Kinetics and morphologies of syndiotactic polystyrene crystallized isothermally over a wide temperature range
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Time-resolved Fourier transform infrared (FTIR) spectroscopy was used to investigate the crystallization kinetics of syndiotactic polystyrene (sPS) for the first time, and the
results were compared with those obtained with differential scanning calorimetry (DSC) and depolarized light scattering (DPLS). Isothermal crystallization either from the melt by cooling or
from the glass by heating was used to determine the temperature (_T_c) dependence of the crystallization rate (_k_). The derived values of _k_ were in good agreement with the results
obtained with other tools over the accessible _T_c ranges 250−262 °C and 110−135 °C for melt and cold crystallization, respectively. Based on the derived _k_ and the crystal growth rates
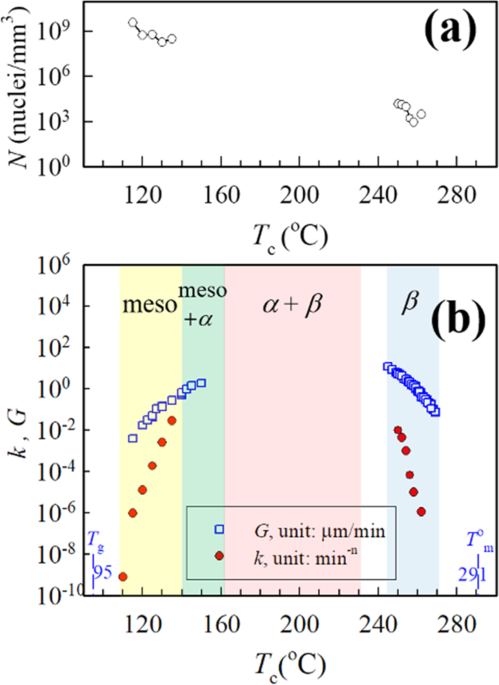
obtained from DPLS and optical microscopy (OM), the density of primary nucleation was readily calculated. The magnitudes of the nucleation densities in the cold-crystallized samples were
~5−6 orders higher than those of the melt-crystallized samples despite the similar _k_ values. The novelty of our work lies in revealing that the volume-filling spherulites of the
cold-crystallized sPS had modulated structure, reminiscent of spinodal decomposition. Thus, the nucleation pathway for cold crystallization is relevant to spinodal-assisted nucleation, which
significantly enhances the nucleation density. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EXPLOSIVE FIBONACCI-SEQUENCE GROWTH INTO UNUSUAL SECTOR-FACE MORPHOLOGY IN POLY(L-LACTIC ACID)
CRYSTALLIZED WITH POLYMERIC DILUENTS Article Open access 02 July 2020 UPPER CRITICAL SOLUTION TEMPERATURE POLYMER ASSEMBLIES VIA VARIABLE TEMPERATURE LIQUID PHASE TRANSMISSION ELECTRON
MICROSCOPY AND LIQUID RESONANT SOFT X-RAY SCATTERING Article Open access 10 June 2023 THE SIZE OF CRITICAL SECONDARY NUCLEI OF POLYMER CRYSTALS DOES NOT DEPEND ON SUPERSATURATION Article
Open access 22 April 2025 REFERENCES * Sorrentino A, Vittoria V. In Syndiotactic polystyrene, John Wiely & Sons, Inc., 2009, p.155. * Krishnan V, Joseph AM, Peethambharan SK, Howd EB.
Nanoporous crystalline aerogels of syndiotactic polystyrene: polymorphism, dielectric, thermal, and acoustic properties. Macromolecules. 2021;54:10605–15. Article CAS Google Scholar * Woo
EM, Sun YS, Yang CP. Polymorphism, thermal behavior, and crystal stability in syndiotactic polystyrene vs. its blends. Prog Polym Sci. 2001;26:945–83. Article CAS Google Scholar * Gowd
EB, Tashiro K, Ramesh C. Structural phase transitions of syndiotactic polystyrene. Prog Polym Sci. 2009;34:280–315. Article CAS Google Scholar * Petraccone V, Auriemma F, Poggetto FD, De
Rosa C, Guerra G, Corradini P. On the structure of the mesomorphic form of syndiotactic polystyrene. Makromol Chem. 1993;194:1335–45. Article CAS Google Scholar * Auriemma F, Petraccone
V, Poggetto FD, De Rosa C, Guerra G, Manfredi C, et al. Mesomorphic form of syndiotatctic polystyrene as composed of small imperfect crystals of the hexagonal (α) crystalline form.
