Modulation of immune checkpoint regulators in interferon γ induced urothelial carcinoma and activated t-lymphocyte cells by cytostatics
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

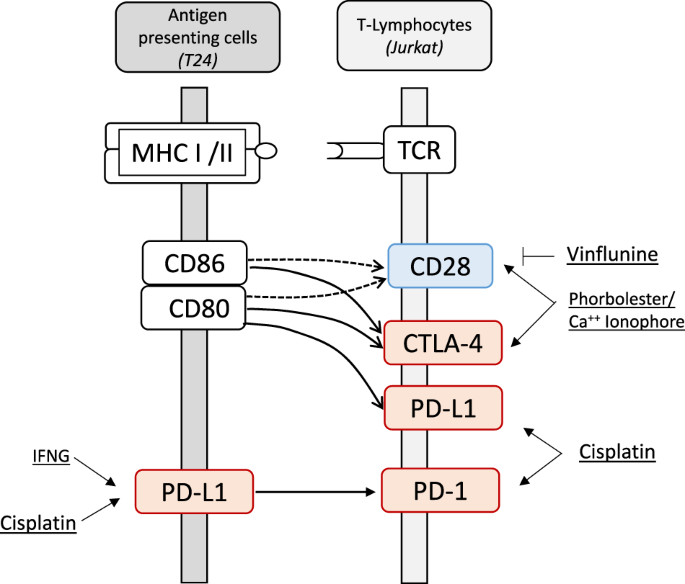
ABSTRACT Exploring the regulation of co-inhibitory (PD-1, PD-L1, CTLA-4) and co-stimulatory (CD28) genes by chemotherapeutic drugs is important for combined immune checkpoint blockade (ICB)
therapy. ICB interferes with T-cell receptor and major histocompatibility complex (MHC) signaling by antibody drugs directed against the co-inhibitors. Here, we analyzed urothelial (T24)
cell line with respect to cytokine signaling by interferon γ (IFNG) and the leukemia lymphocyte (Jurkat) cell line with respect to T-cell activation as mimicked by phorbolester and calcium
ionophore (pma/iono). Alongside, we considered possible intervention with the chemotherapeutics gemcitabine, cisplatin and vinflunine. Noteworthy, cisplatin significantly induced PD-L1-mRNA
in naïve and IFNG treated cells whereas gemcitabine and vinflunine had no effect on PD-L1-mRNA. At the protein level, PD-L1 showed typical induction in IFNG treated cells. In Jurkat cells,
cisplatin significantly induced PD-1-mRNA and PD-L1-mRNA. Pma/iono administration did not alter PD-1-mRNA and PD-L1-mRNA but significantly increased CTLA-4-mRNA and CD28-mRNA levels where
vinflunine suppressed the CD28-mRNA induction. In sum, we demonstrated that certain cytostatic drugs being relevant for the therapy of urothelial cancer, affect co-inhibitory and
co-stimulatory modulators of immune signaling with potential impact for perspective combined ICB therapy of patients. SIMILAR CONTENT BEING VIEWED BY OTHERS DIRECT AKT ACTIVATION IN
TUMOR-INFILTRATING LYMPHOCYTES MARKEDLY INCREASES INTERFERON-Γ (IFN-Γ) FOR THE REGRESSION OF TUMORS RESISTANT TO PD-1 CHECKPOINT BLOCKADE Article Open access 02 November 2022 TRAMETINIB
IMPROVES TREG SELECTIVITY OF ANTI-CCR4 ANTIBODY BY REGULATING CCR4 EXPRESSION IN CTLS IN ORAL SQUAMOUS CELL CARCINOMA Article Open access 15 December 2022 INTERLEUKIN-10 SUPPRESSION ENHANCES
T-CELL ANTITUMOR IMMUNITY AND RESPONSES TO CHECKPOINT BLOCKADE IN CHRONIC LYMPHOCYTIC LEUKEMIA Article 17 March 2021 INTRODUCTION The immune checkpoint blockade therapy (ICB) targets
signaling between T-cell receptor (TCR) and major histocompatibility complex (MHC) by antibody drugs and is applied for the treatment of a growing number of malignancies including urothelial
carcinoma [1]. The immune response of T-cells is balanced through crosstalk of co-inhibitors such as PD-1, PD-L1, CTLA-4 and co-stimulator CD28 that signals between antigen-presenting cells
or neoantigen-harboring tumor cells and T-cells involving CD80 and CD86 as further interacting molecules. Co-inhibitors counteracts co-stimulators and shift T-lymphocytes from the
activation state towards the anergy and exhaustion state [2]. In those states, the immune responses become downregulated in the tumor microenvironment or local lymph nodes [3]. ICB drugs
reverse the co-inhibition and restore T-cell effector function. PD-1, CTLA-4 and CD28 are expressed predominantly in T-lymphocytes. PD-L1 is found in higher abundance in tumor cells and
antigen presenting cells [2]. Obviously, the regulation of the corresponding genes for PD-1, PD-L1, CTLA-4 and CD28 by cytostatic drugs is of potential interest when combining ICB and
chemotherapy. In this scenario, we focused on chemotherapeutic drugs that are recommended for the treatment of urothelial cancer such as cisplatin, vinflunine and gemcitabine [4]. As cell
targets, we analyzed particularly the urothelial cell line (T24) and the immune cell leukemia T-lymphocyte (Jurkat) cell line. In T24 cells, cytokine signaling by interferon γ (IFNG) [5] and
in Jurkat cells phorbolester/ionomycine (PMA/Iono) a mimic for T-cell activation were tested for interference with the cytostatic drugs [6]. MATERIALS AND METHODS Adherent urothelial cell
