Clonal dynamics in osteosarcoma defined by rgb marking
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Osteosarcoma is a type of bone tumour characterized by considerable levels of phenotypic heterogeneity, aneuploidy, and a high mutational rate. The life expectancy of osteosarcoma
patients has not changed during the last three decades and thus much remains to be learned about the disease biology. Here, we employ a RGB-based single-cell tracking system to study the
clonal dynamics occurring in a de novo-induced murine osteosarcoma model. We show that osteosarcoma cells present initial polyclonal dynamics, followed by clonal dominance associated with
adaptation to the microenvironment. Interestingly, the dominant clones are composed of subclones with a similar tumour generation potential when they are re-implanted in mice. Moreover,
individual spontaneous metastases are clonal or oligoclonal, but they have a different cellular origin than the dominant clones present in primary tumours. In summary, we present evidence
that osteosarcomagenesis can follow a neutral evolution model, in which different cancer clones coexist and propagate simultaneously. SIMILAR CONTENT BEING VIEWED BY OTHERS MULTI-COLOR
CLONAL TRACKING REVEALS INTRA-STAGE PROLIFERATIVE HETEROGENEITY DURING MAMMARY TUMOR PROGRESSION Article 12 October 2020 NON-GENETIC DETERMINANTS OF MALIGNANT CLONAL FITNESS AT SINGLE-CELL
RESOLUTION Article 08 December 2021 MULTIFUNCTIONAL BARCODING WITH CLONMAPPER ENABLES HIGH-RESOLUTION STUDY OF CLONAL DYNAMICS DURING TUMOR EVOLUTION AND TREATMENT Article 12 July 2021
INTRODUCTION Osteosarcoma (OS) is the most common malignant solid tumour that affects bones. The disease presents a bimodal distribution with increased incidence during the second decade of
life; OS represents more than 10% of solid cancer cases in adolescents (15–19 years old)1. The paediatric incidence window reflects the biology of the disease; there is a correlation between
skeletal growth, height, and disease appearance. Moreover, OS usually originates in the extremities of long bones, close to the metaphyseal plate, which is also the anatomical location of
bone growth2. Almost 75% of OS is highly malignant, and due to disease aggressiveness, it has typically extended beyond the bone into nearby musculoskeletal structures at detection1,2.
Tumour biopsies showing mesenchymal cells producing osteoid and/or irregular woven bone are categorized as OS. The histologic finding of this incomplete osteogenic process is a requirement
for tumour diagnosis even if other cell subtypes directly derived from the tumour are present. This pathological definition is used because the aetiology of OS is mostly unknown. Genetic
disorders, such as Li–Fraumeni syndrome (_TP53_ germline mutation) and familial Retinoblastoma (_RB1_ germline mutation), are risk factors for osteosarcoma3,4. The Pediatric Cancer Genome
Project (PCGP) identified frequent germline mutations of the _TP53_ gene in OS, similar to the 50% _TP53_ mutation rate of childhood cancers5,6, and whole genome and whole exome sequencing
revealed that alterations in the p53 and Rb pathways are more frequent in OS than previously thought7,8. Therefore, these syndromes are mainly associated with mutations of genes that
participate in genome integrity maintenance and chromosomal stability. Unlike many sarcomas, which are characterized by specific chromosome translocations, OS exhibits a complex karyotype
with high genomic and chromosomal instability;9 it is also characterized by multiple rearrangements across the genome, kataegis, and chromothripsis8,10,11,12. Malignant tumours typically
comprise a heterogeneous pool of cells that differ in terms of morphology, phenotype, gene expression, metabolism, immunogenicity, proliferation, and metastatic potential13,14. Many models
have been postulated to explain the clonal dynamics that drive cancer disease and the generation of heterogeneity14,15. The competitive linear model of clonal cancer evolution proposed by
Nowell16 and the cancer stem cell hypothesis were the first models describing cancer evolution17,18,19. Later, other authors suggested that these two models were not mutually exclusive
because cancer stem cells could be the unit of selection during cancer initiation and progression. A switch from differentiation to self-renewal, supported by the niche, can generate
compartment amplification, in which cancer stem cell units can also undergo independent evolution13,20,21. With the advent of cancer genome studies, branched phylogenies were adopted to
describe cancer evolution22,23,24,25. Additionally, the sequential accumulation of genetic alterations was recently questioned due to evidence indicating macroevolutionary events14,26. Other
authors have rejected clonal dominance in favour of a big bang model of clonal diversity, in which different clonal cancer populations are generated early in tumourigenesis and coexist with
neutral evolution dynamics27,28. In this context, the ecological interaction between tumour subclones29,30,31 and the dynamics of contingency, convergence, and parallel evolution are
implicated in tumour growth14. In the current view of the cancer ecosystem, non-genetic determinants also contribute to tumour growth. The interaction between tumour cells and the
microenvironment, differentiation programs, factors such as hypoxia, and especially the immune system represent crucial players in cancer development14,21. Another largely unexplored field
of clonal cancer dynamics concerns metastatic development. From the seed and soil hypothesis and the preferential diffusion pathway of some tumours, the modern definition of a pre-metastatic
niche highlights the importance of the microenvironment in metastatic cell tropism to seed-specific organs32. Some studies have shown a monoclonal pattern of metastatic seeding, but others
have reported a polyclonal signature for this process33. A model that exhaustively describes cancer growth is extremely important because this knowledge has many practical implications in
the clinic. Especially in the field of personalized medicine, the clonal homogeneity of a primary tumour and heterogeneity of metastatic cells represent relevant variables for designing a
therapeutic strategy. A single tumour biopsy may be insufficient to provide representative information of the total genetic and molecular variability present in the primary tumour.
