Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 direct trial
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Short-term fasting protects tumor-bearing mice against the toxic effects of chemotherapy while enhancing therapeutic efficacy. We randomized 131 patients with HER2-negative stage
II/III breast cancer, without diabetes and a BMI over 18 kg m−2, to receive either a fasting mimicking diet (FMD) or their regular diet for 3 days prior to and during neoadjuvant
chemotherapy. Here we show that there was no difference in toxicity between both groups, despite the fact that dexamethasone was omitted in the FMD group. A radiologically complete or
partial response occurs more often in patients using the FMD (OR 3.168, _P_ = _0.039_). Moreover, per-protocol analysis reveals that the Miller&Payne 4/5 pathological response,
indicating 90–100% tumor-cell loss, is more likely to occur in patients using the FMD (OR 4.109, _P_ = _0.016_). Also, the FMD significantly curtails chemotherapy-induced DNA damage in
T-lymphocytes. These positive findings encourage further exploration of the benefits of fasting/FMD in cancer therapy. Trial number: NCT02126449. SIMILAR CONTENT BEING VIEWED BY OTHERS
FASTING-MIMICKING DIET AND HORMONE THERAPY INDUCE BREAST CANCER REGRESSION Article 15 July 2020 FASTING MIMICKING DIET IN MICE DELAYS CANCER GROWTH AND REDUCES IMMUNOTHERAPY-ASSOCIATED
CARDIOVASCULAR AND SYSTEMIC SIDE EFFECTS Article Open access 08 September 2023 COMBINED INTERMITTENT FASTING AND ERK INHIBITION ENHANCE THE ANTI-TUMOR EFFECTS OF CHEMOTHERAPY VIA THE
GSK3Β-SIRT7 AXIS Article Open access 25 August 2021 INTRODUCTION Extensive preclinical evidence suggests that short-term fasting and fasting mimicking diets (FMDs) can protect healthy cells
against the perils of a wide variety of stressors, including chemotherapy, simultaneously rendering cancer cells more vulnerable to chemotherapy and other therapies1,2,3,4,5. Essentially,
fasting causes a switch in healthy cells from a proliferative state towards a maintenance and repair state. Malignant cells, in contrast, seem to be unable to enter this protective state
because of oncoprotein activity, and therefore fail to adapt to nutrient scarce conditions. Instead, fasting deprives proliferating cancer cells of nutrients, growth and other factors, which
renders them more sensitive to cancer therapy and increases cell death1,3. The phenomenon by which normal but not cancer cells become protected to toxins is termed differential stress
resistance (DSR)2,3 whereas the specific sensitization of cancer cells to stress is called Differential Stress Sensitization (DSS)1,6. Declines of plasma levels of insulin like growth
factor-1 (IGF-1), insulin and glucose are among the mediators of the effects of fasting on cancer cells, as these factors can promote growth and prevent apoptosis1,2,3,4,6,7. Fasting periods
of at least 48 h are required to induce a robust decrease in circulating glucose, IGF-1 and insulin levels6,8. A very low calorie, low protein FMD was developed for its ability to cause
metabolic effects on various starvation response markers similar to those caused by water-only fasting, while reducing the burden associated with a water only fast9,10. Small clinical
studies showed that fasting as an adjunct to chemotherapy is safe and well tolerated, while it may reduce its toxicity11,12,13,14. This multicentre, open label, randomized DIRECT study was
designed to evaluate the impact of an FMD on toxicity as well as on the radiological and pathological response to chemotherapy for breast cancer. RESULTS PATIENT CHARACTERISTICS From
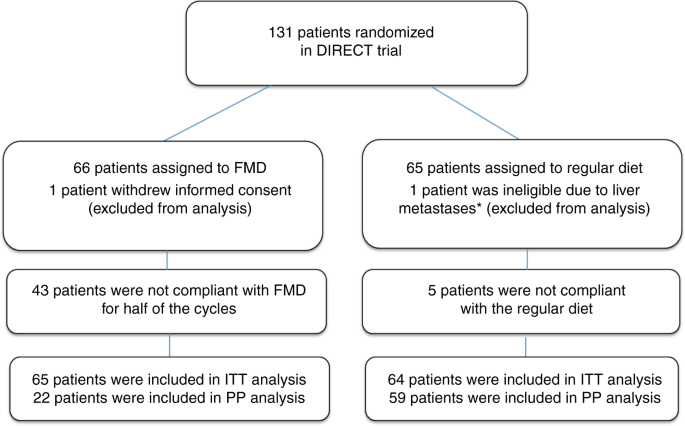
February 2014 to January 2018, 131 patients were randomized (see consort diagram, Fig. 1). One patient withdrew informed consent before starting with chemotherapy and one patient was
ineligible because of liver metastases, which were diagnosed a day after randomization. Of the 129 patients, 65 received FMD as an adjunct to chemotherapy and 64 patients used their regular
diet. Thirty patients received FEC-T chemotherapy and 99 AC-T. Patient characteristics were equally distributed between groups (Table 1 and Supplementary Table 2). INTERIM ANALYSIS Because
the overall (both arms) pCR turned out to be significantly lower (11.7%) than anticipated (which would require the recruitment of twice as many participants to be able to detect the
hypothesized pCR difference between both arms in a subsequent phase III study), in addition to the worse-than-expected compliance, the Data Safety Monitoring Board advised to dispense with
the phase III study. Therefore, we here present the results of the phase II study. COMPLIANCE Fifty three out of 65 patients (81.5%) completed the first FMD cycle, whereas over 50% completed
2 FMD cycles, which could be sufficient to impact the tumor response to chemotherapy in view of the effects of only one or a few FMD cycles in enhancing the efficacy of chemotherapy in
mice15. 22 out of 65 patients (33.8%) used the FMD for at least 4 cycles (all AC or FEC cycles), and 20.0% of the patients complied during all cycles of chemotherapy (Supplementary Table 3).