lines and the suspension T-cell derived Jurkat cell line Jurkat, (DSMZ No. ACC282) were cultured according to protocols by DSMZ, Braunschweig, Germany and as decribed [7]. In subsets, IFNG
(10 ng/ml) (R&D Systems) was added to urothelial T24 cells (DSMZ no.: ACC 376) and phorbol 12-myristate 13-acetate (pma) (Sigma) (100 nM) with ionomycin calcium salt (iono) (Sigma) (100
nM) to Jurkat cells [6] for 24 h. The chemotherapeutic drugs were added for 24 h in doses based on literature: Gemcitabine hydrochlorid (1 µM) [8], Cis-Diamminelplatinum (II) dichloride (100
µM) [9], Vinflunine (ChemScene) 10 (µM) [10]. Procedures of RNA and protein isolation, quantitative real time RT-PCR and Western-blot has been described previously by our laboratory [7].
The DNA sequences of forward (+) and reverse primers (−) are subsequently listed: PD-L1 (CD274) (+) GCGAATTACTGTGAAAGTCAATGCC, (−) TGGTCACATTGAAAAGCTTCTCCTC; PD1 (PDCD1) (+)
GGCCGCACGAGGGACAATAG, (−) AGGAAAGACAATGGTGGCATACTCC; STAT1 (+) ATGATGAACTAGTGGAGTGGAAGCG, (−) CTCTGAATGAGCTGCTGGAAAAGAC; CD28 (+) ATTGGGCAATGAATCAGTGACATTC, (−) AAGCTATAGCAAGCCAGGACTCCAC;
CTLA4 (+) AACCTCACTATCCAAGGACTGAGGG, (−) AGCATTTTGCTCAAAGAAACAGCTG; β-actin (ACTB) (+) TATCCAGGCTGTGCTATCCCTGTAC, (−) TTCATGAGGTAGTCAGTCAGGTCCC. The antibodies were as follows: PD-L1 #13684
(Cell signaling), PD-1, host goat (AF1086, R&D Systems); (LDHA #3558, Cell signaling). Data were analysed with MS-Office Excel and Graphpad Prism Version 9.50. RESULTS Screening of
several urothelial cell lines displayed different levels of PD-L1-mRNA each with typical induction by IFNG (Fig. 1A). For further analysis of the chemotherapeutic drugs, we selected the T24
cells with high basic levels of PD-L1-mRNA. Cisplatin significantly induced PD-L1-mRNA in naïve and IFNG treated cells whereas gemcitabine and vinflunine had no effect on PD-L1-mRNA in both
groups (Fig. 1B). In accordance, the IFNG signaling mediator STAT1-mRNA matched the changes of PD-L1-mRNA both in the control and IFNG treated cells and by cisplatin (Fig. 1C). At the
protein level, PD-L1 showed typical induction in the IFNG treated samples. Adding cisplatin or vinflunine lead to further PD-L1 accumulation in the control group and in the IFNG treated
group (Fig. 1D). The time kinetic revealed strongest upregulation of PD-L1-mRNA by cisplatin and vinflunine after 24 h (Fig. 1E). Similarly, PD-L1 protein peaked at 24 h of cisplatin or
vinflunine treatment but here the effects on PD-L1 protein appeared stronger with vinflunine (Fig. 1F). Next, we analyzed Jurkat cells treated by gemcitabine, cisplatin and vinflunine. We
tested control cells and pma/iono treated cells for mimicking T-cell activation. Cisplatin exerted significant induction of PD-1-mRNA and PD-L1-mRNA whereas Pma-iono treatment did not result
in changes of PD-1-mRNA and PD-L1-mRNA (Fig. 2A, B). In addition, we analyzed the co-inhibitor CTLA-4 and the co-stimulator CD28 (Fig. 2C, D). Strikingly, pma-iono treatment significantly
increased CTLA-4-mRNA and CD28-mRNA levels whereas, gemcitabine, cisplatin and vinflunine had no effect on CTLA4-mRNA. Of note, vinflunine suppressed the pma-iono induced increase of
CD28-mRNA. In addition to mRNA data, an exemplary Western blot of PD-1 and PD-L1 (Fig. 2E, F) reveal that PD-1 protein appears stronger than PD-L1 protein particularly when comparing the
Jurkat PD-L1 protein level (Fig. 2F) with those in T24 cells (Fig. 1D). DISCUSSION This study investigated combined effects of IFNG signaling and T-cell activation with cytostatic drugs for
gene regulation of immune checkpoint modulators. CHEMOTHERAPEUTIC DRUGS AND IMMUNE SIGNALING IN CANCER AND T-CELLS The cisplatin effects on PD-L1 observed here add to related studies
performed on various malignancies. Cisplatin induced PD-L1-mRNA in lung cancer cells and in tumor tissue of cisplatin treated patients [11]. As a relevant mechanism, our data suggest that
cisplatin acts to some extent via STAT1 the crucial downstream mediator of IFNG signaling [5]. The subsequent consequences of pro-inflammatory IFNG signaling for tumor progression are