Macromolecules. 1993;26:3772–7. Article CAS Google Scholar * Wu FS, Woo EM. Comparison of crystallization kinetics of miscible blends of syndiotactic polystyrene with atactic polystyrene
or poly(l,4-dimethyl-p-phenylene oxide). Polym Eng Sci. 1999;39:825–32. Article CAS Google Scholar * Wu TM, Hsu SF, Chien CF, Wu JY. Isothermal and nonisothermal crystallization kinetics
of syndiotactic polystyrene/clay nanocomposites. Polym Eng Sci. 2004;44:2288–97. Article CAS Google Scholar * Chen B, Torkelson JM. Rigid amorphous fraction and crystallinity in
cold-crystallized syndiotactic polystyrene: characterization by differential scanning calorimetry. Polymer. 2021;230:124044. Article CAS Google Scholar * Cimmino S, Di Pace E, Martuscelli
E, Silvestre C. Syndiotactic polystyrene-based blends: crystallization and phase structure. Polymer. 1993;34:2799–803. Article CAS Google Scholar * Wang C, Chen CC, Cheng YW, Liao WP,
Wang ML. Simultaneous presence of positive and negative spherulites in syndiotactic polystyrene and its blends with atactic polystyrene. Polymer. 2002;43:5271–9. Article CAS Google Scholar
* De Rosa C, De Ballesteros OR, Di Gennaro M, Auriemma F. Crystallization from the melt of α and β forms of syndiotactic polystyrene. Polymer. 2003;44:1861–70. Article Google Scholar *
Su CH, Jeng U, Chen SH, Lin SJ, Wu WR, Chuang WT, et al. Nanograin evolution in cold crystallization of syndiotactic polystyrene as illustrated via in-situ small/wide-angle X-ray scattering
and differential scanning calorimetry. Macromolecules. 2009;42:6656–64. Article CAS Google Scholar * Wang C, Liao WP, Wang ML, Lin CC. Miscible blends of syndiotactic polystyrene and
atactic polystyrene. part 2. depolarized light scattering studies and crystal growth rates. Polymer. 2004;45:973–81. Article CAS Google Scholar * Wang C, Lin CC, Chu CP. Crystallization
and morphological features of syndiotactic polystyrene induced from glassy state. Polymer. 2005;46:12595–606. Article CAS Google Scholar * Yu J, Asai S, Sumita M. Time-resolved FTIR study
of crystallization behavior of melt-crystallized poly(phenylene sulfide). J Macromol Sci Phys. 2000;B39:279–96. Article CAS Google Scholar * Kimura T, Ezure H, Tanaka S, Ito E. In situ
FTIR spectroscopic study on crystallization process of isotactic polystyrene. J Polym Sci Polym Phys Ed 1998;36:1227–33. Article CAS Google Scholar * Duan Y, Zhang J, Shen D, Yan S. In
situ FTIR studies on the cold crystallization process and multiple melting behavior of isotactic polystyrene. Macromolecules. 2003;36:4874–9. Article CAS Google Scholar * Wu HD, Wu ID,
Chang FC. Characterization of crystallization in syndiotactic polystyrene thin film samples. Macromolecules. 2000;22:8915–7. Article Google Scholar * Jiang Q, Zhao Y, Zhang C, Yang J, Wang
D. Investigation on the overlapping bands of syndiotactic polystyrene by using 2D-IR spectroscopy. J Mol Struct. 2016;1124:98–102. Article CAS Google Scholar * Kiflie Z, Piccarolo S,
Brucato V, Baltá-Calleja FJ. Role of thermal history on quiescent cold crystallization of PET. Polymer. 2002;43:4487–93. Article CAS Google Scholar * Wang ML. Crystallization and
morphology of syndiotactic/atactic polystyrene blends, Master thesis, National Cheng Kung University, Tainan, Taiwan, May 2002. * Painter P, Sobkowiak M, Park Y. Vibrational relaxation in
atactic polystyrene: an infrared spectroscopic study. Macromolecules. 2007;40:1730–7. Article CAS Google Scholar * Wang C, Hsu YC, Lo CF. Melting behavior and equilibrium melting
temperatures of syndiotactic polystyrene in α and β crystalline forms. Polymer. 2001;42:8447–60. Article CAS Google Scholar * Phillips PJ, Tseng HT. Influence of pressure on
crystallization in poly(ethylene terephthalate). Macromolecules. 1989;22:1649–55. Article CAS Google Scholar * Androsch R, Schick C. Crystal nucleation of polymers at high supercooling of
the melt. Adv Polym Sci. 2017;276:257–88. Article CAS Google Scholar * Wunderlich B. Macromolecular Physics; Academic: New York, 1973; Vol. 1. * Androsch R, Di Lorenzo ML. Crystal