The main reason for non-adherence to the FMD was dislike of distinct components of the diet, perhaps induced by chemotherapy. In the regular diet group, 5 (7.8%) patients were not compliant
(they decided to fast during one or more cycles of chemotherapy). INTENTION TO TREAT (ITT) ANALYSIS Data on toxicity are shown in Supplementary Table 4. Grade III/IV toxicity, scored during
all cycles of chemotherapy, was not significantly different between the FMD group (75.4%) and the regular diet group (65.6%). No grade V toxicity occurred. The percentage of patients who
discontinued chemotherapy did not significantly differ between groups (27.7% FMD vs 23.8% control, _P_ = 0.580). Notably, while side effects were similar in both arms, patients in the FMD
arm did not receive dexamethasone before the AC chemotherapy cycles. The radiological response and pathological response according to Miller and Payne are shown in Fig. 2 and Supplementary
Table 5. The overall pCR rate was 11.7% and did not differ between the two groups (10.8% in FMD group versus 12.7% in control group; OR 0.830, 95% CI 0.282–2.442, _P_ = 0.735).
Interestingly, the radiologically complete or partial response, as measured by MRI or ultrasound before surgery, occurred approximately 3 times more often in the FMD group compared to the
control group in univariate (OR 2.886, 95% CI 1.012–8.227, _P_ = 0.047) and multivariate (OR 3.168, 95% CI 1.062–9.446, _P_ = 0.039) analyses. Accordingly, the proportion of patients with
stable or progressive disease was 2.5 fold lower in the FMD group (11.3%) than in the control group (26.9%, Fig. 2). The FMD affected various metabolic and endocrine parameters in the ITT
analysis (Supplementary Table 6). At day −1/ 0 pre-chemotherapy, plasma insulin was significantly lower in the FMD group (_P_ = 0.004), while a trend for lower plasma glucose levels was
observed in the FMD group (_P_ = 0.062). Urine ketone bodies were higher in the FMD group versus the control group (_P_ < 0.0001). Data on global QoL and the distress thermometer are
shown in Fig. 3 and Supplementary Fig. 2, respectively. QoL was not significantly different between both groups in terms of global QoL (_P_ = 0.841) and overall distress (_P_ = 0.674). PER
PROTOCOL (PP) ANALYSIS A PP analysis was done to substantiate the effects of FMD on toxicity and efficacy of chemotherapy. Specifically, patients who were compliant with the FMD for at least
half of the cycles were compared with those who were less compliant, and with the compliant control patients (i.e., the patients in the control group who did not fast on their own
initiative). Toxicity data of the PP analysis are shown in Table 2. Grade III/IV toxicity did not differ between FMD compliant patients (_n_ = 22) _vs_. control (_n_ = 59) group. In the PP
analysis, the pCR rate did not differ between the compliant FMD patients (13.6%) and controls (12.1%, OR 1.150, 95% CI 0.269–4.911, _P_ = 0.850, Supplementary Table 5). However, the Miller
and Payne pathological response 4/5 (90–100% tumor cell loss) occurred more often in patients using FMD in both univariate (OR 3.194, 95% CI 1.115–9.152, _P_ = 0.031) and multivariate
analyses (OR 4.109, 95% CI 1.297–13.02, _P_ = 0.016, Fig. 2) than in the control group. Furthermore, the more FMD cycles completed, the more patients had either a complete or partial
radiological response to therapy (_P_ for trend = 0.035, Fig. 4). Both analyses were adjusted for hormone receptor status, TNM stage, BMI and chemotherapy regimen. In the PP analysis (Fig. 5
and Supplementary Table 5), glucose was significantly lower in the compliant FMD group compared with the regular diet group before the first cycle and halfway therapy (_P_ = 0.006 and _P_ =
0.042, respectively). Insulin was significantly lower in the compliant FMD group compared with the control group before the first cycle and halfway therapy (_P_ = 0.001 and _P_ < 0.001,
respectively_)_. IGF-1 was significantly lower halfway therapy in patients who were compliant to the FMD in comparison to control patients (_P_ = 0.025). Ketone bodies were positive in most
of the compliant FMD patients (93.3%) and rarely positive in the compliant control group (8.1%, _P_ < 0.0001). The level of γ-H2AX intensity are reported in Supplementary Table 6. Only