multipart since diverse actions meet [12] and must therefore be seen in the specific context of disorder. IFNG enhances MHC-I thereby favoring neo-antigen presentation of tumor cells.
Conversely, immune escape is facilitated by induction of immune checkpoints. Furthermore, IFNG dependent induction of cell cycle arrest, as well as, apoptosis have been reported. Apart from
IFNG signaling, several other pathways have been demonstrated to induce PD-L1. The cGAS/STING pathway has been assigned a critical role for cisplatin- induced PD-L1 in ovarian cancer [13]. A
downstream arm of cGAS/STING pathway converge with the NF-kb pathway that targets PD-L1 promoter as well [14]. In a further study, cisplatin dependent PD-L1 induction has been attributed to
the ERK1/2 and AP1 signaling pathway as demonstrated in several urothelial cell lines [15]. In the Jurkat T-cell model, the induction of CD28 and CTLA4 during T-cell activation [3] could be
mimicked by activation of PKC pathway and intracellular Ca++ accumulation supporting this pharmacologic intervention as relevant trigger. The cisplatin-dependent upregulation of PD-1-mRNA
along with PD-L1-mRNA in Jurkat cells, defines targets related to different signaling pathways. For PD-1 induction, IL-2 and TGF-β1 signaling [16] were demonstrated as relevant rather than
IFNG for PD-L1 [5] were demonstrated as relevant. The selective downregulation of CD28-mRNA by vinflunine in Jurkat cells indicates a further branch possibly affecting combined or sequential
therapy outcomes. Of note, a recent experimental study compared combined therapy of anti-PD-1 therapy with either cisplatin or gemcitabine in lung and pancreatic cancer models and patients
tissue samples [17]. Interestingly, cisplatin but not gemcitabine acted synergistically with PD-1 blockade therapy by increased T cell infiltration with release of antitumor cytokines
involving the triggered cGAS/STING pathway. The cisplatin enhanced PD-1-mRNA levels, as observed here in the Jurkat T-cell model, may mechanistically add to the therapeutic beneficial
effects in that study [17] since higher PD-1 levels may favor anti-PD-1 blockade therapy. PLATINUM-BASED DRUGS IN UROTHELIAL CANCER As a platinum-based drug, we focused on cisplatin that is
the most common applied drug member from the first generation. Alternatively, carboplatin is employed for advanced urothelial cancer patients who are ineligible for cisplatin. Carboplatin
displays less systemic toxicity and is administered in patients with poor performance status such as those with restricted renal function. Pharmacologically, cisplatin and carboplatin bind
to DNA via intra- and interstranded crosslinks causing DNA damage thereby triggering cell cycle block and apoptosis. Chemically, carboplatin has a ‘slower leaving group release’ when
reacting with nucleophiles such as N7-guanine in DNA and this is attributed to less myelosuppressive related side effects. The common pharmacologic action of cisplatin and carboplatin
suggests similar regulation of PD-L1 and PD-1. Variations in “non-canonical” actions targeting molecules beyond DNA, on the other hand, could differentially affect PD-L1 and PD-1 expression,
an as yet undefined and speculative mechanism [18]. COMBINED AND SEQUENTIAL (MAINTENANCE) THERAPY BY CHEMOTHERAPY AND ICB OF PATIENTS WITH UROTHELIAL CANCER In a phase 3 trial of metastatic
urothelial cancer (IMvigor130) [19], combined chemotherapy with ICB by atezolizumab displayed a favorable safety profile. Whereas, a benefit in terms of patients’ overall survival could not
be demonstrated. In another trial of first-line therapy for advanced urothelial carcinoma (KEYNOTE-361), combined chemotherapy with pembrolizumab was not superior to chemotherapy alone in
treatment efficacy [20]. Noteworthy, when applied sequentially after first line chemotherapy, ICB with avelumab significantly improved overall survival of patients with advanced urothelial