nucleation in glass poly(L-lactic acid). Macromolecules. 2013;46:6048–56. Article CAS Google Scholar * Fillon B, Wittmann JC, Lotz B, Thierry A. Self-nucleation and recrytsallization of
isotactic polypropylene (alpha phase) investigated by differential scanning calorimetry. J Polym Sci Polym Phys Ed. 1993;31:1383–93. Article CAS Google Scholar * Hoffman J, Davis G,
Lauritzen J. The rate of crystallization of linear polymers with chain folding. In: Hannay NB, editor. Treatise on solid chemistry, vol. 3 New York: Plenum Press; 1976, p. 497–614. * Suzuki
T, Kovacs AJ. Temperature dependence of spherulitic growth rate of isotactic polystyrene. Polym J. 1970;1:82–100. Article CAS Google Scholar * Ferry JD. Viscoelastic properties of
polymers, 3rd edition, 1980, p. 277–89. The shift factor aT is expressed by log \(a_T = - c_1^0(T - T_0)/(c_2^0 + T - T_0)\), with _T_0 = 373 K, \(c_1^0\) = 12.7, and \(c_2^0\) = 49.8 for
the aPS. * Huang CL, Chen YC, Hsiao TJ, Tsai JC, Wang C. Effect of tacticity on viscoelastic properties of polystyrene. Macromolecules. 2011;44:6155–61. Article CAS Google Scholar *
Turnbull D, Fisher JC. Rate of nucleation in condensed systems. J Chem Phys. 1949;17:71–73. Article CAS Google Scholar * Tang X, Chen W, Li L. The tough journey of polymer
crystallization: battling with chain flexibility and connectivity. Macromolecules. 2019;52:3575–91. Article CAS Google Scholar * Kaji K, Nishida K, Kanaya T, Matsuba G, Konishi T, Imai M.
Spinodal crystallization of polymers: crystallization from the unstable melt. Adv Polym Sci. 2005;191:187–240. Article CAS Google Scholar * Koberstein J, Russell TP, Stein RS. Total
integrated light scattering intensity from polymeric solids. J Polym Sci Polym Phys Ed. 1979;17:1719–30. Article CAS Google Scholar * Stein RS, Rhodes MB. Photographic light scattering by
polyethylene films. J Appl Phys. 1960;31:1873–84. Article CAS Google Scholar * Pogodina NV, Winter HH. Polypropylene crystallization as a physical gelation process. Macromolecules.
1998;31:8164–72. Article CAS Google Scholar * Olmsted PD, Poon WK, McLeish TCB, Terrill NJ, Ryan AJ. Spinodal-assisted crystallization in polymer melts. Phys Rev Lett. 1998;81:373–6.
Article CAS Google Scholar * Strobl G. Crystallization and melting of bulk polymers: New observations, conclusions and a thermodynamic scheme. Prog Polym Sci. 2006;31:398–442. Article
CAS Google Scholar * Yeh GSY, Geil PH. Crystallization of polyethylene terephthalate from the glassy state. J Macromol Sci Phys. 1967;B1:235–49. Article Google Scholar * Nakaoki T,
Kobayashi M. Local conformation of glassy polystyrenes with different stereoregularity. J Mol Struct. 2003;655:343–9. Article CAS Google Scholar * Muthukumar M. Molecular modelling of
nucleation in polymers. Philos Trans R Soc Lond. 2003;A361:539–56. Article Google Scholar Download references ACKNOWLEDGEMENTS This research has been supported by the Ministry of Science
and Technology of Taiwan (MOST 109-2221-E-006-202-MY3). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemical Engineering, National Cheng Kung University, Tainan, 701, Taiwan,
ROC Chun-Yu Lo & Chi Wang Authors * Chun-Yu Lo View author publications You can also search for this author inPubMed Google Scholar * Chi Wang View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Chi Wang. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer
Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Lo, CY., Wang, C. Kinetics and morphologies of syndiotactic polystyrene crystallized isothermally over a wide temperature range. _Polym J_ 55, 761–773 (2023).
https://doi.org/10.1038/s41428-023-00775-8 Download citation * Received: 10 October 2022 * Revised: 27 January 2023 * Accepted: 20 February 2023 * Published: 28 March 2023 * Issue Date: July
2023 * DOI: https://doi.org/10.1038/s41428-023-00775-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
