Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Small molecule polyamines are abundant in all life forms and participate in diverse aspects of cell growth and differentiation. Spermidine/spermine acetyltransferase (SAT1) is the
rate-limiting enzyme in polyamine catabolism and a primary genetic risk factor for suicidality. Here, using genome-wide screening, we find that SAT1 selectively controls nicotinic
acetylcholine receptor (nAChR) biogenesis. SAT1 specifically augments assembly of nAChRs containing α7 or α4β2, but not α6 subunits. Polyamines are classically studied as regulators of ion
channel gating that engage the nAChR channel pore. In contrast, we find polyamine effects on assembly involve the nAChR cytosolic loop. Neurological studies link brain polyamines with
neurodegenerative conditions. Our pharmacological and transgenic animal studies find that reducing polyamines enhances cortical neuron nAChR expression and augments nicotine-mediated
neuroprotection. Taken together, we describe a most unexpected role for polyamines in regulating ion channel assembly, which provides a new avenue for nAChR neuropharmacology. SIMILAR
CONTENT BEING VIEWED BY OTHERS STRUCTURE AND MECHANISM OF HUMAN VESICULAR POLYAMINE TRANSPORTER Article Open access 03 May 2025 ALLOSTERIC MODULATORS OF M1 MUSCARINIC RECEPTORS ENHANCE
ACETYLCHOLINE EFFICACY AND DECREASE LOCOMOTOR ACTIVITY AND TURNING BEHAVIORS IN ZEBRAFISH Article Open access 28 June 2024 _LEVO_-TETRAHYDROPALMATINE INHIBITS Α4Β2 NICOTINIC RECEPTOR
RESPONSE TO NICOTINE IN CULTURED SH-EP1 CELLS Article 12 July 2021 INTRODUCTION Neuronal nicotinic acetylcholine receptor (nAChR) ion channels mediate behavioral, cognitive and autonomic
effects of acetylcholine and addictive properties of nicotine1,2,3,4,5,6. The nAChR family comprises nine alpha (α2-10) and three beta (β2-4) subunits that combine to form an array of
pentameric cation channels throughout the brain and peripheral nervous systems. The major nACh receptors in brain are α7 homomers, α4β2 heteromers, and α6-containing heteromers. These
receptors are targets of numerous approved and experimental medicines for diverse conditions including Alzheimer’s disease, Parkinson’s disease, and neuropathic pain7,8. Whereas nAChRs are
compelling drug targets, progress is hampered because most receptor subtypes do not functionally express in the non-neuronal cell lines used for screening9,10. We previously identified
NACHO, a four-pass transmembrane protein that serves as a client-specific chaperone for assembly of most neuronal nAChRs11,12,13. In brain, NACHO is essential for function of α7 receptors,
and additional proteins, Ric-314 and certain Bcl-2 family members15, synergize with NACHO to enhance α7 assembly and surface trafficking. NACHO also promotes assembly of α4β2 receptors,
though Ric-3 and Bcl-2 proteins have minimal impact on α4β2. Here, we sought factors that conspire with NACHO to enhance function of α4β2 receptors, the most abundant nAChR subtype in brain.