and therefore ICB can be recommended as maintenance therapy (JAVELIN Bladder 100) [21]. To date, these studies have not definitively ruled out differences in treatment outcomes of patients
with ICB between cisplatin and carboplatin. The presented experimental data from cancer and immune cells may provide a hint as to how chemotherapeutic drugs can interfere with ICB. Most
strikingly, cisplatin interfered with gene regulatory pathways that target immune checkpoints in cancer or immune cells and thereby is connected with ICB. Restrictively, the time period
considered in the cell culture studies (24 h) with combined addition of chemotherapeutics, interferon γ or pharmacologic T-cell activation is shorter than the time period (>weeks) of
chemotherapeutic treatment of patients. Our cell experimental strategy aimed to simulate the tumor microenvironment. During tumor progression, intercellular signaling between tumor cells and
infiltrating immune cells occurs dynamically and over an extended period of time. These apparent differences point to the limitations of this cell biology study. In conclusion, we
demonstrated that certain chemotherapeutic drugs that are relevant for the therapy of urothelial cancer can affect distinct co-inhibitory and co-stimulatory mediators of immune cell
signaling [11]. Strikingly, among these are cisplatin and vinflunine. The observed shifts in mRNA levels of immune signaling proteins suggest possible impact on immune checkpoint blockage
therapy that may be of importance for investigation of tumor models and relevant patients. DATA AVAILABILITY All data generated or analysed during this study are included in this published
article. REFERENCES * Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309–37.
https://doi.org/10.1016/j.cell.2021.09.020. Article CAS PubMed PubMed Central Google Scholar * Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious
diseases. Eur J Immunol. 2017;47:765–79. https://doi.org/10.1002/eji.201646875. Article CAS PubMed Google Scholar * Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities,
differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. https://doi.org/10.1097/COC.0000000000000239. Article CAS PubMed PubMed Central Google Scholar *
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020
guidelines. Eur Urol. 2021;79:82–104. https://doi.org/10.1016/j.eururo.2020.03.055. Article CAS PubMed Google Scholar * Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H,
Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–201. https://doi.org/10.1016/j.celrep.2017.04.031. Article CAS
PubMed PubMed Central Google Scholar * Lee JY, Choi A-Y, Oh YT, Choe W, Yeo E-J, Ha J, et al. AMP-activated protein kinase mediates T cell activation-induced expression of FasL and COX-2
via protein kinase C theta-dependent pathway in human Jurkat T leukemia cells. Cell Signal. 2012;24:1195–207. https://doi.org/10.1016/j.cellsig.2012.01.015. Article CAS PubMed Google
Scholar * Hänze J, Kessel F, Di Fazio P, Hofmann R, Hegele A. Effects of multi and selective targeted tyrosine kinase inhibitors on function and signaling of different bladder cancer cells.
Biomed Pharmacother. 2018;106:316–25. https://doi.org/10.1016/j.biopha.2018.06.110. Article PubMed Google Scholar * Kerr M, Scott HE, Groselj B, Stratford MRL, Karaszi K, Sharma NL, et
al. Deoxycytidine kinase expression underpins response to gemcitabine in bladder cancer. Clin Cancer Res. 2014;20:5435–45. https://doi.org/10.1158/1078-0432.CCR-14-0542. Article CAS PubMed
PubMed Central Google Scholar * Sarin N, Engel F, Kalayda GV, Mannewitz M, Cinatl J, Rothweiler F, et al. Cisplatin resistance in non-small cell lung cancer cells is associated with an
abrogation of cisplatin-induced G2/M cell cycle arrest. PLoS One. 2017;12:e0181081. https://doi.org/10.1371/journal.pone.0181081. Article PubMed PubMed Central Google Scholar *
Kruczynski A, Etiévant C, Perrin D, Chansard N, Duflos A, Hill BT. Characterization of cell death induced by vinflunine, the most recent Vinca alkaloid in clinical development. Br J Cancer.
