Inorganic pyrophosphatase 1: a key player in immune and metabolic reprogramming in ankylosing spondylitis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

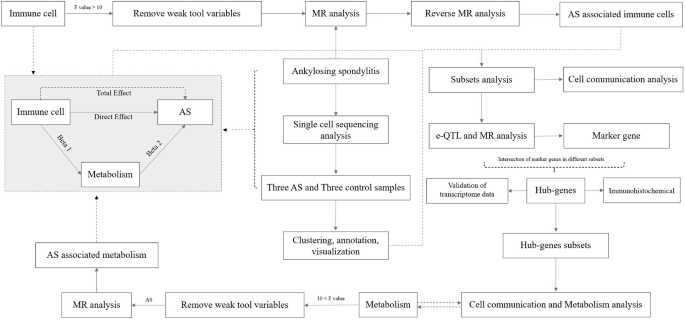
ABSTRACT The relationships among immune cells, metabolites, and AS events were analyzed via Mendelian randomization (MR), and potential immune cells and metabolites were identified as risk
factors for AS. Their relationships were subjected to intermediary MR analysis to identify the final immune cells and metabolites. The vertebral bone marrow blood samples from three patients
with and without AS were subjected to 10× single-cell sequencing to further elucidate the role of immune cells in AS. The key genes were screened via expression quantitative trait loci
(eQTLs) and MR analyses. The metabolic differences between the two groups were compared through single-cell metabolism analysis. Two subgroups of differentiated (CD)8+ memory T cells and
naive B cells were obtained from the combined results of intermediary MR analysis and AS single-cell analysis. After the verification of key genes, inorganic pyrophosphatase 1 (PPA1) was
identified as the hub gene, as it is differentially expressed in CD8+ memory T cells and can affect the metabolism of T cells in AS by affecting the expression of ferulic acid (FA)4 sulfate,
which participates in the cellular immunity in AS. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS
Access through your institution Subscribe to this journal Receive 6 digital issues and online access to articles $119.00 per year only $19.83 per issue Learn more Buy this article * Purchase
on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE SHARED ROLE OF NEUTROPHILS IN ANKYLOSING SPONDYLITIS AND ULCERATIVE COLITIS
Article 25 July 2024 ANALYSIS OF M6A REGULATORS RELATED IMMUNE CHARACTERISTICS IN ANKYLOSING SPONDYLITIS BY INTEGRATED BIOINFORMATICS AND COMPUTATIONAL STRATEGIES Article Open access 01
February 2024 SINGLE-CELL RNA SEQUENCING REVEALS MULTIPLE IMMUNE CELL SUBPOPULATIONS PROMOTE THE FORMATION OF ABNORMAL BONE MICROENVIRONMENT IN OSTEOPOROSIS Article Open access 27 November
2024 DATA AVAILABILITY The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. REFERENCES * Drosos AA,
Venetsanopoulou AI, Voulgari PV. Axial Spondyloarthritis: Evolving concepts regarding the disease’s diagnosis and treatment. Eur J Intern Med. 2023;117:21–7.
https://doi.org/10.1016/j.ejim.2023.06.026. Article CAS PubMed Google Scholar * McGonagle D, Ramonda R, Scagnellato L, Scriffignano S, Weddell J, Lubrano E. A strategy towards
disentangling treatment refractory from misdiagnosed axial Spondyloarthritis. Autoimmun Rev. 2024;23(1):103405. https://doi.org/10.1016/j.autrev.2023.103405. Article PubMed Google Scholar
* Barnett R, Gaffney K, Sengupta R. Diagnostic delay in axial spondylarthritis: A lost battle? Best Pract Res Clin Rheumatol. 2023;37(3):101870. https://doi.org/10.1016/j.berh.2023.101870.
Article PubMed Google Scholar * van Gaalen FA, Rudwaleit M. Challenges in the diagnosis of axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2023;37(3):101871.
https://doi.org/10.1016/j.berh.2023.101871. Article PubMed Google Scholar * Xie J, Xu J, Chen H. Regulatory mechanisms of miR-212-3p on the secretion of inflammatory factors in
monocyte-macrophages and the directed differentiation into osteoclasts in ankylosing spondylitis. Aging. 2023;15(22):13411–21. https://doi.org/10.18632/aging.205249. Article CAS PubMed
PubMed Central Google Scholar * Feng X, Wang C, Ji B, Qiao J, Xu Y, Zhu S, et al. CD_99 G1 neutrophils modulate osteogenic differentiation of mesenchymal stem cells in the pathological
process of ankylosing spondylitis. Ann Rheum Dis. 2024;83(3):324–34. https://doi.org/10.1136/ard-2023-224107. Article CAS PubMed Google Scholar * Komech EA, Koltakova AD, Barinova AA,
Minervina AA, Salnikova MA, Shmidt EI, et al. TCR repertoire profiling revealed antigen-driven CD8+ T cell clonal groups shared in synovial fluid of patients with spondyloarthritis. Front
Immunol. 2022;13:973243. https://doi.org/10.3389/fimmu.2022.973243. Article CAS PubMed PubMed Central Google Scholar * Garrido-Mesa J, Brown MA. T cell repertoire profiling and the
mechanism by which HLA-B27 causes ankylosing spondylitis. Curr Rheumatol Rep. 2022;24(12):398–4. https://doi.org/10.1007/s11926-022-01090-6. Article CAS PubMed PubMed Central Google
Scholar * Rosine N, Fogel O, Koturan S, Rogge L, Bianchi E, Miceli-Richard C. T cells in the pathogenesis of axial spondyloarthritis. J Bone Spine. 2023;90(6):105619.
https://doi.org/10.1016/j.jbspin.2023.105619. Article CAS Google Scholar * Wilbrink R, Spoorenberg A, Verstappen G, Kroese FGM. B cell involvement in the pathogenesis of ankylosing
spondylitis. Int J Mol Sci. 2021;22(24):13325. https://doi.org/10.3390/ijms222413325. Article CAS PubMed PubMed Central Google Scholar * Wang S, Yang N, Zhang H. Metabolic dysregulation
of lymphocytes in autoimmune diseases. Trends Endocrinol Metab: TEM. 2024;35(7):624–37. https://doi.org/10.1016/j.tem.2024.01.005. Article CAS PubMed Google Scholar * Wang PF, Jiang F,
Zeng QM, Yin WF, Hu YZ, Li Q, et al. Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis. J Neuroinflammation. 2024;21(1):28.
https://doi.org/10.1186/s12974-024-03016-8. Article PubMed PubMed Central Google Scholar * Li J, Zhao M, Luo W, Huang J, Zhao B, Zhou Z. B cell metabolism in autoimmune diseases:
signaling pathways and interventions. Front Immunol. 2023;14:1232820. https://doi.org/10.3389/fimmu.2023.1232820. Article CAS PubMed PubMed Central Google Scholar * Mora VP, Loaiza RA,
Soto JA, Bohmwald K, Kalergis AM. Involvement of trained immunity during autoimmune responses. J Autoimmun. 2023;137:102956. https://doi.org/10.1016/j.jaut.2022.102956. Article CAS PubMed
Google Scholar * Xu Y, Chen Y, Zhang X, Ma J, Liu Y, Cui L, et al. Glycolysis in innate immune cells contributes to autoimmunity. Front Immunol. 2022;13:920029.
https://doi.org/10.3389/fimmu.2022.920029. Article CAS PubMed PubMed Central Google Scholar * Hu JQ, Yan YH, Xie H, Feng XB, Ge WH, Zhou H, et al. Targeting abnormal lipid metabolism of
T cells for systemic lupus erythematosus treatment. Biomed Pharmacother = Biomedecine Pharmacotherapie. 2023;165:115198. https://doi.org/10.1016/j.biopha.2023.115198. Article CAS PubMed
Google Scholar * Gan PR, Wu H, Zhu YL, Shu Y, Wei Y. Glycolysis, a driving force of rheumatoid arthritis. Int Immunopharmacol. 2024;132:111913. https://doi.org/10.1016/j.intimp.2024.111913.
Article CAS PubMed Google Scholar * Ferreté-Bonastre AG, Martínez-Gallo M, Morante-Palacios O, Calvillo CL, Calafell-Segura J, Rodríguez-Ubreva J, et al. Disease activity drives
divergent epigenetic and transcriptomic reprogramming of monocyte subpopulations in systemic lupus erythematosus. Ann Rheum Dis. 2024;83(7):865–78. https://doi.org/10.1136/ard-2023-225433.
Article CAS PubMed Google Scholar * Fu JY, Huang SJ, Wang BL, Yin JH, Chen CY, Xu JB, et al. Lysine acetyltransferase 6A maintains CD4(+) T cell response via epigenetic reprogramming of
glucose metabolism in autoimmunity. Cell Metab. 2024;36(3):557–74.e. https://doi.org/10.1016/j.cmet.2023.12.016. Article CAS PubMed Google Scholar * Cribbs AP, Terlecki-Zaniewicz S,
Philpott M, Baardman J, Ahern D, Lindow M, et al. Histone H3K27me3 demethylases regulate human Th17 cell development and effector functions by impacting on metabolism. Proc Natl Acad Sci
USA. 2020;117(11):6056–66. https://doi.org/10.1073/pnas.1919893117. Article CAS PubMed PubMed Central Google Scholar * Chen Y, Song J, Ruan Q, Zeng X, Wu L, Cai L, et al. Single-cell
sequencing methodologies: from transcriptome to multi-dimensional measurement. Small Methods. 2021;5(6):e2100111. https://doi.org/10.1002/smtd.202100111. Article CAS PubMed Google Scholar
* Chen C, Wang P, Zhang RD, Fang Y, Jiang LQ, Fang X, et al. Mendelian randomization as a tool to gain insights into the mosaic causes of autoimmune diseases. Autoimmun Rev.
2022;21(12):1032. https://doi.org/10.1016/j.autrev.2022.103210. Article CAS Google Scholar * Zhou C, Liang T, Jiang J, Zhang Z, Chen J, Chen T, et al. Immune cell infiltration-related
clinical diagnostic model for Ankylosing Spondylitis. Front Genet. 2022;13:949882. https://doi.org/10.3389/fgene.2022.949882. Article CAS PubMed PubMed Central Google Scholar * Chen Y,
Lu T, Pettersson-Kymmer U, Stewart ID, Butler-Laporte G, Nakanishi T, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet.
2023;55(1):44–53. https://doi.org/10.1038/s41588-022-01270-1. Article CAS PubMed PubMed Central Google Scholar * Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, et al. Author
Correction: Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(11):1266. https://doi.org/10.1038/s41588-020-00718-6. Article CAS PubMed
Google Scholar * Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D..et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7.
https://doi.org/10.7554/eLife.34408. * Mensah-Kane J, Schmidt AF, Hingorani AD, Finan C, Chen Y, van Duijvenboden S, et al. No clinically relevant effect of heart rate increase and heart
rate recovery during exercise on cardiovascular disease: a mendelian randomization analysis. Front Genet. 2021;12:569323. https://doi.org/10.3389/fgene.2021.569323. Article PubMed PubMed
Central Google Scholar * Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med.
2015;34(21):2926–40. https://doi.org/10.1002/sim.6522. Article Google Scholar * Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships
inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. https://doi.org/10.1038/s41588-018-0099-7. Article CAS PubMed PubMed Central
Google Scholar * Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023.
Wellcome open Res. 2019;4:186. https://doi.org/10.12688/wellcomeopenres.15555.3. Article PubMed Google Scholar * Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid
instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. https://doi.org/10.1093/ije/dyv080. Article PubMed PubMed Central Google
Scholar * Gala H, Tomlinson I. The use of Mendelian randomisation to identify causal cancer risk factors: promise and limitations. J Pathol. 2020;250(5):541–54.
https://doi.org/10.1002/path.5421. Article PubMed Google Scholar * Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harbor Persp Medi. 2021;11(2):a038984
https://doi.org/10.1101/cshperspect.a038984. Article CAS Google Scholar * Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, et al. A survey of best practices for
RNA-seq data analysis. Genome Biol. 2016;17:13. https://doi.org/10.1186/s13059-016-0881-8. Article CAS PubMed PubMed Central Google Scholar * Mauro D, Thomas R, Guggino G, Lories R,
Brown MA, Ciccia F. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol. 2021;17(7):387–404. https://doi.org/10.1038/s41584-021-00625-y. Article CAS PubMed
Google Scholar * Voruganti A, Bowness P. New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology. 2020;161(2):94–102. https://doi.org/10.1111/imm.13242.
Article CAS PubMed PubMed Central Google Scholar * Pedersen SJ, Maksymowych WP. The pathogenesis of ankylosing spondylitis: an update. Curr Rheumatol Rep. 2019;21(10):58.
https://doi.org/10.1007/s11926-019-0856-3. Article CAS PubMed Google Scholar * McCann C, Kerr EM. Metabolic reprogramming: a friend or foe to cancer therapy?. Cancers. 2021;13(13):3351.
https://doi.org/10.3390/cancers13133351. Article CAS PubMed PubMed Central Google Scholar * Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to
cancer. Protein Cell. 2022;13(12):877–919. https://doi.org/10.1007/s13238-021-00846-7. Article CAS PubMed Google Scholar * Halper-Stromberg A, Jabri B. Maladaptive consequences of
inflammatory events shape individual immune identity. Nat Immunol. 2022;23(12):1675–86. https://doi.org/10.1038/s41590-022-01342-8. Article CAS PubMed Google Scholar * Chou WC,
Rampanelli E, Li X, Ting JP. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol Immunol. 2022;19(3):337–51. https://doi.org/10.1038/s41423-021-00780-y. Article
CAS PubMed Google Scholar * Raniga K, Liang C. Interferons: Reprogramming the metabolic network against viral infection. Viruses. 2018;10(1):36. https://doi.org/10.3390/v10010036. *
Thimmappa PY, Vasishta S, Ganesh K, Nair AS, Joshi MB. Neutrophil (dys)function due to altered immuno-metabolic axis in type 2 diabetes: implications in combating infections. Hum cell.
2023;36(4):1265–82. https://doi.org/10.1007/s13577-023-00905-7. Article CAS PubMed PubMed Central Google Scholar * Marrocco A, Ortiz LA. Role of metabolic reprogramming in
pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front Immunol. 2022;13:936167. https://doi.org/10.3389/fimmu.2022.936167. Article CAS PubMed PubMed Central
Google Scholar * Li F, Liu H, Zhang D, Ma Y, Zhu B. Metabolic plasticity and regulation of T cell exhaustion. Immunology. 2022;167(4):482–94. https://doi.org/10.1111/imm.13575. Article CAS
PubMed Google Scholar * Guimarães ES, Marinho FV, de Queiroz N, Antunes MM, Oliveira SC. Impact of STING Inflammatory Signaling during Intracellular Bacterial Infections. Cells.
2021;11(1):74. https://doi.org/10.3390/cells11010074. * Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J
Am Soc Nephrol. 2016;27(11):3253–65. https://doi.org/10.1681/asn.2016010098. Article PubMed PubMed Central Google Scholar * Zhang Y, Wang J, Yu C, Xia K, Yang B, Zhang Y, et al. Advances
in single-cell sequencing and its application to musculoskeletal system research. Cell Prolif. 2022;55(1):e13161. https://doi.org/10.1111/cpr.13161. Article CAS PubMed Google Scholar *
Yang P, Huang H, Liu C. Feature selection revisited in the single-cell era. Genome Biol. 2021;22(1):321 https://doi.org/10.1186/s13059-021-02544-3. Article PubMed PubMed Central Google
Scholar * Barreiro-Sisto U, Fernández-Fariña S, González-Noya AM, Pedrido R, Maneiro M. Enemies or Allies? Hormetic and apparent non-dose-dependent effects of natural bioactive antioxidants
in the treatment of inflammation. Int J Mol Sci 2024;25(3):1892. https://doi.org/10.3390/ijms25031892. * Wu J, Zhou F, Fan G, Liu J, Wang Y, Xue X, et al. Ferulic acid ameliorates
acetaminophen-induced acute liver injury by promoting AMPK-mediated protective autophagy. IUBMB life. 2022;74(9):880–95. https://doi.org/10.1002/iub.2625. Article CAS PubMed Google
Scholar * Wu J, Xue X, Fan G, Gu Y, Zhou F, Zheng Q, et al. Ferulic acid ameliorates hepatic inflammation and fibrotic liver injury by inhibiting PTP1B activity and subsequent promoting
AMPK phosphorylation. Front Pharmacol. 2021;12:754976. https://doi.org/10.3389/fphar.2021.754976. Article CAS PubMed PubMed Central Google Scholar * Ye L, Hu P, Feng LP, Huang LL, Wang
Y, Yan X, et al. Protective effects of ferulic acid on metabolic syndrome: a comprehensive review. Molecules (Basel, Switzerland). 2022;28(1):281. https://doi.org/10.3390/molecules28010281.