compliant patients were included. γ-H2AX intensity increased 3 h after chemotherapy in both groups for each cell type due to chemotherapy. The increase in DNA damage after chemotherapy was
significantly less in CD45+ CD3+ T-lymphocytes from patients who had FMD as compared to patients using regular diet (_P_ = 0.045, Fig. 6). DISCUSSION This is the first randomized controlled
study evaluating the effects of an FMD on toxicity and efficacy of chemotherapy in patients with cancer. The results suggest that an FMD significantly reinforces the effects of neoadjuvant
chemotherapy on the radiological and pathological tumor response in patients with HER2 negative early breast cancer. The ITT analysis reveals an increase of patients with a radiologically
complete or partial response and a reduction of patients with radiologically stable/progressing disease in the FMD group compared to the control group. The PP analysis shows a beneficial
effect of the FMD on the pathological response according to Miller and Payne. The more cycles of FMD were adhered to, the higher the percentage of Miller and Payne scores 4/5 (documenting
>90% tumor cell loss) in the surgical specimen (Fig. 4). By chance, the percentage of patients with a triple negative tumor randomized to receive the FMD was double the percentage of
those in the control group (Table 1). pCR is more likely to occur in case of triple negative tumors16,17. However, triple negative tumors were significantly less common in patients who
complied with the FMD than in those who did not, while the response of the tumor to chemotherapy was clearly more favorable in compliant patients (Supplementary table 2). Moreover, the
positive effects of the FMD persisted after adjustment for the receptor status of the tumor. These facts suggest that it was the FMD rather than the hormone receptor status which determined
the better response of the tumor in agreement with the extensive pre-clinical data. Patients using the FMD as an adjunct to chemotherapy did not experience more grade III/IV adverse events
than patients who did not follow a diet, despite the fact that they were not prescribed dexamethasone in concert with FEC/AC. This suggests that the FMD may obviate the need for
dexamethasone in the prevention of the side effects of chemotherapy. Importantly, DNA damage in T-lymphocytes was less in patients who received the FMD in combination with chemotherapy
compared to those receiving chemotherapy while on a regular diet, suggesting that the FMD protected these cells against the induction of DNA damage by chemotherapy. The study was meant to be
a phase II/III study to evaluate the effects of the FMD on toxicity and efficacy of chemotherapy, respectively. However, a pre-defined interim analysis revealed a lower than anticipated
overall pCR rate in the combined arms (albeit similar to the pCR rate in a similar trial of the same BOOG group18), necessitating the recruitment of twice as many participants to reliably
judge the impact of the FMD on this primary outcome measure. Because this would prolong the study period and require additional funds, the DSMB advised to stop and report the results at the
completion of phase II. Remarkably, the phase II study, involving only 131 patients, was sufficient to show benefits of the FMD in sensitizing breast cancer cells to chemotherapy, with
efficacy demonstrated both at the clinical and pathology levels. Pre-clinical data, that has been accumulating for over 10 years, indicates that fasting can protect cancer-bearing mice
against the side effects of chemotherapy3, while sensitizing the tumor to its toxic effects1,2. Even one or a few cycles of FMD by itself can inhibit the progression of a wide variety of
cancers and increase the therapeutic efficacy of chemotherapy in mice1,15, but can also prime breast cancer and other tumor cell types to an attack by immune cells15. Accordingly, in spite
of the fact that many patients could not adhere to the dietary regimen during all cycles of chemotherapy, our intention to treat analysis reveals benefits in terms of tumor response. Only a