These neuronal α4β2 receptors mediate diverse physiological actions of acetylcholine and underlie nicotine dependence16. Genome-wide cDNA screening for protein enhancers of α4β2 function
identified, quite surprisingly, a single standout clone that encodes spermidine/spermine acetyltransferase (SAT1), the rate limiting enzyme for polyamine catabolism. By degrading polyamines,
SAT1 promotes α4β2 function to an even greater extent than does NACHO. Polyamines are abundant polycations, including spermidine and spermine, that play multiple roles in cell growth,
differentiation and survival17. The interplay between their synthesis by ornithine decarboxylase-1 (ODC1) and their degradation by SAT1 controls polyamine levels. ODC1 is amongst the most
dynamically regulated of all human proteins, and ODC1 is a drug target in oncology and infectious disease17,18. SAT1 transcription is also highly-regulated, and its acetylation of polyamines
promotes their cellular export18. Interestingly, numerous large genomic studies link polymorphisms in SAT1 with suicidal behavior19. In neurons, polyamines play important roles in synaptic
transmission by conferring inward rectification to certain potassium channels, AMPA receptors and nACh receptors20,21,22. Polyamines also participate in the pathogenesis of neurodegenerative
disorders17 and the excitotoxicity associated with cerebral ischemia23. We now find that polyamines also control assembly of neuronal α4β2 and α7 receptors. By contrast, polyamines do not
modulate assembly of α6β4 nAChRs, AMPA receptors or any other ion channel tested. Whereas polyamines classically regulate channel gating by occluding the ion pore, polyamine regulation of
nAChR assembly instead relies on negatively charged residues within the α4 or α7 cytosolic loop. Neuropharmacology studies using wild-type and NACHO knockout mice show that lowering
polyamine levels selectively upregulates cerebrocortical α4β2 and α7 levels and enhances the neuroprotective properties of nicotine. These studies identify an unexpected role for polyamines
in controlling ion channel biogenesis and suggest new strategies in neuropharmacology. RESULTS GENOME-WIDE SCREENING IDENTIFIES SAT1 AS AN ENHANCER OF NACHR To identify novel regulators of
α4β2 receptors, we co-transfected human embryonic kidney 293T cells (HEK293T) with plasmids encoding α4 and β2 subunits along with individual constructs from a 5943-cDNA clone library from
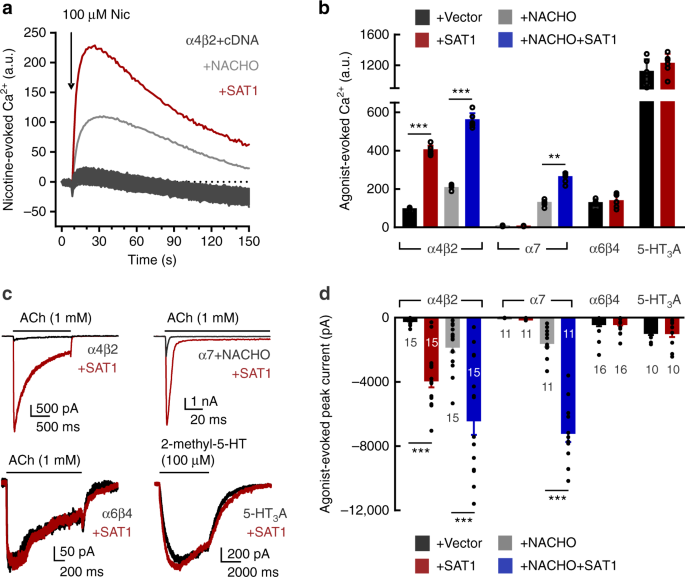
the Broad Institute24. Transfected cells were stimulated with nicotine (100 µM) and intracellular Ca2+ was quantified with a fluorescence imaging plate reader (FLIPR) (Fig. 1a). As
previously shown11, α4β2 alone produced a small nicotine-evoked Ca2+ signal, and co-transfection with NACHO significantly enhanced this (Fig. 1a). High throughput screening identified a
single clone that profoundly augmented the nicotine-induced Ca2+ response—to levels much higher than NACHO; this clone encoded spermidine/spermine N1-acetyltransferase (SAT1) (Fig. 1a). We
evaluated the effect of SAT1 on other nAChRs and other Cys-loop receptors both by FLIPR and electrophysiology. Consistent with our screening results, SAT1 dramatically increased ACh-evoked
currents from α4β2 (Fig. 1c, d). SAT1 also synergized with NACHO to further enhance the α4β2-mediated Ca2+ influx (Fig. 1b, d), suggesting that SAT1 and NACHO employ different mechanisms.