Additionally, the implication of heterogeneity in the management of patients presenting with metastatic disease represents a significant challenge. The general approach, driven by the
assumption of close similarity between a primary tumour and metastases, has been to analyse the primary tumour and avoid more invasive biopsies at metastatic locations. This approach
restricted the estimation of how many different clones can constitute a tumour or metastasize to an organ. Moreover, metastatic disease is a time-dependent process; nevertheless, little is
known about its timing, the changes in the clonal composition over time, and the degree of independent evolution between primary tumour and metastases. To study the events driving
osteosarcomagenesis, here, we focus on the clonal dynamics that occur during the formation, development, and progression of a murine model of in vitro transformed mesenchymal progenitor
cells (MPCs). We previously reported the transformation of MPCs by deleting the _Tp53_ and _Rb_ genes. These MPCs, if inoculated in the proper orthotopic or ectopic ceramic-based
osteoinductive microenvironment, efficiently recapitulate OS formation34. In this study, we used a single-cell tracking technique to study the in vivo OS clonal dynamics during tumour
formation and progression. Based on lentiviral transduction with vectors coding for three different fluorescent proteins (Cerulean, Venus, and Cherry) as a marking approach (Lenti LeGO-RGB
vectors), we developed a protocol in which each individual OS cell displays a different colour of the rainbow spectrum. These cells were used to interrogate the clonal evolution-related
questions in in vivo orthotopic, ectopic, and metastatic tumourigenesis studies. In our studies we show that osteosarcomagenesis can follow a neutral evolution model; different clones can
coexist and propagate over time and only some of them become locally dominant invading the adjacent microenvironment. Metastatic disease also presents signs of polyclonality, where
metastatic clones can be distinct from the dominant clones present in the primary tumour. In summary, our study offers an overview of the clonal dynamics in OS development. RESULTS EFFICIENT
AND STABLE RGB MARKING OF MURINE MPCS To generate RGB (red–green–blue) multi-coloured cells, we used three lentiviral vectors Cherry (red), Venus (green), and Cerulean (blue), which express
different fluorescent proteins. The RGB marking of murine _p53__−/−__Rb__−/−_ bone marrow-derived MPCs (BM-MPCs) was achieved by transducing cells with multiplicity of infection (MOI)
corresponding to equimolar transduction efficiency per vector of 50%. Correct cell line transduction was validated based on fluorescent colour saturation and the variability of colour
mixing. As verified by confocal microscopy, an optimal colour spectrum was obtained using an MOI of 0.75 per vector, whereas excessively high MOIs resulted in poor colour mixing
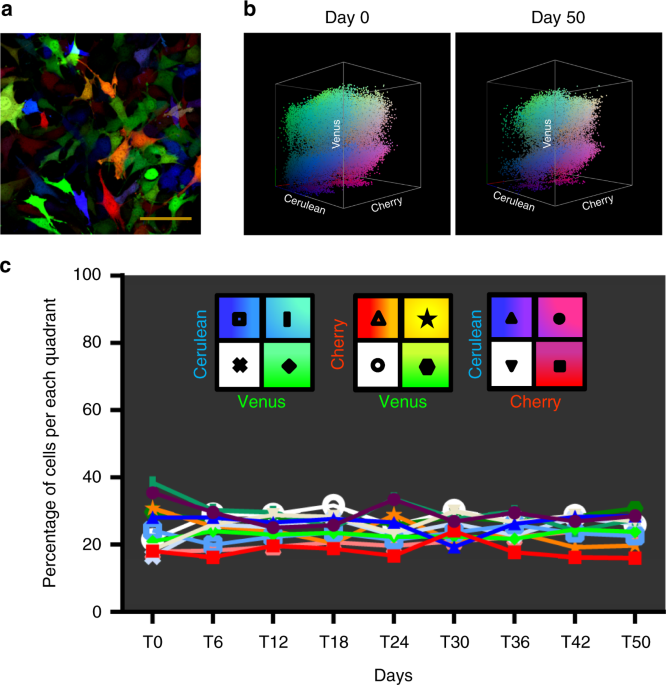
(Supplementary Figure 1a). These multi-coloured cells, designated RAINBONE cells in this text, showed a wide range of colours, with each colour representing a different clone (Fig. 1a). To
optimize flow cytometry analysis, monoclonal cell lines were obtained by the limiting dilution of RAINBONE cells, and an oligoclonal mix was further generated by mixing six of these
monoclonal lines. As shown in Supplementary Figure 1b, each monoclonal cell line presented a narrow peak of fluorescence, whereas the oligoclonal mix was composed of a combination of
discrete peaks (Supplementary Figure 1c). In contrast, polyclonal RAINBONE cells displayed a broad fluorescent distribution generated by the integration of signals from a multitude of clonal
populations (Supplementary Figure 1d). Three-dimensional (3D) visualization, which was accomplished by plotting the three fluorescent variables in a Cartesian plot (_x_,_y_,_z_), or 3D
plot, increased clonal discrimination. Thus, monoclonal or oligoclonal cell lines could be easily identified, whereas heterogeneous RAINBONE cells covered the three axes and their possible
fluorescent colour combinations. Clonal heterogeneity and the stability of fluorescent markers of RAINBONE cells were studied and quantified during 50 days of in vitro culture by flow
cytometry (Supplementary Figure 2; Fig. 1b, c). Visual stochastic network embedding (viSNE) (Supplementary Figure 3a) and spanning-tree progression analysis of density-normalized events
(SPADE) (Supplementary Movie 1) were also applied to study clonal heterogeneity over time. Our results showed that the multicolour spectrum of RAINBONE cells was stable and that clonal
heterogeneity was maintained during in vitro culture. TUMOUR HETEROGENEITY IN OSTEOSARCOMAS It was previously shown that murine _p53__−/−__Rb__−/−_ BM-MPCs can generate OS if implanted
ectopically in the proper osteoinductive microenvironment34. Therefore, we tested the nature of subclonal interaction in tumour growth. RAINBONE cells were further transduced with an
ff-Luciferase lentiviral vector, and unmarked _p53__−/−__Rb__−/−_ BM-MPCs were also transduced to calculate an optimal MOI and obtain >80% transduction efficiency (Supplementary Figure
4). Limiting dilution clones obtained with RAINBONE cells and a clonal mix of increasing clonal composition were embedded in ceramic scaffolds and implanted subcutaneously in vivo in
NOD.Cg-Prkdcscid-Il2rgtm1Wjl/SzJ (NSG) mice_;_ luciferase activity was used to directly quantify tumourigenicity and tumour growth kinetics (Supplementary Figure 5a). Seven out of 7 randomly
selected monoclonal cell lines were tumourigenic (100% incidence), further revealing a tendency to grow faster if compared to OS generated by a clonal mixture of either 5 or 10 different
limiting dilution clones or the pool of RAINBONE cells. Overall, OS tumour growth was slower at increasing clonal complexity and each clone shows a heterogeneous growth kinetic if implanted
alone. Furthermore, around 40% of RAINBONE cells formed colonies at in vitro cell transformation assays (Supplementary Figure 5b). In summary, this experiment confirmed the competitive
nature of subclonal populations and further indicated that the Ad-Cre deletion of _Tp53_ and _Rb_ is a strong transforming event for murine MPCs which are composed of a pool of heterogeneous
transformed cells. Given their competitive nature, the clonal composition of RAINBONE-generated OS was studied in vivo for short (25 days) and long (50 days) periods. An experimental
workflow schematic is shown in Fig. 2a. After 25 days, RAINBONE cells developed tumour masses with highly vascularized areas surrounding the ceramics. Histologically, tumours developed
heterogeneously, with both rich bone matrix deposition areas and fibroblastic regions (Fig. 2b). At this stage of development, tumour cells showed no clonal dominance by flow cytometry (Fig.
2c) or confocal studies (Fig. 2d, e and Supplementary Figure 6a). Instead, tumours were characterized by large areas of multi-coloured cells suggesting a polyclonal composition. viSNE
(Supplementary Figure 3b) and SPADE analysis (Supplementary Figure 3c) also confirmed clonal heterogeneity. Genome insertion site analysis by linear amplification-mediated polymerase chain
reaction (LAM-PCR) indicated strong amplification of different long terminal repeat (LTR)-genome junctions (Fig. 2f and Supplementary Figure 7), further supporting a polyclonal tumour
composition. Spectral karyotyping (SKY) analysis identified a high level of genomic heterogeneity among cancer cells, which presented a tetraploid karyotype with high levels of aneuploidy,
some large deletions, and non-clonal translocations (Supplementary Figure 8 and Supplementary Table 1). In summary, our data demonstrate that at an early stage, OS can be composed of
coexistent, heterogeneous, competing cancer populations. However, this competition is not associated with a strong clonally selective event. CLONAL EVOLUTION IN OSTEOSARCOMA PROGRESSION To
further explore the dynamics of OS progression, we extended tumour development and clonal studies. In contrast to the previous results, RAINBONE tumours showed changes in their clonal growth
after a longer period (50 days). The central tumour mass maintained a polyclonal composition, but the tumour periphery showed abundant expansion of a few clones (Fig. 3a and Supplementary
Figure 6b). A heterogeneous clonal composition was confirmed by LAM-PCR (Fig. 3c and Supplementary Figure 7), and flow cytometry detected the enrichment of some discrete populations. This
result was also confirmed by SPADE and viSNE analysis (Fig. 3e and Supplementary Figure 3b–c). Histologically, extracompartmental areas showed increased cellularity with scant bone matrix
deposition, whereas an osteogenic phenotype was maintained in the ceramic region (Supplementary Figure 6b). Of note, clones having the ability to grow outside the ceramic region were
different in each animal tumour (Fig. 3e). Despite these areas being apparently monoclonal, a few clones could also be identified, supporting the heterogeneous nature of these regions (Fig.