few, generally small clinical studies have evaluated the potential of fasting to improve cancer treatment11,12,13,14, primarily focusing on feasibility and toxicity of treatment. Just two of
these trials were randomized11,12,13,14, but the results were in line with those of the current study. Previously, we reported reduced hematological toxicity and DNA damage in circulating
mononuclear cells in a small group of women who fasted for 24 h prior to (neo)adjuvant chemotherapy for breast cancer11. A second randomized study revealed improved QoL and less fatigue in
breast- and ovarian cancer patients, who fasted for 60 h around the time of chemotherapy14. Yet another study reported a trend towards less grade 3–4 neutropenia and reduced DNA damage in
leukocytes in patients who fasted for 48–72 as compared to 24 h around the time of platinum-based chemotherapy for a variety of malignancies12. Finally, fasting for variable time periods may
reduce adverse events of chemotherapy, which was suggested by a case series of 10 patients with various cancer types13. These data agree with the current data, showing that the FMD is safe
and effective as an adjunct to chemotherapy, at least in patients with a normal body mass index at inclusion. Our data should be cautiously interpreted, particularly those of the PP
analysis, which bears the risk of selection bias. However, the ITT analysis confirms the positive impact of the FMD on the radiological response, whilst clearly showing a trend in support of
the PP positive effect on the pathological response. Moreover, due to self-selection bias patients in the control group decided to fast on their own initiative, which may have decreased the
positive impact of the FMD in the ITT analysis. In conclusion, the results of this study are the first to suggest that FMD cycles are safe and effective as an adjunct to chemotherapy in
women with early breast cancer. These findings together with preclinical data encourage further exploration of the benefits of fasting/FMD in patients receiving a wide range of cancer
therapies. METHODS STUDY DESIGN AND PATIENTS This is a randomized, controlled, observer-blind study. Eligible patients from 11 Dutch centers had histologically confirmed diagnosis of
HER2-negative, stage II/III (cT1cN + or ≥T2 any cN, cM0) early breast cancer, adequate bone marrow reserve (white blood counts >3.0 × 109/L, absolute neutrophil count ≥1.5 × 109/L and
platelet count ≥100 × 109/L), adequate liver function (bilirubin ≤1.5 × upper limit of normal (UNL) range, ALAT and/or ASAT ≤2.5× UNL, Alkaline Phosphatase ≤5× UNL), adequate renal function
(calculated creatinine clearance ≥50 mL min−1), normal cardiac function, a WHO performance state 0–2, age ≥18 years, BMI >19 kg m−2, absence of diabetes mellitus, absence of allergies for
FMD content, and signed informed consent. The study (NCT02126449) was conducted in accordance with the Declaration of Helsinki (October 2013) and approved by the Ethics Committee of the
Leiden University Medical Center in agreement with the Dutch law for medical research involving human subjects. DRUGS Women received 8 cycles of neo-adjuvant AC-T chemotherapy (4 cycles
doxorubicin 60 mg m−2 and cyclophosphamide 600 mg m−2 intravenously (i.v.)), followed by 4 cycles of T (docetaxel 100 mg m−2 i.v.), or 6 cycles of neo-adjuvant FEC-T chemotherapy, consisting
of 3 cycles of 5-fluorouracil, epirubicin and cyclophosphamide at a dose of 500, 100 and 500 mg m−2 i.v., respectively), followed by 3 cycles of T (docetaxel 100 mg m−2 i.v.), all q 3
weeks. The anti-emetic agents granisetron (1 mg i.v.) or ondansetron (8 mg i.v.) were administered prior to chemotherapy. Dexamethasone (8 mg i.v.) was administered shortly before
chemotherapy for all cycles in the control group, whereas it was omitted during the AC or FEC courses in the FMD group, as dexamethasone may counteract the endocrine and metabolic effects of
dietary intervention in the FMD group19. INTERVENTION Women were randomized in a 1:1 ratio to receive the FMD (Xentigen™) or regular diet for 3 days prior to and on the day of each cycle of
chemotherapy. The FMD is a 4-day plant-based low amino-acid substitution diet, consisting of soups, broths, liquids and tea (Supplementary Fig. 3). Calorie content declined from day 1
