (2018). Article
CAS Google Scholar * Liang, Y. et al. Cigarette smoke exposure promotes differentiation of CD4+ T cells toward Th17 cells by CD40-CD40L costimulatory pathway in mice. _Int. J. chronic
Obstr. Pulm. Dis._ 13, 959–968 (2018). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This study was funded by grants from National Natural Science Foundation of China
(grant number 81770041 and 81660007 and 81800037). AUTHOR INFORMATION Author notes * These authors contributed equally: Hui Zhang, Shi-Lin Qiu AUTHORS AND AFFILIATIONS * Department of
Respiratory and Critical Care Medicine, the First Affiliated Hospital of Guangxi Medical University, Nanning, China Hui Zhang, Shi-Lin Qiu, Qi-Ya Tang, Xiu Zhou, Jian-Quan Zhang, Zhi-Yi He,
Jing Bai, Mei-Hua Li, Jing-Min Deng, Yi Liang & Xiao-Ning Zhong Authors * Hui Zhang View author publications You can also search for this author inPubMed Google Scholar * Shi-Lin Qiu
View author publications You can also search for this author inPubMed Google Scholar * Qi-Ya Tang View author publications You can also search for this author inPubMed Google Scholar * Xiu
Zhou View author publications You can also search for this author inPubMed Google Scholar * Jian-Quan Zhang View author publications You can also search for this author inPubMed Google
Scholar * Zhi-Yi He View author publications You can also search for this author inPubMed Google Scholar * Jing Bai View author publications You can also search for this author inPubMed
Google Scholar * Mei-Hua Li View author publications You can also search for this author inPubMed Google Scholar * Jing-Min Deng View author publications You can also search for this author
inPubMed Google Scholar * Yi Liang View author publications You can also search for this author inPubMed Google Scholar * Xiao-Ning Zhong View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS Z.H. and Q.S.L. drafted the article. T.Q.Y., Z.X., B.J., L.M.H. and D.J.M. performed the experiments and contributed to acquisition of data.
Z.J.Q. and H.Z.Y. analysed the data. L.Y. designed the study. Z.X.N. designed the study and revised the article. CORRESPONDING AUTHOR Correspondence to Xiao-Ning Zhong. ETHICS DECLARATIONS
CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. Edited by Y. Shi SUPPLEMENTARY INFORMATION ONLINE SUPPLEMENT FOR FULL DETAILS OF METHODS RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, H., Qiu, SL., Tang, QY. _et al._ Erythromycin
suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. _Cell Death Dis_ 10, 678 (2019). https://doi.org/10.1038/s41419-019-1909-2 Download citation *
Received: 01 April 2019 * Revised: 25 July 2019 * Accepted: 26 July 2019 * Published: 12 September 2019 * DOI: https://doi.org/10.1038/s41419-019-1909-2 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