Whereas SAT1 alone did not rescue homomeric α7 function, SAT1 powerfully synergized with NACHO to increase α7 mediated currents (Fig. 1b–d). In contrast, SAT1 had no significant effect on
α6β4 or 5-HT3A receptor function (Fig. 1b–d). SAT1, a small cytosolic protein, is the rate-limiting enzyme for polyamine catabolism (Supplementary Fig. 1). Together with polyamine oxidase,
SAT1 acetylates higher-order polyamines converting them to inactive forms that are transported out of cells18. By lowering polyamine levels, SAT1 effects on nAChRs could reflect
disinhibition, as polyamines can negatively affect nAChR gating22. This seemed unlikely for two reasons, First, α6-containing nAChRs are more sensitive to polyamine inhibition of gating than
are α725 whereas we find the opposite sensitivity to SAT1 in our functional assays (Fig. 1b–d). Second, polyamines do not block nAChRs at the hyperpolarized membrane potentials we used for
patch clamp studies22. As an alternative mechanism, we asked whether SAT1 increases nAChR surface expression. To assess this, we utilized extracellular HA-tagged subunits that allow
detection of surface receptors without disrupting channel function11. Strikingly, SAT1 boosted surface levels of both α4β2 and α7 (Fig. 2a, b). SAT1 also augmented surface trafficking atop
the effects of the α7 protein chaperones Ric-3, Bcl-XL, and Mcl-1, as well as the α7 chemical chaperone/orthosteric antagonist, methyllycaconitine (MLA) (Supplementary Fig. 2a, b) implying
that receptor upregulation by SAT1 involves a mechanism distinct from any previously described. SAT1 PROMOTES ASSEMBLY OF NACHRS BY CATALYZING POLYAMINES To determine whether effects on
nAChRs involve the catalytic activity of SAT1, we constructed a Tyr140Phe mutant that abolishes SAT1 enzyme activity26. This mutant SAT1 (mutSAT1) did not change surface expression of α4β2
or α7 receptors in the presence or absence of NACHO (Fig. 2a, b). Ornithine decarboxylase 1 (ODC1) is the rate-limiting enzyme for polyamine synthesis (Supplementary Fig. 1), and
difluoromethylornithine (DFMO) is an ODC1 active site inhibitor27 that depletes cellular polyamines18. Like SAT1 co-transfection, DFMO pretreatment augmented surface expression of α4β2 and
synergized with NACHO to further enhance α4β2 and α7 surface receptors (Fig. 2a, b). Pursuing the opposite tact, we found that co-transfection with the polyamine biosynthetic enzymes ODC1
and adenosylmethionine decarboxylase 1 (AMD1) diminished nAChR surface staining (Supplementary Fig. 3a, b). Neither catabolic (SAT1 co-transfection or DFMO pretreatment) nor anabolic (ODC1
and AMD1 co-transfection) polyamine manipulations affected surface expression of the 5-HT3A receptor (Fig. 2a, b; Supplementary Fig. 3a, b). We asked next whether polyamines influence nAChR
assembly, which can be probed with orthosteric ligands, such as [3H]epibatidine, that only bind at the interface between folded subunits28. Remarkably, either SAT1 or DFMO enhanced
[3H]epibatidine binding to α4β2 or α7 receptors (Fig. 2c). Furthermore, ODC1 and AMD1 reduced ligand binding to these receptors (Supplementary Fig. 3c). N1,N11-bis-(ethyl)-norspermine
(BenSpm) is a stable polyamine analog and SAT1 inhibitor26,29, and treatment of cells with 10 µM BenSpm specifically reversed the effects of SAT1 on nAChR surface expression (Fig. 2d). By
contrast, incubating cells with spermine, which does not penetrate cells and is rapidly degraded by SAT1, did not affect nAChR surface expression (Fig. 2d). Taken together, we find that
polyamines, independent of their established role in ion channel gating, enhance nAChR function by promoting surface expression and augmenting receptor assembly. POLYAMINES REGULATE NACHRS
ASSEMBLY VIA THEIR CYTOSOLIC LOOP Certain neuronal nAChRs display blunted ion flow at depolarized potentials. This inward rectification is mediated by polyamine binding to pore-lining
glutamate residues in the nAChR transmembrane domain 2 (TM2) corresponding to Glu247 in α422,30. It was previously shown that mutating this acidic residue to alanine (α4E247A) relieves
polyamine block of α4β2 channels in _Xenopus laevis_ oocytes22. Loss of negative charge in the α4E247A also reduces calcium permeability through the mutant receptor. Accordingly, we found
that a E247A α4 mutant (Fig. 3a) co-transfected with β2 evinced minimal nicotine-evoked Ca2+ influx in HEK293T cells (Fig. 3b), and this was unaffected by preincubation with DFMO or
co-transfection with SAT1. By contrast, DFMO or SAT1 enhanced surface expression of α4E247A/β2 similar to wildtype α4β2 (Fig. 3c, d). These data establish distinct mechanisms for polyamine
regulation of nAChR gating and trafficking. We next mutated Trp156 (Fig. 3e) in the α4 ligand-binding domain30. As expected, when co-transfected with β2, this α4W156A mutant was functionally
inactive and did not bind to [3H]epibatidine (Fig. 3f, g). By contrast, this mutant showed typical surface staining enhancement upon co-transfection with SAT1 cDNA (Fig. 3h, i) indicating
that polyamine regulation is independent of agonist binding. To identify nAChR regions responsible for regulation, we generated chimeras of α4 with α6, as surface expression of the latter is
not regulated by polyamines (Fig. 4a). We co-transfected these α4/α6 chimeras with β4 and quantified both receptor surface expression and nicotine-evoked Ca2+ signaling (Fig. 4). SAT1
co-transfection enhanced a chimera containing the α6 N-terminal and α4 C-terminal region but did not affect the converse chimera (Fig. 4a–c), which implies that polyamine regulation involves
the channel or the large cytosolic loop, which occurs between TM3 and TM4. We next constructed a pair of chimeras that swapped only this cytosolic loop. Quantification of receptor surface
expression and function showed that the α4 TM3-TM4 cytosolic loop is both necessary and sufficient for SAT1-dependent regulation of these nAChRs (Supplementary Fig. 4). Similarly, exchanging
the TM3-TM4 loop of homomeric α7 receptor with that of α6 (Supplementary Fig. 4d) abolished SAT1-mediated upregulation of alpha-bungarotoxin (α-Bgt) binding sites on α7 (Supplementary Fig.
4e, f), which demonstrates that the intracellular loop determines polyamine-regulation of both α4 and α7 nAChR assembly. Previous studies noted that, within the T3-TM4 loop, a subdomain
proximal to TM4 contains conserved positively and negatively charged residues31. Interestingly, we observed certain negatively charged residues (Glu and Asp) in this subdomain that are
conserved between α7 and α4 but diverge in α6 to uncharged Asn (Supplementary Fig. 5a, b)31. Mutating those Glu and Asp to Ala in α7 (E438A or D451A) and α4 (E569A or D582A) blocked
SAT1-mediated upregulation of α7 assembly (Supplementary Fig. 5c, d) and α4β2 surface expression (Supplementary Fig. 5 e, f). By contrast, these mutations did not blunt effects of Ric-3 on
α7 or NACHO on α4β2. Replacing the corresponding Asn residues (N434 or N447) to Glu within the α6 cytosolic loop conferred moderate but significant SAT1 and DFMO sensitivity to α6β4
receptors (Supplementary Fig. 5g, h). These experiments identify negatively charged residues within the TM3-TM4 cytosolic loop that can mediate polyamine regulation of nAChR assembly and
surface expression. Future studies are needed to fully elucidate these mechanisms. POLYAMINES REGULATE NACHR GATING AND ASSEMBLY INDEPENDENTLY To further distinguish mechanisms for polyamine
control of nAChR assembly and gating, we compared effects of the membrane-permeant polyamine analog BenSpm with philanthotoxin-343 (PhTx-343), which cannot enter cells and engages the
channel pore via a long polyamine tail25,32. Neither BenSpm nor PhTx-343 preincubation changed basal surface levels of α4β2. Whereas BenSpm abrogated SAT1-mediated enhancement of surface
α4β2, PhTx-343 did not (Fig. 5a, b). By contrast, both BenSpm and PhTx-343 preincubation abolished nicotine-evoked Ca2+ with or without SAT1 co-transfection (Fig. 5c). These data demonstrate
that only cell-permeant polyamines can control of nAChR surface trafficking. To more completely elucidate these mechanisms, we compared effects of BenSpm and PhTx-343 on α4-containing and
α6-containing receptors, as SAT1 regulates trafficking of α4β4 but not α6β4 (Fig. 4b). As expected, pre-treatment with BenSpm, but not PhTx-343, reversed SAT1-mediated enhancement of α4β4
surface expression and neither had effect on α6β4 surface expression (Fig. 5d, e). By contrast, both BenSpm and PhTx-343 application abolished nicotine-evoked Ca2+ signaling from either α4β4
or α6β4 (Fig. 5f). We next evaluated effects of BenSpm and PhTx-343 on the α6/α4 chimeric constructs, which swap the extracellular N-terminal or TM3-TM4 cytosolic loop domains. As
predicted, the α4 cytosolic loop was necessary and sufficient for BenSpm pre-treatment to reverse SAT1-mediated enhancement of receptor surface expression, and PhTx-343 did not alter surface
expression for any chimera (Fig. 5e and Supplementary Fig. 6d, e). By contrast, acute application of either BenSpm or PhTx-343 blocked nicotine-evoked Ca2+-signaling from all receptor
chimeras in a concentration-dependent manner (Fig. 5f, Supplementary Fig. 6a–c). These molecular biological and pharmacological studies establish distinct mechanisms and protein domains for
polyamine control of nAChR trafficking and gating. POLYAMINES REGULATE ASSEMBLY OF NEURONAL NACHRS We next explored whether polyamines upregulate endogenous nAChRs in neurons. Accordingly,
we treated cultured rat cortical neurons with DFMO and quantified α7 nAChR surface expression with fluorescent α-Bgt. As a positive control, we incubated neurons with 100 μM nicotine33 and
found a significant increase in surface α-Bgt binding sites (Fig. 6a, b). Similarly, treating neurons with DFMO robustly increased surface α-Bgt binding (Fig. 6a, b). By contrast, neither
DFMO nor nicotine altered surface expression of the AMPA-type glutamate receptor subunit GluA1 (Fig. 6a, b). Preincubating neurons with DFMO also increased the nicotine-evoked Ca2+ signal
(Fig. 6c), an effect that was comparable to the enhancement seen following nicotine pretreatment. These nicotine-evoked Ca2+ responses reflect α7 activity, as they require the α7-specific
positive allosteric modulator (PAM) PNU-1205961534 and were abolished by the α7-specific inhibitor α-Bgt (Fig. 6c). [3H]Epibatidine binding in brain largely reflects α4β2 receptors and was
absent in cerebral cortex of α4 knockout mice35. Importantly, incubating cortical neurons with DFMO or transducing them with a lentivirus expressing SAT1 enhanced levels of [3H]epibatidine
binding (Fig. 6d), indicating an increased α4β2 receptor assembly. To confirm polyamine regulation of neuronal α4β2, we transduced cortical neurons with α4-expressing and β2-expressing
lentiviruses. Levels of [3H]epibatidine binding to the transduced neuronal membranes were ~20 times higher than untransduced neurons, and this was further increased with DFMO pretreatment
(Fig. 6d). Importantly, neither DFMO preincubation nor SAT1 transduction altered binding sites for the GABA receptor ligand [3H]flunitrazepam (Fig. 6d). Taken together, these data show that
polyamines regulate assembly of neuronal nAChR but not AMPA or GABA receptors. POLYAMINES MODULATE NICOTINE-MEDIATED NEUROPROTECTION Nicotine has well-established neuroprotective properties
and can mitigate excitotoxicity in vivo36 and in vitro37,38. This neuroprotection is mediated both by α739 and α4β2 nAChRs38,40. To assess the functional consequence of polyamine
upregulation of receptor assembly, we asked whether this nicotine-mediated neuroprotection could be enhanced by reducing cellular polyamine levels. We induced excitotoxicity by challenging
cultured neurons for one hour with glutamate (30 µM) and quantified cell survival and cytosolic cytochrome C (CytC), which is a trigger for apoptosis41,42. We also surface-labeled α7
receptors with fluorescent α-Bgt. In line with previous reports, glutamate challenge induced excitotoxity and increased cytosolic CytC (Supplementary Fig. 7a–c). Co-application of 100 μM
nicotine reduced excitotoxicity, but glutamate did not alter surface expression of α7 (Supplementary Fig. 7b, d). On the other hand, neurons pretreated with either DFMO or nicotine showed a
three-fold increase in surface α-Bgt-binding sites (Supplementary Fig. 7a, d) and a concordant reduction of glutamate-induced CytC release (Fig. 6e; Supplementary Fig. 7c). Nicotine or DFMO
preincubation also augmented nicotine-mediated protection from glutamate-mediated excitotoxic cell death (Fig. 6e and Supplementary Fig. 7b). To more directly link the neuroprotective
effects of DFMO with nAChRs, we studied NACHO KO mice, which have dramatically reduced nAChR function in brain12. We found that nicotine blunted glutamate-induced cell death in neurons from
wild-type but not NACHO KO mice (Fig. 7a–c). Fitting with results from our rat cortical neuron experiments, we found that pre-treating wild-type mouse neurons with DFMO increased surface
α-Bgt staining (Fig. 7e) and mitigated glutamate-induced increases in cytosolic CytC (Fig. 7d). By contrast, DFMO pre-treatment did not augment α-Bgt binding levels in NACHO KO neurons.
Accordingly, DFMO preincubation did not confer subsequent nicotine-induced protection from excitotoxic neuronal death in NACHO KO neurons (Fig. 7c). Collectively, these experiments show that
polyamines regulate functional assembly of nAChRs, which promotes neuroprotection in wild-type, but not NACHO KO neurons. DISCUSSION This study identifies an unexpected role for polyamines
in controlling assembly of neuronal α4β2 and α7 receptors. Our genome-wide analysis found that SAT1 increases assembly of α4β2 receptors to a much greater extent than any other protein
screened, including the nicotinic receptor chaperone NACHO. Polyamine-regulated assembly is specific for certain nAChRs, is distinct from other nAChR chaperone mechanisms, and requires
negatively charged amino acids within the α4 or α7 cytosolic loop. Blocking polyamine synthesis with DFMO upregulates neuronal α4β2 and α7 surface levels and promotes nicotine-mediated
neuroprotection, which provides a new angle for drug discovery. The biogenesis of pentameric nAChRs is a complex and tightly-regulated process43. Seminal studies in the 1980s found that
nicotine and other orthosteric ligands upregulate brain nACh protein levels, and this likely participates in nicotine dependence44,45. In _C. elegans_, Ric-3 is required for efficient
assembly of worm nAChRs46; in mammalian brain, NACHO is required for α711 and many other nAChRs12. Additional proteins synergize with NACHO for assembly of specific mammalian nAChRs. Ric-314
and certain Bcl-2 family proteins15 work with NACHO to promote function of α7, whereas BARP, LAMP5, and SULT2B1 conspire with NACHO to enhance function of α6-containing receptors13. Our
discovery of SAT1 regulation identifies the polyamine pathway as an additional controlling mechanism for α4β2 and α7 receptors. These multiple layers of nAChR regulation utilize distinct
mechanisms. In the case of α7, NACHO mediates subunit oligomerization11. Subsequent regulation by nicotine, Ric-3, and Bcl-2 proteins synergizes with NACHO to promote protein folding,
receptor surface expression, and channel function14,15. Nicotine and other orthosteric ligands promote assembly through α7’s ACh binding domain45 whereas Bcl-2 proteins15 and polyamines
engage the receptor cytosolic loop. However, polyamine regulation involves a distinct mechanism, as we find SAT1 augments receptor assembly atop NACHO, nicotinic ligand, Ric-3, or Bcl-2
family proteins. It is intriguing to ask why nAChRs—but not other receptors in the Cys-loop superfamily including 5-HT3 and GABAA receptors—require accessories for functional expression. One
possibility is that multiple assembly mechanisms provide regulatory nodes for controlling nAChR function. NACHO transcription is upregulated by physiological stimuli47, and Bcl-2 family
protein levels are dynamically-induced during developmental and pathological processes48. Furthermore, protein accessories may also determine nAChR cellular localization. The α6-containing
nAChRs specifically concentrate at presynaptic terminals of specific monoaminergic neurons, and this may be enabled by the lysosomal protein, LAMP5, which displays a similar restricted
distribution49. Polyamines are ubiquitous in biology and play multiple roles in cell growth, survival, and differentiation17. In neurons, polyamines control gating of several important ion
channels. Cytosolic polyamines confer inward rectification to certain potassium channels, AMPA receptors, and nAChRs20,21,22. Elegant biophysical studies showed that neuronal depolarization
draws cytosolic polyamines into the channel pore, which precludes ion flow20. This polyamine site is accessible to extracellular philanthotoxins50, which have long polyamine tails that can
engage the channel. Our studies decisively establish that regulation of α7 and α4β2 assembly by polyamines is distinct from their classical role in controlling ion channel gating. First, we
find that SAT1 increases α4β2 and α7 function even when recorded at hyperpolarized potentials that preclude nAChR gating control by cellular polyamines22 (Fig. 1c, d). Second, SAT1 promotes
assembly of non-functional α4β2 receptors that cannot bind ACh (Fig. 3e–i). Third, extracellular philanthotoxin acutely blocks α4β2 receptor function but philanthotoxin does not affect
receptor assembly (Fig. 5a–c). Fourth, chronic but not acute application of a cell-permeable polyamine analog, BenSpm, reverses the effects of SAT1 on α7 and α4β2 receptor assembly and
function (Fig. 2d). Interestingly, we find that SAT1 does not augment function of α6-containing nAChRs. Taking advantage of this α-subunit specificity, our α6/α4 chimeras determine that
polyamine regulation of channel assembly involves the α4 cytosolic loop. Again, this is distinct from the pore region that determines polyamine control of rectification. As α7 and α4β2 are
the most abundant nAChRs in human brain and control diverse aspects synaptic signaling and plasticity, polyamine regulation of their assembly has important physiological and
pathophysiological implications. Polyamine levels in neurons are dynamically regulated over both short-time and long-time scales. Acutely, synaptic transmission increases synthesis of
polyamines, which modulate integrative neuronal properties by reducing AMPA receptor currents51 and provide an excitability buffer by negatively regulating Na+ channels52. Long-lasting
changes in synaptic transmission associated with pathological processes such as epilepsy dramatically upregulate ODC153, which our study predicts would downregulate nAChR assembly. Indeed,
α4-containing nAChRs levels are decreased in piriform cortex of kindled mice54. Polyamines and nAChRs share compelling links to neuropsychiatric disorders. Numerous genetic, genomic, and
biochemical studies identify alterations in the polyamine pathway in major depressive disorder and suicide55. An SNP in the promoter region of SAT1 that reduces expression is associated with
suicide in a French-Canadian founder population55. Postmortem studies find reduced SAT1 protein and mRNA levels in precentral gyrus and cortical frontal lobe56. Also, polyamine levels
increase during anxiety episodes, and this polyamine stress response pathway19 could suppress nAChR assembly. Fitting with this, preclinical and early clinical studies suggest that α7 or
α4β2 agonists can improve depressive behavior in animal models and in depressed patients57. Numerous observations also link polyamine and nAChR alterations with cognitive and
neurodegenerative processes. Spermine synthase mutations cause Snyder-Robinson syndrome characterized by mental retardation and juvenile myoclonic epilepsy58, and these central symptoms are