(~1200 kcal), to days 2–4 (~200 kcal). Moreover, the carbohydrates/proteins/fats energy ratio was approximately 3.5/1/2 on the first day, while complex carbohydrates were the main
macronutrient (>80 energy%) the other subsequent 3 days. Patients were allowed to eat the diet components at any time of the designated day. RANDOMIZATION, MASKING AND DATA STORAGE
Patients were centrally randomized at the LUMC datacenter through block randomization with various block sizes stratified by stage (II versus III), estrogen receptor status (positive versus
negative), BMI (<25 kg m−2 versus >25 kg m−2) and chemotherapy regimen (AC-T versus FEC-T). The web based relational database management system ProMISe
(https://www.msbi.nl/promise/ProMISe.aspx) was used for data storage and exchange. BLOOD SAMPLING Venous blood samples were drawn prior to each chemotherapy administration (pre-chemotherapy
on day −1 or day 0). Compliance with the diet was estimated by the following parameters: insulin, glucose, and IGF-1 (measured in a 9 mL serum-separating tube). All samples were analyzed by
the accredited clinical laboratories of the participating centers. The effect of FMD on chemotherapy-induced DNA damage in peripheral blood mononuclear cells (PBMCs) was examined in a side
study. Sodium heparinized venous blood samples (9 mL) were collected for prior to the first cycle of chemotherapy and three hours after start of chemotherapy. TOXICITY AND EFFICACY The
primary endpoint of the phase II and phase III parts of the study were grade III/IV toxicity and pathological complete response (pCR), respectively. Toxicity was documented by the physician
and graded according to the Common Terminology Criteria for Adverse Events version4.03 (CTCAE v.4.03). Pathological complete response (pCR) was defined as the absence of residual invasive
cancer within the breast and lymph nodes16, excluding isolated tumor cells (ITC). Secondary endpoints included radiological response and pathological response according to the Miller and
Payne (Supplementary Table 1)16. Histopathology was centrally revised by one pathologist (DC), who was blinded to which treatment the patient received. Clinical response was measured by MRI
or ultrasound of the breast halfway and at the end of therapy, according to RECIST1.120. QUALITY OF LIFE (QOL) Global health was assessed with the EORTC QLQ-C3021 before therapy (after
randomization), halfway therapy, at the end of therapy and at six months follow-up. Higher scores (0–100 scale) on the functional scales indicate a better QoL. Psychosocial distress was
measured with the distress thermometer22, with an 11‐point range from 0 (no distress) to 10 (extreme distress). Patients were asked to circle the number that best described the overall
distress they experienced in the past week at 3 timepoints: halfway therapy, at the end of therapy and at six months follow-up. DNA DAMAGE: ISOLATION OF PBMCS AND Γ-H2AX STAINING PBMCs were
isolated using Ficoll-Amidotrizoaat (Pharmacy LUMC) gradient centrifugation according to the standard operating procedure of the Medical Oncology department of LUMC. Isolated PBMCs were
carefully resuspended and 3 times washed in PBS (B. Braun, Melsungen, Germany). Samples were fixed in 1.5% formaldehyde and permealized in ice-cold pure methanol. Cells were washed 3 times
in staining buffer (PBS with 5% bovine serum albumin (BSA, Sigma, St Louis, USA)) and stained for 30 min on ice with anti-CD45-PerCP-Cy5.5 (BD Bioscience, Breda, the Netherlands), clone 2D1
anti-CD3-PE (BD, clone SK7), anti-CD14-AF700 (BD, clone M5E2), anti-CD15-PE CF594 (BD, clone W6D3) and anti-γ-H2AX-AF488 (Biolegend, clone 2F3), followed by another washing step and
resuspension in PBS. Per experiment we used 1,000,000 cells or more when available. The cell acquisition was performed immediately after the staining procedure on the flow cytometer (BD LSR
Fortessa Flow Cytometer analyzer, BD Bioscience, Breda, The Netherlands) and data were analyzed using BD FACS Diva Software version 6.2. The CD45+ cells were gated, after which the CD3+
T-lymphocytes, CD3− non-T cells (also harboring B lymphocytes) or CD14+ CD15− monocytes were analyzed for the geomean (as measure for the intensity) of γ-H2AX (Supplementary Fig. 2).
STATISTICAL ANALYSIS The primary endpoint of phase II of the study was grade III/IV toxicity. Based on trials with similar neo-adjuvant chemotherapy17,23,24, the statistical power analysis
revealed that a total number of 128 patients (64 patients in each arm) was required to be able to detect a 50% reduction of grade III/IV adverse effects with 80% power using a nominal
significance level of 3.06% . The primary endpoint of the phase III part of the study was pathological complete response (pCR). We estimated the overall pCR rate to amount to 18%, based on
studies examining similar third generation chemotherapy17,18,23. Our sample size calculation revealed that we would require a total number of 212 patients (106 per treatment arm). An interim
analysis, focusing on feasibility and adverse events, was planned after completion of the phase II part of the protocol by 128 patients and was approved by the Ethics Committee of the
Leiden University Medical Center. Early stopping rules included significantly more or unacceptable adverse events in either group. A data safety monitoring board conducted the interim
analysis. Survival data will be reported after 5 years follow-up. All parameters were tested for normality using a Kolmogorov-Smirnov test, with Bonferroni adjustment when evaluated in
subgroups. Normally distributed parameters, if necessary after log transformation, were summarized as mean (and standard error of the mean (SEM)) and compared using independent or paired
samples _t_-tests when appropriate. The non-normally distributed parameters were summarized as median (and 25th and 75th percentiles) and compared using a Mann-Whitney test for independent
groups or Wilcoxon signed rank test for paired groups. The effect of FMD on efficacy of chemotherapy was analyzed using logistic regression, yielding univariate and multivariate odds ratios
(ORs), 95% confidence intervals (CIs), and _P_-values. Multivariate analyses were adjusted for stratification factors25. ER status, BMI, stage of disease and chemotherapy regimen. The
Armitage’s trend test was used to test an association between an ordinal variable and two categories. Mean changes in QoL from baseline to halfway, end of therapy and 6 months follow-up were
assessed in linear mixed models with 95% CIs. All tests were 2-tailed with a significance level of 0.05. All data were analyzed using IBM SPSS Statistics for Windows (Version 23.0. Armonk,
NY: IBM Corp). REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY All study data are
presented in the manuscript and supplementary materials. The source data underlying Tables 1 and 2, Figs. 2–6, Supplementary Fig. 1 and Supplementary Tables 2–7 are provided as a Source Data
file. Additional raw data that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Lee, C. et al. Fasting cycles retard
growth of tumors and sensitize a range of cancer cell types to chemotherapy. _Sci. Transl. Med._ 4, 124ra127 (2012). Article Google Scholar * Di Biase, S. & Longo, V. D.
Fasting-induced differential stress sensitization in cancer treatment. _Mol. Cell Oncol._ 3, e1117701 (2016). Article Google Scholar * Raffaghello, L. et al. Starvation-dependent
differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. _Proc. Natl. Acad. Sci. USA_ 105, 8215–8220 (2008). Article ADS CAS Google Scholar *
Huisman, S. A. et al. Fasting protects against the side-effects of irinotecan treatment but does not abrogate anti-tumor activity in mice. _Br. J. Pharm._ 173, 804–814 (2015). Article
Google Scholar * de Groot, S., Pijl, H., van der Hoeven, J. J. M. & Kroep, J. R. Effects of short-term fasting on cancer treatment. _J. Exp. Clin. Cancer Res._ 38, 209 (2019). Article
Google Scholar * Thissen, J. P., Ketelslegers, J. M. & Underwood, L. E. Nutritional regulation of the insulin-like growth factors. _Endocr. Rev._ 15, 80–101 (1994). CAS PubMed Google
Scholar * Pollak, M. Insulin, insulin-like growth factors and neoplasia. _Best. Pr. Res. Clin. Endocrinol. Metab._ 22, 625–638 (2008). Article CAS Google Scholar * Cahill, G. Jr., Felig,
P., Owen, O. & Wahren, J. Metabolic adaptation to prolonged starvation in man. _Nord. Med._ 83, 89 (1970). PubMed Google Scholar * Brandhorst, S. et al. A periodic diet that mimics
fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. _Cell Metab._ 22, 86–99 (2015). Article CAS Google Scholar * Nencioni, A., Caffa, I.,
Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. _Nat. Rev. Cancer_ 18, 707–719 (2018). Article CAS Google Scholar * de Groot, S. et
al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. _BMC Cancer_ 15, 652 (2015). Article
Google Scholar * Dorff, T. B. et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. _BMC Cancer_ 16, 360 (2016). Article Google Scholar * Safdie, F. M.
et al. Fasting and cancer treatment in humans: a case series report. _Aging_ 1, 988–1007 (2009). Article Google Scholar * Bauersfeld, S. P. et al. The effects of short-term fasting on
quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. _BMC Cancer_ 18, 476 (2018). Article Google Scholar * Di
Biase, S. et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. _Cancer Cell_ 30, 136–146 (2016). Article Google Scholar * Ogston, K. N. et al. A new
histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. _Breast_ 12, 320–327 (2003). Article Google Scholar * von
Minckwitz, G. et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast
cancer: the GEPARDUO study of the German Breast Group. _J. Clin. Oncol._ 23, 2676–2685 (2005). Article Google Scholar * Charehbili, A. et al. Addition of zoledronic acid to neoadjuvant
chemotherapy does not enhance tumor response in patients with HER2-negative stage II/III breast cancer: the NEOZOTAC trial (BOOG 2010-01). _Ann. Oncol._ 25, 998–1004 (2014). Article CAS
Google Scholar * Hickish, T. et al. Glucose intolerance during adjuvant chemotherapy for breast cancer. _J. Natl. Cancer Inst._ 101, 537 (2009). Article Google Scholar * Eisenhauer, E. A.
et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). _Eur. J. Cancer_ 45, 228–247 (2009). Article CAS Google Scholar * Aaronson, N. K. et al.
The European Organization for research and treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. _J. Natl. Cancer Inst._ 85,
365–376 (1993). Article CAS Google Scholar * Tuinman, M. A., Gazendam-Donofrio, S. M. & Hoekstra-Weebers, J. E. Screening and referral for psychosocial distress in oncologic practice:
use of the distress thermometer. _Cancer_ 113, 870–878 (2008). Article Google Scholar * Vriens, B. E. et al. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as
neoadjuvant treatment in patients with breast cancer. _Eur. J. Cancer_ 49, 3102–3110 (2013). Article CAS Google Scholar * Martin, M. et al. Adjuvant docetaxel for node-positive breast
cancer. _N. Engl. J. Med._ 352, 2302–2313 (2005). Article ADS CAS Google Scholar * Kahan, B. C. & Morris, T. P. Reporting and analysis of trials using stratified randomisation in
leading medical journals: review and reanalysis. _BMJ_ 345, e5840 (2012). Article Google Scholar Download references ACKNOWLEDGEMENTS We are greatly indebted to the patients for
participating in this study, and their physicians for including the patients: E. Göker (Alexander Monro Hospital), A.J.M. Pas (‘t Langeland Hospital) A.H. Honkoop (Isala). This work was
supported by grants from Pink Ribbon (2012.WO31.C155) and Amgen (20139098). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medical Oncology, Leiden University Medical Center,
P.O. Box 9600, 2300 RC, Leiden, The Netherlands Stefanie de Groot, Rieneke T. Lugtenberg, Marij J. P. Welters, Ilina Ehsan, Johanneke E. A. Portielje, Jacobus J. M. van der Hoeven, Johan W.
R. Nortier, Judith R. Kroep, Johanneke E. A. Portielje & Judith R. Kroep * Department of Pathology, Leiden University Medical Center, P.O. Box 9600, 2300 RC, Leiden, The Netherlands
Danielle Cohen & Vincent T. H. B. M. Smit * Department of Human Genetics, Leiden University Medical Center, P.O. Box 9600, 2300 RC, Leiden, The Netherlands Maaike P. G. Vreeswijk *
Department of Medical Oncology, Medical center Leeuwarden, P.O. Box 888, 8901 NR, Leeuwarden, The Netherlands Hiltje de Graaf & Hiltje de Graaf * Department of Medical Oncology, Amphia,
P.O. Box 90157, 4800 RL, Breda, The Netherlands Joan B. Heijns & Joan B. Heijns * Department of Medical Oncology, Haga hospital, P.O. Box 40551, 2504 LN, Den Haag, The Netherlands
Johanneke E. A. Portielje & Johanneke E. A. Portielje * Department of Medical Oncology, Viecuri, 5912BL, Venlo, The Netherlands Agnes J. van de Wouw & Agnes J. van de Wouw *
Department of Medical Oncology, Deventer hospital, P.O. Box 5001, 7416 SE, Deventer, The Netherlands Alex L. T. Imholz, Lonneke W. Kessels, Alex L. T. Imholz & Lonneke W. Kessels *
Department of Medical Oncology, Noordwest hospital group, location Alkmaar, P.O. Box 501, 1815 JD, Alkmaar, The Netherlands Suzan Vrijaldenhoven & Suzan Vrijaldenhoven * Department of
Medical Oncology, Hospital Gelderse vallei, 6710 HN, Ede, The Netherlands Arnold Baars & Arnold Baars * Department of Surgery, Leiden University Medical Center, P.O. Box 9600, 2300 RC,
Leiden, The Netherlands Elma Meershoek-Klein Kranenbarg & Marjolijn Duijm-de Carpentier * Department of Medical Statistics and Bioinformatics, Leiden University Medical Center, P.O. Box
9600, 2300RC, Leiden, The Netherlands Hein Putter * Longevity Institute, School of Gerontology, and Department of Biological Sciences, University of Southern California, Los Angeles, CA,
90089, USA Valter D. Longo * IFOM FIRC Institute of Molecular Oncology, Via Adamello 16, Milan, Italy Valter D. Longo * Department of Endocrinology, Leiden University Medical Center, P.O.
