Dendritic cell migration in inflammation and immunity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

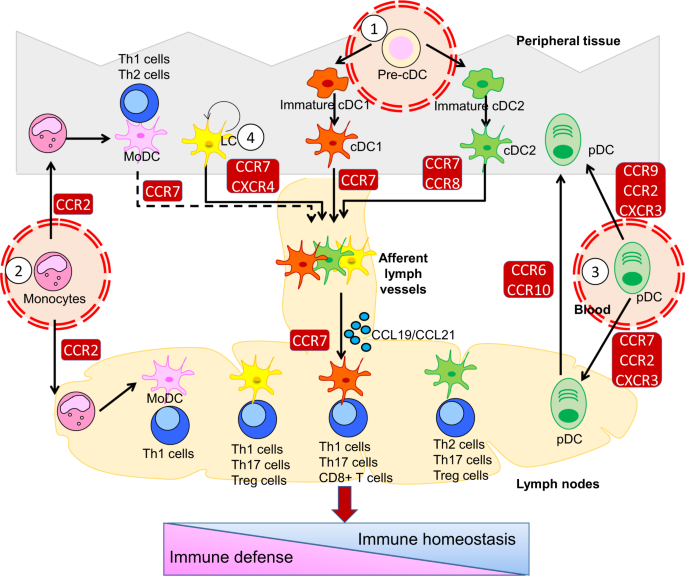
Dendritic cells (DCs) are the key link between innate immunity and adaptive immunity and play crucial roles in both the promotion of immune defense and the maintenance of immune tolerance.
The trafficking of distinct DC subsets across lymphoid and nonlymphoid tissues is essential for DC-dependent activation and regulation of inflammation and immunity. DC chemotaxis and
migration are triggered by interactions between chemokines and their receptors and regulated by multiple intracellular mechanisms, such as protein modification, epigenetic reprogramming,
metabolic remodeling, and cytoskeletal rearrangement, in a tissue-specific manner. Dysregulation of DC migration may lead to abnormal positioning or activation of DCs, resulting in an
imbalance of immune responses and even immune pathologies, including autoimmune responses, infectious diseases, allergic diseases and tumors. New strategies targeting the migration of
distinct DC subsets are being explored for the treatment of inflammatory and infectious diseases and the development of novel DC-based vaccines. In this review, we will discuss the migratory
routes and immunological consequences of distinct DC subsets, the molecular basis and regulatory mechanisms of migratory signaling in DCs, and the association of DC migration with the
pathogenesis of autoimmune and infectious diseases.
This work was supported by grants from the National Natural Science Foundation of China (32070903, 31870909, and 81788101).
National Key Laboratory of Medical Immunology & Institute of Immunology, Second Military Medical University, Shanghai, China
Department of Immunology, Center for Immunotherapy, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing, China
Anyone you share the following link with will be able to read this content:
