The impact of learning on perceptual decisions and its implication for speed-accuracy tradeoffs
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT In standard models of perceptual decision-making, noisy sensory evidence is considered to be the primary source of choice errors and the accumulation of evidence needed to overcome
this noise gives rise to speed-accuracy tradeoffs. Here, we investigated how the history of recent choices and their outcomes interact with these processes using a combination of theory and
experiment. We found that the speed and accuracy of performance of rats on olfactory decision tasks could be best explained by a Bayesian model that combines reinforcement-based learning
with accumulation of uncertain sensory evidence. This model predicted the specific pattern of trial history effects that were found in the data. The results suggest that learning is a
critical factor contributing to speed-accuracy tradeoffs in decision-making, and that task history effects are not simply biases but rather the signatures of an optimal learning strategy.
SIMILAR CONTENT BEING VIEWED BY OTHERS TRIAL-HISTORY BIASES IN EVIDENCE ACCUMULATION CAN GIVE RISE TO APPARENT LAPSES IN DECISION-MAKING Article Open access 22 January 2024 TEMPORAL
REGULARITIES SHAPE PERCEPTUAL DECISIONS AND STRIATAL DOPAMINE SIGNALS Article Open access 17 August 2024 MULTIPLE TIMESCALES OF LEARNING INDICATED BY CHANGES IN EVIDENCE-ACCUMULATION
PROCESSES DURING PERCEPTUAL DECISION-MAKING Article Open access 08 June 2023 INTRODUCTION Evidence accumulation is an important core component of perceptual decision-making that mitigates
the effects of environmental uncertainty by combining information through time1,2,3,4,5,6,7,8. Theoretical models based on a random diffusion-to-bound (Drift-diffusion models—DDMs) have been
successful in modeling critical aspects of psychophysical decision tasks, capturing the dependence of accuracy (psychometric) and reaction time (chronometric) functions. These models have
been tested both by searching for neural activity corresponding to model variables1,9,10,11,12,13, and the exploration of more sophisticated task designs and modeling6,14. One widely
observed but not well-understood phenomenon is that different kinds of decisions appear to benefit from accumulation of evidence over different time scales. For example, monkeys performing
integration of random dot motion1 and rats performing a click train discrimination task6 can integrate evidence for over one second. But rats performing an odor mixture categorization task
fail to benefit from odor sampling beyond 200–300 ms14,15. A possible explanation is that neural integration mechanisms are specific to a given species and sensory modality. However, even
animals performing apparently similar odor-based decision tasks can show very different integration time windows16,17. Changes in speed-accuracy tradeoff (SAT)2,11,18, which could change the
height of the decision bound, have been proposed as a possible explanation for differences seen across similar studies. However, manipulation of motivational parameters failed to increase
the integration window in odor categorization, suggesting that other factors must limit decision accuracy14. In DDMs, the chief source of uncertainty is stochasticity in incoming sensory
evidence, modeled as Gaussian white noise around the true mean evidence rate19,20. It is this rapidly fluctuating noise that accounts for the benefits of temporal integration. The nature and
implications of other sources of variability have also been considered6,8,19,20,21,22, including variability in starting position21, non-accumulation time20 and threshold19. A potentially
important source of variability is trial-by-trial fluctuations in the mean rate of evidence accumulation. Such fluctuations would correspond to uncertainty in the mapping of sensory data
onto evidence for a particular choice9,23. This mapping could be implemented as the strength of weights between sensory representations into action values9. A combination of weights would
then represent a classification boundary between sensory stimuli24. Weight fluctuations would introduce errors that, unlike rapid fluctuations, could not be mitigated by temporal integration
and would therefore curtail its benefits14,25. Such “category boundary” variability (not to be confused with the stopping “bound” in accumulation models) might affect differently particular
decision tasks, being particularly important when the stimulus-to-action map must be learned de novo14,25. The effects of reward-history on choices in perceptual tasks, although commonly
observed26,27,28,29, have been considered suboptimal biases because each trial is in fact independent of the preceding trials. Here, we hypothesized that such biases are instead signatures
of an optimal learning strategy that is adapted to natural dynamic environments30. Intuitively, an optimal learning agent must always use both priors (history of stimuli, choices and
rewards) and current sensory information in proportion to their confidence31,32. Under this view, an optimal choice policy and learning algorithm that uses accumulation of evidence and
reward statistics to infer choices and update its stimulus-choice mapping—a Bayesian drift-diffusion model—can be derived32. Here, to test this model, we compared performance of rats in two
odor-guided decision tasks: (1) an odor identification task in which the difficulty was increased by lowering stimulus concentration and (2) an odor mixture categorization task15, in which
the difficulty was increased by making the stimuli closer to a category boundary. We hypothesized that performance in the second task would be dominated by uncertainty in the stimulus-choice
mapping and therefore benefit less from sensory integration. Indeed, we observed that the change in reaction times over a given range of accuracy was much smaller in the mixture
categorization task. We found that standard diffusion-to-bound models could fit performance on either task alone, but not simultaneously. However, the optimal Bayesian-DDM model could fit
both tasks simultaneously and out-performed simpler models with and without alternative learning rules. Critically, the introduction of learning predicted a history-dependence of
trial-by-trial choice biases whose specific pattern was indeed observed in the data. These findings suggest that “errors” in many psychophysical tasks are not due to stochastic noise, but
rather to suboptimal choices driven by optimal learning algorithms that are being tested outside the conditions in which they evolved33. RESULTS DIFFERENT SPEED-ACCURACY TRADEOFFS IN TWO
DIFFERENT OLFACTORY DECISION TASKS We trained Long Evans rats on two different versions of a two-alternative choice (2AC) olfactory reaction time task. We refer to these as two “tasks”, but
they were identical in all aspects except for the nature of the presented stimulus (Fig. 1). In the first task, “odor identification”, a single pure odor was presented in any given trial. We
manipulated difficulty by diluting odors over a range of 3 log steps (1000-fold, liquid dilution) (Fig. 2a). Thus, absolute concentration determined the difficulty. In the second task,
“odor categorization”, mixtures of two pure odors were presented with a fixed total concentration but at four different ratios15 (Fig. 2b). The distance of the stimulus to the category
boundary (50/50, iso-concentration line), determined the difficulty, with lower contrasts corresponding to more difficult trials. E.g., 56/44 and 44/56 stimuli (12% contrast) were more
difficult than 80/20 and 20/80 (60% contrast). Note that the easiest stimuli (10−1 dilution and 100% contrast) were identical between the two tasks. In a given session, the eight stimuli
from one of the two tasks were presented in randomly interleaved order. To ensure that any differences in performance were due to the manipulated stimulus parameters, all comparisons were
done using the same rats performing the two tasks on different days with all other task variables held constant (Supplementary Fig. 1). We quantified performance using accuracy (fraction of
correct trials) and odor sampling duration, a measure for reaction time (RT)14,15 (Fig. 1, Supplementary Fig. 2). We observed that rats performing the two tasks showed marked differences in
how much RTs increased as task difficulty increased (Fig. 2c–f). For the identification task, RTs increased substantially (112 ± 3 ms; mean ± SEM, _n_ = 4 rats; _F_(3,31) = 44.04, _P_ <
10−7; Fig. 2d), whereas for the categorization task the change was much smaller (31 ± 3 ms; _F_(3,31) = 2.61, _P_ = 0.09, ANOVA) (Fig. 2f), despite the fact that the accuracy range was
similar. To control for the possibility that a smaller performance range for the categorization task accounted for differences in SAT, we re-ran this task with two sets of stimuli with
harder, lower contrast stimuli. This yielded a range of accuracies as broad as those in the identification task yet still only resulted in 41 ± 24 and 50 ± 19 ms changes in RT (Supplementary
Fig. 3). Therefore, the difference observed in SAT for odor identification vs. mixture categorization was not due to differences in the range of task difficulties. CONSTRUCTION OF A
DIFFUSION-TO-BOUND MODEL FOR OLFACTORY DECISIONS In order to explore which variables might be constraining the rats’ performance, we fit the data using DDMs (Fig. 3a). In a 2-AFC task with
free response time, trading off the cost of accumulating evidence with reward rate becomes paramount. With adequately tuned decision thresholds, DDMs are known to implement the optimal
tradeoff strategy across a wide range of tasks, including those used here34,35,36. We implemented a DDM composed of sensory, integration and decision layers. The sensory layer implements a
transformation of odor concentrations into momentary evidence. Perceptual intensity in olfaction37,38, as in other modalities2,6 can be well-described using a power law. We therefore defined
the mean strength of sensory evidence _μ_ for each odor using a power law of the odor concentrations, $$\mu _i\left( {c_i} \right) = kc_i^\beta$$ (1) where _k_ and _β_ are free parameters2.
We constrained _k_ and _β_ to be identical between the two odors (stereoisomers with identical vapor pressures and similar intensities15,39,40,41). Evidence at each time step is drawn from
a normal distribution \(m_i(t):N(\mu _i,\sigma )\), where \(\sigma = 1\) is the standard deviation of the variability corrupting the true rate, _μ__i_. The integration layer, which also
consists of two units, integrates the noisy evidence over time independently for each odor. The last step of the model consists of a unit that takes the difference between the integrated
inputs. If this difference exceeds a given bound, _θ_ or _–θ_, the model stops and makes a choice according to the hit bound: left for _θ_, right for _–θ_. Finally, we allowed for a
time-dependent decrease in bound height (“collapsing”), _τ_, mimicking an urgency signal35,42 (Methods). DIFFUSION-TO-BOUND MODEL FAILS TO FIT BOTH TASKS SIMULTANEOUSLY To explain our
behavioral data with the standard DDM, we developed a series of different fitting procedures. All involved maximizing a log-likelihood function for a data set of 22,208 (identification),
19,947 (categorization) or 42,155 (both) trials using simulations over 100,000 trials (Methods). The overall quality of each fit is shown in Supplementary Fig. 4. The first procedure was to
test whether we could predict the behavioral data of the categorization task using the fitted parameters from the identification task. The model captured both accuracy and RTs in the
identification task (Fig. 3b, solid lines). However, when the same model was run on the categorization task, the model correctly predicted the range of RT’s in the data, but strongly
overestimated the animals’ accuracy at low contrasts (Fig. 4c, solid lines). Therefore, as a second procedure, we attempted to fit the model to the categorization task and predict the
identification task. This was also unsuccessful: the model fit the categorization data well (Fig. 3c, dashed lines) but failed to capture either accuracy or RTs in the identification task
(Fig. 3b, dashed lines). A third procedure, simultaneous fitting both data sets, also failed in describing both tasks successfully (Supplementary Fig. 5). Thus it was not possible to
accurately fit the standard DDM to both tasks using a single set of parameters. Accurate fits to both tasks were only possible if we allowed parameters to be fit independently. DIFFERENCES
IN SAT ARE NOT DUE TO CONTEXT DEPENDENT STRATEGIES Motivational variables can modulate performance and reaction time in perceptual tasks. For example, variables like reward rate35 or
emphasis for accuracy vs. speed2,11 can have an effect on observed SATs, by modulating decision criteria. Because the two tasks were run in separate sessions, we considered the possibility
that rats changed these criteria between sessions. To address this, we devised a “mixture identification” task in which we interleaved the full set of stimuli from the two tasks as well as
intermediate mixtures (Fig. 4a). On any given session, 8 randomly chosen stimuli out of the 32 possible were presented. Consistent with the previous observations, RTs in this joint task were
significantly affected by concentration but not by mixture contrast (Fig. 4b, c; two-way ANOVA (_F_(3,48) = 8.69, _P_ < 10−3 vs. 0.94, _P_ = 0.42)). There was no significant interaction
between concentration and contrast (_F_(9,48) = 0.28, _P_ > 0.9). Each individual rat showed a significant effect of odorant concentration (ANOVA for each rat: _F_1(3,15) = 78.66, _P_1
< 10−6; _F_2(3,15) = 14.66, _P_2 < 10−3; _F_3(3,15) = 204.91, _P_3 < 10−7; _F_4(3,15) = 27.86, _P_4 < 10−4), whereas only two showed a significant effect of mixture contrast
(_F_1(3,15) = 1.14, _P_1 = 0.39; _F_2(3,15) = 0.52, _P_2 = 0.67; _F_3(3,15) = 9.6, _P_3 < 0.01; _F_4(3,15) = 6.47, _P_4 < 0.05). These results indicate that the differences in the
relation between accuracy and RT in the previous data set are not due to changes in decision criteria across sessions. As expected from the failure of standard model to fit the previous
data, the standard DDM model could not explain these data either (Supplementary Fig. 6). DDM WITH STIMULUS-DEPENDENT BAYESIAN LEARNING FITS PERFORMANCE ACROSS BOTH TASKS Until now, we have
been considering a standard DDM that assumes all behavioral uncertainty comes from rapid variability in incoming sensory evidence. However, it is well known that subjects’ choices are
sensitive to the recent history of rewards28,43,44,45, and that reward expectation can influence performance and RTs14,46,47. One possible explanation for the overestimate of accuracy in the
categorization task is therefore that choices and trial outcomes produce on-going fluctuations in the animals’ mapping from odors to choices through a process resembling reinforcement
learning. Such fluctuations would produce uncertainty in classification of stimuli near the category boundary that could not be rescued by integration during a trial14,25. To develop this
idea, we asked how optimal subjects ought to use trial history (stimuli, choices, and rewards) to update their “belief” about the category boundary under the assumption that it is volatile
(i.e. that the true mapping from stimuli to correct choices varies stochastically across trials). Although the full Bayesian optimal strategy is intractable, we were able derive a
near-optimal strategy that yields behavioral performance indistinguishable from optimal32 (Methods). This resulted in a DDM with stimulus-dependent Bayesian learning, which we refer to as
“Bayes-DDM” (Fig. 5a). The Bayes-DDM is the same as the standard DDM but augmented with weights that transform the stimulus input into evidence: $$e_i = w_is_i(t),$$ (2) which is then
combined with bias _b_ to form a net evidence $$e(t) = w_1s_1(t) + w_2s_2(t) + b.$$ (3) In this equation, the weights _w__i_ and the bias _b_ define, respectively, the slope and offset of
the category boundary. After each trial, we updated the stimulus weights _w__i_ using a tractable approximation to the Bayes-optimal learning rule, $$\Delta {\boldsymbol{w}} = \alpha
_{\mathrm{w}}({\boldsymbol{s}},t){\boldsymbol{\Sigma }}_{\mathrm{w}}{\boldsymbol{s}},$$ (4) where \({\boldsymbol{\Sigma }}_{\mathrm{w}}\) is the weight covariance matrix (also learned;
Methods), that quantifies the current weight uncertainty, and \(\alpha _{\mathrm{w}}({\boldsymbol{s}},t)\) the learning rate. This learning rule introduces three new parameters that describe
the learner’s assumptions about how the weights change and influence the learning rate _α_ (Methods). We fitted the Bayes-DDM to the data by maximizing the log-likelihood of both olfactory
decision tasks simultaneously2. In the absence of a closed-form analytical solution19, we generated mean RTs, choices and trial-to-trial choice biases by numerically simulating a sequence of
100,000 trials for each combination of tested parameters (Methods). In contrast to the standard DDM, the Bayes-DDM produced a very good simultaneous fit of the both tasks (Fig. 5b, c). As a
further test, we also assessed whether the model could fit the behavioral results for the merged (interleaved) task (Fig. 4). To do so, we fitted the model to the 32 stimuli from the
interleaved condition. We found that the model indeed provided a good qualitative match to this data set as well (Fig. 5d). Therefore, by making the additional assumption that subjects
assume a volatile category boundary and make trial by trial adjustments accordingly, we were able to arrive at a model that captured our entire data set. BAYES-DDM SUCCESSFULLY PREDICTS
TRIAL-BY-TRIAL CONDITIONAL CHANGES IN CHOICE BIAS The Bayes-DDM model can be considered as a hypothesis concerning the form of trial-to-trial biases that we expect to be sufficient to
explain the data. Crucially, the specific predictions of this model can be tested against behavioral variables that were not directly fit. That is, we can check whether the form of the
trial-to-trial biases in the experimental data is in fact compatible with the form and magnitude of the learning we introduced. We observed quite large effects of trial history. Figure 6
shows the average psychometric choice functions (Fig. 6a, b, dashed lines) and psychometric choice functions conditioned on the previous odor stimulus (Fig. 6a, b, solid lines, with
different stimulus difficulties separated by quadrants, as indicated). Note that only cases in which the previous trial was rewarded are included. To quantify the impact of a previous trial,
we calculated the difference in the average choice bias conditional upon the trial being correct and a given stimulus being delivered relative to the overall average choice bias
(Δ_C_B(_x_); Methods). Note that Δ_C_B(_x_) is a measure of the amount of learning induced by a past trial, as measured the fractional change in choice probability, with Δ_C_B(_x_) > 0
indicating a greater likelihood of repeating a choice in the same direction as the prior trial, Δ_C_B(_x_) < 0 a choice in the opposite direction. Because Δ_C_B(_x_) was symmetric for
left/right stimuli, we plot Δ_C_B(_x_) collapsed over stimuli of equal difficulty (Fig. 6c, d; uncollapsed data plotted in Supplementary Fig. 7; individual rats shown in Supplementary Fig.
8). These analyses showed that rats have a tendency to repeat a choice in the same direction that was rewarded in the previous trial (“win-stay”), but the stimulus-dependent analysis
revealed a qualitative difference between the two tasks with respect to how past stimuli impacted choice bias. For the identification task, the influence of the previous trial was largely
stimulus-independent (Fig. 6c, one-way ANOVA, _F_(3,12) = 2.0, _P_ = 0.17). For the categorization task (Fig. 6d), in contrast, that influence showed a graded dependence on the stimulus,
being larger for a difficult previous choice than for an easier one (_F_(3,12) = 25.4, _P_ < 10−5). We also conducted this analysis for incorrect trials but, due to the small numbers of
trials, the data were too variable to draw any firm conclusions (Supplementary Figs. 9, 10). Remarkably, for both tasks the predictions of Bayes-DDM closely matched the data. For the
categorization task, as expected, the model captured the strong dependence of Δ_C_B(_x_) on stimulus difficulty (Fig. 6d). For the identification task, the model was able to capture the
relative lack of stimulus dependence of Δ_C_B(_x_) (Fig. 6c). These results can be understood by considering that the Bayes-DDM depends on both the accumulated inputs _S_ and decision time
_t_, reflecting a form of decision confidence32 (Methods). In tasks like ours, with a varying difficulty, harder trials are associated with later choices and come with a lower decision
confidence48. On correct easy trials, learning is smaller when the animal’s confidence is high. This makes sense: if the animal is correct and highly confident, there is little reason to
adjust the weights. The relative lack of stimulus dependence of Δ_C_B(_x_) in the identification task is explained by a low signal-to-noise ratio for difficult trials, implying that the
sensory component of Eq. 4 will be low. Thus, there is a larger contribution of the stimulus-independent term (the bias—_b_) in updating choice bias (Methods). Although the dependence of
decision confidence on decision time suggests the possibility of a dependence of Δ_C_B(_x_) on RT, we found that bias learning washes out the confidence–RT relationship, such that neither
data nor the Bayes-DDM model feature a strong modulation of learning by RT (Supplementary Fig. 11). COMPARISON WITH OTHER LEARNING RULES The optimal Bayes-DDM learning rule takes a complex
form involving multiple terms whose respective roles are not immediately clear. In order to gain some insight into why this rule captures the animals’ behavior, and whether confidence has a
role, we fitted several variations of our model. We first fitted a model without learning but in which the weights are drawn on every trial from a multivariate Gaussian distribution whose
mean is set to the optimal weights (\(1/\sqrt 2\), \(- 1/\sqrt 2\) and 0) and whose variance is a free parameter. Interestingly, this model could fit the psychometric and chronometric curves
in both tasks. However, the model failed to show sequential effects since the weights are redrawn independently on every trial (Supplementary Fig. 12). Model comparison confirmed that this
model performs considerably worse than Bayes-DDM (Fig. 7). DDMs weight the difference between the two evidences. To control for a possible alternative integration process, we implemented two
different versions of an LCA model49 (Methods) in which absolute evidences for each side are integrated over time, and the two integrators inhibit each other. These models did a good job of
explaining both psych- and chronometric curves. but failed to replicate the changes in choice bias seen in the data (Supplementary Figs. 13, 14). This also suggests that a learning process
must be at play. Next, we tried a model with a limited form of learning in which the optimal learning rule is applied only to the bias while the sensory weights are set to their optimal
values. It has been argued that sequential effects can be captured by variations in the bias28. This model had a BIC score comparable to the optimal model (Fig. 7) and captured the flat
profile of the identification task, thus suggesting that sequential effects in this task are due to bias fluctuations. However, this model failed to account for the profile of sequential
effects in the categorization task (Supplementary Fig. 15). Although the data show sequential effects inversely proportional to the difficulty of the previous trial, this model predicted a
flat profile (Supplementary Fig. 15g). Conversely, we fitted a model in which the sensory weights, but not the bias, are adjusted on every trial according to the optimal learning rule. This
model fit the psychometric and chronometric curves reasonably well (Supplementary Fig. 16). However, in contrast to the previous model, this one captured the sequential effects in the
categorization task but not in the identification task (Supplementary Fig. 16d). Moreover, the BIC score for this model was far worse than Bayes-DDM. In addition, we fitted a model that
adjusts only the bias on a trial by trial basis, but with randomly fluctuating sensory weights (Supplementary Fig. 17). This model retained the sequential effects seen in the identification
task, but failed to produce those of the categorization task (Supplementary Fig. 17g). Taken together, these modeling results suggest that the learning-induced bias fluctuations support the
sequential effects in identification, whereas the learning-induced weight fluctuations support the sequential effects in categorization. As a final model, we explored a simpler, heuristic
implementation of the Bayes-DDM rule using a delta rule that is modulated by decision confidence. For this purpose, we used a standard DDM with learning rules of the form: $$\Delta
{\boldsymbol{w}} = \alpha \left( {\lambda - \frac{{\theta _{t = T}}}{{\theta _{t = 0}}}} \right){\boldsymbol{s}}$$ (5) $$\Delta b = \alpha _{\mathrm{b}}\left( {\lambda - \frac{b}{{\theta _{t
= 0}}}} \right)$$ (6) where _θ__t_=0 is the value of bound at the beginning of the trial, whereas \(\theta _{t = T}\) is the value of the bound at the time of the decision, _λ_ is the
correct choice (1 or −1), and _α_ and _α_b are the weight and bias learning rates. The modulation of learning by confidence is due to the term \(\frac{{\theta _{t = T}}}{{\theta _{t =
0}}}\). The collapsing bound causes this ratio to decrease with elapsed time. Critically, elapsed time is inversely proportional to confidence in DDMs when the difficulty of the task is
unknown and varies from trial to trial35,48. Therefore, for incorrect trials, the error term in this learning rule is decreasing as confidence decreases over time, which is to say the model
learns less when it is less confident. Interestingly, for correct trials, the relationship is inverted, as the model learns more strongly when less confident, which makes intuitive sense: in
confident correct trials there is no more information to be gained. Ultimately, this rule is only an approximation to the optimal rule. Nonetheless, Bayesian model comparison revealed that
this learning rule accounts for our experimental data nearly as well as the full optimal learning rule, thus indicating that the rat’s behavior is consistent with a confidence weighted
learning rule (Fig. 7, Supplementary Fig. 18, RL-DDM). The results of Bayesian model comparisons are often sensitive to the way extra parameters are penalized. We found this not to be the
case in our data, as the ranking of the models remained the same whether we use AIC, AICc or BIC. Moreover, our conclusions held whether we fitted individual animals separately, or as if
obtained from a single ‘meta-rat’ (Figs. 6, 7 and Supplementary Fig. 4). FLUCTUATIONS IN CATEGORY BOUNDARY DEGRADE ODOR CATEGORIZATION PERFORMANCE MORE THAN IDENTIFICATION Finally, we sought
to gain insight into how category boundary learning works in conjunction with stimulus integration to explain the difference between identification and categorization performance. To do so,
we calculated “inferred” drift rates (_μ_) for that trial by taking the actual accumulated evidence (before weight multiplication) and divided by integration time. This allowed us to
visualize the combined effects of stochastic noise and boundary (weight) fluctuations (Methods) (Fig. 8). Here, for all panels we plot the evidence for the two options (_μ_1, _μ_2) against
one another, so that the ideal category boundary is the diagonal (black line). In Fig. 8a, b, we show all the stimuli for the standard DDM, whereas Fig. 8c–f focus on only one of the hardest
stimuli. Figure 8c, d shows the standard, non-learning DDM fit to the identification task and tested on both. Where accuracy should be similar for the two tasks, it can be seen that this
model generates too few errors for the categorization task (compare the low fraction of red dots (error) to blue dots (correct) in Fig. 8d vs. c). In Fig. 8e, f, we re-ran the same trials,
using “frozen noise”, but simulating a fluctuating bound comparable to the Bayes-DDM with optimal learning (Methods). Here the meaning of the dot colors is different: trials that did not
change classification are gray, trials that became incorrect are red, and trials that became correct are blue. It can be seen that weight fluctuations changed the classification of very few
trials in the identification task (Fig. 8e) but changed a substantial fraction in the categorization task (Fig. 8f), the majority of which became errors (red). The difference in effects on
the two tasks can be understood by considering that stimulus weights have a multiplicative effect on evidence strength. Thus, stimulus weight fluctuations correspond to rotations around the
origin and are larger for larger stimulus values. Therefore, high concentration mixtures, which are far from the origin, are much more susceptible to these fluctuations than low
concentration stimuli (Fig. 8e). In contrast, variability in the bias, _b_, affect the intercept of the bound, giving rise to additive effects that are similar no matter the magnitude of the
evidence and therefore affect the two tasks in a similar way (Supplementary Fig. 19). DISCUSSION Our results demonstrate that rats show different speed-accuracy tradeoffs (SAT) depending on
the task at hand. When challenged to identify odors at low concentrations, rats show a significant increase of reaction time (RT) that is accompanied by performance degradation (Fig. 2c,
d). In contrast, when the challenge is to categorize mixtures of two odors in different proportions, rats show only a small increase in RT (Fig. 2e, f). We used a standard drift-diffusion
model (DDM) to show that this difference cannot be explained by stimulus noise (Fig. 3) even with the addition of reward-dependent choice biases (Supplementary Fig. 15). We therefore
introduced a Bayesian learning process, the kind theorized to drive stimulus-response learning optimally in dynamic environments32. With the combination of these three factors—stimulus
noise, reward bias and categorical boundary learning—the resulting “Bayes-DDM” not only fit the average performance data (Fig. 6b–e), but also predicted the choice biases on the recent
history of stimuli, choices and rewards (Fig. 6f, g). Furthermore, Bayes-DDM was able to fit the performance over an interpolated stimulus space combining both tasks (Fig. 6d), ruling out
differences in strategies between the two tasks and arguing that rats used the same decision-making system while identifying and categorizing odors. We found that odor categorization
performance is more susceptible to category boundary fluctuations than identification (Fig. 8) which in turn implies that the categorization task benefits less from longer temporal
integration. Indeed, additional Bayes- and RL-DDM simulations showed that performance remains almost unaltered in mixture categorization with an increase of integration threshold,
contrasting with what would be predicted for odor identification (Supplementary Fig. 20). This agrees with the observation that one sniff (the minimal unit of olfactory sampling time for
animals such as a rat) is enough for maximum performance in mixture categorization15. Weight fluctuations, which impair performance in a trial-by-trial basis, cannot be filtered out within
the integration process. On the other hand, the identification task is mostly affected by stimulus noise, which is reflected within the diffusion process, and thus benefits much more from
integration. We thus conclude that the observation of different SATs is due to different computational requirements in the two tasks. Previous studies have explored dynamic stimulus learning
using signal detection theory52–58], but none has attempted to accounting for the impact of learning on evidence accumulation and RTs. Frank and colleagues combined reinforcement learning
with DDMs in the RL-DDM model50,51,52. However, in this model the integration and learning processes are completely separable and did not interact. Our results show that learning can
interact with evidence accumulation and that this can be detrimental for psychophysical performance. We suggest that this takes place because animals adopt a strategy that is optimized under
the assumption of a dynamic environment, whereas the actual environment is static. The continual, performance-hindering, learning we observed is striking considering that our task itself
doesn’t change over months of testing. This was not due to incomplete learning, as performance was stable over the analyzed data (Supplementary Figs. 21, 22). The psychophysics-like
experimental paradigm is indeed highly artificial in the sense that outcomes and states are crystallized. It is unlikely that this would be the case in a more naturalistic environment,
where, due to environmental dynamics, odors could signal different outcomes, rewards and states over time. A normal, ever-changing environment would imply adaptability and never-ending
learning as the optimal strategy. This strategy becomes suboptimal in a static environment, but this may be a small price to pay compare to the cost of stopping learning erroneously when the
world is actually dynamic. These results are consistent with a recent proposal that suboptimal inference, as opposed to internal noise, is a major source of behavioral variability33. In
this case, the apparent suboptimal inference is the result of assuming that the world is dynamic when, in fact, it is static. METHODS ANIMAL SUBJECTS Four Long Evans rats (200–250 g at the
start of training) were trained and tested in accordance with European Union Directive 2010/63/EU. All procedures were reviewed and approved by the animal welfare committee of the
Champalimaud Centre for the Unknown and approved by the Portuguese Veterinary General Board (Direcção Geral de Veterinária, approval 0421/000/000/2019). Rats were pair-housed and maintained
on a normal 12 h light/dark cycle and tested during the daylight period. Rats were allowed free access to food but were water-restricted. Water was available during the behavioral session
and for 20 min after the session at a random time as well as on non-training days. Water availability was adjusted to ensure animals maintained no <85% of ad libitum weight at any time.
TESTING APPARATUS AND ODOR STIMULI The behavioral apparatus for the task was designed by Z.F.M. in collaboration with M. Recchia (Island Motion Corporation, Tappan, NY). The behavioral
control system (BControl) was developed by Z.F.M., C. Brody (Princeton University) in collaboration with A. Zador (Cold Spring Harbor Laboratory). The behavioral setup consisted of a box (27
× 36 cm) with a panel containing three conical ports (2.5 cm diameter, 1 cm depth). Each port was equipped with an infrared photodiode/phototransistor pair that registered a digital signal
when the rat’s snout was introduced into the port (“nose poke”), allowing us to determine the position of the animal during the task with high temporal precision. Odors were delivered from
the center port and water from the left and right ports. Odor delivery was controlled by a custom made olfactometer designed by Z.F.M. in collaboration with M. Recchia (Island Motion
Corporation, Tappan, NY). During training and testing the rats alternated between two different boxes. The test odors were _S_-(+) and _R_-(−) stereoisomers of 2-octanol, chosen because they
have identical vapor pressures and similar intensities. In the odor identification task, difficulty was manipulated by using different concentrations of pure odors, ranging from 10−4 to
10−1 (v/v). The different concentrations were produced by serial liquid dilution using an odorless carrier, propylene glycol (1,2-propanediol). In the odor mixture categorization task, we
used binary mixtures of these two odorants at different ratios, with the sum held constant: 0/100, 20/80, 32/68, 44/56 and their complements (100/0, etc.). Difficulty was determined by the
distance of the mixtures to the category boundary (50/50), denoted as “mixture contrast” (e.g., 80/20 and 20/80 stimuli correspond to 60% mixture contrast). Choices were rewarded at the left
choice port for odorant A (identification task) or for mixtures A/B > 50/50 (categorization task) and at the right choice port for odorant B (identification task) or for mixtures A/B
< 50/50 (categorization task). In both tasks, the set of eight stimuli were randomly interleaved within the session. During testing, the probability of each stimulus being selected was
the same. For the experiment in Figs. 2, 3, 5 and 6, only mixtures with a total odor concentration of 10−1 were used. For the experiment in Fig. 4, we used the same mixture contrasts with
total concentrations ranging from 10−1 to 10−4 prepared using the diluted odorants used for the identification task. In each session, four different mixture pairs were pseudo-randomly
selected from the total set of 32 stimuli (8 contrasts at 4 different total concentrations). Thus, for this task, a full data set comprised 4 individual sessions. For all the different
experiments, four of the eight stimuli presented in each session were rewarded on the left (odorant A, for identification; A/B > 50/50, for categorization) and the other four were
rewarded on the right (odorant B, for identification; A/B < 50/50, for categorization). Each stimulus was presented with equal probability and corresponded to a different filter in the
manifold. For the experiments in Supplementary Fig. 3, we used two different sets of mixture ratios: 0/100, 17/83, 33.5/66.5, 50/50 in one experiment and 0/100, 39/61, 47.5/52.5, 49.5/50.5
in the second experiment. In the experiment using 50/50 mixture ratios, we used two filters both with the mixture 50/50, one corresponding to the left-rewarded stimulus and the other one to
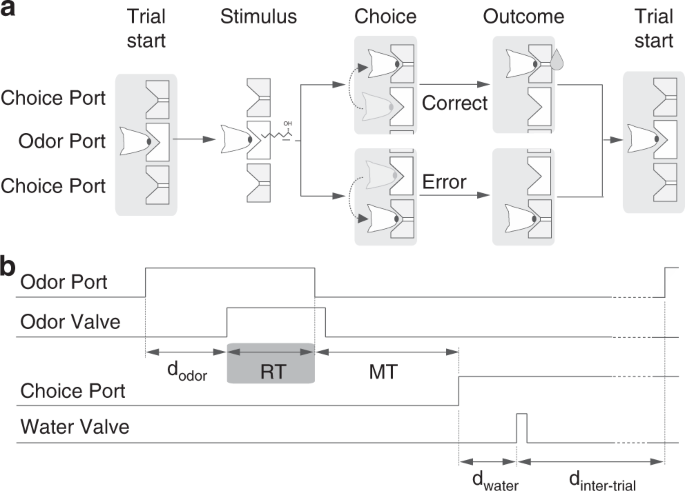
the right-rewarded stimulus. Thus, for the 50/50 mixtures, rats were rewarded randomly, with equal probability for both sides. REACTION TIME PARADIGM The timing of task events is illustrated
in Fig. 1. Rats initiated a trial by entering the central odor-sampling port, which triggered the delivery of an odor with delay (_d_odor) drawn from a uniform distribution with a range of
[0.3, 0.6] s. The odor was available for up to 1 s after odor onset. Rats could exit from the odor port at any time after odor valve opening and make a movement to either of the two reward
ports. Trials in which the rat left the odor sampling port before odor valve opening (~4% of trials) or before a minimum odor sampling time of 100 ms had elapsed (~1% of trials) were
considered invalid. Odor delivery was terminated as soon as the rat exited the odor port. Reaction time (the odor sampling duration) was calculated as the difference between odor valve
actuation until odor port exit (Fig. 1) minus the delay from valve opening to odor reaching the nose. This delay was measured with a photo ionization detector (mini-PID, Aurora Scientific,
Inc) and had a value of 53 ms. Reward was available for correct choices for up to 4 s after the rat left the odor sampling port. Trials in which the rat failed to respond to one of the two
choice ports within the reward availability period (~1% of trials) were also considered invalid. For correct trials, water was delivered from gravity-fed reservoirs regulated by solenoid
valves after the rat entered the choice port, with a delay (_d_water) drawn from a uniform distribution with a range of [0.1, 0.3] s. Reward was available for correct choices for up to 4 s
after the rat left the odor sampling port. Trials in which the rat failed to respond to one of the two choice ports within the reward availability period (0.5% of trials) were also
considered invalid. Reward amount (_w_rew), determined by valve opening duration, was set to 0.024 ml and calibrated regularly. A new trial was initiated when the rat entered odor port, as
long as a minimum interval (_d_inter-trial), of 4 s from water delivery, had elapsed. Error choices resulted in water omission and a “time-out” penalty of 4 s added to _d_inter-trial.
Behavioral accuracy was defined as the number of correct choices over the total number of correct and incorrect choices. Invalid trials (in total 5.8 ± 0.8% of trials, mean ± SEM, _n_ = 4
rats) were not included in the calculation of performance accuracy or reaction times (odor sampling duration or movement time). TRAINING AND TESTING Rats were trained and tested on three
different tasks: (1) a two-alternative choice odor identification task; (2) a two-alternative choice odor mixture categorization task15; and (3) a two-alternative choice “odor mixture
identification” task. The same rats performed all three tasks and all other task variables were held constant. The training sequence consisted of: (I) handling (2 sessions); (II) water port
training (1 session); (III) odor port training, in which a nose poke at the odor sampling port was required before water was available at the choice port. The required center poke duration
was increased from 0 to 300 ms (4–8 sessions); (IV) introduction of test odors at a concentration of 10−1, rewarded at left and right choice ports according to the identity of the odor
presented (1–5 sessions); (V) introduction of increasingly lower concentrations (more difficult stimuli) (5–10 sessions); (VI) training on odor identification task (10–20 sessions); (VII)
testing on odor identification task (14–16 sessions); (VIII) training on mixture categorization task (10–20 sessions); (IX) testing on mixture categorization task (14–15 sessions); (X)
testing on mixture identification task (12–27 sessions) (Supplementary Fig. 1). During training, in phases V and VI, we used adaptive algorithms to adjust the difficulty and to minimize bias
of the animals. We computed an online estimate of bias: $$b_t = (1 - \tau )C_t + \tau b_{t - 1}$$ (7) where _b__t_ is the estimated bias in the current trial, _b__t−1_ is the estimated bias
in the previous trial, _C__t_ is the choice of the current trial (0 if right, 1 if left) and _τ_ is the decay rate (_τ_ = 0.05 in our experiments). The probability of being presented with a
right-side rewarded odor _p_ was adjusted to counteract the measured bias using: $$p_{\mathrm{R}} = 1 - \frac{1}{{1 + e^{{\textstyle{{\left( {b_t - b_0} \right)} \over \gamma }}}}}$$ (8)
where _b_0 is the target bias (set to 0.5), and _γ_ (set to 0.25) describes the degree of non-linearity. Analogously, the probability of a given stimulus difficulty was dependent on the
performance of the animal, i.e., the relative probability of difficult stimuli was set to increase with performance. Performance was calculated in an analogous way as (1) at the current
trial but _c__t_ became _r__t_—the outcome of the current trial (0 if error, 1 if correct). A difficulty parameter, δ, was adjusted as a function of the performance, $$\delta _{t + 1} = - 1
+ \frac{2}{{1 + e^{{\textstyle{{\left( {p_t - p_0} \right)} \over \gamma }}}}}$$ (9) where _p_0 is the target performance (set to 0.95). The probability of each stimulus difficulty,
\(\varphi\), was drawn from a geometric cumulative distribution function (GEOCDF, Matlab) $$\varphi _{t + 1} = \frac{{1 - {\mathrm{GEOCDF}}\left( {i,|\delta _{t + 1}|} \right)}}{{\mathop
{\sum}\nolimits_{j = 1}^N {1 - {\mathrm{GEOCDF}}\left( {j,|\delta _{t + 1}|} \right)} }}$$ (10) where _N_ is the number of stimulus difficulties in the session, and takes a value from 2 to 4
(when _N_ = 1, i.e. only one stimulus difficulty, this algorithm is not needed); _i_ corresponds to the stimulus difficulty and is an integer from 1 to 4 (when _δ_ > 0, the value 1
corresponds to the easiest stimuli and 4 to the most difficult one, and vice-versa when _δ_ < 0). In this way, when |_δ_| is close to 0, corresponding to an average performance close to
0.95, the distribution of stimuli was close to uniform (i.e. all difficulties are equally likely to be presented). When performance is greater, then the relative probability of difficult
trials increased; conversely, when the performance is lower, the relative probability of difficult trials decreased. Training phases VI and VIII were interrupted for both tasks when number
of stimulus difficulties _N_ = 4 and difficulty parameter δ stabilized on a session-by-session basis. Each rat performed one session of 90–120 min per day (250–400 trials), 5 days per week
for a period of ~120 weeks. During testing, the adapting algorithms were turned off and each task was tested independently. The data set was collected only after performance was stable
(Supplementary Fig. 21) during periods in which the animals showed stable accuracy and left/right bias on both tasks (Supplementary Figs. 21, 22). Throughout the test period, there was
variability in accuracy and bias across sessions, but there was no correlation between these performance metrics and session number (accuracy: Spearman’s rank correlation _ρ_ = −0.066, _P_ =
0.61 for identification, _ρ_ = 0.16, _P_ = 0.24 for categorization; bias: _ρ_ = 0.104, _P_ = 0.27 for both tasks, identification: _ρ_ = 0.093, _P_ = 0.48, categorization: _ρ_ = 0.123, _P_ =
0.37). SESSION BIAS AND CHOICE BIAS The psychometric curves are obtained by fitting the function, _ψ_(_x_): $$\psi (x) = l^L + (1 - l^L - l^R)\Phi (x|\mu ,\sigma )$$ (11) where _x_ is the
stimulus, _Φ_(_x_|_μ_,_σ_) is the cumulative function of a Gaussian with mean and variance, _μ_ and _σ_, and _l__L_ and _l__R_ are the right and left lapse rates. The parameters are fitted
by minimizing the square distance between _ψ_(_x_) and the empirical fraction of right-ward choices for each stimulus value _x_ through fminsearch (Matlab)28,37: To quantify how a reward and
its interaction with stimulus difficulty impacts choice bias, we also fitted psychometric curves for the current trial _T_, conditioned on the response and difficulty of the previous
trials, _T_−1: $$\psi \left( {x_T{\mathrm{|}}C_{T - 1} = R,d_{T - 1}} \right) = l_T^L + (1 - l_T^L - l_T^R)\Phi \left( {x_T{\mathrm{|}}\mu _T,\sigma _T;C_{T - 1} = R,d_{T - 1}} \right)$$
where _C_(_T_−1) = _R_ means that the animal made a correct, right-ward choice on the previous trial. The difficulty variable _d_(_T_−1) indicates that the stimulus in the previous trial
took the value _x_(_T_−1) or \(x_{T - 1}^\dagger \) corresponding to difficulty level _d_(_T_−1). For instance, in the identification task, the conditions in which [A]=10−1 or [B] = 10−1
corresponds to the same difficult level. Difficulty corresponds to the x-axis on Fig. 6c–d and Supplementary Figs. 7 and 8). We then quantified the change in choice bias as a function of the
difficulty on the previous trial as follow: $$\Delta C_b^c\left( {d_{T - 1}} \right) = \frac{1}{2}\left( {\psi \left( {x_T = I{\mathrm{|}}C_{T - 1} = R,d_{T - 1}} \right) - \psi \left( {x_T
= I{\mathrm{|}}C_{T - 1} = L,d_{T - 1}} \right)} \right)$$ (12) where _I_ is the indifference point of the unconditioned psychometric curve in Eq. 11, that is, the stimulus value for which
the rat chooses left or right with equal probability, _ψ_(_x_ = _I_) = 0.5 (Fig. 6a–b, solid black line). For trials following an error, we first define the following psychometric curve:
$$\psi \left( {x_{T - 2}|F_{T - 1} = R,d_{T - 1}} \right) = l_{T - 2}^L + \left( {1 - l_{T - 2}^L - l_{T - 2}^R} \right) \\ \Phi \left( {x_{T - 2}|\mu _{T - 2},\sigma _{T - 2},F_{T - 1} =
R,d_{T - 1}} \right)$$ where _F_(_T_−1) = _R_ refers to an incorrect, right-ward response in the previous trial. This expression corresponds to the psychometric curve for two trials back but
conditioned on the response and difficulty one trial back. We then define the change in choice bias as: $$\Delta C_b^f\left( {d_{T - 1}} \right) = \frac{1}{2}\left( {\Delta C_b^f\left(
{F_{T - 1} = R,d_{T - 1}} \right) - \Delta C_b^f\left( {F_{T - 1} = L,d_{T - 1}} \right)} \right)$$ (13) where $$\Delta C_b^f\left( {F_{T - 1} = R,d_{T - 1}} \right) = \psi \left( {x_T =
I|F_{T - 1} = R,d_{T - 1}} \right) - \psi \left( {x_{T - 2} = I|F_{T - 1} = R,d_{T - 1}} \right)$$ $$\Delta C_b^f\left( {F_{T - 1} = L,d_{T - 1}} \right) = \psi \left( {x_T = I|F_{T - 1} =
L,d_{T - 1}} \right) - \psi \left( {x_{T - 2} = I|F_{T - 1} = L,d_{T - 1}} \right)$$ where I is the same indifference point as for choice biases after correct trials. We here conditioned on
two trials back to avoid biases introduced by long bouts of incorrect trials. For correct trials, our results are qualitatively similar, irrespective of whether we used Eq. 12 or Eq. 13. For
error trials, the use of Eq. (13) over Eq. (12) had a major impact on the bias estimates and revealed a win stay loose shift strategy for our rats. MODEL DRIFT-DIFFUSION MODEL FOR
DECISION-MAKING For a given stimulus with concentrations _c_A and _c_B, we define the accumulated evidence at time _t_, _e_(_t_). The diffusion process has the following properties: at time
_t_ = 0, the accumulated combined evidence is zero, _e_(0) = 0; and the momentary evidence _m__i_ is a random variable that is independent at each time step. We discretize time in steps of
0.1 ms and run numerical simulations of multiple runs/trials. For each new time step _t_ = _n_Δ_t_, we generate a new momentary evidence draw: $$m_i(t) = m_i(n\Delta t) = N\left( {kc_i^\beta
,\sigma } \right)$$ (14) that is, through a normally distributed random generator with a mean of \(kc_i^\beta\), in which we define _k_ as the sensitive parameter, and _β_ as the exponent
parameter. _σ_ defines the amount of noise in the generation of momentary evidences. We set _σ_ to 1, making \(kc_i^\beta\) equivalent to the signal to noise ratio for a particular stimuli
and respective combination of _c_oncentrations (_c_A_, c_B). Integrated evidences (_s_1, _s_2) are simply the integration of the momentary evidences over time $$s_i(t) = \int \nolimits_{\tau
= 0}^t {m_i(\tau )d\tau }$$ (15) We translate this in our discretized version as a cumulative sum at all time steps, effectively being: $$s_i(n\Delta t) = \mathop {\sum}\limits_{j = 0}^n
{m_i(j\Delta t)}$$ (16) We then define the decision variable accumulated evidence as: $$e(t) = w_1s_1(t) + w_2s_2(t) + b$$ (17) or in its discretized version: $$e(n\Delta t) = w_1s_1(n\Delta
t) + w_2s_2(n\Delta t) + b$$ (18) where \(w_1\) and \(w_2\) are model-dependent combination weights on the accumulated evidence, and \(b\) is an a priori decision bias (\(w_1 = 1/\sqrt 2
;w_2 = - 1/\sqrt 2 ;b = 0\) for optimal decisions; \(\sqrt 2\) scaling ensures \(||{\boldsymbol{w}}|| = 1\)). Together, these parameters define slope and offset of the category boundary,
which determines the mapping between accumulated evidence and associated choices. We also define the (accumulation) decision bound _θ_(_t_) and make it in most models collapsing over time
through either a linear or an exponential decay. Thus, at time step _n_Δ_t_ the bound is either $$\theta (t) = \theta (n\Delta t) = \theta _{t = 0} + \theta _{{\mathrm{slo}}}n\Delta t$$ (19)
where we define \(\theta _{t = 0}\) as the bound height at the starting point of integration _t_ = 0 and \(\theta _{{\mathrm{slo}}} \le 0\) as its slope, or $$\theta (t) = \theta (n\Delta
t) = \theta _{t = 0}e^{ - n\Delta t/\tau }$$ (20) where \(\tau \ge 0\) is the bound height’s mean lifetime. The collapse parameters \(\theta _{{\mathrm{slo}}}\) and _τ_ define the level of
urgency in a decision, the smaller it becomes, the more urgent a given decision will become, given rise to more errors35,42. For models with non-collapsing boundaries, we used \(\theta (t) =
\theta _{t = 0}\), independent of time. For models with collapsing boundaries, they collapsed linearly, except for RL-DDM, where they collapse exponentially. Decisions are triggered once
the accumulated evidence, _e_(_t_), crosses one of the two decision boundaries \(\left\{ {\theta (t), - \theta (t)} \right\}\). To simulate these decisions, we first simulated a
one-dimensional diffusion model that directly uses _e_(_t_) as the diffusing “particle”, and from this reconstructed the higher-dimensional accumulated momentary evidences
\({\boldsymbol{s}}(t) = \left( {s_1(t),s_2(t)} \right)^T\). For the one-dimensional simulation, we used a momentary Gaussian evidence with drift \(w_1kc_1^\beta + w_2kc_2^\beta\) and
diffusion variance \(w_1^2 + w_2^2\) (both per unit time step), corresponding to the moments of _e_(_t_) − _b_. We reintroduce the bias _b_ by shifting the boundaries to \(\left\{ {\theta
(t) - b, - \theta (t) - b} \right\}\). For non-collapsing boundaries, we simulated accumulation boundary crossings using a recently developed, fast, and unbiased method53. For collapsing
boundaries, we simulated these boundary crossing by Euler integration in \(\Delta t = 0.001s\) time steps, and set the final _e_(_t_) to lie on the crossed boundary to avoid overshooting
that might arise due to time discretization. In both cases, we defined the decision time _t_d as the time when crossing occurred, and the choice in trial _k_ by $$C_k = {\mathrm{choice}} =
\left\{ {{\mathrm{left}},\,e(t_{\mathrm{d}}) \, > \, 0\,{\mathrm{right}},\,e(t_{\mathrm{d}}) \, < \, 0} \right\}$$ (21) To recover the higher-dimensional accumulated momentary
evidences at decision time, _S_(_t__d_), we sampled those from the two-dimensional Gaussian \({\boldsymbol{s}}(t_{\mathrm{d}})|e(t_{\mathrm{d}}),t_{\mathrm{d}} \sim N\left( {(c_1^\beta
,c_2^\beta )^T\,k\,t,{\boldsymbol{I}}\,t} \right)\) (i.e., unbounded diffusion), subject to the linear decision boundary constraint \({\boldsymbol{w}}^T{\boldsymbol{s}}\left(
{t_{\mathrm{d}}} \right){\vec{\boldsymbol{s}}} + b = e(t_{\mathrm{d}})\), using the method described in ref. 54. In order to capture <100% accuracy in easy trials and systematic and
consistent choice biases, we introduced an additional “lapse” component with lapse rate _l_r and bias b to the model. The lapse rate _l_r determined the probability with which the choice is
not determined by the diffusion model, but is instead drawn from a Bernoulli distribution that chooses “right” with probability _l_r and “left” with probability 1 − _l_r. Model fits revealed
small lapse rates close to 0.05 (Supplementary Tables 1 and 2). These lapse rates are typically needed for this type of models and have been hypothesized in the past to be due to effects of
attention and/or exploration55. Lastly, the reaction time for a particular trial was simulated by adding a normally distributed non-decision time variable with mean _t_ND and standard
deviation 0.1_t__d_ to the decision time arising from the diffusion model simulations2, $$t_{\mathrm{r}} = t_{\mathrm{d}} + t_{{\mathrm{ND}}} + \eta _{{\mathrm{ND}}},$$ (22) where \(\eta
_{{\mathrm{ND}}}|t_{\mathrm{d}} \sim N(0,(0.1\,t_{\mathrm{d}})^2)\) models the stochasticity of the non-decision time. Without weight and bias learning (that is, when fixing \(w_1 = 1/\sqrt
2 ;w_2 = - 1/\sqrt 2 ;b = 0\)), the base model with a non-collapsing has the following six parameters: sensitivity (_k_), exponent (_β_), non-decision time mean (_t_ND), initial bound height
(_θ__t=_0), lapse rate (_l_r), and bias (_b_). A collapsing bound introduces one additional parameter, which is the boundary slope (\(\theta _{{\mathrm{slo}}}\)) for linearly collapsing
boundaries, or the boundary mean lifetime (_τ_) for exponentially collapsing boundaries. DRIFT-DIFFUSION MODEL WITH BAYESIAN REWARD BIAS AND STIMULUS LEARNING—BAYES-DDM The following
provides an overview of the Bayesian model that learns stimulus combination weights, reward biases, or both. A complete description of the model and its derivation can be found in ref. 32.
We first focus on weight learning, and then describe how to apply the same principles to bias learning. The model assumes that there are true, latent combination weights \({\boldsymbol{w}}^
\ast\) that the decision-maker cannot directly observe, but aims to infer based on feedback on the correctness of his/her choices. To ensure continual learning, these latent weights are
assumed to slowly change across consecutive trials _k_ and _k_ + 1 according to a first-order autoregressive process, $${\boldsymbol{w}}^{ \ast (k + 1)}|{\boldsymbol{w}}^{ \ast (k)} \sim
N\left( {\gamma _{\mathrm{w}}{\boldsymbol{w}}^{ \ast (k)},\sigma _{\mathrm{w}}^2{\boldsymbol{I}}} \right),$$ (23) with weight “leak” \(0 \le \gamma _{\mathrm{w}} < 1\), ensuring that
weights remain bounded, and weight diffusion variance \(\sigma _{\mathrm{w}}^2\), ensuring a continual, stochastic weight change. This process has zero steady-state mean and a steady-state
variance of \(\sigma _{\mathrm{w}}^2/(1 - \gamma _{\mathrm{w}}^2)\) for each of the true weight components, which we used as the decision-maker’s prior \(p({\boldsymbol{w}})\) over the
inferred weight vector _W_. For each sequence of trials that we simulated, the decision-maker starts with this prior in the first trial and updates its belief about the weight vector in each
subsequent trial in two steps. We describe these two steps in light of making a choice in trial _k_, receiving feedback about this choice, updating one’s belief, and then moving on to the
next trial \(k + 1\). Before the first step in trial \(k\), the decision-maker holds the “prior” belief \(p\left(
{{\boldsymbol{w}}^{(k)}|{\mathrm{all}}\,{\mathrm{past}}\,{\mathrm{information}}} \right) = p\left( {{\boldsymbol{w}}^{(k)}} \right)\) that is implicitly conditional on all feedback received
in previous trials \(1, \cdots ,k - 1\). The decision-maker then observes some sensory evidence, accumulates this evidence, commits to choice \(C_k\) with decision time \(t_k\) and
accumulated momentary evidences \({\boldsymbol{s}}(t_{\mathrm{d}})\). After this, the correct choice \(C_k^ \ast \in \left\{ { - 1,1} \right\}\) (-1 for “left”, 1 for “right”) is revealed,
which, in our 2-AFC setup is the same as telling the decision-maker if choice \(C_k\) was correct or incorrect. The Bayes-optimal way to update one’s belief about the true weights upon
receiving this feedback is given by Bayes’ rule, $$p\left( {{\boldsymbol{w}}^{(k)}|C_k^ \ast ,{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right) \propto p\left( {C_k^ \ast
|{\boldsymbol{w}}^{(k)},{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right)p\left( {{\boldsymbol{w}}^{(k)}} \right).$$ (24) Unfortunately, the functional form of the likelihood
\(p\left( {C_k^ \ast |{\boldsymbol{w}}^{(k)},{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right)\) does not permit efficient sequential updating of this belief, but we have shown
elsewhere32 that we can approximate the above without considerable performance loss by assuming that the posterior (and, by induction, also the prior) is Gaussian. Using prior parameters
\(p\left( {{\boldsymbol{w}}^{(k)}} \right) = N( {{\boldsymbol{w}}^{(k)}\left| {\mu _{\mathrm{W}}^{(k)}} \right.,\Sigma _{\mathrm{w}}^{(k)}} )\) and posterior parameters \(p\left(
{{\boldsymbol{ w}}^{(k)}|C_k^ \ast ,{\boldsymbol{ s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right) = N( {{\boldsymbol{w}}^{(k)}| {{\mathbf{\upmu}} _{\mathrm{w}}^{ + (k)}} ,{\mathbf{\Sigma
}}^{\prime{(k)}}_{\mathbf{w}}})\) yields the update equations $${\mathbf{\upmu }}_{\mathrm{w}}^{+(k)} = {\mathbf{\upmu }}_{\mathrm{w}}^{(k)} + \alpha _{\mathrm{w}}\left(
{{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right)C_k^ \ast {\boldsymbol{\Sigma }}_{\mathrm{w}}^{(k)}{\boldsymbol{s}}(t_{\mathrm{d}})$$ (25) $${\boldsymbol{\Sigma
}}^{\prime{(k)}}_{\mathbf{w}} = {\boldsymbol{\Sigma }}_{\mathrm{w}}^{(k)} + \alpha _{{\mathrm{cov}}}\left( {s(t_{\mathrm{d}}),t_{\mathrm{d}}} \right)\,\left( {\left( {{\boldsymbol{\Sigma
}}_{\mathrm{w}}^{(k)^{ - 1}} + {\tilde{\boldsymbol{s}}}{\tilde{\boldsymbol{s}}}^T} \right)^{ - 1} - {\boldsymbol{\Sigma }}_{\mathrm{w}}^{(k)}} \right),$$ (26) with learning rates $$\alpha
_{\mathrm{w}}\left( {{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right) = \frac{{N\left( {g|0,1} \right)}}{{\Phi (g)\sqrt {1 + {\tilde{\boldsymbol{s}}}^T{\boldsymbol{\Sigma
}}_{\mathrm{w}}^{(k)}{\tilde{\boldsymbol{s}}}} }},$$ (27) $$\alpha _{{\mathrm{cov}}}\left( {{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right) = \alpha _{\mathrm{w}}\left(
{{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right)^2 + \alpha _{\mathrm{w}}\left( {{\boldsymbol{s}}(t_{\mathrm{d}}),t_{\mathrm{d}}} \right)\,g,$$ (28) $$g = \frac{{C_k^ \ast \mu
_{\mathrm{w}}^{(k)^T}{\tilde{\boldsymbol{s}}}}}{{\sqrt {1 + {\tilde{\boldsymbol{s}}}^T{\boldsymbol{\Sigma }}_{\mathrm{w}}^{(k)}{\tilde{\boldsymbol{s}}}} }},$$ (29) $${\tilde{\boldsymbol{s}}}
= \frac{{{\boldsymbol{s}}(t_d)}}{{\sqrt {t_{\mathrm{d}} + \sigma _{\mathrm{e}}^{ - 2}} }},$$ (30) where \(\Phi ( \cdot )\) is the cumulative function of a standard Gaussian, and where
\(\sigma _{\mathrm{e}}^2\) is a variance that describes the distribution of decision difficulties (e.g., odor intensities) across trials, and which we assume to be known by the
decision-maker. In the above, _g_ turns out to be a quantity that is closely related to the decision confidence in trial _k_. Furthermore, both learning rates, \(\alpha _{\mathrm{w}}\) and
\(\alpha _{{\mathrm{cov}}}\) are strongly modulated by this confidence, as follows: they are small for high-confidence correct decisions, moderate for low-confidence decisions irrespective
of correctness, and high for high-confidence incorrect choices. A detailed derivation, together with more exploration of how learning depends on confidence is provided in ref. 32. Once the
posterior parameters have been computed, the second step follows. This step takes into account that the true weights change across consecutive trials, and is Bayes-optimally captured by the
following parameter updates: $$\mu _{\mathrm{w}}^{(k + 1)} = \gamma _{\mathrm{w}}\mu _{\mathrm{w}}^{ + \,\,\,\,\,\,(k)},$$ (31) $$\mathbf{\Sigma}_{\mathrm{w}}^{(k + 1)} = \gamma
_{\mathrm{w}}^2 {\mathbf{\Sigma }}^{\prime{(k)}}_{\mathbf{w}} + \sigma _{\mathrm{w}}^2I.$$ (32) These parameters are then used in trial _k_ + 1. Overall, the Bayesian weight learning model
has two adjustable parameters (in addition to those of the base decision-making model): the assumed weight leak (\(\gamma _{\mathrm{w}}\)) and weight diffusion variance (\(\sigma
_{\mathrm{w}}^2\)) across consecutive trials. Let us now consider how similar principles apply to learning the bias term. For this, we again assume a true underlying bias _b_* that changes
slowly across consecutive trials according to $$b^{ \ast (k + 1)}|b^{ \ast (k)} \sim N\left( {\gamma _{\mathrm{w}}b^{ \ast (k)},\sigma _{\mathrm{b}}^2} \right),$$ (33) where the leak
\(\gamma _{\mathrm{w}}\) is the same as for _W_*, but the diffusion \(\sigma _{\mathrm{b}}^2\) differs. As we show in ref. 32, the bias can be interpreted as a per-trial a priori bias on the
correctness on either choice, which brings it into the realm of probabilistic inference. More specifically, this bias can be implemented by extending the, until now two-dimensional,
accumulated momentary evidences \({\boldsymbol{s}}(t_{\mathrm{d}})\) in each trial, by an additional, constant element. An analogous extension of _W_ adds the bias term to them, until now
two-dimensional, weight vector. Then, we can perform the same Bayesian updating of the, now three-dimensional, weight vector parameters as described weights, to learn weights and the bias
simultaneously. The only care we need to take is to ensure that, in the second step, the covariance matrix elements associated with the bias are updated with diffusion variance \(\sigma
_{\mathrm{b}}^2\) rather than \(\sigma _{\mathrm{w}}^2\). Overall, a Bayesian model that learns both weights and biases has three adjustable parameters: the assumed weight and bias leak
(\(\gamma _{\mathrm{w}}\)), the weight diffusion variance (\(\sigma _{\mathrm{w}}^2\)), and the bias diffusion variance (\(\sigma _{\mathrm{b}}^2\)). A Bayesian model that only learns the
bias has two adjustable parameters: the assumed bias leak (\(\gamma _{\mathrm{w}}\)), and the bias diffusion variance (\(\sigma _{\mathrm{b}}^2\)). DRIFT-DIFFUSION MODEL WITH HEURISTIC
REWARD BIAS AND STIMULUS LEARNING—RL-DDM Rather than using the Bayesian weight and bias update equations in their full complexity, we also designed a model that captures their spirit, but
not their details. This model does not update a whole distribution over possible weights and biases, but instead only works with point estimates, which take values \({\boldsymbol{w}}^{(k)}\)
and \(b^{(k)}\) in trial \(k\). After feedback \(C_k^ \ast \in \left\{ { - 1,1} \right\}\) (as before, −1 for “left”, 1 for “right”), the model updates the weight according to
$${\boldsymbol{w}}^{ + (k)} = {\boldsymbol{w}}^{(k)} + \alpha \left( {C_k^ \ast - \frac{{e(t_{\mathrm{d}})}}{{\theta _{t = 0}}}} \right){\boldsymbol{s}}(t_{\mathrm{d}}),$$ (34) where
\(\alpha\) is the learning rate. Note that, for rapid decisions (i.e., \(t_{\mathrm{d}} \approx 0\)), we have \(|e(t_{\mathrm{d}})| \approx \theta _{t = 0}\), such that the residual term in
brackets is zero for correct choices, such that learning only occurs for incorrect choices. For slower choices and collapsing boundaries, we will have \(|e(t_{\mathrm{d}})| < \theta _{t =
0}\), such that the residual will be non-zero even for correct choices, promoting weight updates for both correct and incorrect choices. Considering that decision confidence in the Bayesian
model is generally lower for slower choices, this learning rule again promotes learning rates weighted by confidence: fast, high-confidence choices result in no weight updates for correct
choices, and large weight updates for incorrect choices, whereas low, low-confidence choices promote moderate updates irrespective of the correctness of the choice, just as for the
Bayes-optimal updates. To ensure a constant weight magnitude, the weights are subsequently normalized by $${\boldsymbol{w}}^{(k + 1)} = \frac{{{\boldsymbol{w}}^{ + \left( k
\right)}}}{{\|{\boldsymbol{w}}^{ + (k)}}\|},$$ (35) to form the weights for trial \(k + 1\). Bias learning takes a similar flavor, using the update equation $$b^{(k + 1)} = b^{(k)} + \alpha
_{\mathrm{b}}\left( {C_k^ \ast - \frac{{b^{(k)}}}{{\theta _{t = 0}}}} \right),$$ (36) where \(\alpha _{\mathrm{b}}\) is the bias learning rate. In contrast to weight learning, this update
equation does not feature any confidence modulation, but was nonetheless sufficient to capture the qualitative features of the data. Overall, this learning model added two adjustable
parameters to the base decision-making model: the weight learning rate (_α_), and the bias learning rate (\(\alpha _{\mathrm{b}}\)). ALTERNATIVE LEARNING HEURISTICS To further investigate
whether a confidence-modulated learning rate was required, we designed models that did not feature such confidence weighting. For weight learning, they used the delta rule
$${\boldsymbol{w}}^{ + \left( k \right)} = {\boldsymbol{w}}^{(k)} + \alpha \left( {C_k^ \ast - C_k} \right)\frac{{\theta (t_{\mathrm{d}})}}{{\theta _{t =
0}}}{\boldsymbol{s}}(t_{\mathrm{d}}),$$ (37) where _α_ is the learning rate, and whose weight updated is, as before, followed by the normalization \({\boldsymbol{w}}^{(k + 1)} =
{\boldsymbol{w}}^{ + (k)}/\|{\boldsymbol{w}}^{ + (k)}\|\). Here, we assume the same encoding of make choice \(C_k\) and correct choice \(C_k^ \ast\), that is, \(C_k \in \left\{ { - 1,1}
\right\}\) (−1 for “left”, 1 for “right”), such that the residual in brackets is only non-zero if the choice was incorrect. In that case, the learning rate is modulated by boundary height,
but no learning occurs after correct choices. The bias is learned similarly, using $$b^{(k + 1)} = b^{(k)} + \alpha \left( {C_k^ \ast - C_k} \right)\frac{{\theta (t_{\mathrm{d}})}}{{\theta
_{t = 0}}}.$$ (38) Overall, this results in one adjustable parameter in addition to the base decision-making model: the learning rate (_α_). DRIFT-DIFFUSION MODEL WITH REWARD BIAS AND
STIMULUS WEIGHT FLUCTUATIONS To test whether random weight and bias fluctuations are sufficient to capture the across-task differences, we also fit a model that featured such fluctuations
without attempting to learn these weights from feedback. Specifically, we assumed that, in each trial, weights and biases where drawn from $${\boldsymbol{w}}^{(k)} \sim N\left( {(1, -
1)^T/\sqrt 2 ,\sigma _{{\mathrm{rw}}}^2{\boldsymbol{I}}} \right),\,b^{(k)} \sim N\left( {0,\sigma _{{\mathrm{rb}}}^2} \right),$$ (39) which are normal distributions centered on the optimal
weights and bias values, but with (co)variances \(\sigma _{{\mathrm{rw}}}^2I\) and \(\sigma _{{\mathrm{rb}}}^2\). We adjusted these (co)variances to best match the data, leading to two
adjustable parameters in addition to those of the base decision model: the weight fluctuation variance (\(\sigma _{{\mathrm{rw}}}^2\)) and the bias fluctuation variance (\(\sigma
_{{\mathrm{rb}}}^2\)). LEAKY, COMPETING ACCUMULATOR MODEL—LCA To test whether a non-learning two-component race model with mutual inhibition is able to fit both tasks with the same set of
parameters, we implemented a leaky, competing accumulator model49. In this model, two accumulators, \(s_1\) and \(s_2\) accumulate evidence according to $${\mathrm{d}}s_i = \left(
{kc_i^\beta - \tau ^{ - 1}s_i - w_{{\mathrm{inh}}}s_{3 - i}} \right){\mathrm{d}}t + {\mathrm{d}}W_i,$$ (40) where _τ_ is the leak time constant, \(w_{{\mathrm{inh}}}\) is the mutual
inhibition weight, and \(W_i\) is a Wiener process. The accumulators start at \(s_1\left( 0 \right) = s_2\left( 0 \right) = 0\), are lower-bounded by \(s_1\left( t \right) \ge 0\) and
\(s_2\left( t \right) \ge 0\), and accumulate evidence until the first of the two reaches the decision threshold \(\theta (t)\), triggering the corresponding choice. The model was simulated
by Euler integration in 0.1 ms time steps, and lapses and biases were implemented as for the other model. We fitted two variants, one with a time-invariant boundary, \(\theta \left( t
\right) = \theta _0\), with a total of eight parameters, and one with a time-variant boundary, \(\theta \left( t \right) = \theta _0 + \theta _{{\mathrm{slo}}}t\), with nine parameters.