2002;86:143–50. https://doi.org/10.1038/sj.bjc.6600025. Article CAS PubMed PubMed Central Google Scholar * Fournel L, Wu Z, Stadler N, Damotte D, Lococo F, Boulle G, et al. Cisplatin
increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019;464:5–14. https://doi.org/10.1016/j.canlet.2019.08.005. Article CAS
PubMed Google Scholar * Zaidi MR. The interferon-gamma paradox in cancer. J Interferon Cytokine Res. 2019;39:30–8. https://doi.org/10.1089/jir.2018.0087. Article CAS PubMed PubMed
Central Google Scholar * Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation
profiles. Oncogene. 2019;38:2380–93. https://doi.org/10.1038/s41388-018-0581-9. Article CAS PubMed Google Scholar * Antonangeli F, Natalini A, Garassino MC, Sica A, Santoni A, Di Rosa F.
Regulation of PD-L1 expression by NF-κB in cancer. Front Immunol. 2020;11:584626. https://doi.org/10.3389/fimmu.2020.584626. Article CAS PubMed PubMed Central Google Scholar * Tsai
T-F, Lin J-F, Lin Y-C, Chou K-Y, Chen H-E, Ho C-Y, et al. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway. Biosci Rep.
2019;39:BSR20190362. https://doi.org/10.1042/BSR20190362. Article CAS PubMed PubMed Central Google Scholar * Li Q, Wang Y, Jia W, Deng H, Li G, Deng W, et al. Low-dose anti-angiogenic
therapy sensitizes breast cancer to PD-1 blockade. Clin Cancer Res. 2020;26:1712–24. https://doi.org/10.1158/1078-0432.CCR-19-2179. Article CAS PubMed Google Scholar * Glorieux C, Xia X,
You X, Wang Z, Han Y, Yang J, et al. Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer. J Adv Res. 2022;40:109–24.
https://doi.org/10.1016/j.jare.2021.12.005. Article CAS PubMed Google Scholar * Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer.
2021;21:37–50. https://doi.org/10.1038/s41568-020-00308-y. Article CAS PubMed Google Scholar * Galsky MD, Arija JÁA, Bamias A, Davis ID, Santis M de, Kikuchi E, et al. Atezolizumab with
or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–57.
https://doi.org/10.1016/S0140-6736(20)30230-0. Article CAS PubMed Google Scholar * Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY-S, et al. Pembrolizumab alone or
combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:931–45.
https://doi.org/10.1016/S1470-2045(21)00152-2. Article CAS PubMed Google Scholar * Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for
advanced or metastatic urothelial carcinoma. N. Engl J Med. 2020;383:1218–30. https://doi.org/10.1056/NEJMoa2002788. Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS We thank Susanne Lingelbach for excellent medical laboratory technical assistance. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION
Author notes * These authors contributed equally: Jörg Hänze, Johannes Schulte-Herbrüggen. AUTHORS AND AFFILIATIONS * Department of Urology, Philipps-University Marburg, Marburg, Germany
Jörg Hänze, Johannes Schulte-Herbrüggen & Rainer Hofmann * Department of Radiotherapy and Radiooncology, Faculty of Medicine, Philipps-University Marburg, Marburg, Germany Axel Hegele *
Urological Center Mittelhessen, DRK Hospital Biedenkopf, Biedenkopf, Germany Axel Hegele Authors * Jörg Hänze View author publications You can also search for this author inPubMed Google
Scholar * Johannes Schulte-Herbrüggen View author publications You can also search for this author inPubMed Google Scholar * Rainer Hofmann View author publications You can also search for
this author inPubMed Google Scholar * Axel Hegele View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS JH and JSH designed, acquired data,
interpreted the results, drafted and revised the work. RH conceived the work. AH conceived and drafted the work. CORRESPONDING AUTHOR Correspondence to Jörg Hänze. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Hänze, J., Schulte-Herbrüggen, J., Hofmann, R. _et al._ Modulation of immune checkpoint regulators in interferon γ induced urothelial carcinoma and activated
T-lymphocyte cells by cytostatics. _Genes Immun_ 24, 149–153 (2023). https://doi.org/10.1038/s41435-023-00203-0 Download citation * Received: 15 November 2022 * Revised: 05 April 2023 *
Accepted: 14 April 2023 * Published: 03 May 2023 * Issue Date: June 2023 * DOI: https://doi.org/10.1038/s41435-023-00203-0 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