3d). Explanted tumour cells from these peripheral regions were sorted by fluorescence-activated cell sorting (FACS) based on their fluorescence fingerprint (Fig. 3f). As expected, these
clones showed a few discrete insertion sites in a pattern similar to the control monoclonal populations obtained by in vitro limiting dilution (Fig. 3g; Supplementary Figure 7 and
Supplementary Table 2). SKY analysis identified high genomic heterogeneity among cancer cells even when they were of monoclonal origin (Supplementary Figure 8 and Supplementary Table 1).
Additionally, for these clones, the clone-to-clone relationship presented a competitive nature; 4 out of 4 monoclonal tumours in secondary transplantation exhibit faster growth compared to
an oligoclonal mix composed of the previous tumour (Supplementary Figure 5c). However, subclonal competition did not lead to clonal extinction; all clones remained present, as indicated by
flow cytometry analysis (Supplementary Figure 5d–left). Three out of 4 oligoclonal tumours showed the dominance of the G11 clone (turquoise arrow), whereas one tumour was mostly formed by
the R7 population (pink arrow), which surprisingly showed faster growth when implanted alone (Supplementary Figure 5c). Furthermore, in vitro growth assays indicated that in experimental
conditions with no space competition and an equal nutrient supply, the R9 clones grew faster (Supplementary Figure 5d–right); nevertheless, this clone was infrequently identified in the in
vivo assay (Supplementary Figure 5d–left). In summary, these experiments demonstrate that clonal evolution is associated with the ability to grow in a new microenvironment at a late stage of
disease. Furthermore, this phenotype is acquired by different dominant clones that present karyotypic heterogeneity and different in vitro or in vivo growth rates. In this case, we
confirmed a regional and selective clonal dominance while a highly polyclonal area remains present. NEUTRAL AND SELECTIVE DYNAMICS OF ORTHOTOPIC OS DEVELOPMENT Tumour formation at the
orthotopic site by our experimental _p53__−/−__Rb__−/−_ BM-MPCs was previously demonstrated to efficiently recapitulate the main features of OS, including metastatic disease34. Therefore,
RAINBONE cells were also inoculated in the proximal tibia of immunodeficient mice, and tumour development was evaluated by bioluminescence and X-ray imaging. Mice showed radiographic
characteristics compatible with intramedullar bone deposition and cortical bone osteolysis (Fig. 4a, d). At 50 days after RAINBONE cells were implanted, mice were killed due to limb function
loss. Confocal microscopy indicated great colour heterogeneity in the tumour. Cells of different clonal origins were found in the medullar space infiltrating the compact bone, growing at
the endosteum, and even as sparse single cells at perivascular locations (Fig. 4b). Increased pseudo-trabecular bone formation was promoted by cells of different clonal origins and also
evidenced by colourful osteoblastic rimming (Fig. 4c). In some mice, tumours produced a strong periosteal reaction with structures resembling Codman triangles and presented a soft tissue
mass development over the bone surface (Fig. 4d). These areas were composed of few dominant clones with increased invasiveness of the adjacent musculoskeletal tissues (Fig. 4e). Tumours
frequently destroyed the metaphyseal plate (Fig. 4f) and developed outside of the medullar cavity in large monoclonal globular-shaped soft tissue masses, which also presented low-frequency
infiltrating clones of different clonal origins (Fig. 4e, g). Altogether, the results suggest that clonal heterogeneity is a common growth dynamic in our OS models and that there are signs
of clonal evolution in the late phases, a characteristic that is primarily associated with the ability to grow in a new microenvironment. Furthermore, we show that orthotopic and
osteoinductive ectopic models do not differ substantially in terms of clonal evolution; each develops some clones that are able to expand extracompartmentally in late-stage disease.
METASTATIC DISEASE IS DRIVEN BY POLYCLONAL SEEDING OF LUNGS Bioluminescent and X-ray imaging were employed in the orthotopic model as diagnostic high-sensitivity techniques. Bioluminescence
revealed the formation of multiple metastatic nodules in the lungs, and this was further confirmed by histological analysis (Fig. 5a and Supplementary Figure 6c). Metastatic dissemination
appeared heterogeneous, with different clonal development dynamics. Metastases were usually heterogeneous, presenting different sizes and clonal origins (Fig. 5b). In total, 146 metastases
were quantified for monoclonality or oligoclonality and measured (Fig. 5c). Monoclonal seeding was more frequent; however, excluding small micrometastases (<200 µm), which could represent
a dormant state, the nodules showing significant growth were both monoclonal and oligoclonal (Fig. 5d). Furthermore, metastatic clones did not correspond to the dominant clones present in
the primary OS tumour (Fig. 5e and Supplementary Figure 9a). Assuming that heterogeneous nodules could originate from the lung homing of a cluster of cells or by secondary clones homing into
a pre-existing nodule, we decided to test the ability of in vivo tested metastatic clones (Supplementary Figure 9b) to seed pre-existing metastases. We induced RAINBONE tumours, and
bioluminescence was used to follow tumour growth and effective lung engraftment (Fig. 5f). Individual metastatic clones were inoculated intravenously (i.v.) when there was evidence of
spontaneous metastatic disease (50 days). After 10 days of i.v. inoculation, lungs were dissected and processed for confocal studies. Only four out of 75 (5.3%) lung metastatic nodules
analysed presented the characteristic fluorescent fingerprint of the i.v. inoculated clones, indicating the low homing ability of metastatic clones into pre-existing metastatic lung nodules
(Fig. 5g). Our data indicate that metastatic disease is highly heterogeneous and that different clones develop in the lungs with signs of polyclonal seeding. Moreover, the aggressiveness of
dominant clones at the primary site does not correlate with increased metastatic clonal frequency. DOMINANT CLONES ARE COMPOSED OF HOMOGENEOUS SUBCLONES As described above, RAINBONE tumours
induced subcutaneously in osteogenic implants present spatially dominant clone development at late-stage disease (50 days). We wanted to study the grade of heterogeneity of these dominant
clones, including the hypothetical presence of cancer stem cells responsible for sustaining tumour repopulation. Therefore, we designed an experiment (Fig. 6a) in which dominant clones were
sorted by FACS and monoclonal populations were established (Fig. 6b, c); three populations were further decoloured using adenoviral vectors expressing Cre recombinase. Fluorescent marker
loss was assessed by flow cytometry (Fig. 6d), and we did not observe resistance to Ad-Cre recombination; three out of 3 clones showed almost pure fluorescent marker loss (Supplementary
Figure 10 and Supplementary Table 3). Decoloured cells underwent a second round of RGB colouring using lentiviral Gene Ontology (LeGO)-RGB lentiviral vectors, generating RAINBONE-2 cells
(Fig. 6e, f), which were implanted in NSG mice. In these secondary tumours, we observed a strong reduction in bone matrix content and faster tumour development (15 days) compared to primary
RAINBONE tumours. Confocal study and flow cytometry with secondary tumours showed a polyclonal contribution to tumour development (Fig. 6g, h). Then, RAINBONE-2 explanted cells underwent
further tertiary transplantation in NSG mice. Again, these tertiary tumours showed the same polyclonal heterogeneity (Fig. 6i, j). The viSNE and SPADE analysis also confirmed the
heterogeneous subclonal composition (Supplementary Figure 11). Our results indicate that dominant clones are formed by a homogeneous equilibrium of subclones with similar tumour regeneration
potential. DISCUSSION Single-cell studies and massive genome sequencing techniques have allowed the tracking of tumour development14. The results of these studies provide better
understanding of cancer as a heterogeneous disease and highlight differences in the growth patterns of specific tumour types. Here, we used a single-cell tracking technique based on
fluorescent protein expression using lentiviral vectors (Lenti LeGO-RGB vectors). Due to the variety of integration sites and vector copy number, when these vectors are used in the
appropriate combination, they mark each individual cell with a different colour of the rainbow spectrum that is then transmitted to derived progeny. This technology is a powerful tool for
clonal cell studies in vitro and in vivo35,36,37 as it represents an unbiased approach for studying tumour physiology; it does not require any preselected marker and allows the direct study
of tumour clones and progeny in the spatial organization of the tissue. Lenti LeGO-RGB marking has been successfully used to clonally track in vivo metastatic mammary adenocarcinoma38,
pancreatic adenocarcinoma39, and neuroendocrine carcinoma35, and it has also been used in combination with mass spectrometry39. These findings support the value of RGB marking in tumour
heterogeneity studies. However, this approach has only been tested in well-established carcinoma cell lines. Murine _p53__−/−__Rb__−/−_ BM-MPCs employed for generating RAINBONE cells were
not isolated from pre-existing tumours but were transformed in vitro prior to inoculation into mice. Our double-hit model allows murine cell transformation with the establishment of a
heterogeneous cell population of transformed MPCs. In our conditions, RAINBONE cells present malignant features and are efficiently transformed by _p53_ and _Rb_ loss. This result is in
contrast with previous studies reporting a low tumour-initiating potential for mesenchymal lineage cells. However, in our studies we employed severe immunodeficient NSG mice and implanted
cells in an orthotopic and ectopic bone-like microenvironment, thus excluding some of the harsh conditions that most likely affected other studies. Indeed, the tumour-initiating cell
frequency of melanoma cells can reach 25% of the total population when using a cell matrix or less immunocompetent mice40. Given the high tumour-initiating potential, our model represents a
powerful tool to test the cohabitation of different cancer clones and the possible dynamics of competition among them (see further discussion below). Our artificial condition represents a
model with which we can test clonal evolution, the existence of selective events, or even the neutral dynamics of growth. Furthermore, these cells hypothetically have not been shaped by the
tumour microenvironment and have never experienced the growth dynamics and selective pressure occurring during in vivo tumour development. All these characteristics make RAINBONE cells an
interesting model of in vivo primary tumour generation, allowing us to test different hypotheses about clonal dynamics and dispersal forces occurring during osteosarcomagenesis. Our
experience with RGB marking is that it represents a very powerful technology; nevertheless, some technical difficulties were extremely challenging. We were unable to rapidly isolate a
specific monoclonal population by FACS sorting RAINBONE tumours. This limitation, produced by a loss of definition in flow cytometric data, was due to the equal representation of different
clonal populations with overlapping flow cytometric fingerprints. Furthermore, in some cases it was difficult to obtain a pure clonal sorting, and some very low-frequency clones of different
colours appeared in culture. We tried different flow cytometers, services, and users, and we solved this problem by resorting clones after a short in vitro amplification. We rationalized
that sorting multiple clonal populations at the same time can affect process efficiency and purity. In conclusion, with the employment of different microscopic and genetic techniques, we
avoided the misinterpretation of results, and the technical limitations did not greatly hinder the main objective of the study. In evolutionary theories, competition is a long and steady
principle that is continuously occurring in ecological systems, such as cancer41,42. From an early stage of cancer development, tumour cells compete for limited resources (nutrients and
oxygen) to the point of saturation21 and encounter a strongly selective microenvironment (pH changes, immune system, geographic barriers, and chemotherapy) that further limits their
development14. In this context, dispersal forces could also play a key role in tumour progression;43 populations of spreading cells could reach untapped resources, increasing distance from
competitors and thus reducing cell–cell interaction44. Dispersal forces could also explain the metastatic process, a paradoxical outcome of tumour evolution that is not related to cell
survival the way that other tumour hallmarks are (apoptosis, immune evasion, etc.)45,46. The main basis of the neutral theory is the neutral outcome of this process of competition, in which
different species (cancer clones) mainly coexist and the acquisition of new genomic traits is mostly neutral. This model contrasts with the reiterative positive selection postulated by the
Darwinian model of evolution. According to the Darwinian model, the acquisition of new phenotypic traits gives an increased replicative fitness to a new species with the continuous
extinction of the unfit ones. Nevertheless, the neutral and competitive models are not completely in antithesis, and neutral evolution also allows positive selection27,47. The difference
between the two models is mostly concerning the frequency of the positive selective events, which are defined as rare in the neutral theory. Positive selection is mostly relegated to strong
microenvironmental changes, chemotherapy, immunotherapy, metastatic spread, and during the first stage of tumour evolution27,48. Therefore, after the accumulation of genomic alteration that
initiates tumour growth, cancer cells expand neutrally and accumulate extensive genomic heterogeneity. In line with the neutral model, within a short time of tumour evolution (25 days), OS
tumours systemically presented histological heterogeneity together with a polyclonal distribution of tumour cells, thus not resembling a strongly selective linear model of clonal cancer
evolution16. As in Nature, clonal competition is also occurring in our model, in which the dynamics of competition among cancer clones represent forces able to slow down tumour growth.
Nevertheless, the outcome of this competition is not resulting in clonal selection. We observed that different clones characterized by high genomic heterogeneity contributed with
proliferating or differentiating cells in the tumour (Fig. 7a). This behaviour is in agreement with a neutral evolution pattern and lacks evidence of a real selective advantage gain. In
contrast, at late-stage evolution (50 days), tumours showed a different organization; large extracompartmental monoclonal areas arose adjacent to the osteoinductive area generated in the
hydroxyapatite/tricalcium phosphate (HA/TCP) compartment, presumably as a consequence of the effective pressure for clonal selection caused by different microenvironmental conditions. In
this sense, clonal selection seems to be mostly associated with an adaptation to new specific spatial/microenvironmental determinants (Fig. 7a). It is important to consider that one
parameter in the staging system of musculoskeletal tumours is the ability to grow extracompartmentally, which is also associated with a worse prognosis. The fact that different clones within
the same tumour are able to grow extracompartmentally provides evidence of a parallel evolution model among cancer cells. Additionally, in agreement with a contingency evolution context,
our data suggest that starting from a pool of transformed cells, the chance of becoming a clone with this phenotype is not pre-established but that constraints can lead to convergence on
this possible outcome. In fact, each specific tumour in each animal shows different dominant clones. Karyotype analysis of these clonal populations revealed extended karyotype variability
among cells, which is compatible with a divergent pathway of cancer evolution and in accord with branched models. At the subclonal level, these cells show a high tumour-initiating potential,
which is also in agreement with a secondary and tertiary level of coexistence (Fig. 7a). However, it is common to observe low-frequency clones infiltrating the dominant clonal population in
these extracompartmental regions, which adds a new level of heterogeneity. This type of pattern strongly resembles the dynamics of collaboration and/or parasitism that were proposed in
other reports49. In summary, our study demonstrates that different dynamics simultaneously participate during tumour evolution, but we support the idea that the clinical relevance of tumour
evolution should not be restricted only to dominant clones. In support of this concept, a clinical report in osteosarcoma described how some low-frequency clones detected at diagnosis could
be responsible for tumour relapse, thus underlining the importance of low-frequency populations of cancer cells and the less fruitful branches of tumour evolution50. Metastatic seeding is
another malignant feature of OS disease that can follow different dynamics. Some interesting reports, also employing multicolour lineage tracing, have started to highlight unknown mechanisms
occurring during metastatic dissemination. Thus, the monoclonal or polyclonal dynamics of metastatic spread, collective dissemination, and reseeding were reported in different models of
carcinomas51,52,53. Interestingly, some authors demonstrated that clonal cooperativity in cancer dissemination may play a primary role in improving the chances of engraftment at distant
sites. In a model of pancreatic cancer, polyclonal clusters of cancer cells actively colonized distant organs, representing a cooperative strategy, and a reduction of cluster formation also
reduced metastatic potential45. In our OS model, we observed the development of different monochromatic and oligochromatic nodules, in accord with a polyclonal seeding (Fig. 7b), and a
parallel progression model in lung colonization. Furthermore, the existence of oligoclonal metastases raises new questions about their origin and their intermetastatic subclonal dynamics
because different clones can still coexist. Given the inefficiency of metastatic clones to home in pre-existing metastases, oligoclonal nodules do not seem to be the outcome of a secondary
seeding wave, or reseeding (Fig. 7b). This result could be explained by the existence of a local microenvironment that allows the homing of monoclonal or oligoclonal seeds at the beginning
of the disease and that impedes the engraftment of new clones once perturbed. In summary, our data question the competitive linear model in metastatic evolution and indicate that clonal
dynamics occurring in metastatic disease do not differ from the dynamics at the primary site. In conclusion, tumour evolution is thought to be caused by clonal competition and selection,
which would lead to aggressive clone development in the fight for the survival of the fittest. By contrast, we present evidence that osteosarcomagenesis can follow the dynamics of neutral
evolution, in which different cancer clones coexist and propagate simultaneously over time. Clonal biodiversity seems to be an important feature in our model. This equilibrium is maintained
until the disease progresses to a more aggressive form that is associated with the invasion of an adjacent microenvironment where dominant clones appear. Distant lung polyclonal seeding also
results in the spatial dominance of many clones, which can be distinct from the dominant clones in the primary tumour. In summary, our study offers an overview of the clonal dynamics and
relevance of dispersal forces in OS development; this knowledge is useful for understanding tumour biology and may improve clinical practice and therapeutic design. METHODS CELL LINES Murine
BM-MPCs were isolated from transgenic FVB mice bearing _Tp53_ and _Rb_ genes flanked by LoxP sites. Gene deletion was achieved in vitro by the adenoviral transduction of Cre recombinase
gene under the control of cytomegalovirus promoter to obtain transformed _p53__−/−__Rb__−/−_ BM-MPCs. Successful gene knockdown was confirmed by genomic PCR and western blot34.
_p53__−/−__Rb__−/−_ BM-MPCs underwent lentiviral RGB marking in vitro. Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum, 1%
penicillin/streptomycin, and 1% Glutamax and were routinely tested for mycoplasma presence (MycoAlert-Mycoplasma Detection kit, LONZA). RGB LENTIVIRAL VECTORS AND RGB MULTICOLOUR MARKING
LeGO-RGB lentiviral vectors were used as colour-guided clonal cell trackers. LeGO vectors were kindly provided by Dr. Kristoffer Riecken, University Medical Center Hamburg, Germany36,37,54.
LeGO-Cer2 (Addgene: 27388), LeGO-V2 (Addgene: 27340), and LeGO-C2 (Addgene: 27339) plasmids were employed to produce lentiviral vectors coding for blue, green, and red fluorescent proteins,
respectively. Supernatant was collected 48 h after transfection and concentrated by ultracentrifugation. Lentiviral particle mixtures were added to the _p53__−/−__Rb__−/−_ BM-MPCs and
incubated overnight to generate RGB multicolour-marked murine _p53__−/−__Rb__−/−_ BM-MPCs, which were named RAINBONE cells. The RGB colour mix was achieved using a MOI of 0.75, which
corresponds to an equimolar transduction efficiency of 50% per lentiviral vector 3 days after transduction. Six monoclonal cell lines were derived from RAINBONE cells by in vitro limiting
dilutions. Single-cell plating efficiency and clonal purity were assessed by confocal fluorescence microscopy and flow cytometry, respectively. LeGO-RGB vectors also contain additional loxP
sites which allow the elimination of fluorescent proteins using a Cre recombinase. Three different FACS sorted RAINBONE clones were decoloured by the in vitro adenoviral transduction of Cre
recombinase. Decoloured clones underwent lentiviral recolouring. These recoloured clonal populations represent the RAINBONE-2 cells that underwent secondary and tertiary in vivo tumour
generation. FLOW CYTOMETRY ANALYSIS AND CELL SORTING Cells were resuspended in phosphate-buffer saline (PBS) for flow cytometric study. Fluorescence signal distribution was analysed using a
BD LSRFortessa (BD Bioscience) cell analyser. Cerulean fluorescent protein was excited at 405 nm and detected with a 450/50 bandpass filter, Venus was excited at 488 nm and detected with a
530/30 bandpass filter, and Cherry was excited at 561 nm and detected with a 610/20 bandpass filter. Discrete cell populations developed in osteogenic implants were further sorted using a
iCyt SY3200 Cell Sorter (SONY). Cerulean fluorescent protein was excited at 405 nm and detected with a 525/50 bandpass filter, Venus was excited at 488 nm and detected with a 525/50 bandpass
filter, and Cherry was excited at 532 nm and detected with a 615/30 bandpass filter. Sorted populations were expanded in vitro for a short period, and sorting purity was verified by flow
cytometry and confocal microscopy. Flow cytometry data were analysed with FlowJO software (FlowJo LLC). UNSUPERVISED VISUALIZATION ANALYSIS OF CLONAL ARCHITECTURE FCS files were loaded in
the Cytobank website (https://premium.cytobank.org) to perform different types of unsupervised analysis of the clonal architecture of the samples. Samples were gated to analyse the
mononuclear cell fraction. The clustering of sample events was performed, taking into account only the intensity of Cerulean, Venus, and Cherry channels. For this aim, a viSNE map was
generated; this approach uses _t_-distributed stochastic neighbour embedding (t-SNE) algorithms55. The generated results are provided in two-dimensional scatter plots and show the intensity
of the three fluorescent channels analysed. A SPADE algorithm was used to extract population hierarchies and visualize individual clones in a tree-like structure56. SPADE performs
density-dependent down-sampling to equally represent rare and abundant populations and then performs agglomerative clustering while taking into account the intensity of selected channels. In
this case, SPADE was used to cluster and represent the data as 200 different clones. FLUORESCENT MICROSCOPY ANALYSIS In vitro confocal microscopic studies of RAINBONE cells were performed
by seeding cells in multichambers. After overnight incubation, slides were washed with PBS and fixed with 4% formalin or 1% paraformaldehyde (PFA) for 1 min. After fixation, slides were
washed again with PBS and mounted with ProLong. In the case of explanted primary tumours and lungs, samples were processed for histologic staining and confocal fluorescence analysis by
cryosectioning. Samples were fixed overnight in 4% formalin or 1% PFA and decalcified for 72 h prior to inclusion in optimal cutting temperature Tissue-Tek. All processes were performed in
the dark at room temperature. The 8 µm slides were defrosted and stained according to histologic standards or pre-warmed, hydrated in PBS for 2 min, and then mounted using ProLong for
confocal microscopy studies. A confocal multispectral TCS-SP5 (Leica Microsystems) microscope was employed in this study. Representative images were obtained by maximum projection of a
10-layer stack of 8 µm-thick sections. Images were processed using LAS AF (Leica Microsystems). For lung seeding quantification, metastatic nodules were screened to identify the fluorescent
fingerprint of i.v. inoculated FACS clones. A colocalization study was performed with ImageJ to ensure the presence of fluorescent markers specific to the inoculated clone and to exclude
ambiguous cells. Macroscopic fluorescence and/or brightfield image maps were acquired with TCS-SP5 (Leica Microsystems) and AxioScan.Z1 (Zeiss). LAS AF and ZEN 2.3 (blue edition) were
employed for image processing. MOUSE MODELS All procedures and animal care were performed at the National Institute of Health Carlos III (ISCIII) with the approval of the Institutional
Animal Research and Welfare Ethics Committee according to the EU Directive for animal experiments in a specific pathogen-free environment. Experiments were performed using 8–10-week-old NSG
mice. A minimum sample size of 4 mice per each experimental group was established; this size was chosen in accordance with 3 Rs (Replacement, Reduction, and Refinement) rule for animal
experimentation, ensuring sufficient statistical power in dichotomous studies. Inclusion or exclusion criteria were pre-established and represent the physiological status of the animal at
final experimental point. OS development was induced using two different procedures. For the orthotopic inoculation into the bone marrow space of the proximal tibia, cells were resuspended
in PBS, filtered through a 70 µm nylon filter, and concentrated to 7.5 × 106 cells/ml. Surgery was performed by bending the mouse leg at 90° to drill the tip of the tibia with a 25 G needle
and depositing the cell suspension in the medullar space (1.5 × 105 cells/mouse) with a 27 G needle. For ectopic osteogenic implants, 40 mg of ceramic powder (60% hydroxyapatite/40%
tricalcium phosphate beta) with a surface microporosity less than 10 μm (Biomatlante) was deposited in a 50-ml falcon tube and washed with 1 ml of culture medium. The cell suspension was
mixed with ceramic powder (1.5 × 105/implant), centrifuged at 1200 rpm for 5 min in a centrifuge with a swinging bucket rotor, and incubated overnight. Culture medium was carefully removed,
and cells with ceramic powder were bound in a fibrin clot for subcutaneous implantation34. For bioluminescent studies, RAINBONE cells were transduced at MOI 5 with lentiviral particles
carrying the firefly luciferase gene. Lentiviral vectors were produced using a phR-SIN-SFFV-pLuc-IRES-GFP transfer vector57 after the deletion of GFP. This vector was employed to quantify
tumour growth in vivo without affecting the RGB marking. In vivo bioluminescent quantification was performed using an IVIS Lumina3 image system (Perkin Elmer). Mice were anesthetized with 2%
isoflurane and were imaged in ventral positions 1 min after the intravenous administration of 100 µl of a D-luciferin solution (12.5 mg/ml in PBS). Data were analysed using Living Image
software (Perkin Elmer). Quantification was performed by subtracting mouse background average radiance from the ROI average radiance of primary tumours and thorax. For subcutaneous in vivo
growth kinetic studies, normalization was calculated as fold change to day 1 after implantation or by the basal cell line luminescence (SCLL) measured in vitro. SCLL was calculated as
luciferase activity per µg of protein. For orthotopic studies, the normalization factor is represented by the average radiance 4 h post inoculation. For animal experimentation, researchers
were not blind; researchers involved in flow cytometry data acquisition and LAM-PCR analysis were blind. SKY ANALYSIS RAINBONE cells and explanted tumour cells underwent molecular
cytogenetic analysis. Cultured adherent cells were treated with colchicine (0.5 μg/ml) for 4 h at 37 °C and routinely harvested. Metaphases were prepared using a conventional cytogenetic
protocol for methanol/acetic acid (3:1)-fixed cells. Slides were prepared from the fixed material and hybridized using the SKY method according to the manufacturer’s protocol (Applied
Spectral Imaging). Images were acquired with an SD300 Spectra Cube (Applied Spectral Imaging) mounted on a Axioplan microscope (Zeiss) using a custom-designed optical filter, SKY-1 (Chroma
Technology). Up to 15 metaphase cells were captured and analysed for each cell line when possible. GENOMIC INSERTION SITE ANALYSIS BY LAM-PCR Lentiviral integration site analysis was
performed by a modified LAM-PCR58,59. This method amplifies the DNA around the viral/host junction and identifies the adjacent host DNA by sequencing. A single-stranded copy of the
proviral-host junction was made by linear extension from a biotinylated viral LTR primer; this single-stranded junction fragment was trapped and isolated on streptavidin-coated magnetic
beads, and a second strand was generated by random-primed polymerization. The host sequence was cut at the nearest _Rsa_I or _Hea_III restriction site and ligated to an anchor primer; the
junction region was amplified by nested PCR. The complexity of the population of integration sites was initially monitored by running PCR products on 4–20% polyacrylamide gel. Individual
insertion sites were identified by cloning and sequencing individual bands. The non-viral sequence from each band was used to search the mouse genome using a BLAST search on the University
of California Santa Cruz Genome Browser. Uncropped polyacrylamide gels images are presented in Supplementary Figure 12. STATISTICAL ANALYSIS Data were graphed with GraphPad Prism (GraphPad
Software) and Excel service pack (Microsoft software). Statistical analyses were performed by GraphPad Prism (GraphPad Software). Data are expressed as the means ± SD unless otherwise
specified. In boxplots graphs, centre line indicates median, bounds of box indicate 25th and 75th percentiles, and whiskers indicate minimum and maximum. Correlation between two parameters
was estimated by Pearson's coefficient of correlation, by two-tailed tests, and with a confidence interval of 95%. _P_ values less than 0.05 were considered statistically significant.
