Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Despite their rapidly-expanding therapeutic potential, human pluripotent stem cell (hPSC)-derived cell therapies continue to have serious safety risks. Transplantation of
hPSC-derived cell populations into preclinical models has generated teratomas (tumors arising from undifferentiated hPSCs), unwanted tissues, and other types of adverse events. Mitigating
these risks is important to increase the safety of such therapies. Here we use genome editing to engineer a general platform to improve the safety of future hPSC-derived cell transplantation
therapies. Specifically, we develop hPSC lines bearing two drug-inducible safeguards, which have distinct functionalities and address separate safety concerns. In vitro administration of
one small molecule depletes undifferentiated hPSCs >106-fold, thus preventing teratoma formation in vivo. Administration of a second small molecule kills all hPSC-derived cell-types, thus
providing an option to eliminate the entire hPSC-derived cell product in vivo if adverse events arise. These orthogonal safety switches address major safety concerns with pluripotent
cell-derived therapies. SIMILAR CONTENT BEING VIEWED BY OTHERS SUFFICIENCY FOR INDUCIBLE CASPASE-9 SAFETY SWITCH IN HUMAN PLURIPOTENT STEM CELLS AND DISEASE CELLS Article 23 July 2020 A
VERSATILE POLYPHARMACOLOGY PLATFORM PROMOTES CYTOPROTECTION AND VIABILITY OF HUMAN PLURIPOTENT AND DIFFERENTIATED CELLS Article 03 May 2021 GENOME EDITING USING CRISPR/CAS9 TO TREAT
HEREDITARY HEMATOLOGICAL DISORDERS Article 09 March 2021 INTRODUCTION Increasing numbers of human pluripotent stem cell (hPSC)-derived cell therapies have been transplanted into patients,
with over 30 ongoing or completed clinical trials for multiple indications, including spinal cord injury, macular degeneration, and type 1 diabetes1. The breadth of these clinical trials
highlights the promise of hPSC-derived cell therapies. However, hPSC-based therapies present unique safety risks compared to adult-derived cell therapies2,3. To realize the potential of
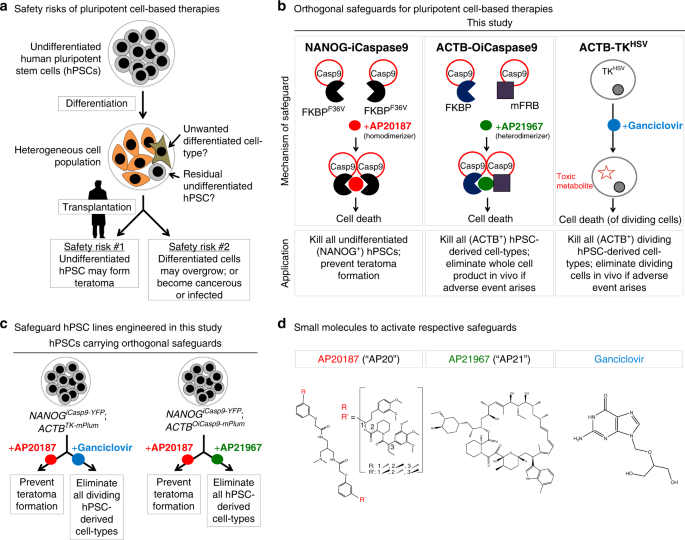
hPSC-derived therapies, strategies to mitigate these unique risks need to be further developed. These risks fall into two main categories (Fig. 1a). First, hPSC differentiation often yields
a heterogeneous cell population4,5, and even a small number of residual undifferentiated hPSCs (10,000 or even fewer) can form a teratoma in vivo6,7. If billions of hPSC-derived cells are to
be transplanted into a patient, even 0.001% remaining hPSCs might be therapeutically unacceptable; thus a 5-log depletion of undifferentiated hPSCs will be critical5. Indeed,
transplantation of certain hPSC-derived liver8 and pancreatic9,10,11 populations yielded teratomas in animal models3, which would be concerning if they similarly arose in human patients.
Second, differentiated cell-types of the wrong lineage can, upon transplantation, generate tumors or unwanted tissues altogether. For example, transplantation of PSC-derived neural
populations into animal models generated tumors12,13,14 or cysts15 in some cases. Indeed hPSCs have also been reported to acquire certain genetic abnormalities in culture (e.g., _TP53_
mutations or _BCL2L1_ amplifications)16,17,18, some of which induce their differentiated progeny to form tumors in vivo13. These safety issues may be further exacerbated as hPSCs are
engineered to be hypoimmunogenic in order to minimize their rejection by patients’ immune systems19,20. Notably, if hPSC-derived hypoimmunogenic cells become malignantly transformed or
virally infected, they may not be adequately controlled by the recipient’s immune system. In such cases, an inducible system to eliminate all transplanted hPSC-derived cells would be a
valuable tool to reduce these risks. To mitigate both of these safety risks for hPSC-based cell therapies, here we develop orthogonal systems to selectively kill undifferentiated hPSCs or to
efficiently eliminate the entire cell product if necessary (Fig. 1b–d). All three of these genetically encoded safety systems (_NANOG__iCaspase9_, _ACTB__OiCaspase9_, and _ACTB__TK_) are
specifically inserted into endogenous genes, and enable us to ablate hPSC-derived cell populations upon small molecule administration both in vitro and in vivo. RESULTS SELECTIVELY KILLING
UNDIFFERENTIATED HPSCS BY _NANOG_ _ICASPASE9_ We addressed the safety concern that trace numbers of undifferentiated hPSCs can form teratomas in vivo6,8,9,10,11 (Figs. 1 and 2a). Others have
described surface markers that identify undifferentiated hPSCs (e.g., SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, and PODXL1)21,22 as well as genetic kill-switches (based on expression of the
_CDK1_23, _TERT_24, _SCD1_25, _SURVIVIN/BIRC5_26,27, and _SOX2_28 genes) to kill such cells. However, the efficacy of all such systems depends on whether expression of these marker genes is
specific to pluripotent cells. We found that all of these previously reported markers were expressed by undifferentiated hPSCs as well as by cells that had been differentiated into endoderm
(liver progenitors29,30), mesoderm (bone progenitors31), and ectoderm (forebrain progenitors32) (Supplementary Fig. 1a–c, Fig. 2b). Hence, previous marker-based strategies to deplete
pluripotent cells are not specific and would also deplete the therapeutic product consisting of differentiated cells. Indeed we found that the small-molecule SURVIVIN inhibitor YM155 (which
was previously reported to kill undifferentiated hPSCs26,27) was inhibitory to the growth of both undifferentiated and differentiated hPSCs (Supplementary Fig. 1d), consistent with broad
expression of _SURVIVIN_ across undifferentiated and differentiated hPSCs (Supplementary Fig. 1a). This emphasizes the importance of selectively depleting undifferentiated hPSCs to create a
safe differentiated cell product that could then be safely transplanted with a significantly decreased risk of teratoma formation. We assayed the expression of multiple pluripotency
transcription factors33 and found that _NANOG_ was the most specific to the pluripotent state (Fig. 2b). _NANOG_ is crucial for pluripotency in human and mouse and its expression is largely
restricted to pluripotent cells in vivo34,35,36,37. Indeed we found that _NANOG_ was expressed by undifferentiated hPSCs but was sharply downregulated within 24 h of ectoderm differentiation
and within 48 h of endoderm or mesoderm differentiation in vitro (Fig. 2b). We therefore developed a specific and simple system to track whether cells were in a pluripotent state (_NANOG_+)
and to link this to controllable elimination of such cells via apoptosis. We exploited Cas9 RNP (ribonucleoprotein)/AAV6-based genome editing38 to knock-in an inducible Caspase9
(_iCaspase9_) cassette39 and a fluorescent reporter (_YFP_) immediately downstream of the _NANOG_ coding sequence (Fig. 2c, Supplementary Fig. 2a), thus creating a _NANOG__iCasp9-YFP_
knock-in allele while leaving the _NANOG_ coding sequence intact, as _NANOG_ is critical for pluripotency34,35,37. The _NANOG_, _iCaspase9_, and _YFP_ genes are all transcribed together from
the _NANOG__iCasp9-YFP_ allele but are separated by T2A self-cleaving peptides40 such that after translation, they are expressed as three separate proteins. _iCaspase9_ encodes a
Caspase9-FKBPF36V fusion protein that, after dimerization with the small molecule AP20187 (hereafter called “AP20”), induces cell-intrinsic, rapid, and irreversible apoptosis (Fig. 1b)39.