Box 9600, 2300 RC, Leiden, The Netherlands Hanno Pijl * BOOG Study Center, P.O. Box 9236, 1006 AE, Amsterdam, The Netherlands A. Elise van Leeuwen-Stok * Department of Medical Oncology,
Alexander Monro Hospital, 3723 MB, Bilthoven, The Netherlands Emine Göker * Department of Medical Oncology, ‘t Langeland Hospital, 2725 NA, Zoetermeer, The Netherlands Anke J. M. Pas *
Department of Medical Oncology, Isala hospital, 8025 AB, Zwolle, The Netherlands Aafke H. Honkoop Authors * Stefanie de Groot View author publications You can also search for this author
inPubMed Google Scholar * Rieneke T. Lugtenberg View author publications You can also search for this author inPubMed Google Scholar * Danielle Cohen View author publications You can also
search for this author inPubMed Google Scholar * Marij J. P. Welters View author publications You can also search for this author inPubMed Google Scholar * Ilina Ehsan View author
publications You can also search for this author inPubMed Google Scholar * Maaike P. G. Vreeswijk View author publications You can also search for this author inPubMed Google Scholar *
Vincent T. H. B. M. Smit View author publications You can also search for this author inPubMed Google Scholar * Hiltje de Graaf View author publications You can also search for this author
inPubMed Google Scholar * Joan B. Heijns View author publications You can also search for this author inPubMed Google Scholar * Johanneke E. A. Portielje View author publications You can
also search for this author inPubMed Google Scholar * Agnes J. van de Wouw View author publications You can also search for this author inPubMed Google Scholar * Alex L. T. Imholz View
author publications You can also search for this author inPubMed Google Scholar * Lonneke W. Kessels View author publications You can also search for this author inPubMed Google Scholar *
Suzan Vrijaldenhoven View author publications You can also search for this author inPubMed Google Scholar * Arnold Baars View author publications You can also search for this author inPubMed
Google Scholar * Elma Meershoek-Klein Kranenbarg View author publications You can also search for this author inPubMed Google Scholar * Marjolijn Duijm-de Carpentier View author
publications You can also search for this author inPubMed Google Scholar * Hein Putter View author publications You can also search for this author inPubMed Google Scholar * Jacobus J. M.
van der Hoeven View author publications You can also search for this author inPubMed Google Scholar * Johan W. R. Nortier View author publications You can also search for this author
inPubMed Google Scholar * Valter D. Longo View author publications You can also search for this author inPubMed Google Scholar * Hanno Pijl View author publications You can also search for
this author inPubMed Google Scholar * Judith R. Kroep View author publications You can also search for this author inPubMed Google Scholar CONSORTIA DUTCH BREAST CANCER RESEARCH GROUP (BOOG)
* Hiltje de Graaf * , Joan B. Heijns * , Johanneke E. A. Portielje * , Agnes J. van de Wouw * , Alex L. T. Imholz * , Lonneke W. Kessels * , Suzan Vrijaldenhoven * , Arnold Baars * , Emine
Göker * , Anke J. M. Pas * , Aafke H. Honkoop * , A. Elise van Leeuwen-Stok * & Judith R. Kroep CONTRIBUTIONS J.R.K., H.a.P., J.W.R., M.P.G.V., J.J.M.H., V.T.H.B.M. and V.D.L.
contributed to studyconcept/design, J.R.K., R.T.L., D.C., M.J.P.W., I.E., H.d.G., J.B.H., J.E.A.P., A.J.v.d.W., A.L.T.I., L.W.K., S.V., A.B., E.M.K.K., M.D.C. and S.d.G. contributed to data
acquisition. H.e.P. and S.d.G. contributed to statistical analysis. S.d.G., H.a.P., J.R.K. and V.D.L. contributed to manuscript preparation. All authors contributed toward data analysis,
drafting and revising the paper and agree to be accountable for all aspects of the work. CORRESPONDING AUTHOR Correspondence to Judith R. Kroep. ETHICS DECLARATIONS COMPETING INTERESTS
V.D.L. has equity interest in L-Nutra. H.P. has shares in a company that invested in L-Nutra. The remaining authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW
INFORMATION _Nature Communications_ thanks Cheng Cheng Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are
available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION PEER REVIEW FILE REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE de Groot, S., Lugtenberg, R.T., Cohen, D. _et al._ Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the
multicentre randomized phase 2 DIRECT trial. _Nat Commun_ 11, 3083 (2020). https://doi.org/10.1038/s41467-020-16138-3 Download citation * Received: 17 December 2019 * Accepted: 14 April 2020
* Published: 23 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16138-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