One-way or two-way analysis of variance (ANOVA) with Bonferroni post-testing was used to compare significant differences for more than two groups. For multiple comparisons, a confidence
interval of 95% was adopted, and only _P_ values lower than 0.05 were considered statistically significant. _*P_ < 0.05, _**P_ < 0.01, _***P_ < 0.001, and _****P_ < 0.0001 were
deemed statistically significant. DATA AVAILABILITY Datasets generated and analysed in the study are available from the corresponding author upon reasonable request. REFERENCES *
ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. _Ann. Oncol._ 25 (SUPPL. 3), iii113–iii123 (2014).
Article Google Scholar * Savage, S. A. & Mirabello, L. Using epidemiology and genomics to understand osteosarcoma etiology. _Sarcoma_ 2011, 548151 (2011). Article Google Scholar *
Gianferante, D. M., Mirabello, L. & Savage, S. A. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. _Nat. Rev. Endocrinol._ 13, 480–491 (2017).
Article CAS Google Scholar * Gorlick, R. Current concepts on the molecular biology of osteosarcoma. _Cancer Treat. Res._ 152, 467–478 (2009). Article Google Scholar * Zhang, J. et al.
Germline mutations in predisposition genes in pediatric cancer. _New Engl. J. Med._ 373, 2336–2346 (2015). Article CAS Google Scholar * Downing, J. R. et al. The Pediatric Cancer Genome
Project. _Nat. Genet._ 44, 619–622 (2012). Article CAS Google Scholar * Chen, X. et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma.
_Cell Rep._ 7, 104–112 (2014). Article CAS Google Scholar * Perry, J. A. et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma.
_Proc. Natl Acad. Sci. USA_ 111, E5564–E5573 (2014). Article CAS Google Scholar * Martin, J. W., Squire, J. A. & Zielenska, M. The genetics of osteosarcoma. _Sarcoma_ 2012, e627254
(2012). Article Google Scholar * Lorenz, S. et al. Unscrambling the genomic chaos of osteosarcoma reveals extensive transcript fusion, recurrent rearrangements and frequent novel TP53
aberrations. _Oncotarget_ 7, 5273–5288 (2015). PubMed Central Google Scholar * Kovac, M. et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency.
_Nat. Commun._ 6, 8940 (2015). Article CAS Google Scholar * Kuijjer, M. L., Hogendoorn, P. C. W. & Cleton-Jansen, A.-M. Genome-wide analyses on high-grade osteosarcoma: making sense
of a genomically most unstable tumor. _Int. J. Cancer_ 133, 2512–2521 (2013). CAS PubMed Google Scholar * Gay, L., Baker, A.-M. & Graham, T. A. Tumour cell heterogeneity. _F1000Res_
5, 238 (2016). Article Google Scholar * McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. _Cell_ 168, 613–628 (2017). Article CAS
Google Scholar * Mel Greaves: Cancer through the lens of evolution. _Trends Cancer_ 2, 539–541 (2016). * Nowell, P. C. The clonal evolution of tumor cell populations. _Science_ 194, 23–28
(1976). Article ADS CAS Google Scholar * Furth, J., Kahn, M. C. & Breedis, C. The transmission of leukemia of mice with a single cell. _Am. J. Cancer_ 31, 276–282 (1937). Google
Scholar * Pierce, G. B. & Wallace, C. Differentiation of malignant to benign cells. _Cancer Res._ 31, 127–134 (1971). CAS PubMed Google Scholar * Bonnet, D. & Dick, J. E. Human
acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. _Nat. Med._ 3, 730–737 (1997). Article CAS Google Scholar * Kreso, A. & Dick,
J. E. Evolution of the cancer stem cell model. _Cell Stem Cell_ 14, 275–291 (2014). Article CAS Google Scholar * Greaves, M. & Maley, C. C. Clonal evolution in cancer. _Nature_ 481,
306–313 (2012). Article ADS CAS Google Scholar * Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. _New Engl. J. Med._ 366, 883–892
(2012). Article CAS Google Scholar * Bashashati, A. et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling.
_J. Pathol._ 231, 21–34 (2013). Article CAS Google Scholar * Gerlinger, M. et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing.
_Nat. Genet._ 46, 225–233 (2014). Article CAS Google Scholar * Zhang, J. et al. Intra-tumor heterogeneity in localized lung adenocarcinomas delineated by multi-region sequencing.
_Science_ 346, 256–259 (2014). Article ADS CAS Google Scholar * Gerlinger, M. et al. Cancer: evolution within a lifetime. _Annu. Rev. Genet._ 48, 215–236 (2014). Article CAS Google
Scholar * Williams, M. J., Werner, B., Barnes, C. P., Graham, T. A. & Sottoriva, A. Identification of neutral tumor evolution across cancer types. _Nat. Genet._ 48, 238–244 (2016).
Article CAS Google Scholar * Sottoriva, A. et al. A Big Bang model of human colorectal tumor growth. _Nat. Genet._ 47, 209–216 (2015). Article CAS Google Scholar * Rosenthal, R.,
McGranahan, N., Herrero, J. & Swanton, C. Deciphering genetic intratumor heterogeneity and its impact on cancer evolution. _Annu. Rev. Cancer Biol._ 1, 223–240 (2017). Article Google
Scholar * Tabassum, D. P. & Polyak, K. Tumorigenesis: it takes a village. _Nat. Rev. Cancer_ 15, 473–483 (2015). Article CAS Google Scholar * Marusyk, A. & Polyak, K. Tumor
heterogeneity: causes and consequences. _Biochim. Biophys. Acta_ 1805, 105–117 (2010). CAS PubMed Google Scholar * Liu, Y. & Cao, X. Characteristics and significance of the
pre-metastatic niche. _Cancer Cell_ 30, 668–681 (2016). Article CAS Google Scholar * Turajlic, S. & Swanton, C. Metastasis as an evolutionary process. _Science_ 352, 169–175 (2016).
Article ADS CAS Google Scholar * Rubio, R. et al. Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. _Stem Cells_ 32, 1136–1148 (2014).
Article CAS Google Scholar * Weber, K. et al. RGB marking facilitates multicolor clonal cell tracking. _Nat. Med._ 17, 504–509 (2011). Article CAS Google Scholar * Weber, K.,
Thomaschewski, M., Benten, D. & Fehse, B. RGB marking with lentiviral vectors for multicolor clonal cell tracking. _Nat. Protoc._ 7, 839–849 (2012). Article CAS Google Scholar *
Gomez-Nicola, D., Riecken, K., Fehse, B. & Perry, V. H. In-vivo RGB marking and multicolour single-cell tracking in the adult brain. _Sci. Rep._ 4, 7520 (2014). Article ADS CAS Google
Scholar * Coffey, S. E., Giedt, R. J. & Weissleder, R. Automated analysis of clonal cancer cells by intravital imaging. _Intravital_ 2, https://doi.org/10.4161/intv.26138(2013). *
Abramowski, P. et al. Combined application of RGB marking and mass spectrometric imaging facilitates detection of tumor heterogeneity. _Cancer Genom. Proteom._ 12, 179–187 (2015). CAS
Google Scholar * Quintana, E. et al. Efficient tumor formation by single human melanoma cells. _Nature_ 456, 593–598 (2008). Article ADS CAS Google Scholar * Patel, M. S., Shah, H. S.