hPSCs should not be able to silence this knock-in _NANOG__iCasp9-YFP_ system, because if they downregulated endogenous _NANOG_ expression, they would no longer be pluripotent37. Importantly,
we inserted the _NANOG__iCasp9-YFP_ allele into both _NANOG_ loci to prevent the emergence of escape cells (e.g., if a pluripotent cell stochastically used only one allele of _NANOG_ to
support its growth and pluripotent state41). Genomic sequencing confirmed successful biallelic targeting of the _NANOG_ locus, without off-target integration into the _NANOGP8_ pseudogene
(Supplementary Fig. 2a). _NANOG__iCasp9-YFP_ hPSCs maintained normal pluripotency marker expression (Supplementary Fig. 2b and c), karyotype (Supplementary Fig. 2d) and the ability to
differentiate into endoderm, mesoderm, and ectoderm cells (Supplementary Fig. 1c). NANOG protein and mRNA were expressed at normal levels in _NANOG__iCasp9-YFP_ hPSCs (Supplementary Fig. 2b
and c), showing that insertion of the _iCasp9-YFP_ cassette downstream of the _NANOG_ gene did not noticeably affect _NANOG_ expression. The _NANOG__iCasp9-YFP_ allele faithfully paralleled
endogenous _NANOG_ expression: YFP and _iCaspase9_ mRNA were uniformly expressed by undifferentiated _NANOG__iCasp9-YFP_ hPSCs, but both were extinguished upon endoderm, mesoderm, or
ectoderm differentiation (Fig. 2d, e, Supplementary Fig. 2e). The _NANOG__iCasp9-YFP_ knock-in allele remained active in undifferentiated hPSCs even after long-term culture, demonstrating
that it was not silenced (Supplementary Fig. 2f). After successfully engineering the cells, we tested whether the _NANOG__iCasp9-YFP_ system could specifically ablate undifferentiated hPSCs
without eliminating differentiated cells (the potential therapeutic cell product). AP20 treatment activated iCaspase9 in undifferentiated _NANOG__iCasp9-YFP_ hPSCs and eliminated them, while
sparing their differentiated progeny (Fig. 3). This system was effective (depleting undifferentiated hPSCs 1.75 × 106-fold), sensitive (activated by 1 nM AP20), and specific (sparing >
95% of differentiated bone, liver, or forebrain progenitors). Twenty-four hours treatment with 1 nM of AP20 led to a 1.75 × 106-fold depletion of undifferentiated hPSCs (as assayed across
seven independent experiments; Fig. 3a). This _NANOG__iCasp9-YFP_ system thus enables greater than the 5-log reduction of hPSCs anticipated to be needed to ensure safety of a cell product
with a billion differentiated cells. It also demonstrates quantitative killing of hPSCs exceeding prior reported systems, which generally deplete undifferentiated hPSCs by 1-log or
less22,24,25,26,27. AP20 was remarkably potent (IC50 = 0.065 nM (Supplementary Fig. 3a, b)) and rapid (even 12 h of treatment sufficed to eliminate hESCs (Supplementary Fig. 3c)). 1 nM AP20
was the optimal dose to activate the _NANOG__iCasp9-YFP_ system (Fig. 3a), as higher AP20 concentrations downregulated _NANOG_ (Supplementary Fig. 3b). AP20 is structurally related to FK506
(which is also a BMP agonist)42, and BMP activation is known to downregulate _NANOG_ in hPSCs37. Given that very small numbers of hPSCs (~10,000) are sufficient to form teratomas in vivo6,
we tested the lower bounds of this system to test whether any rare hPSCs survived drug treatment and whether they could form teratomas. We pre-treated 5 × 105 hPSCs with control media or 1
nM AP20 for 24 h prior to subcutaneous transplantation into the left and right dorsal flanks of NOD-SCID _Il2rg_−/− (NSG) mice to form teratomas (Fig. 3b). Past studies primarily assessed
teratoma formation through visual inspection of subcutaneous nodules23,24,25,27. However, here we used bioluminescent imaging to determine if micro-teratomas might form, because
_AkaLuciferase_-based bioluminescent imaging is more quantitative and sensitive; it can detect a single cell in vivo43. Bioluminescent imaging revealed that 0/19 of mice transplanted with
AP20-treated hPSCs formed teratomas, whereas 19/19 of mice transplanted with control-treated hPSCs formed teratomas (_N_ = 3 independent experiments; Fig. 3b). Taken together, the ability to
prevent the formation of even microscopic teratomas is an important step towards developing safer pluripotent cell-derived therapies. Importantly, 24-h treatment with AP20 specifically
eliminated undifferentiated hPSCs while sparing differentiated hPSC-derived tissue progenitors: >95% of _NANOG__iCasp9-YFP_ hPSC-derived day-6 liver progenitors29,30, bone progenitors31,
and forebrain progenitors32 all remained viable (Fig. 3c, Supplementary Fig. 3d), and expression of differentiation markers was not substantially affected (Supplementary Fig. 3e). This is
consistent with the absence of NANOG expression in each of these hPSC-derived tissue progenitor populations (Fig. 2b, e). The _NANOG__iCasp9-YFP_ system also specifically eliminated
undifferentiated hPSCs within heterogeneous cell populations. To simulate a cell-manufacturing failure, we generated day-5 hPSC-derived bone (sclerotome) progenitors and deliberately
introduced 10% undifferentiated hPSCs (Fig. 3d). Treatment with AP20 for the last 24 h of differentiation led to a >10-fold decrease in NANOG-YFP+ cells (monitored by virtue of the YFP
encoded by the _NANOG__iCasp9-YFP_ allele) (Fig. 3d). The surviving NANOG-YFP+ cells were compromised and were no longer pluripotent, as upon FACS purification and continued culture in hPSC
media, they did not form colonies within the limit of detection of our assay (Fig. 3d). Similar results were observed when mixing hPSCs and sclerotome cells at different ratios
(Supplementary Fig. 3f). In conclusion, AP20 treatment of _NANOG__iCasp9-YFP_ hPSCs provides an effective, sensitive, rapid, and selective means to eliminate undifferentiated hPSCs without
eliminating differentiated progeny. ELIMINATING HPSC-DERIVED CELL POPULATIONS IN VIVO BY _ACTB_ _TK_ While the _NANOG__iCasp9-YFP_ system reduces teratoma risk, this is not the only concern
for hPSC-derived cell therapies, as differentiated PSC-derived cell-types can uncontrollably proliferate in vivo, as observed for neural tumors12,13,14. The _NANOG__iCasp9-YFP_ system would
not be an effective safeguard for this type of adverse event. We thus developed an orthogonal drug-inducible safeguard to curb the growth of, or eliminate, all transplanted cells in vivo if
overgrowing, unwanted, or damaging tissues/cells are detected post-transplantation (Fig. 4a). This system could also be used to eliminate transplanted hPSC-derived cells once their
therapeutic effect was achieved, thus allowing a living drug to have a controllable endpoint. To this end, in the _NANOG__iCasp9-YFP_ hPSC line, we knocked-in a second drug-inducible
kill-switch (herpes simplex virus-derived thymidine kinase (_TK_))44 and a fluorescent reporter (_mPlum_) into a constitutively expressed gene (_ACTB_ [_BETA-ACTIN_]), thus creating a
_ACTB__TK-mPlum_ knock-in allele (Fig. 4b, Supplementary Fig. 4a). The _ACTB_, _TK_, and _mPlum_ genes are all transcribed together from this allele but are separated by T2A self-cleaving
peptides40 such that after translation, they are expressed as three separate proteins. TK phosphorylates ganciclovir to a nucleotide analog that competes with ddGTP which, after
incorporation into DNA during replication, results in chain termination, consequently killing dividing cells44. Ganciclovir treatment should therefore eliminate all hPSC-derived dividing
cell-types, irrespective of their lineage or differentiation status (since the _ACTB_ gene is ubiquitously expressed and is essential for proliferation; Supplementary Fig. 4b). The
proliferation, pluripotency marker expression, karyotype, and differentiation potential of _ACTB__TK-mPlum_;_NANOG__iCasp9-YFP_ hPSCs was not perturbed (Fig. 4c, Supplementary Figs. 1c and
2b–d). The ACTBTK-mPlum cassette was highly expressed in undifferentiated hPSCs as well as hPSC-derived endoderm, mesoderm, and ectoderm tissue progenitors (Fig. 4c, d), paralleling _ACTB_
mRNA expression (Supplementary Fig. 4b). Because TK is expressed under the control of the endogenous _ACTB_ locus, our system should evade silencing, unlike previous transgenes driven by
exogenous viral promoters45,46. Indeed, given that _ACTB_ is generally an essential gene47, if the allele was silenced, the cell would not proliferate and would die. We found that
ganciclovir treatment completely eliminated _ACTB__TK-mPlum__;NANOG__iCasp9-YFP_ hPSCs (Fig. 5a) and substantially eliminated within 24 h of drug exposure their derivative liver, bone, and
forebrain progenitors (Fig. 5b). The differing efficacy of _ACTB__TK-mPlum_ in undifferentiated and differentiated hPSCs may relate to the differing proliferative rates of these lineages. To
eliminate all cells independent of their division rate, we developed a separate kill-switch (_ACTB__OiCaspase9_; described in a subsequent section [Figs. 1 and 6]). We tested the
_ACTB__TK-mPlum_ safeguard in an in vivo model in which growth of hPSC-derived tissues had to be suppressed or even potentially eliminated (Fig. 5c). In this model system, we subcutaneously
transplanted undifferentiated 106 _ACTB__TK-mPlum__;NANOG__iCasp9-YFP_ hPSCs into the left and right dorsal flanks of NSG mice, which formed teratomas in 3 weeks (Fig. 5c, Supplementary Fig.
4c). Starting at 3 weeks post-transplantation, we treated transplanted mice with ganciclovir every day. This ablated any detectable teratomas as measured by bioluminescent imaging: by week
7 post-transplantation (i.e., 4 weeks after initiating ganciclovir administration), 10/10 of control mice harbored detectable teratomas, whereas 0/10 ganciclovir-treated mice had detectable
teratomas (Fig. 5c, Supplementary Fig. 4c). The complete elimination of teratomas by our _ACTB__TK-mPlum_ system contrasts with results obtained with a past _CDK1__TK-mCherry_ system, which
incompletely suppressed teratoma growth23. Finally, we confirmed that the _NANOG__iCasp9-YFP_ system (which is activated by AP20) was still operational in the dual-safeguard
_NANOG__iCasp9-YFP__;ACTB__TK-mPlum_ hPSC line. To demonstrate this, we pre-treated the dual _NANOG__iCasp9-YFP__;ACTB__TK-mPlum_ hPSCs with 1 nM AP20 before transplantation (to activate the
_NANOG__iCasp9-YFP_ system), which prevented them from forming teratomas in vivo (Fig. 5c, Supplementary Fig. 4c). In sum, this series of experiments demonstrate the power of the
_ACTB__TK-mPlum_ safety switch: treatment with ganciclovir can be used to eliminate undesired hPSC-derived cell populations in vivo. However, the TK system took a prolonged amount of time
(~1 month) to eliminate teratomas (Fig. 5c, Supplementary Fig. 4c) and it principally kills dividing cells (although bystander cells may also be indirectly eliminated)44. A different tool to
rapidly kill the entire hPSC-derived cell product (not just dividing cells) would thus be a further advance, and is described below. ENGINEERING AN ORTHOGONAL ICASPASE9 To rapidly kill the
entire hPSC-derived cell product, we created an orthogonal iCaspase9-based killing system that would be compatible with our _NANOG__iCasp9-YFP_ system (Fig. 1), which specifically eliminates
undifferentiated hPSCs. While iCaspase9 dimerization is induced by AP20 (resulting in apoptosis)39, we engineered a variant of iCaspase9 that can be activated by a second orthogonal small
molecule that is not AP20 (Figs. 1b and 6a). This orthogonal iCaspase9 (henceforth, OiCaspase9) comprises Caspase9 fused to both a mutant FRB domain and a FKBP domain; these two domains are
dimerized by a different small molecule (AP21967, hereafter called “AP21”)48 (Fig. 6a). To implement and test this OiCaspase9 system, we knocked it into the _ACTB_ gene in
_NANOG__iCasp9-YFP_ hPSCs (Fig. 6b; Supplementary Fig. 5a), thus generating _ACTB__OiCasp9-mPlum_;_NANOG__iCasp9-YFP_ hPSCs that were karyotypically normal (Supplementary Fig. 5b). In the
_ACTB__OiCasp9-mPlum_ knock-in allele, the _ACTB_, _OiCasp9_, and _mPlum_ genes are separated by T2A self-cleaving peptides40, such that they are transcribed together but are expressed as
three separate proteins (Fig. 6b). DIRECTLY KILLING ALL HPSC-DERIVED CELLS BY _ACTB_ _OICASPASE9_ In this dual-safeguard (_ACTB__OiCasp9-mPlum_;_NANOG__iCasp9-YFP_) hPSC line, we could kill
all hPSC-derived cell-types (whether undifferentiated or differentiated liver, bone, and neural progenitors) through treatment with AP21, which activated the ACTBOiCasp9-mPlum kill-switch
(Fig. 6c–e). Alternatively, we could selectively kill undifferentiated hPSCs through treatment with AP20, which activated the NANOGiCasp9-YFP kill-switch (Fig. 6e). Importantly, iCaspase9
and OiCaspase9 did not cross-react: AP21 selectively activated OiCaspase9 (but not iCaspase9; Fig. 6d, Supplementary Fig. 5c and d). Reciprocally, AP20 specifically activated iCaspase9 (but
not OiCaspase9; Fig. 6e). Therefore, iCaspase9 and OiCaspase9 constitute orthogonal kill-switches, providing a toolkit to inducibly kill distinct cell subsets in response to different
contingencies: for instance, (1) selectively eliminating undifferentiating hPSCs to reduce teratoma risk using AP20 (which activates NANOGiCasp9-YFP) or (2) killing all hPSC-derived
cell-types if an adverse event arises using AP21 (which activates ACTBOiCasp9-mPlum) (Fig. 1a). We demonstrated that the ACTBOiCasp9-mPlum kill-switch was functional and effective in vivo,
and that it was orthogonal to the NANOGiCasp9-YFP system. To mimic an adverse event wherein the hPSC-derived cell product had to be destroyed, 106 _ACTB__OiCasp9-mPlum_;_NANOG__iCasp9-YFP_
hPSCs were subcutaneously transplanted into the left and right dorsal flanks of NSG mice to form teratomas over the course of 4 weeks (Fig. 6f, Supplementary Fig. 5e and f). 4 weeks
post-transplantation, we delivered a single in vivo injection of AP21 (which activates ACTBOiCasp9-mPlum), which completely eliminated any detectable teratomas within 3 days (Fig. 6f,
Supplementary Fig. 5e and f). Even after 4 further weeks, teratomas did not re-emerge, showing that this single AP21 injection fully eliminated teratomas within the sensitive limit of
bioluminescence detection (Fig. 6f; Supplementary Fig. 5e and f). Conversely, in vitro treatment of _ACTB__OiCasp9-mPlum_;_NANOG__iCasp9-YFP_ hPSCs with AP20 (which activates
NANOGiCasp9-YFP) killed undifferentiated hPSCs in vitro, and thus prevented teratomas from forming at all (Fig. 6f; Supplementary Fig. 5e and f). Taken together, the OiCaspase9 cell-ablation
system is superior to the aforementioned TK system in two major ways. First, the ACTBOiCasp9-mPlum safeguard eliminated teratomas more rapidly in vivo (~3 days, with a single AP21
injection; Fig. 6f; Supplementary Fig. 5e and f) than the ACTBTK-mPlum system (~4 weeks, with daily ganciclovir injections; Fig. 5c; Supplementary Fig. 4c). This can be ascribed to their
differing mechanisms-of-action: OiCaspase9 activates the apoptotic pathway to rapidly kill cells, whereas TK inhibits DNA replication, thus principally killing only dividing cells. Second,
OiCaspase9 comprises native human proteins and thus should not be immunogenic. This contrasts with the viral protein TK44; patients have immunologically rejected TK-expressing cell
therapies49. DISCUSSION Improving the safety of hPSC-derived cell therapies is an important priority in order to make such therapies available to a broad range of patients for diverse
indications3, including those diseases (e.g., non-oncologic diseases) with current therapies that work but are not ideal, in which minimizing risk of a hPSC-derived therapy is essential. In
preclinical models, hPSC-derived cell populations have been reported to form teratomas8,9,10,11, other types of tumor12,13,14, or cysts15 with varying frequencies. Another safety risk is
embodied by the tendency of hPSCs to acquire certain genetic mutations upon culture16,17,18; such mutations have been shown to lead to tumor formation by their differentiated progeny in
vivo13. A less recognized but still important potential application of therapeutic safeguards is for hypoimmunogenic hPSC-based cell products19,20. Upon transplantation, if hypoimmunogenic
cells become cancerous or infected, they may not be adequately controlled by patients’ immune systems. Here we report a general platform to improve the potential safety of hPSC-derived cell
therapies, with the aim of mitigating two safety risks that beleaguer this otherwise-promising family of cell therapies. We engineered hPSCs with dual orthogonal safeguards with different
functionalities and that can be activated in response to different contingencies. The first safety switch (_NANOG__iCasp9_) provides a method to substantially reduce the risk of teratoma
formation _prior_ to transplantation. Specifically, it enables the in vitro depletion of teratoma-forming cells from a therapeutic hPSC-derived cell product by >106-fold using the AP20
drug. This degree of depletion would create a safety buffer for cell products of >1 billion cells or more to be transplanted without the potential toxicity of teratoma formation (given
that 10,000 hESCs are sufficient to form a teratoma in mouse models6). By contrast, the second orthogonal safety switch (either _ACTB__TK_ or _ACTB__OiCasp9_) provides two different ways
(treatment with GCV or AP21, respectively) to rapidly eliminate the entire cell product—not just pluripotent cells—if needed. The _ACTB__OiCasp9_ system more rapidly eliminates cells in vivo
and is likely less immunogenic than the virus-derived _ACTB__TK_ system49. One might choose to eliminate the cell product because it either had led to adverse events or because it had
served its therapeutic purpose and was no longer needed. Importantly, both of these orthogonal safety switches are integrated into the same hPSC line (the
_ACTB__TK-mPlum_;_NANOG__iCasp9-YFP_ hPSC line and _ACTB__TK-mPlum_;_NANOG__iCasp9-YFP_ hPSC line), allowing us to use different small molecules to activate distinct safeguards in response
to different contingencies. The drugs used to activate our safety switches we describe (ganciclovir and AP20) are safely used in patients44,50, suggesting the clinical translatability of our
proposed safety assurance systems. Any marker-based strategy to deplete unwanted cells—such as the ones we report here—are only as effective as the specificity of the chosen marker gene.
_NANOG_ is a highly specific marker for pluripotent cells in vivo34,35,36, which we also show here in vitro. Nonetheless, _NANOG_ is still expressed in rare differentiated lineages (e.g.,
primordial germ cells51). The _NANOG__iCasp9_ safeguard, therefore, would not be applicable to such hPSC-derived cell products. Recently a _SOX2__iCasp9_ system was developed to eliminate
pluripotent cells28. However _SOX2_ is a less-specific marker of pluripotent cells, as it is also expressed in a variety of differentiated lineages including ectodermal (e.g., neural)52 and
anterior endoderm (e.g., liver)53 progenitors (Fig. 2b). A _TERT__TK_ system was also developed to eliminate unwanted, hPSC-derived lineages24, but this system would only be effective in the
specialized scenario that the unwanted cell-type was _TERT_+ but the desired, therapeutic cell-type was _TERT_−. A variety of non-genetic means5 also exist to ablate pluripotent cells,
including small molecules (targeting _SCD1_ and _SURVIVIN_)25,26,27, cytotoxic antibodies (targeting _PODXL1_)22, and culture media54,55 as well as extracellular matrices56 that
preferentially eliminate pluripotent cells. However, _SCD1_, _SURVIVIN_, and _PODXL1_ are expressed in both undifferentiated and differentiated hPSCs (Supplementary Fig. 1a), underscoring
the importance of specificity for a cell-ablation system. The dual orthogonal genome-edited hPSC lines we generated are not suitable for clinical use because they contain foreign fluorescent
protein markers and were not manufactured using Good Manufacturing Practices. However such safeguards can be engineered into other hPSC lines with clinically relevant markers (such as
truncated versions of NGFR, EGFR, CD19, or CD20) because the Cas9 RNP/AAV6 system we used to genetically engineer these lines is highly efficient and specific across a range of hPSC lines38.
Moreover, the RNP/AAV6 genome-editing system is so efficient in hPSCs38 that selectable markers might not even be needed to efficiently identify clones containing biallelic integrations of
both of these safeguard systems. While the use of dual safeguards address two important safety concerns for hPSC-derived cell therapies, it would also be possible to engineer cells by genome
editing using only one of the systems as well, as they are independent of each other and utilize different genetic loci for their activity. Finally, our safety systems are precisely knocked
into endogenous loci within hPSCs (by contrast to past efforts to randomly insert them using lentiviral transgenes45,46), thus reducing the risk of insertional mutagenesis or ectopic
silencing of these safety systems. Avoiding transgene silencing should enhance the efficacy of the safeguard system, and avoiding insertional mutagenesis should provide additional safety to
the genetically engineered cell product. METHODS HPSC CULTURE H9 hPSCs (WiCell)57 were used throughout this entire study, and were validated to be mycoplasma negative and karyotypically
normal. Undifferentiated hPSCs were cultured feeder-free in mTeSR1 media (StemCell Technologies) on cell-culture plates that had been pre-coated with Matrigel (Corning) or Geltrex (Gibco)
basement membrane matrices. Each day, mTeSR1 media was changed and hPSC cultures were visually inspected with care to avoid any spontaneous differentiation. When partially confluent, hPSCs
were serially passaged as small clumps by removing mTeSR1, and then adding an EDTA solution (Versene [Gibco]) for 7 min at room temperature to partially dissociate them. Subsequently, EDTA
was removed, fresh mTeSR1 was added, and then hPSCs were scraped off of the plate using a cell scraper and then transferred to new Matrigel-coated or Geltrex-coated plates in mTeSR1 media,
in accord with WiCell’s Feeder Independent culture protocol. GENOME EDITING OF HPSCS To engineer H9 hPSCs carrying the NANOGiCasp9-YFP, ACTBTK-mPlum or ACTBOiCasp9-mPlum systems, we inserted
genetic cassettes into the endogenous _NANOG_ or _ACTB_ genes using the Cas9 RNP (ribonucleoprotein)/AAV6 strategy38. The main conceit of the Cas9 RNP/AAV6 strategy is to electroporate
hPSCs with RNP complexes carrying an engineered, high-specificity HiFi Cas9 variant58 (Integrated DNA Technologies) complexed with chemically modified sgRNAs59 (Synthego); simultaneously,
AAV6 vectors carrying genetic templates for homologous recombination are also concurrently delivered38. To this end, 24 h prior to editing, H9 hPSCs were first treated with 10 μM ROCK
inhibitor (Y-27632) to enhance their survival upon future single-cell dissociation. Second, hPSCs at 70–80% confluence were dissociated into single cells by incubating them with Accutase
(Life Technologies) for several minutes at 37 °C. Then, ROCK inhibitor-supplemented mTeSR1 media was added to neutralize the dissociating agent. Afterwards, dissociated hPSCs were counted.
Concurrent to cell dissociation, the Cas9 RNP complex was prepared38. The RNP complex was formed by combining 5 μg of HiFi Cas958 (Integrated DNA Technologies) and 1.75 μg of sgRNA for 10
min at room temperature, which was then diluted with 20 μL of P3 Primary Cell Solution (Lonza). To electroporate Cas9 RNP complex into hPSCs, 500,000 hPSCs were mixed with the nucleofection
solution containing the aforementioned Cas9/sgRNA RNP. hPSCs were then electroporated in a 16-well Nucleocuvette Strip, using the 4D Nucleofector system (Lonza) with the CA137
electroporation code38. Following electroporation, cells were plated into one well of a Matrigel-coated 24-well plate containing 500 μL of mTeSR1 media supplemented with 10 μM Y-27632. AAV6
donor vector containing donor constructs for homologous recombination was then directly added to the hPSCs at a 100K multiplicity of infection (MOI). Cells were then incubated with AAV6
donor vector at 37 °C for 24 h. mTeSR1 + 10 μM Y27632 was changed 24 h post-editing; after 48 h, mTeSR1 alone was used (without Y27632). After Cas9 RNP/AAV6 editing, single hPSCs were
expanded as clonal lines for genomic sequencing to confirm successful knock-ins. Altogether, we engineered three safety systems: _NANOG__iCasp9-YFP_, _ACTB__TK-mPlum_, and
_ACTB__OiCasp9-mPlum_ in H9 hPSCs. SGRNA FOR GENOME EDITING The _NANOG_ and _ACTB_ synthetic sgRNAs were purchased from Synthego with chemically modified nucleotides at the three terminal
positions at both the 5′ and 3′ ends59. Modified nucleotides contained 2′-O-methyl 3′-phosphorothioate59. sgRNAs were designed to target the ends of the _NANOG_ and _ACTB-_coding sequences.
The genomic sgRNA target sequences, with the PAM sequence in bold, were: _NANOG_: 5′-ACTCATCTTCACACGTCTTCAGG-3′ _ACTB:_ 5′-CCGCCTAGAAGCATTTGCGGCGG_-_3′ CONSTRUCTION OF DONOR VECTORS FOR
HOMOLOGOUS RECOMBINATION Genetic cassettes encoding the respective safety switches and flanking homology arms for homologous recombination (_NANOG__iCasp9-YFP_, _ACTB__TK-mPlum_, and
_ACTB__OiCasp9-mPlum_) were cloned into the pAAV-MCS plasmid (Agilent Technologies), which contains AAV2 inverted terminal repeat (ITR) sequences. Vectors were designed to replace the stop
codon of each respective gene (_NANOG_ or _ACTB_) and to insert each respective safety switch system immediately downstream of the coding sequence of each gene, in lieu of the stop codon.
DNA cloning was performed using the NEBuilder® HiFi DNA Assembly Cloning Kit to create donor vectors for homologous recombination. DNA sequences are provided for the _NANOG__iCasp9-YFP_
construct (Supplementary Data 1), _ACTB__TK-mPlum_ construct (Supplementary Data 2), _ACTB__OiCasp9-mPlum_ construct (Supplementary Data 3) as well as the backbone vector (Supplementary Data
4). Note that while the donor vector backbone for homologous recombination contains AAV2 ITR sequences (Supplementary Data 4), it is pseudotyped with AAV6 capsid proteins (described below)
to produce AAV6 particles38. PRODUCTION OF AAV6 DONOR VECTORS Plasmids were grown in _E. coli_ (NEB® Stable Competent _E. Coli_ (Cat# C3040I) and produced using Invitrogen’s Endotoxin-Free
Maxi Plasmid Purification Kit (Cat# A33073). Following DNA purification, 50 million 293FT cells (Life Technologies) were plated in 15 cm2 dishes. The cells were transfected the next day
using 120 μL (1 mg/mL) of PEI (MW 25K) (Polysciences), 6 μg of donor plasmid for homologous recombination (described above), and 22 μg pDGM6 (which carried AAV6 capsid [cap] gene, AAV2
replication [rep] gene, and adenoviral helper genes) (gift from D. Russell)38. 72 h post-transfection, cells were harvested and purified using the Takara AAVpro Purification Kit (Cat. 6666)
according to the manufacturer’s protocol. AAV6 vector titer was determined using digital droplet (ddPCR) to measure vector genome concentration. SEEDING HPSCS FOR DIRECTED DIFFERENTIATION
hPSCs were grown to near-confluency at which point they were dissociated into single cells or very small clumps using Accutase (Gibco). Cells were seeded onto Matrigel-coated or
Geltrex-coated 12-well plates at a density of ~25,000 cells/cm2 in mTeSR1 supplemented with the ROCK inhibitor thiazovivin (1 μM, Tocris; to enable the survival of dissociated hPSCs). The
next day after seeding, cells were washed once with DMEM/F12 (to remove all traces of mTeSR1 media) and subsequently, differentiation media was added. Differentiation media—whose composition
is detailed below—was changed every 24 h. Whenever the new differentiation media composition was different from that of the previous day, the cells were briefly washed with DMEM/F12 (to
remove any trace of the previous differentiation signals) before adding the new differentiation media. HPSC DIFFERENTIATION INTO LIVER BUD PROGENITORS hPSCs were sequentially differentiated
towards anteriormost primitive streak, definitive endoderm, and then liver bud progenitors within 6 days with the following media compositions on each day of differentiation29,30: Day 1:
CDM2 base media supplemented with 100 ng/mL Activin A + 3 μM CHIR99021 + 20 ng/mL FGF2 + 50 nM PI-103. Day 2: CDM2 base media supplemented with 100 ng/mL Activin A + 250 nM LDN-193189 + 50
nM PI-103. Day 3: CDM3 base media supplemented with 20 ng/mL FGF2 + 30 ng/mL BMP4 + 75 nM TTNPB + 1 μM A-83-01. Day 4–6: CDM3 base media supplemented with 10 ng/mL Activin A + 30 ng/mL BMP4
+ 1 μM Forskolin. The composition of CDM2 basal medium29,31 is: 50% IMDM + GlutaMAX (Thermo Fisher, 31980-097) + 50% F12 + GlutaMAX (Thermo Fisher, 31765-092) + 1 mg/mL polyvinyl alcohol
(Sigma, P8136-250G) + 1% v/v chemically defined lipid concentrate (Thermo Fisher, 11905-031) + 450 μM 1-thioglycerol (Sigma, M6145-100ML) + 0.7 μg/mL recombinant human insulin (Sigma,
11376497001) + 15 μg/mL human transferrin (Sigma, 10652202001) + 1% v/v penicillin/streptomycin (Thermo Fisher, 15070-063). Polyvinyl alcohol was brought into suspension by gentle warming
and magnetic stirring, and the media was sterilely filtered (through a 0.22 μm filter) prior to use. The composition of CDM3 basal medium30 is: 45% IMDM + GlutaMAX (Thermo Fisher, 31980-097)
+ 45% F12 + GlutaMAX (Thermo Fisher, 31765-092) + 10% KnockOut serum replacement (Thermo Fisher, 10828028) + 1 mg/mL polyvinyl alcohol (Sigma, P8136-250G) + 1% v/v chemically defined lipid
concentrate (Thermo Fisher, 11905-031) + 1% v/v penicillin/streptomycin (Thermo Fisher, 15070-063). Polyvinyl alcohol was brought into suspension by gentle warming and magnetic stirring, and
the media was sterilely filtered (through a 0.22 μm filter) prior to use. HPSC DIFFERENTIATION INTO BONE PROGENITORS hPSCs were sequentially differentiated towards anterior primitive
streak, paraxial mesoderm, and sclerotome (bone) progenitors within 6 days with the following media compositions on each day of differentiation31: Day 1: CDM2 base media supplemented with 30
ng/mL Activin A + 4 μM CHIR99021 + 20 ng/mL FGF2 + 100 nM PIK90. Day 2: CDM2 base media supplemented with 1 μM A83-01 + 250 nM LDN-193189 + 3 μM CHIR99021 + 20 ng/mL FGF2. Day 3: CDM2 base
media supplemented with 1 μM A83-01 + 250 nM LDN-193189 + 1 μM XAV939 + 500 nM PD0325901. Day 4–6: CDM2 base media supplemented with 1 μM C59 + 5 nM SAG 21K. HPSC DIFFERENTIATION INTO
FOREBRAIN PROGENITORS hPSCs were differentiated into forebrain progenitors with the following media composition for all 6 days of differentiation32: Days 1–6: DMEM/F12 supplemented with 1%
N2 (Gibco) + 1% B27 without RA (Gibco) + 1% GlutaMAX (Gibco) + 500 nM LDN-193189 + 3 μM SB-431542 + 1 μM XAV939. TERATOMA FORMATION 10 million _NANOG__iCasp9-YFP_ hPSCs (or various other
genetically modified hPSC lines) were seeded in a Geltrex-coated 15-cm dish, in mTeSR1 supplemented with 1 μM thiazovivin (and, when applicable, 1 nM AP20187). 24 h later, cells were then
dissociated by treatment with TrypLE Express for 5 min at 37 °C. Dissociated cells in TrypLE Express were diluted 1:10 in DMEM/F12, pelleted and resuspended in 1 mL of a 1:1 mixture of
mTeSR1 and Matrigel per original 15-cm dish (~10,000 cells/μL for untreated groups). Tubes were kept on ice until transplant. 6–10-week-old immunodeficient NOD-SCID _Il2rg_−/− (NSG)
mice—both males and females—were used for all experiments. Mice were anesthetized during transplantation using isoflurane. 100 μL of cell suspension (~1 million cells) was injected
subcutaneously into each of the right and left dorsal flanks of the mouse. Teratoma growth was monitored throughout the duration of the experiment via visual inspection and bioluminescent
imaging. ACTIVATION OF NANOGICASP9-YFP SAFETY SYSTEM The NANOGiCasp9-YFP safety system was activated by administering AP20187 to uniform populations of a single cell-type, or alternatively,
heterogeneous cell populations containing multiple cell-types. In the first scenario, uniform populations of _NANOG__iCasp9-YFP_ hPSCs or their differentiated progeny were treated with
AP20187 (1 nM, or other doses as indicated) for 24 h to deplete pluripotent cells in vitro. AP20187 was added to the appropriate media for each cell-type: mTeSR1 for undifferentiated hPSCs
or differentiation media for differentiated cells (composition of differentiation media is described above). Depletion of hPSCs was then quantified by multiple in vivo and in vitro assays,
as described below. In the second scenario, we used AP20187 to kill undifferentiated hPSCs within a heterogeneous cell population. To this end, we simulated a cell-therapy manufacturing
error: undifferentiated hPSCs were deliberately spiked into a differentiated cell population. Specifically, 1 × 106 _NANOG__iCasp9-YFP_ hPSCs were dissociated with Accutase and mixed with 1
× 105 _NANOG__iCasp9-YFP_ hPSC-derived day 5 sclerotome cells. This mixed cell population was seeded in sclerotome media (CDM2 base media + 1 μM C59 + 5 nM SAG 21 K [described above]) + 100
ng/mL FGF2 (to help undifferentiated hPSCs survive) + 10 μM Y-27632 (to help single, dissociated hPSCs adhere and survive), in the presence or absence of 1 nM AP20187 for 1 h. For the
remaining 23 h, ROCK inhibitor was removed; that is, the heterogeneous cell populations were cultured in sclerotome media + 100 ng/mL FGF2 in the presence or absence of AP20187. QUANTIFYING
CELL DEATH INDUCED BY NANOGICASP9-YFP SYSTEM To quantify cell death after AP20187 treatment of undifferentiated or differentiated _NANOG__iCasp9-YFP_ hPSCs carrying various safety systems,
we used multiple independent assays. First, we performed a clonal assay for surviving hPSC colonies. To this end, hPSCs were dissociated into single cells with Accutase (Thermo Fisher) and 1
× 106 cells were plated per well of a six-well plate that was pre-coated with Matrigel. To enhance single-cell survival, hPSCs were plated in mTeSR1 supplemented with ROCK inhibitor 10 μM
Y-27632 for 1 h (in the presence or absence of AP20187 at the indicated concentrations). ROCK inhibitor was then withdrawn for the remaining 23 h of culture; that is, hPSCs were cultured in
mTeSR1 (in the presence or absence of AP20187). Subsequently, AP20187 was withdrawn altogether and hPSCs were cultured with mTeSR1 for 1 week, to allow any surviving hPSCs to regrow and to
form clonal colonies, which were then scored (i.e., 1 surviving colony after AP20187 treatment of 1 × 106 hPSCs indicated survival of 1 out of 106 cells). Second, we performed a cell count
assay. To this end, hESCs were dissociated with EDTA and 5 × 105 cells were plated per well of a six-well plate that was pre-coated with Matrigel. Cells were seeded in mTeSR1 + ROCK
inhibitor 10 μM Y-27632 (in the presence or absence of AP20187 at the indicated concentrations) for 24 h. Subsequently, cells were dissociated and the number of viable cells were counted
using the Bio-Rad TC20™ Automated Cell Counter (trypan blue exclusion). Third, we performed an Alamar Blue assay for metabolically active cells. To this end, hESCs were cultured in mTeSR1
(in the presence or absence of AP20187 at the indicated concentrations). After 24 h of AP20187 treatment, mTeSR1 media with Alamar Blue (concentration based on manufacturer’s protocol) was
changed for both untreated and treated samples. A control well containing media + Alamar Blue was used to assess blank wells and to therefore to measure and subtract fluorescence noise.
Fourth, we performed flow cytometric quantification of viable cells. To this end, _NANOG__iCasp9-YFP_ hESCs were dissociated into single cells with Accutase and 1 × 106 cells were plated per
well in a six-well plate pre-coated with Matrigel. To enhance single-cell survival, hPSCs were plated in mTeSR1 supplemented with ROCK inhibitor 10 μM Y-27632 for 1 h (in the presence or
absence of AP20187 at the indicated concentrations). ROCK inhibitor was then withdrawn for the remaining 23 h of culture; that is, hPSCs were cultured in mTeSR1 (in the presence or absence
of AP20187). Subsequently, to quantify the percentage of surviving cells, the cultures were dissociated with TrypLE Express. Cells in TrypLE Express were diluted 1:10 in DMEM/F12 and
centrifuged (pelleted) at 500×_g_ for 5 min. Each cell pellet was resuspended in FACS buffer (PBS + 1 mM EDTA [Invitrogen] + 2% v/v FBS [Atlanta Bio] + 1% penicillin/streptomycin [Gibco])
supplemented with DAPI (1:10,000, Biolegend) to discriminate live vs. dead cells. YFP+ (i.e., NANOG+) cells were analyzed (Beckman Coulter CytoFlex Analyzer) to count live cells for both
untreated and AP20187-treated groups. In some experiments, YFP+ (i.e., NANOG+) cells were sorted (BD FACS Aria II) and cultured in mTeSR1 to test whether they were actually still living and
could form hPSC colonies. Colony counting assay was performed in seven independent experiments and data shown in Fig. 3a represents the average colony number across all experiments. All
other AP20187 treatment experiments for further validation (Alamar Blue proliferation assay, cell counting, and flow cytometry of viable cells) was performed in one independent experiment
with three biological replicates assessed per experiment. ACTIVATION OF THE ACTBTK-MPLUM SAFETY SYSTEM _ACTB__TK-mPlum_;_NANOG__iCasp9-YFP_ hPSCs or their differentiated progeny were treated
with Ganciclovir (2 μM, or other doses as indicated) for 24 h in vitro. Ganciclovir was added to the appropriate media for each cell-type: mTeSR1 for undifferentiated hPSCs or
differentiation media for differentiated cells (composition of differentiation media is described above). To activate the ACTBTK-mPlum system in vivo, 106
_ACTB__TK-mPlum_;_NANOG__iCasp9-YFP_ hPSCs were subcutaneously transplanted into the left and right dorsal flanks of NSG mice (i.e., 106 cells per flank), with the goal of forming teratomas
(as described in greater detail above). After 3 weeks of transplantation (during which overt teratomas formed), mice were intraperitoneally treated with ganciclovir (50 mg/kg) daily for 4
additional weeks. QUANTIFYING CELL DEATH INDUCED BY ACTBTK-MPLUM SYSTEM _NANOG__iCasp9-YFP_;_ACTB__TK-mPlum_ hPSCs (5 × 105 cells) were plated and treated with ganciclovir (GCV) at varying
concentrations (0.5–2 μM) for 24 h in mTeSR1; subsequently, GCV was withdrawn and hPSCs were cultured in mTeSR1 alone for 6 further days. Three days post-GCV treatment, cell death was
observed in hPSCs. At the end of 6 days of culture in mTeSR1 alone, the number of surviving live cells was counted. Each ganciclovir treatment was performed in one independent experiment,
with three biological replicates assessed per experiment. ACTIVATION OF THE ACTBOICASP9-MPLUM SAFETY SYSTEM _ACTB__OiCasp9-mPlum__;NANOG__iCasp9-YFP_ hPSCs or their differentiated progeny
were treated with AP21967 (1 nM, or other doses as indicated) for 24 h in vitro. AP21967 was added to the appropriate media for each cell-type: mTeSR1 for undifferentiated hPSCs or
differentiation media for differentiated cells (composition of differentiation media is described above). To activate the ACTBOiCasp9-mPlum system in vivo, 106 _ACTB__OiCasp9-mPlum__;
NANOG__iCasp9-YFP_ hPSCs were subcutaneously transplanted into the left and right dorsal flanks of NSG mice (i.e., 106 cells per flank), with the goal of forming teratomas (as described in
greater detail above). After 4 weeks of transplantation (during which overt teratomas formed), mice were intraperitoneally treated with a single dose of AP21967 (10 mg/kg). QUANTIFYING CELL
DEATH INDUCED BY ACTBOICASP9-MPLUM SYSTEM _ACTB__OiCasp9-mPlum_;_NANOG__iCasp9-YFP_ hPSCs or their differentiated progeny were treated with AP21967 (1 nM or the indicated concentration) for
24 h in the respective culture media (mTeSR1 for hPSCs or respective differentiation media for hPSC-derived differentiated lineages). The number of surviving cells was then quantified by
manual cell counting or the alamar blue assay. RNA EXTRACTION To collect RNA for quantitative PCR (qPCR), undifferentiated or differentiated hPSCs were lysed in 350 μL of RLT Plus Buffer and
RNA was extracted using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s protocol. REVERSE TRANSCRIPTION AND QPCR Three hundred naograms of total RNA was reverse
transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. qPCR was performed in 384-well format31 on a
QuantStudio 5 qPCR machine (Thermo Fisher). Expression of all genes was first normalized to the levels of the reference gene _YWHAZ_, and then plotted relative to the levels of _YWHAZ_
(i.e., 1.0 = equivalent to _YWHAZ_). Forward and reverse primer sequences used to detect the expression of the respective genes are provided in Supplementary Table 1. All qPCR experiments
were performed once. For each qPCR experiment, two biological replicates (two different wells in the same culture plate) were analyzed; qPCR was then performed on two technical replicates
for each biological replicate (i.e., 2 biological replicates × 2 technical replicates = 4 replicates altogether). Plotted qPCR data represents the average of the 4 replicates, and standard
error is shown. FLOW CYTOMETRY Undifferentiated and differentiated hPSCs were dissociated by incubation in TrypLE Express (Gibco) for 5 min at 37 °C. Subsequently, dissociated cells in
TrypLE Express were diluted 1:10 in DMEM/F12 and centrifuged (pelleted) at 500×_g_ for 5 min. Each cell pellet was resuspended in FACS buffer (PBS + 1 mM EDTA [Invitrogen] + 2% v/v FBS
[Atlanta Bio] + 1% penicillin/streptomycin [Gibco]) supplemented with fluorophore-conjugated primary antibodies. Antibody catalog numbers and staining concentrations are listed in
Supplementary Table 2. Antibody staining occurred for 30 min on ice protected from light. After staining, cells were washed twice with FACS buffer and resuspended in 200 μL FACS buffer with
DAPI (1:10,000, Biolegend) for live/dead discrimination. Flow cytometry was performed on a Beckman Coulter CytoFlex analyzer (Stanford Stem Cell Institute FACS Core). For data analysis,
cells were gated based on forward and side scatter with height and width used for doublet discrimination. Subsequently, live cells that were negative for DAPI were gated for all marker
analyses and calculations of population frequency. Flow cytometry of undifferentiated hPSCs was performed across three independent experiments, with two biological replicates per experiment
(for both Figs. 2d and 4c, respectively). All other flow cytometry experiments were performed once, with two biological replicates per experiment. INTRACELLULAR FLOW CYTOMETRY
Undifferentiated hPSCs were dissociated by incubation in TrypLE Express (Gibco) for 5 min at 37 °C. Subsequently, dissociated cells in TrypLE Express were diluted 1:10 in DMEM/F12 and
centrifuged (pelleted) at 500×_g_ for 5 min. Cells were subsequently fixed, permeabilized and stained using the Inside Stain Kit (Miltenyi Biotec #130-090-477) as per the manufacturer’s
protocol. Antibody catalog numbers and staining concentrations are listed in Supplementary Table 2. Flow cytometry was performed on a Beckman Coulter CytoFlex analyzer (Stanford Stem Cell
Institute FACS Core). For data analysis, cells were gated based on forward and side scatter with height and width used for doublet discrimination. Subsequently, cells were gated for all
marker analyses and calculations of population frequency. Each intracellular flow cytometry experiment was performed once, with two biological replicates assessed per experiment. GENERATION
OF _AKALUC_-EXPRESSING HPSCS PiggyBac donor plasmid (pPB_CAG_AkaLuc_Puro) was constructed by starting from pPB_CAG_rtTAM2_IN (ref. 60) and then replacing rtTAM2_IN with AkaLuc and Puro,
using In-Fusion® HD Cloning Plus (Takara Bio). AkaLuciferase refers a highly sensitive luciferase variant optimized for intravital bioluminescent imaging, which affords the capability to
detect single cells in vivo under certain circumstances43. PiggyBac transposition was used to deliver the construct into undifferentiated hPSCs for bioluminescent imaging experiments.
BIOLUMINESCENT IMAGING Twenty minutes prior to imaging, mice were injected intraperitoneally with 100 μL of 15 mM AkaLumine HCl (otherwise known as TokeOni [Aobious])43 dissolved in H2O.
Mice were anesthetized using isoflurane and placed in the imaging chamber of either an IVIS Spectrum or SII Lago-X bioluminescent imaging machine. Imaging parameters were kept constant
throughout the duration of each experiment with no images reaching saturation (Binning = 4, FStop = 1.2, exposure time = 10 s). Subsequent image analysis was done in Aura with regions of
interest (ROIs) drawn for each mouse to calculate total flux (photons/s) in order to quantify teratoma growth over time. Each bioluminescence imaging experiment was performed once. Taken
together, we performed three independent experiments (shown in Figs. 3b, 5c, and 6c): For the experiment shown in Fig. 3b, four mice were transplanted with hPSCs and four mice were
transplanted with AP20187-treated hPSCs. For the experiment shown in Fig. 5c, 10 mice were transplanted with hPSCs, 10 mice were transplanted with AP20187-treated hPSCs, and 10 mice were
transplanted with hPSCs and then treated with Ganciclovir in vivo. Complete data for all mice across all timepoints is shown in Supplementary Fig. 4c. For the experiment shown in Fig. 6f,
five mice were not transplanted, five mice were transplanted with AP20187-treated hPSCs, five mice were transplanted with hPSCs and then treated with AP21967 in vivo and five mice were
transplanted with hPSCs and left untreated. Complete data for all mice across all timepoints is shown in Supplementary Fig. 5f. ALKALINE PHOSPHATASE STAINING Alkaline phosphatase staining
was done using the Alkaline Phosphatase Staining Kit (Red) (ab242286, Abcam) using the manufacter’s protocol. In brief, hPSCs were washed with PBS, fixed, and stained for 20 min using the
alkaline phosphatase kit. IMAGING Fluorescent images were taken using the BZ-X710 All-in-One Fluorescence Microscope (Keyence) or the EVOS FL cell imaging system (Thermo Fisher). KARYOTYPE
ANALYSIS Karyotype analysis was performed by the Stanford University Cytogenetics Lab. hPSCs growing in Matrigel-coated T25 flasks were dissociated for analysis. Chromosomes were analyzed
using the GTW banding method. Twenty metaphase cells were analyzed, all of which were concluded to have a normal karyotype (46, XY). Two karyotyping experiments were performed throughout
this study. IMMUNOSTAINING hPSCs or their differentiated progeny were fixed in 4% paraformaldehyde for 15 min; permeabilized in 0.2% Triton X-100 in PBS; and then blocked with blocking
buffer (0.1% Triton-X and 2% FBS in PBS). For primary staining, anti-NANOG (RRID: AB_10559205), anti-SOX2 (RRID:AB_2195767), and anti-TWIST1 (RRID:AB_883292) antibodies were diluted 200-fold
with blocking buffer and stained at 4 °C overnight. Cells were then washed three times and then secondary staining was performed with 1:500 diluted Cy5 Donkey Anti-Rabbit IgG (RRID:
AB_2340607) for 1 h. Cells were washed again and DAPI (RRID: AB_2629482) staining was used on the third wash. For live staining, cells were treated with 5 µg/mL of Hoescht (Invitrogen™ Cat#
H3569) for 30 min, washed with PBS, and then imaged using the EVOS FL cell imaging system. Immunofluorescence experiments were performed once. All catalog numbers for antibodies and other
reagents are provided in Supplementary Table 3. GENOTYPING OF GENETICALLY EDITED HPSCS To confirm successful genetic targeting of the _NANOG_ and _ACTB_ loci, genomic DNA was isolated from
_NANOG__iCasp9-YFP_;_ACTB__TK-mPlum_ hPSCs using QuickExtract DNA Extraction Solution (Epicentre) following the manufacturer’s instructions. Then, genomic PCR was performed using Phusion
Green HSII Master Mix (Thermo Fisher) and the primer sequences listed in Supplementary Table 4. For DNA sequencing of the targeted alleles, PCR amplicons were gel-extracted and submitted for
Sanger sequencing through MCLab (South San Francisco, CA, USA). Off-target editing events were predicted for each sgRNA by COSMID46 tool (http://crispr.bme.gatech.edu). Based on these
predictions, we identified _NANOGP8_ as a possible off-target locus and analyzed this possibility using primers detailed in Supplementary Table 4. Each genomic PCR experiment was performed
once, with one biological sample assessed per experiment. REGULATORY AND INSTITUTIONAL REVIEW All animal experiments were conducted in accord with experimental protocols approved by the
Stanford Administrative Panel on Laboratory Animal Care (APLAC). All hPSC experiments were conducted in accord with experimental protocols approved by the Stanford Stem Cell Research
Oversight (SCRO) committee. STATISTICS No statistical methods were used to determine sample size. For animal experiments, no statistical methods were used to determine sample size. In each
animal experiment, all animals were analyzed (none were excluded), and data for each individual animal is shown in Figs. 3b, 5c, 6c, Supplementary Fig. 4c, Fig. 6f and Supplementary Fig. 5f.