MODEL FITTING We found the best-fitting parameters for each model by log-likelihood maximization2. Due to collapsing bounds and (for some models) sequential updates of weights and biases, we
could not directly use previous approaches that rely on closed-form analytical expressions19 for fitting diffusion models with non-collapsing boundaries. Instead, for any combination of
parameters, we simulated the model responses to a sequence of 100,000 trials with stimulus sequence statistics matching those of the rodent experiments for the conditions that we were
interested in fitting. Please see “Drift-diffusion model for decision-making” for details on how these simulations were performed. The simulated responses were used to compute summary
statistics describing model behavior, which were subsequently used to evaluate the log-likelihood of these parameters in the context of the animals’ observed behavior. We computed the
log-likelihood in two ways, first by ignoring sequential choice dependencies, and second by taking such dependencies into account. All model simulations were performed as described further
above. We did not explicitly simulate the stochasticity of the non-decision time, but instead included this stochasticity as an additional noise-term in the likelihood function (not
explicitly shown below). To describe how we computed the likelihood of model parameters \(\phi\) without taking sequential dependencies into account, let index \(m\) denote the different
task conditions (i.e., a set of odor concentrations for odors A and B), and let \(n_m\) be the numbers of observed trials for this condition in the rodent data that we are modeling. For each
condition \(m\), we approximate the response time distributions by Gaussians, using \(t_m\) and \(\sigma ^2_{{\mathrm{t}},m}\) to denote the mean response time and variance observed in the
animals’ behavior (across trials). Furthermore, let \(P_{c,m}\) be the observed probability of making a correct choice in that condition. The corresponding model predictions for parameters
\(\phi\), extracted from model simulations, are denoted \(\bar t_m(\phi )\) and \(\bar P_{{\mathrm{c}},m}(\phi )\). With this, we computed the likelihood of responses times by
$$L_{{\mathrm{t}},m}(\phi ) = N\left( {\bar t_m(\phi )|t_m,\frac{{\sigma ^2_{{\mathrm{t}},m}}}{{n_m}}} \right),$$ (41) which is the probability of drawing the predicted mean reaction time
from a Gaussian centered on the animals’ observed mean and with a variance that corresponds to the standard error of that mean. The likelihood of the choice probabilities was for each
condition computed by $$L_{{\mathrm{c}},m}(\phi ) = \bar P_{{\mathrm{c}},m}(\phi )^{P_{{\mathrm{c}},m}n_m}\left( {1 - \bar P_{{\mathrm{c}},m}(\phi )} \right)^{(1 - P_{{\mathrm{c}},m})n_m},$$
(42) which is the probability of drawing the animals’ observed number of correct and incorrect choices with the choice probabilities predicted by the model. The overall log-likelihood is
found by summing over the per-condition log-likelihoods, resulting in $$LL(\phi ) = \mathop {\sum}\limits_m {\left( {{\mathrm{log}}L_{{\mathrm{t}},m}(\phi ) +
{\mathrm{log}}L_{{\mathrm{c}},m}(\phi )} \right)} .$$ (43) To evaluate the log-likelihood that takes into account sequential choice dependencies, we computed the reaction time likelihoods,
\(L_{{\mathrm{t}},m}(\theta )\), as before, but changed the choice probability likelihood computation as follows. For trials following correct choices, we computed the choice probability
likelihood separately for each stimulus combination given the previous and the current trial, thus taking into account that psychometric curves depend on the stimulus condition of the
previous trial (Fig. 6a, b). Due to the low number of incorrect trials for certain conditions, we did not perform this conditioning on the previous trial’s condition when computing the
choice probability likelihoods after incorrect choices, but instead computed the likelihood across all trials simultaneously. For both ways of computing the log-likelihood, we found the
parameters that maximize this log-likelihood by use of the Subplex algorithm as implemented in the NLopt library (Steven G. Johnson, The NLopt nonlinear-optimization package,
http://ab-initio.mit.edu/nlopt). In some cases, we performed the fits without taking into account the sequential choice dependencies, and then predicted these sequential choice dependencies
from the model fits (e.g., Fig. 6c, d). In other cases (e.g., for some model comparisons), we performed the model fits while taking into account sequential dependencies. The specifics of the
model fits are clarified in the main text. The best model fits and respective parameters can be found in Supplementary Tables 1 and 2. MODEL COMPARISON For comparison between different
models with different number of parameters, we use Bayesian information criterion (BIC) for model selection56. For each model, we calculate the BIC57: $${\mathrm{BIC}} = -
2{\mathrm{ln}}\,(L) + q\Delta {\mathrm{ln}}(n)$$ (44) where _q_ is the number of free parameters fitted by the model and _n_ the number of trials that we fitted. Each model has a BIC
associated to it. We compared different models by first converting the BIC score into a log10-based marginal likelihood, using \(- 0.5{\mathrm{BIC}}/{\mathrm{ln}}(10)\), and then compared
models by computing the log10-Bayes factor as the difference between these marginal likelihoods. These differences dictate the explanatory strength of one model in relation to the other. The
model with the larger marginal likelihood is preferred and the evidence in favor is decisive if the log10 difference exceeds 2. To ensure that our analysis is not driven by the strong
parameter number penalty that BIC applies, we performed the same analysis using the Akaike information criterion (AIC) and its corrected version (AICc), but found qualitatively no change in
the results. All different model comparisons can be found in Supplementary Fig. 4. In Supplementary Fig. 4, we compared the following models. Models denoted simply “DDM” were diffusion
models with optimal weights, \(w_1 = 1/\sqrt 2 ;w_2 = - 1/\sqrt 2\). Models denoted “Bayes-DDM” learned their weights as described in the Bayes-DDM section. The “Random weights” models used
weights that were stochastically and independently drawn in each trial (see Stimulus weight fluctuations section). The “Delta rule” models learned their weights by the delta rule. The “Full
RL-DDM” model used the learning rules described in the RL-DDM section. Only “lapse” variants of these models included the lapse model components. Decision boundaries were constant except for
the “collapsing boundary” model variants. The bias was fixed to \(b = 0\), except for the “Full RL-DDM” model and “bias” variants. In these bias variants, the biases (but not necessarily
the weights, depending on the model) were learned as described in the Bayes-DDM section, except for the “Delta rule” models, for which bias learning was described in the Alternative learning
heuristics section. In Supplementary Fig. 4, all models are compared to the Bayes-DDM model that learns both weights and the bias, includes a lapse model, and has collapsing boundaries.
WEIGHTS FLUCTUATION ANALYSIS As the Bayes-DDM model reaches a decision, it has access to two variables, amount of evidence at the bound and the decision time _t__d_. For better understanding
the dynamics immediately before the multiplication of the weights, we looked at the combination of sensory evidence (_s_1_, s_2) for each simulated trial. For each trial _j_, there is a
noisy sensory evidence trajectory (integration layer from Fig. 6). This means that by the end of trial _j_, we can compute the mean drift rates that gave to rise to a decision: $$\langle
{\mu _i^j} \rangle = \frac{{s_i^j}}{{t_{\mathrm{d}}^j - t_{\mathrm{r}}}}$$ (45) Each group in Fig. 8a, b has been segregated taking into account the Mahalanobis distance, as each line
represents the distance of _D_ = 1 for a particular stimulus set. Considering the integrated evidence of Eq. 13 and combined with the choice function of Eq. 21 we see that $$w_1s_1(t) +
w_2s_2(t) + b = 0$$ (46) Should represent the separation line between the two stimuli, and thus we can rewrite Eq. 46 as: $$s_2(t_{\mathrm{d}}) = - \frac{{w_1}}{{w_2}}s_1(t_{\mathrm{d}}) +
\frac{b}{{w_2}}$$ (47) Considering the straight-line equation \(y = mx + i\), we see that in our integrated evidence plots the boundary separation can be drawn with slope \(m = -
\frac{{w_1}}{{w_2}}\) and intercept \(i = \frac{b}{{w_2}}\). Stimulus weight fluctuation should then have an impact in the slope of the boundary line separating the classification between
left and right stimuli, and _b_ should influence the origin intercept on that stimulus representation (Fig. 8). Considering the data points simulated for 100,000 trials, we analyzed the
effect of slope fluctuation in error rates. That is, how many errors would the model create by having a particular value of _m_, for both the identification and categorization task (Fig. 8).
ANALYSIS All the behavioral and statistical analysis, as well as all fitting, were performed in Matlab®. The different models were implemented and fitted in Julia v1.0.4. REPORTING SUMMARY
Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data that support the findings of this study are
available from the corresponding author upon reasonable request. CODE AVAILABILITY The code that support the findings of this study are available from the corresponding author upon
reasonable request. REFERENCES * Roitman, J. D. & Shadlen, M. N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. _J.
Neurosci._ 22, 9475–9489 (2002). Article CAS PubMed PubMed Central Google Scholar * Palmer, J., Huk, A. C. & Shadlen, M. N. The effect of stimulus strength on the speed and accuracy
of a perceptual decision. _J. Vis._ 5, 376–404 (2005). Article PubMed Google Scholar * Chittka, L., Dyer, A. G., Bock, F. & Dornhaus, A. Bees trade off foraging speed for accuracy.
_Nature_ 424, 388 (2003). Article ADS CAS PubMed Google Scholar * Histed, M. H., Carvalho, L. A. & Maunsell, J. H. R. Psychophysical measurement of contrast sensitivity in the
behaving mouse. _J. Neurophysiol._ 107, 758–765 (2012). Article PubMed Google Scholar * Bowman, N. E., Kording, K. P. & Gottfried, J. A. Temporal integration of olfactory perceptual
evidence in human orbitofrontal cortex. _Neuron_ 75, 916–927 (2012). Article CAS PubMed PubMed Central Google Scholar * Brunton, B. W., Botvinick, M. M. & Brody, C. D. Rats and
humans can optimally accumulate evidence for decision-making. _Science_ 340, 95–98 (2013). Article ADS CAS PubMed Google Scholar * Gold, J. I. & Shadlen, M. N. The neural basis of
decision making. _Annu. Rev. Neurosci._ 30, 535–561 (2007). Article CAS PubMed Google Scholar * Ratcliff, R. & McKoon, G. The diffusion decision model: theory and data for two-choice
decision tasks. _Neural Comput._ 20, 873–922 (2008). Article PubMed PubMed Central MATH Google Scholar * Beck, J. et al. Probabilistic population codes for Bayesian decision making.
_Neuron_ 60, 1142–1152 (2008). Article CAS PubMed PubMed Central Google Scholar * Kiani, R., Hanks, T. D. & Shadlen, M. N. Bounded integration in parietal cortex underlies decisions
even when viewing duration is dictated by the environment. _J. Neurosci._ 28, 3017–3029 (2008). Article CAS PubMed PubMed Central Google Scholar * Hanks, T. D., Ditterich, J. &
Shadlen, M. N. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. _Nat. Neurosci._ 9, 682–689 (2006). Article CAS PubMed PubMed Central Google
Scholar * Erlich, J. C., Brunton, B. W., Duan, C. A., Hanks, T. D. & Brody, C. D. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in
the rat. _eLife_ 4, e05457 (2015). * Hanks, T. D. et al. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. _Nature_ 520, 220–223 (2015). Article ADS CAS
PubMed PubMed Central Google Scholar * Zariwala, H. A., Kepecs, A., Uchida, N., Hirokawa, J. & Mainen, Z. F. The limits of deliberation in a perceptual decision task. _Neuron_ 78,
339–351 (2013). Article CAS PubMed PubMed Central Google Scholar * Uchida, N. & Mainen, Z. F. Speed and accuracy of olfactory discrimination in the rat. _Nat. Neurosci._ 6,
1224–1229 (2003). Article CAS PubMed Google Scholar * Rinberg, D., Koulakov, A. & Gelperin, A. Speed-accuracy tradeoff in olfaction. _Neuron_ 51, 351–358 (2006). Article CAS PubMed
Google Scholar * Abraham, N. M. et al. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. _Neuron_ 44, 865–876 (2004). CAS PubMed
Google Scholar * Khan, R. M. & Sobel, N. Neural processing at the speed of smell. _Neuron_ 44, 744–747 (2004). Article CAS PubMed Google Scholar * Ratcliff, R. A theory of memory
retrieval. _Psychol. Rev._ 85, 59–108 (1978). Article Google Scholar * Ratcliff, R. & Smith, P. L. A comparison of sequential sampling models for two-choice reaction time. _Psychol.
