Staying home or leaving for a party: tissue-dependent choices of tissue-resident memory t cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Access through your institution Buy or subscribe CD8+ T cells differentiate into different types of memory T cells after priming in the lymphoid organs. Central memory T cells selectively
express lymph node homing markers and recirculate between the blood and the secondary lymphoid organs. Effector memory T cells lack lymph node homing capacity, mainly circulate in the blood,
and may enter peripheral tissues. The third subset consists of tissue-resident memory T (Trm) cells that express CD69 and adhesion molecules such as CD11a, CD103, and CD49a that prevent
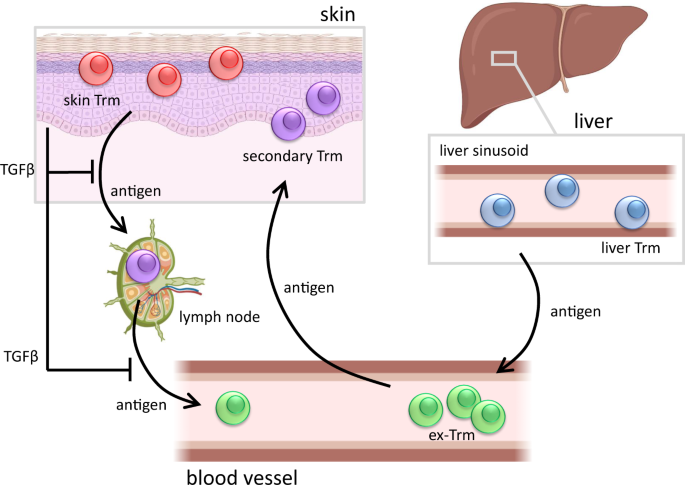
tissue egress. These Trm cells can be found throughout tissues and have important protective functions in infectious diseases as well as in malignancies. While it has been known for quite
some time that Trm cells form rapid local recall responses, more recent studies indicate that upon secondary infection, Trm cells can also return to the blood and differentiate into
circulating memory T-cell subsets [1, 2]. These studies indicate that Trm 1ells retain functional plasticity and that ex-Trm cells may contribute to systemic immunity. Trm populations of
skin, liver, small intestine, lungs, and other tissues are characterized by a specific set of transcription factors, including Runx3, Hobit, and Blimp-1, that control tissue exit [3].
Despite a common transcriptional signature, Trm cells in different tissues exhibit substantial diversity, as illustrated by the differential expression of certain surface receptors. CD103 is
highly expressed by Trm cells in skin, where this TGFβ-induced adhesion receptor mediates binding to epithelial-expressed E-cadherin [4]. In contrast, liver Trm cells do not express CD103
but instead upregulate CD11a, which is essential for lodgment into the sinusoidal endothelium. A recent paper by Mackay and coworkers has shed new light on the diversity of skin and liver
Trm cells and provides evidence that these Trm populations also have a different capacity to differentiate into circulating memory T cells [5]. This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per
issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Behr FM, Parga-Vidal L, Kragten NAM, van Dam TJP, Wesselink TH,
Sheridan BS, et al. Tissue-resident memory CD8(+) T cells shape local and systemic secondary T cell responses. Nat Immunol. 2020;21:1070–81. Article CAS Google Scholar * Fonseca R, Beura
LK, Quarnstrom CF, Ghoneim HE, Fan Y, Zebley CC, et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat Immunol. 2020;21:412–21. Article CAS
Google Scholar * Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes.
Science. 2016;352:459–63. Article CAS Google Scholar * Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident
memory T cells of skin. Nat Immunol. 2013;14:1294–301. Article CAS Google Scholar * Christo SN, Evrard M, Park SL, Gandolfo LC, Burn TN, Fonseca R, et al. Discrete tissue
microenvironments instruct diversity in resident memory T cell function and plasticity. Nat Immunol. 2021;22:1140–51. Article CAS Google Scholar * Beura LK, Wijeyesinghe S, Thompson EA,
Macchietto MG, Rosato PC, Pierson MJ, et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity. 2018;48:327–38 e5. Article CAS Google Scholar * Park
SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol.
2018;19:183–91. Article CAS Google Scholar * Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1
therapy. Nature. 2016;537:417–21. Article CAS Google Scholar * Wijeyesinghe S, Beura LK, Pierson MJ, Stolley JM, Adam OA, Ruscher R, et al. Expansible residence decentralizes immune
homeostasis. Nature. 2021;592:457–62. Article CAS Google Scholar * Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, et al. Liver-resident memory CD8(+) T cells form a front-line
defense against malaria liver-stage infection. Immunity. 2016;45:889–902. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Hematopoiesis, Sanquin Research and Landsteiner Laboratory, Amsterdam UMC, University of Amsterdam, 1066, CX, Amsterdam, The Netherlands Klaas P. J. M. van Gisbergen * Department of
Molecular Cell Biology and Immunology, Cancer Center Amsterdam, Amsterdam Institute for Infection and Immunity, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands Joke
M. M. den Haan Authors * Klaas P. J. M. van Gisbergen View author publications You can also search for this author inPubMed Google Scholar * Joke M. M. den Haan View author publications You
can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Joke M. M. den Haan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE van Gisbergen, K.P.J.M., den Haan, J.M.M. Staying home or leaving for a party:
tissue-dependent choices of tissue-resident memory T cells. _Cell Mol Immunol_ 19, 651–652 (2022). https://doi.org/10.1038/s41423-021-00828-z Download citation * Received: 09 December 2021 *
Accepted: 14 December 2021 * Published: 13 January 2022 * Issue Date: June 2022 * DOI: https://doi.org/10.1038/s41423-021-00828-z SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