Animals were randomly allocated to experimental groups without pre-selection. When collecting animal data, investigators were not blinded to experimental group assignments. REPORTING
SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY Data supporting the findings of this work are
available within the paper and its Supplementary Information files. The datasets generated and analyzed during the current study and any other relevant data are available from the
corresponding author upon request. Source data for Figs. 2, 3, 5, and 6 are available in the Source Data file. REFERENCES * Guhr, A. et al. Recent trends in research with human pluripotent
stem cells: impact of research and use of cell lines in experimental research and clinical trials. _Stem Cell Rep._ 11, 485–496 (2018). Article Google Scholar * Wang, X. et al. Cloning and
variation of ground state intestinal stem cells. _Nature_ 522, 173–178 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Lee, A. S., Tang, C., Rao, M. S., Weissman, I. L.
& Wu, J. C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. _Nat. Med._ 19, 998–1004 (2013). Article CAS PubMed PubMed Central Google Scholar * McKnight,
K., Wang, P. & Kim, S. K. Deconstructing pancreas development to reconstruct human islets from pluripotent stem cells. _Cell Stem Cell_ 6, 300–308 (2010). Article CAS PubMed PubMed
Central Google Scholar * Fowler, J. L., Ang, L. T. & Loh, K. M. A critical look: Challenges in differentiating human pluripotent stem cells into desired cell types and organoids.
_Wiley Interdiscip. Rev.: Dev. Biol._ 113, 891–823 (2019). Google Scholar * Lee, A. S. et al. Effects of cell number on teratoma formation by human embryonic stem cells. _Cell Cycle_ 8,
2608–2612 (2009). Article CAS PubMed Google Scholar * Hentze, H. et al. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies.
_Stem Cell Res._ 2, 198–210 (2009). Article PubMed Google Scholar * Haridass, D. et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem
cell- derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. _Am. J. Pathol._ 175, 1483–1492 (2010). Article CAS Google Scholar * Kroon, E. et al.
Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. _Nat. Biotechnol._ 26, 443–452 (2008). Article CAS PubMed Google
Scholar * Rezania, A. et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. _Diabetes_ 61,
2016–2029 (2012). Article CAS PubMed PubMed Central Google Scholar * Rostovskaya, M., Bredenkamp, N. & Smith, A. Towards consistent generation of pancreatic lineage progenitors from
human pluripotent stem cells. _Philos. Trans. R. Soc. Lond. Ser. B_ 370, 20140365 (2015). Article CAS Google Scholar * Ganat, Y. M. et al. Identification of embryonic stem cell-derived
midbrain dopaminergic neurons for engraftment. _J. Clin. Investig._ 122, 2928–2939 (2012). Article CAS PubMed PubMed Central Google Scholar * Werbowetski-Ogilvie, T. E., Morrison, L.
C., Fiebig-Comyn, A. & Bhatia, M. In vivo generation of neural tumors from neoplastic pluripotent stem cells models early human pediatric brain tumor formation. _Stem Cells_ 30, 392–404
(2012). Article CAS PubMed Google Scholar * Nori, S. et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with
epithelial–mesenchymal transition. _Stem Cell Rep._ 4, 360–373 (2015). Article CAS Google Scholar * Manley, N. C., Priest, C. A., Denham, J., Wirth, E. D. & Lebkowski, J. S. Human
embryonic stem cell-derived oligodendrocyte progenitor cells: preclinical efficacy and safety in cervical spinal cord injury. _Stem Cells Transl. Med._ 6, 1917–1929 (2017). Article CAS
PubMed PubMed Central Google Scholar * Werbowetski-Ogilvie, T. E. et al. Characterization of human embryonic stem cells with features of neoplastic progression. _Nat. Biotechnol._ 27,
91–97 (2009). Article CAS PubMed Google Scholar * Merkle, F. T. et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. _Nature_ 545, 229–233
(2017). Article ADS CAS PubMed PubMed Central Google Scholar * The International Stem Cell Initiative. Screening ethnically diverse human embryonic stem cells identifies a chromosome
20 minimal amplicon conferring growth advantage. _Nat. Biotechnol._ 29, 1132–1144 (2011). * Han, X. et al. Generation of hypoimmunogenic human pluripotent stem cells. _Proc. Natl Acad. Sci.
USA_ 2014, 201902566 (2019). Google Scholar * Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic
recipients. _Nat. Biotechnol._ 116, 1346 (2019). Google Scholar * Draper, J. S., Pigott, C., Thomson, J. A. & Andrews, P. W. Surface antigens of human embryonic stem cells: changes upon
differentiation in culture*. _J. Anat._ 200, 249–258 (2002). Article CAS PubMed PubMed Central Google Scholar * Choo, A. B. et al. Selection against undifferentiated human embryonic
stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. _Stem Cells_ 26, 1454–1463 (2008). Article CAS PubMed Google Scholar * Liang, Q. et al. Linking a cell-division
gene and a suicide gene to define and improve cell therapy safety. _Nature_ 563, 701–704 (2018). Article ADS CAS PubMed Google Scholar * Qadir, M. M. F. et al. A double fail-safe
approach to prevent tumorigenesis and select pancreatic β cells from human embryonic stem cells. _Stem Cell Rep._ 12, 611–623 (2019). Article CAS Google Scholar * Ben-David, U. et al.
Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. _Cell Stem Cell_ 12, 167–179 (2013). Article CAS PubMed
Google Scholar * Bedel, A. et al. Preventing pluripotent cell teratoma in regenerative medicine applied to hematology disorders. _Stem Cells Transl. Med._ 6, 382–393 (2017). Article CAS
PubMed Google Scholar * Lee, M.-O. et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. _Proc. Natl Acad. Sci. USA_ 110, E3281–3290 (2013). Article
CAS PubMed PubMed Central Google Scholar * Wu, Y. et al. Using gene editing to establish a safeguard system for pluripotent stem-cell-based therapies. _ISCIENCE_ 22, 409–422 (2019).
Article PubMed PubMed Central ADS Google Scholar * Loh, K. M. et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage
bifurcations. _Cell Stem Cell_ 14, 237–252 (2014). Article CAS PubMed PubMed Central Google Scholar * Ang, L. et al. A roadmap for human liver differentiation from pluripotent stem
cells. _Cell Rep._ 22, 2190–2205 (2018). Article CAS PubMed PubMed Central Google Scholar * Loh, K. M. et al. Mapping the pairwise choices leading from pluripotency to human bone,
heart, and other mesoderm cell types. _Cell_ 166, 451–467 (2016). Article CAS PubMed PubMed Central Google Scholar * Maroof, A. M. et al. Directed differentiation and functional
maturation of cortical interneurons from human embryonic stem cells. _Cell Stem Cell_ 12, 559–572 (2013). Article CAS PubMed PubMed Central Google Scholar * Loh, K. M. & Lim, B. A
precarious balance: pluripotency factors as lineage specifiers. _Cell Stem Cell_ 8, 363–369 (2011). Article CAS PubMed Google Scholar * Chambers, I. et al. Functional expression cloning
of Nanog, a pluripotency sustaining factor in embryonic stem cells. _Cell_ 113, 643–655 (2003). Article CAS PubMed Google Scholar * Mitsui, K. et al. The homeoprotein Nanog is required
for maintenance of pluripotency in mouse epiblast and ES cells. _Cell_ 113, 631–642 (2003). Article CAS PubMed Google Scholar * Hart, A. H., Hartley, L., Ibrahim, M. & Robb, L.