* Li Y, Sair AT, Zhao W, Li T, Liu RH. Ferulic acid mediates metabolic syndrome via the regulation of hepatic glucose and lipid metabolisms and the insulin/IGF-1 rReceptor/PI3K/AKT pathway
in palmitate-treated HepG2 cells. J Agric food Chem. 2022;70(46):14706–17. https://doi.org/10.1021/acs.jafc.2c05676. Article CAS PubMed Google Scholar * Sun L, Yang Z, Zhao W, Chen Q,
Bai H, Wang S, et al. Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxue Decoction in the treatment of diabetic nephropathy in
type 2 diabetes mellitus. J Ethnopharmacol. 2022;283:114699. https://doi.org/10.1016/j.jep.2021.114699. Article CAS PubMed Google Scholar * Hammad SM, Lopes-Virella MF. Circulating
sphingolipids in insulin resistance, diabetes and associated complications. Int J Mol Sci. 2023;24(18):14015. https://doi.org/10.3390/ijms241814015. * Naquet P, Kerr EW, Vickers SD, Leonardi
R. Regulation of coenzyme A levels by degradation: the ‘Ins and Outs’. Prog lipid Res. 2020;78:101028. https://doi.org/10.1016/j.plipres.2020.101028. Article CAS PubMed PubMed Central
Google Scholar * Yin Y, Wu Y, Zhang X, Zhu Y, Sun Y, Yu J, et al. PPA1 regulates systemic insulin sensitivity by maintaining adipocyte mitochondria function as a novel PPARγ Target Gene.
Diabetes. 2021;70(6):1278–91. https://doi.org/10.2337/db20-0622. Article CAS PubMed Google Scholar * Tavasolian F, Pastrello C, Ahmed Z, Jurisica I, Inman RD. Vesicular traffic-mediated
cell-to-cell signaling at the immune synapse in Ankylosing Spondylitis. Front Immunol. 2022;13:1102405. https://doi.org/10.3389/fimmu.2022.1102405. Article CAS PubMed Google Scholar *
Liu L, Yuan Y, Zhang S, Xu J, Zou J. Osteoimmunological insights into the pathogenesis of ankylosing spondylitis. J Cell Physiol. 2021;236(9):6090–100. https://doi.org/10.1002/jcp.30313.
Article CAS PubMed Google Scholar * Tam HKJ, Robinson PC, Nash P. Inhibiting IL-17A and IL-17F in rheumatic disease: therapeutics help to elucidate disease mechanisms. Curr Rheumatol
Rep. 2022;24(10):310–20. https://doi.org/10.1007/s11926-022-01084-4. Article CAS PubMed PubMed Central Google Scholar * Gracey E, Yao Y, Qaiyum Z, Lim M, Tang M, Inman RD. Altered
cytotoxicity profile of CD8+ T cells in ankylosing spondylitis. Arthritis Rheumatol. 2020;72(3):428–34. https://doi.org/10.1002/art.41129. Article CAS PubMed Google Scholar * Zhang L,
Jarvis LB, Baek HJ, Gaston JS. Regulatory IL4+CD8+ T cells in patients with ankylosing spondylitis and healthy controls. Ann Rheum Dis. 2009;68(8):1345–51.
https://doi.org/10.1136/ard.2008.088120. Article CAS PubMed Google Scholar * Vecellio M, Cohen CJ, Roberts AR, Wordsworth PB, Kenna TJ. RUNX3 and T-bet in immunopathogenesis of
ankylosing spondylitis-novel targets for therapy? Front Immunol. 2018;9:3132. https://doi.org/10.3389/fimmu.2018.03132. Article CAS PubMed Google Scholar * Pedersen SJ, Maksymowych WP.
Beyond the TNF-α inhibitors: new and emerging targeted therapies for patients with axial spondyloarthritis and their relation to pathophysiology. Drugs. 2018;78(14):1397–418.
https://doi.org/10.1007/s40265-018-0971-x. Article CAS PubMed Google Scholar * Sun Y, Yao J, Lu C, Yang N, Han X, Lin H, et al. Cold-inducible PPA1 is critical for the adipocyte browning
in mice. Biochem Biophys Res Commun. 2023;677:45–53. https://doi.org/10.1016/j.bbrc.2023.08.009. Article CAS PubMed Google Scholar * Khan MA. HLA-B*27 and ankylosing spondylitis: 50
years of insights and discoveries. Curr Rheumatol Rep. 2023;25(12):327–40. https://doi.org/10.1007/s11926-023-01118-5. Article CAS PubMed Google Scholar * Braun J, Sieper J. Fifty years
after the discovery of the association of HLA B27 with ankylosing spondylitis. RMD Open. 2023;9(3):e003102. https://doi.org/10.1136/rmdopen-2023-003102. * Brown EM, Nguyen PNU, Xavier RJ.