& Shrivastava, N. c-Myc-dependent cell competition in human cancer cells. _J. Cell Biochem._ 118, 1782–1791 (2017). Article CAS Google Scholar * Giacomo, S. D. et al. Human cancer
cells signal their competitive fitness through MYC activity. _Sci. Rep._ 7, 12568 (2017). Article ADS Google Scholar * Waclaw, B. et al. A spatial model predicts that dispersal and cell
turnover limit intratumour heterogeneity. _Nature_ 525, 261–264 (2015). Article ADS CAS Google Scholar * Amend, S. R., Roy, S., Brown, J. S. & Pienta, K. J. Ecological paradigms to
understand the dynamics of metastasis. _Cancer Lett._ 380, 237–242 (2016). Article CAS Google Scholar * Eichenlaub, T., Cohen, S. M. & Herranz, H. Cell competition drives the
formation of metastatic tumors in a _Drosophila_ model of epithelial tumor formation. _Curr. Biol._ 26, 419–427 (2016). Article CAS Google Scholar * Taylor, T. B., Wass, A. V., Johnson,
L. J. & Dash, P. Resource competition promotes tumour expansion in experimentally evolved cancer. _BMC Evol. Biol._ 17, 268 (2017). Article Google Scholar * Davis, A., Gao, R. &
Navin, N. Tumor evolution: linear, branching, neutral or punctuated? _Biochim. Biophys. Acta_ 1867, 151–161 (2017). CAS PubMed Central Google Scholar * Efremova, M. et al. Targeting
immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. _Nat. Commun._ 9, 32 (2018). Article ADS Google Scholar * Colom, B. & Jones, P. H. Clonal
analysis of stem cells in differentiation and disease. _Curr. Opin. Cell Biol._ 43, 14–21 (2016). Article CAS Google Scholar * Chen, K. S. et al. A novel TP53-KPNA3 translocation defines
a de novo treatment-resistant clone in osteosarcoma. _Cold Spring Harb. Mol. Case Stud._ 2, a000992 (2016). Article Google Scholar * Maddipati, R. & Stanger, B. Z. Pancreatic cancer
metastases harbor evidence of polyclonality. _Cancer Discov._ 5, 1086–1097 (2015). Article CAS Google Scholar * Friedl, P., Locker, J., Sahai, E. & Segall, J. E. Classifying
collective cancer cell invasion. _Nat. Cell Biol._ 14, 777–783 (2012). Article Google Scholar * Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination
of keratin 14-expressing tumor cell clusters. _Proc. Natl Acad. Sci. USA_ 113, E854–E863 (2016). Article CAS Google Scholar * Weber, K., Mock, U., Petrowitz, B., Bartsch, U. & Fehse,
B. Lentiviral gene ontology (LeGO) vectors equipped with novel drug-selectable fluorescent proteins: new building blocks for cell marking and multi-gene analysis. _Gene Ther._ 17, 511–520
(2010). Article CAS Google Scholar * Amir, E. D. et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. _Nat. Biotech._
31, 545–552 (2013). Article CAS Google Scholar * Qiu, P. et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. _Nat. Biotech._ 29, 886–891 (2011).
Article CAS Google Scholar * Garaulet, G. et al. IL10 released by a new inflammation-regulated lentiviral system efficiently attenuates zymosan-induced arthritis. _Mol. Ther._ 21, 119–130
(2013). Article CAS Google Scholar * Harkey, M. A. et al. Multiarm high-throughput integration site detection: limitations of LAM-PCR technology and optimization for clonal analysis.
_Stem Cells Dev._ 16, 381–392 (2007). Article CAS Google Scholar * Schmidt, M. et al. Detection and direct genomic sequencing of multiple rare unknown flanking DNA in highly complex
samples. _Hum. Gene Ther._ 12, 743–749 (2001). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank ISCIII and CNIO flow cytometry and cell sorting units for their
participation in our studies. We are thankful to the CCEH-Fred Hutchinson Cancer Research Center for LAM-PCR service. We acknowledge Raquel Pérez Tavarez, María Blázquez Mesa, Alicia Giménez
Sánchez, Elena Calvo Cazalilla, and Monserrat Arroyo Correas for useful help on the pathology studies; and Teresa Cejalvo, Isabel Cubillo Moreno, and Miguel Angel Rodríguez-Milla for their
contributions in experimental setup. We thank the visual artist Isabella Lacquaniti for her help with drawings and schematics. We are also thankful to the Fondo de Investigaciones Sanitarias
(FIS: PI11/00377 and PI14CIII/00005 to J.G.-C., FIS: CP11/00206 to A.A., and RTICC: RD12/0036/0027 to J.G.-C.), the Madrid Regional Government (CellCAM; P2010/BMD-2420 to J.G.-C.), the
Asociación Pablo Ugarte, and the Asociación Afanion for grants support. AUTHOR INFORMATION Author notes * These authors contributed equally: Arantzazu Alfranca, Javier García-Castro. AUTHORS
AND AFFILIATIONS * Cellular Biotechnology Unit, Instituto de Salud Carlos III (ISCIII), Madrid, 28029, Spain Stefano Gambera, Ander Abarrategi, Álvaro Morales-Molina, Arantzazu Alfranca
& Javier García-Castro * Haematopoietic Stem Cell Lab, The Francis Crick Institute, London, NW1 1AT, UK Ander Abarrategi * Electron and Confocal Microscopy Unit, Instituto de Salud
Carlos III (ISCIII), Madrid, 28029, Spain Fernando González-Camacho * Laboratory of Translational Research in Child and Adolescent Cancer, Vall d’Hebron Hospital, Barcelona, 08035, Spain
Josep Roma * Immunology Department, Hospital Universitario de La Princesa, Madrid, 28006, Spain Arantzazu Alfranca Authors * Stefano Gambera View author publications You can also search for
this author inPubMed Google Scholar * Ander Abarrategi View author publications You can also search for this author inPubMed Google Scholar * Fernando González-Camacho View author
publications You can also search for this author inPubMed Google Scholar * Álvaro Morales-Molina View author publications You can also search for this author inPubMed Google Scholar * Josep
Roma View author publications You can also search for this author inPubMed Google Scholar * Arantzazu Alfranca View author publications You can also search for this author inPubMed Google
Scholar * Javier García-Castro View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conception and design: S.G., A.A. and J.G.-C. Development of
methodology: S.G., A.A. and J.G.-C. Acquisition of data: S.G., F.G., and A.M.-M. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis):
S.G., F.G., A.M.-M., A.A. and J.G.-C. Writing, review, and/or revision of the manuscript: S.G., A.A., A.M.-M., J.R., F.G., A.A. and J.G.-C. Administrative, technical, or material support
(i.e., reporting or organizing data, constructing database): S.G., A.M.-M., J.R., F.G., A.A. and J.G.-C. CORRESPONDING AUTHOR Correspondence to Javier García-Castro. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY MOVIE
1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Gambera, S., Abarrategi, A., González-Camacho, F. _et al._ Clonal dynamics in osteosarcoma defined by RGB marking. _Nat Commun_ 9, 3994 (2018).
https://doi.org/10.1038/s41467-018-06401-z Download citation * Received: 26 November 2017 * Accepted: 03 September 2018 * Published: 28 September 2018 * DOI:
https://doi.org/10.1038/s41467-018-06401-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