Identification, cloning and expression analysis of the pluripotency promoting Nanog genes in mouse and human. _Dev. Dyn._ 230, 187–198 (2004). Article CAS PubMed Google Scholar * Wang,
Z., Oron, E., Nelson, B., Razis, S. & Ivanova, N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. _Cell Stem Cell_ 10, 440–454 (2012).
Article CAS PubMed Google Scholar * Martin, R. M. et al. Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated
homologous recombination. _Cell Stem Cell_ 24, 821–828.e825 (2019). Article CAS PubMed Google Scholar * Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy.
_Blood_ 105, 4247–4254 (2005). Article CAS PubMed PubMed Central Google Scholar * Kim, J. H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human
cell lines, zebrafish and mice. _PLoS ONE_ 6, e18556 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Miyanari, Y. & Torres-Padilla, M.-E. Control of ground-state
pluripotency by allelic regulation of Nanog. _Nature_ 483, 470–473 (2012). Article ADS CAS PubMed Google Scholar * Spiekerkoetter, E. et al. FK506 activates BMPR2, rescues endothelial
dysfunction, and reverses pulmonary hypertension. _J. Clin. Investig._ 123, 3600–3613 (2013). Article CAS PubMed PubMed Central Google Scholar * Iwano, S. et al. Single-cell
bioluminescence imaging of deep tissue in freely moving animals. _Science_ 359, 935–939 (2018). Article ADS CAS PubMed Google Scholar * Fillat, C., Carrió, M., Cascante, A. &
Sangro, B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. _Curr. Gene Ther._ 3, 13–26 (2003). Article CAS
PubMed Google Scholar * Itakura, G. et al. Fail-Safe System against potential tumorigenicity after transplantation of iPSC derivatives. _Stem Cell Rep._ 8, 673–684 (2017). Article CAS
PubMed PubMed Central Google Scholar * Ando, M. et al. A safeguard system for induced pluripotent stem cell-derived rejuvenated T cell therapy. _Stem Cell Rep._ 5, 597–608 (2015). Article
CAS Google Scholar * Shawlot, W., Deng, J. M., Fohn, L. E. & Behringer, R. R. Restricted beta-galactosidase expression of a hygromycin-lacZ gene targeted to the beta-actin locus and
embryonic lethality of beta-actin mutant mice. _Transgen. Res._ 7, 95–103 (1998). Article CAS Google Scholar * Bayle, J. H. et al. Rapamycin analogs with differential binding specificity
permit orthogonal control of protein activity. _Chem. Biol._ 13, 99–107 (2006). Article CAS PubMed Google Scholar * Traversari, C. et al. The potential immunogenicity of the TK suicide
gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. _Blood_ 109, 4708–4715 (2007). Article CAS
PubMed Google Scholar * Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. _N. Engl. J. Med._ 365, 1673–1683 (2011). Article PubMed PubMed Central
Google Scholar * Kobayashi, T. et al. Principles of early human development and germ cell program from conserved model systems. _Nature_ 546, 416–420 (2017). Article ADS CAS PubMed
PubMed Central Google Scholar * Avilion, A. A. et al. Multipotent cell lineages in early mouse development depend on SOX2 function. _Genes Dev._ 17, 126–140 (2003). Article CAS PubMed
PubMed Central Google Scholar * Sherwood, R. I., Chen, T.-Y. A. & Melton, D. A. Transcriptional dynamics of endodermal organ formation. _Dev. Dyn._ 238, 29–42 (2009). Article CAS
PubMed PubMed Central Google Scholar * Tohyama, S. et al. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. _Cell Metab._ 23, 663–674 (2016). Article CAS
PubMed Google Scholar * Tohyama, S. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. _Cell Stem Cell_ 12,
127–137 (2013). Article CAS PubMed Google Scholar * Kirkeby, A. et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for
Parkinson’ disease. _Cell Stem Cell_ 20, 135–148 (2017). Article CAS PubMed PubMed Central Google Scholar * Thomson, J. A. et al. Embryonic stem cell lines derived from human
blastocysts. _Science_ 282, 1145–1147 (1998). Article ADS CAS PubMed Google Scholar * Vakulskas, C. A. et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex
enables efficient gene editing in human hematopoietic stem and progenitor cells. _Nat. Med._ 24, 1–16 (2018). Article CAS Google Scholar * Hendel, A. et al. Chemically modified guide RNAs
enhance CRISPR-Cas genome editing in human primary cells. _Nat. Biotechnol._ 33, 985–989 (2015). Article CAS PubMed PubMed Central Google Scholar * Takashima, Y. et al. Resetting
transcription factor control circuitry toward ground-state pluripotency in human. _Cell_ 158, 1254–1269 (2014). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We thank Volker Wiebking for help with preliminary studies, Timothy Chai and Lay Teng Ang for feedback on the manuscript, and Rayyan Jokhai for assistance with figure
preparation. We also thank Porteus and Loh laboratory members for their generous support, as well as the Stanford Institute for Stem Cell Biology & Regenerative Medicine and the Stanford
Stem Cell FACS Facility for infrastructure support. R.M.M. is supported by the Stanford Stem Cell Biology and Regenerative Medicine Training Grant (T32GM119995). J.L.F. is supported by
National Defense Science and Engineering Graduate (NDSEG) and Stanford Honorary Bio-X Fellowships. M.H.P. is a Chan-Zuckerberg Biohub Investigator and the Laurie Kraus Lacob Faculty Scholar
in Pediatric Translational Medicine. Work on this project in the Porteus lab was supported by a philanthropic gift from the Taube/Koret Foundation. K.M.L. is supported by the NIH Director’s
Early Independence Award (DP5OD024558), Stanford-UC Berkeley Siebel Stem Cell Institute, Stanford Ludwig Center for Cancer Stem Cell Research and Medicine, Stanford Beckman Center and
Anonymous Family, and is a Packard Fellow for Science and Engineering, Pew Scholar, Human Frontier Science Program Young Investigator, and The Anthony DiGenova Endowed Faculty Scholar.
AUTHOR INFORMATION Author notes * These authors contributed equally: Renata M. Martin, Jonas L. Fowler. * These authors jointly supervised this work: Matthew H. Porteus, Kyle M. Loh. AUTHORS
AND AFFILIATIONS * Institute for Stem Cell Biology & Regenerative Medicine, Stanford University School of Medicine, Stanford, CA, 94305, USA Renata M. Martin, Jonas L. Fowler, M. Kyle
Cromer, Benjamin J. Lesch, Ezequiel Ponce, Nobuko Uchida, Toshinobu Nishimura, Matthew H. Porteus & Kyle M. Loh * Department of Pediatrics, Stanford University School of Medicine,
Stanford, CA, 94305, USA Renata M. Martin, M. Kyle Cromer, Benjamin J. Lesch, Ezequiel Ponce, Nobuko Uchida & Matthew H. Porteus * Department of Developmental Biology, Stanford-UC
Berkeley Siebel Stem Cell Institute, Stanford Ludwig Center for Cancer Stem Cell Research and Medicine, Stanford University School of Medicine, Stanford, CA, 94305, USA Jonas L. Fowler &
Kyle M. Loh * ReGen Med Division, BOCO Silicon Valley, Palo Alto, CA, 94303, USA Nobuko Uchida * Department of Genetics, Stanford University School of Medicine, Stanford, CA, 94305, USA
Toshinobu Nishimura Authors * Renata M. Martin View author publications You can also search for this author inPubMed Google Scholar * Jonas L. Fowler View author publications You can also
search for this author inPubMed Google Scholar * M. Kyle Cromer View author publications You can also search for this author inPubMed Google Scholar * Benjamin J. Lesch View author
publications You can also search for this author inPubMed Google Scholar * Ezequiel Ponce View author publications You can also search for this author inPubMed Google Scholar * Nobuko Uchida
View author publications You can also search for this author inPubMed Google Scholar * Toshinobu Nishimura View author publications You can also search for this author inPubMed Google
Scholar * Matthew H. Porteus View author publications You can also search for this author inPubMed Google Scholar * Kyle M. Loh View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS R.M.M., M.K.C., B.J.L., and E.P. genetically engineered hPSC lines carrying safeguard systems. J.L.F. and R.M.M. differentiated hPSCs into specific
cell-types and also validated safeguard systems in vivo and in vitro. N.U. and T.N. contributed to preliminary experiments. CORRESPONDING AUTHORS Correspondence to Matthew H. Porteus or Kyle
M. Loh. ETHICS DECLARATIONS COMPETING INTERESTS M.H.P. serves on the scientific advisory board for CRISPR Tx and Allogene Tx. Neither company had input into the design, execution, data
analysis, or publication of the work presented in this manuscript. N.U. is a current employee of ReGen Med Division, BOCO Silicon Valley. The remaining authors declare no competing
interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer
reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 REPORTING SUMMARY DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES PEER REVIEW FILE
SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Martin, R.M., Fowler, J.L., Cromer, M.K. _et al._ Improving the safety of human pluripotent stem cell therapies using genome-edited orthogonal safeguards. _Nat Commun_ 11,
2713 (2020). https://doi.org/10.1038/s41467-020-16455-7 Download citation * Received: 25 November 2019 * Accepted: 16 April 2020 * Published: 01 June 2020 * DOI:
https://doi.org/10.1038/s41467-020-16455-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