Emerging biochemical, microbial and immunological evidence in the search for why HLA-B∗27 confers risk for spondyloarthritis. Cell Chem Biol. 2024;20:S2451-9456(24)00314-3.
https://doi.org/10.1016/j.chembiol.2024.07.012. * Paley MA, Yang X, Hassman LM, Penkava F, Garner LI, Paley GL, et al. Mucosal signatures of pathogenic T cells in HLA-B*27+ anterior uveitis
and axial spondyloarthritis. JCI insight. 2024;9(16)e174776. https://doi.org/10.1172/jci.insight.174776. Download references FUNDING This study was supported by the National Natural Science
Foundation of China(Grant/Award number: 8236090175), Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation (Grant/Award number: 2023JJA140227), The
“Medical Excellence Award” Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University, and Guangxi Young and Middle aged Teacher’s
Basic Ability Promoting Project(Grant/Award Number: 2023KY0115). AUTHOR INFORMATION Author notes * These authors contributed equally: Tianyou Chen, Chengqian Huang. AUTHORS AND AFFILIATIONS
* The First Affiliated Hospital of Guangxi Medical University, No.6 Shuangyong Road, Nanning, Guangxi, 530021, People’s Republic of China Tianyou Chen, Chengqian Huang, Jiarui Chen, Jiang
Xue, Zhenwei Yang, Yihan Wang, Songze Wu, Wendi Wei, Liyi Chen, Shian Liao, Xiaopeng Qin, Rongqing He, Boli Qin & Chong Liu Authors * Tianyou Chen View author publications You can also
search for this author inPubMed Google Scholar * Chengqian Huang View author publications You can also search for this author inPubMed Google Scholar * Jiarui Chen View author publications
You can also search for this author inPubMed Google Scholar * Jiang Xue View author publications You can also search for this author inPubMed Google Scholar * Zhenwei Yang View author
publications You can also search for this author inPubMed Google Scholar * Yihan Wang View author publications You can also search for this author inPubMed Google Scholar * Songze Wu View
author publications You can also search for this author inPubMed Google Scholar * Wendi Wei View author publications You can also search for this author inPubMed Google Scholar * Liyi Chen
View author publications You can also search for this author inPubMed Google Scholar * Shian Liao View author publications You can also search for this author inPubMed Google Scholar *
Xiaopeng Qin View author publications You can also search for this author inPubMed Google Scholar * Rongqing He View author publications You can also search for this author inPubMed Google
Scholar * Boli Qin View author publications You can also search for this author inPubMed Google Scholar * Chong Liu View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS Conceptualization: Tianyou Chen, Chong Liu; Methodology: Tianyou Chen, Chengqian Huang; Formal analysis and investigation: Jiang Xue; Validation and Writing -
original draft preparation: Jiarui Chen; Writing - review and editing: Tianyou Chen, Chengqian Huang, Chong Liu; Funding acquisition: Chong Liu; Data curation: Zhenwei Yang, Yihan Wang,
Songze Wu; Resources: Wendi Wei, Liyi Chen, Shian Liao; Software: Xiaopeng Qin, Rongqing He; Supervision: Chong Liu; Visualization: Boli Qin. CORRESPONDING AUTHOR Correspondence to Chong
Liu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. CONSENT FOR PUBLICATION All authors are aware of and agree to the publication of this study. ETHICAL
APPROVAL AND CONSENT TO PARTICIPATE The study was approved by the ethics review board of First Affiliated Hospital Of Guangxi Medical University Ethical Review Committee (Approval No.
2024-E205-01) in accordance with the Declaration of Helsinki. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from
all individual patients included in the study. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION SUPPLEMENTARY DOCUMENT SUPPLEMENTARY FIGURE 1 SUPPLEMENTARY FIGURE 2 SUPPLEMENTARY TABLE 1 SUPPLEMENTARY TABLE
2 SUPPLEMENTARY TABLE 3 RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the
author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, T., Huang, C., Chen, J. _et al._ Inorganic pyrophosphatase 1: a key player in immune and metabolic reprogramming in
ankylosing spondylitis. _Genes Immun_ 26, 9–21 (2025). https://doi.org/10.1038/s41435-024-00308-0 Download citation * Received: 23 June 2024 * Revised: 22 October 2024 * Accepted: 31 October
2024 * Published: 07 November 2024 * Issue Date: February 2025 * DOI: https://doi.org/10.1038/s41435-024-00308-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