Rev._ 111, 333–367 (2004). Article PubMed PubMed Central Google Scholar * Mulder, M. J., Wagenmakers, E.-J., Ratcliff, R., Boekel, W. & Forstmann, B. U. Bias in the brain: a
diffusion model analysis of prior probability and potential payoff. _J. Neurosci._ 32, 2335–2343 (2012). Article CAS PubMed PubMed Central Google Scholar * Fründ, I., Wichmann, F. A.
& Macke, J. H. Quantifying the effect of intertrial dependence on perceptual decisions. _J. Vis._ 14, 9 (2014). Article PubMed Google Scholar * Gold, J. I., Law, C.-T., Connolly, P.
& Bennur, S. The relative influences of priors and sensory evidence on an oculomotor decision variable during perceptual learning. _J. Neurophysiol._ 100, 2653–2668 (2008). Article
PubMed PubMed Central Google Scholar * Majaj, N. J., Hong, H., Solomon, E. A. & DiCarlo, J. J. Simple learned weighted sums of inferior temporal neuronal firing rates accurately
predict human core object recognition performance. _J. Neurosci._ 35, 13402–13418 (2015). Article CAS PubMed PubMed Central Google Scholar * Uchida, N., Kepecs, A. & Mainen, Z. F.
Seeing at a glance, smelling in a whiff: rapid forms of perceptual decision making. _Nat. Rev. Neurosci._ 7, 485–491 (2006). Article CAS PubMed Google Scholar * Rescorla, R. A &
Wagner, A R. in _Classical Conditioning II Current Research and Theory_ (eds Black, A. H. & Prokasy, W. F.) Vol. 21, 64–99 (Appleton-Century-Crofts, 1972). * Sutton, R. S. & Barto,
A. G. _Introduction to Reinforcement Learning_ (MIT Press, 1998). * Busse, L. et al. The detection of visual contrast in the behaving mouse. _J. Neurosci._ 31, 11351–11361 (2011). Article
CAS PubMed PubMed Central Google Scholar * Scott, B. B., Constantinople, C. M., Erlich, J. C., Tank, D. W. & Brody, C. D. Sources of noise during accumulation of evidence in
unrestrained and voluntarily head-restrained rats. _Elife_ 4, 1–23 (2015). Article Google Scholar * Summerfield, C., Behrens, T. E. & Koechlin, E. Perceptual classification in a
rapidly changing environment. _Neuron_ 71, 725–736 (2011). Article CAS PubMed PubMed Central Google Scholar * Pouget, A., Drugowitsch, J. & Kepecs, A. Confidence and certainty:
distinct probabilistic quantities for different goals. _Nat. Neurosci._ 19, 366–374 (2016). Article CAS PubMed PubMed Central Google Scholar * Drugowitsch, J., Mendonça, A. G., Mainen,
Z. F. & Pouget, A. Learning optimal decisions with confidence. _Proc. Natl Acad. Sci. USA_ 116, 24872–24880 (2019). * Beck, J. M., Ma, W. J., Pitkow, X., Latham, P. E. & Pouget, A.
Not noisy, just wrong: the role of suboptimal inference in behavioral variability. _Neuron_ 74, 30–39 (2012). Article CAS PubMed PubMed Central Google Scholar * Bogacz, R., Brown, E.,
Moehlis, J., Holmes, P. & Cohen, J. D. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. _Psychol. Rev._ 113,
700–765 (2006). Article PubMed Google Scholar * Drugowitsch, J., Moreno-Bote, R., Churchland, A. K., Shadlen, M. N. & Pouget, A. The cost of accumulating evidence in perceptual
decision making. _J. Neurosci._ 32, 3612–3628 (2012). Article CAS PubMed PubMed Central Google Scholar * Tajima, S., Drugowitsch, J. & Pouget, A. Optimal policy for value-based
decision-making. _Nat. Commun._ 7, 1–12 (2016). Article CAS Google Scholar * Stevens, S. S. _Psychophysics_ (Transaction Publishers, 1975). * Wojcik, P. T. & Sirotin, Y. B. Single
scale for odor intensity in rat olfaction. _Curr. Biol._ 24, 568–573 (2015). Article CAS Google Scholar * Taniguchi, M., Kashiwayanagi, M. & Kurihara, K. Quantitative analysis on odor
intensity and quality of optical isomers in turtle olfactory system. _Am. J. Physiol. Regul. Integr. Comp. Physiol._ 262, R99–R104 (1992). Article CAS Google Scholar * Laska, M.,
Psychologie, M., München, L. & München, D.- Olfactory discrimination ability of human subjects for enantiomers with an isopropenyl group at the chiral center. _Chem. Senses_ 29, 143–152
(2004). Article PubMed Google Scholar * Pierce, J. D., Zeng, X.-N., Aronov, E. V., Preti, G. & Wysocki, C. J. Cross-adaptation of sweaty-smelling 3-methyl-2- hexenoic acid by a
structurally-similar, pleasant-smelling odorant. _Chem. Senses_ 20, 401–411 (1995). Article CAS PubMed Google Scholar * Churchland, A. K. et al. Variance as a signature of neural
computations during decision making. _Neuron_ 69, 818–831 (2011). Article CAS PubMed PubMed Central Google Scholar * Herrnstein, R. J. Formal properties of the matching law. _J. Exp.
Anal. Behav._ 21, 159–164 (1974). Article CAS PubMed PubMed Central Google Scholar * Baum, W. M. Matching, undermatching, and overmatching in studies of choice. _J. Exp. Anal. Behav._
32, 269–281 (1979). Article CAS PubMed PubMed Central Google Scholar * Sugrue, L. P., Corrado, G. S. & Newsome, W. T. Matching behavior and the representation of value in the
parietal cortex. _Science_ 304, 1782–1787 (2004). Article ADS CAS PubMed Google Scholar * Lauwereyns, J., Watanabe, K., Coe, B. & Hikosaka, O. A neural correlate of response bias in
monkey caudate nucleus. _Nature_ 418, 413–417 (2002). Article ADS CAS PubMed Google Scholar * Roesch, M. R. & Olson, C. R. Neuronal activity related to reward value and motivation
in primate frontal cortex. _Science_ 304, 307–310 (2004). Article ADS CAS PubMed Google Scholar * Kiani, R. & Shadlen, M. N. Representation of confidence associated with a decision
by neurons in the parietal cortex. _Science_ 324, 759–764 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Usher, M. & McClelland, J. L. The time course of perceptual
choice: the leaky, competing accumulator model. _Psychol. Rev._ 108, 550–592 (2001). Article CAS PubMed Google Scholar * Cockburn, J., Collins, A. G. E. & Frank, M. J. A
reinforcement learning mechanism responsible for the valuation of free choice. _Neuron_ 83, 551–557 (2014). Article CAS PubMed PubMed Central Google Scholar * Frank, M. J. et al. FMRI
and EEG predictors of dynamic decision parameters during human reinforcement learning. _J. Neurosci._ 35, 485–494 (2015). Article CAS PubMed PubMed Central Google Scholar * Collins, A.
G. E., Albrecht, M. A., Waltz, J. A., Gold, J. M. & Frank, M. J. Interactions among working memory, reinforcement learning, and effort in value-based choice: a new paradigm and selective
deficits in schizophrenia. _Biol. Psychiatry_ 82, 431–439 (2017). Article PubMed PubMed Central Google Scholar * Drugowitsch, J. Fast and accurate Monte Carlo sampling of first-passage
times from Wiener diffusion models. _Sci. Rep._ 6, 1–13 (2016). Article CAS Google Scholar * Simpson, D. P., Turner, I. W. & Pettitt, A. N. Sampling from Gaussian Markov random fields
conditioned on linear constraints. _ANZIAM J._ 48, 1041 (2008). Article MathSciNet MATH Google Scholar * Wichmann, F. & Hill, N. J. The psychometric function: I. Fitting, sampling,
and goodness of fit. _Percept. Psychophys._ 63, 1293–1313 (2001). Article CAS PubMed Google Scholar * Schwarz, G. Estimating the dimension of a model. _Ann. Stat._ 6, 461–464 (1978).
Article MathSciNet MATH Google Scholar * Wit, E., Heuvel, Evanden & Romeijn, J.-W. ‘All models are wrong.’: an introduction to model uncertainty. _Stat. Neerl._ 66, 217–236 (2012).
Article MathSciNet Google Scholar Download references ACKNOWLEDGEMENTS We thank Joseph Paton, Marta Moita, Alfonso Renart, Tim Hanks, Chris Summerfield and the Mainen, and Pouget
Laboratories for helpful discussions on the work presented here. In particular, we would like to thank Jeff Beck and Ingmar Kanitscheider on feedback regarding model conceptualization and
implementation. This work was supported by grants from the Champalimaud Foundation (M.I.V., A.G.M., E.E.J.D., Z.F.M.), European Research Council (Advanced Investigator Grants 250334 and
671251, Z.F.M.), Fundação para a Ciência e a Tecnologia (SFRH/BD/33938/2009 to A.G.M., SFRH/BD/33274/2008 to M.I.V.), Human Frontier Science Program (Grant RGP0027/2010, Z.F.M. & A.P.),
Simons Foundation (Grant 325057, Z.F.M. & A.P.), the University of Geneva (A.P.), the Swiss National Science Foundation (31003A_143707 and 31003A_165831, A.P.), the James S. McDonnell
Foundation (Scholar award in Understanding Human Cognition, grant 220020462, J.D.) and the National Institute of Mental Health (R01MH115554, J.D.). AUTHOR INFORMATION Author notes * These
authors contributed equally: André G. Mendonça, Jan Drugowitsch. * These authors jointly supervised: Alexandre Pouget, Zachary F. Mainen. AUTHORS AND AFFILIATIONS * Champalimaud Research,
Champalimaud Centre for the Unknown, Lisbon, Portugal André G. Mendonça, M. Inês Vicente, Eric E. J. DeWitt & Zachary F. Mainen * Neurobiology Department, Harvard Medical School, Boston,
MA, USA Jan Drugowitsch * University of Geneva, Geneva, Switzerland Alexandre Pouget Authors * André G. Mendonça View author publications You can also search for this author inPubMed Google
Scholar * Jan Drugowitsch View author publications You can also search for this author inPubMed Google Scholar * M. Inês Vicente View author publications You can also search for this author
inPubMed Google Scholar * Eric E. J. DeWitt View author publications You can also search for this author inPubMed Google Scholar * Alexandre Pouget View author publications You can also
search for this author inPubMed Google Scholar * Zachary F. Mainen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.G.M., M.I.V. and
Z.F.M. designed the experiments. A.G.M., J.D., Z.F.M. and A.P. designed the models. M.I.V. conducted the experiments with assistance from A.G.M.. M.I.V., A.G.M., J.D. and E.E.J.D. analyzed
the data. A.G.M. and J.D. implemented the models. A.G.M., J.D., M.I.V., A.P. and Z.F.M. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Zachary F. Mainen. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nautre Communication_ thanks Bruno Averbeck, Paul Miller, and other,
anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mendonça, A.G., Drugowitsch, J.,
Vicente, M.I. _et al._ The impact of learning on perceptual decisions and its implication for speed-accuracy tradeoffs. _Nat Commun_ 11, 2757 (2020).
https://doi.org/10.1038/s41467-020-16196-7 Download citation * Received: 09 March 2019 * Accepted: 01 April 2020 * Published: 02 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16196-7
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