also found in certain patients with mutations in α4 nAChR59. Interestingly, both SAT1 overexpression60 and nicotine36 protect animals from kainate-induced toxicity. Polyamine levels are
elevated17 and nAChR levels are reduced61 in Alzheimer’s disease, which is best treated today with cholinesterase inhibitors that augment nAChR activity. Furthermore, DFMO23 and nicotinic
agonists40 both mitigate neurotoxicity from neuronal ischemia. Our results that blocking ODC1 increases neuronal α4β2 and α7 and promotes nicotine-mediated neuroprotection provide a
mechanistic link for these observations. As such, targeting this polyamine/nAChR assembly pathway provides a new neuropharmacological strategy. METHODS GENES AND MOLECULAR BIOLOGY AND CELL
CULTURE The following genes are studied here: (Human forms) CHRNA4 (NM_000744.6), CHRNB2 (NM_000748.2), CHRNA7 (NM_000746.5), CHRNA6 (NM_004198), CHRNB4 (NM_000750), TMEM35A or NACHO
(NM_021637.2), Ric-3 (NM_024557.5), Bcl-XL (NM_138578.2), Mcl-1 (NM_021960.4), SAT1 (NM_002970.3), ODC1 (NM_002539); (mouse forms) 5HT-3A (NM_013561), AMD1 (NM_009665). Chimeric constructs
used in this study, with residues in the parenthesis are: α6NT/α4 (α61-239/α4243–628), α4NT/α6 (α41–242/α6240–495), α6/α4loop (α6328–465 exchanged to α4331–600), α4/α6loop (α4331–600
exchanged to α6328–465), α7/α6loop (α7328–457 exchanged to α6339–453). In brief, for α6NT/α4 chimera, α4243–628 region and α61–239 was linearized and amplified with PCR. The primers for
α61–239 contained complementary overhangs immediately upstream and downstream of amplified region. PCR products were ligated using In-Fusion HD Cloning Plus kit (Takara, 683910) in
accordance with manufacturer’s protocol. All other chimeras were generated following similar strategy and were confirmed by sequencing. Site-directed mutagenesis was done with two
complementary primer reactions (primer details provided in Supplementary Table 1), and all mutations were confirmed by sequencing. The α7-HA, β2-HA, and β4-HA constructs contained a PSGA
linker and HA tag immediately following the C-terminal residue. HEK293T cells (ATCC® CRL-3216™) were cultured in DMEM medium supplemented with 10% FBS and 1 mM sodium pyruvate. Cells were
seeded at 80-90% confluence and were transiently transfected using FuGENE®6 transfection reagents (Promega Corporation). All functional and expression assays in HEK293T cells were performed
after 48–72 h incubation at 37 °C following transfection, unless stated otherwise. All animal experiments reported here were overseen and approved by an AAALAC accredited institutional
review board. Primary cortical neurons were prepared from E18 rat cortex (supplied by BrainBits®) and E18 mice cortices from wild-type or NACHO knockout mice (TMEM35atm1(KOMP)Vlcg)11
(obtained from the laboratory’s vivarium). Cortices were dissociated using 10 U/mL papain (Worthington) digestion for 10 min, followed by trituration with a 10 mL glass pipette. Neurons were
seeded at 15,000 cells per well (384-well plates) and maintained in NbActiv4® media (BrainBits®). Immunostaining or FLIPR assays were performed at DIV 20 or DIV 13, respectively, following
6 days incubation in nicotine (100 µM) or DFMO (5 mM) as indicated. For radioligand binding assays, neurons were transduced with SAT1 or α4 and β2 encoding lentiviruses (DIV 7) or treated
with DFMO (DIV 14) and harvested on DIV 20. The lentiviral vectors encoding SAT1, α4 and β2 used a PGK promoter for transgene expression. The SAT1-expressing viral particles were packaged by
Vigene Biosciences™, while the α4-expressing and β2-expressing viruses were packaged by VectorBuilder. BROAD CDNA LIBRARY SCREEN The cDNA library used for α4β2 screening was from the Broad
Institute and contains 5943 cDNA clones. This genome-wide screen used a FLIPR assay on transfected HEK293T cells in 384-well plates. Each well was transfected with 60 ng of total cDNA with
the following composition: α4:β2:single Broad gene (2:4:3). Transfections with NACHO (α4:β2:NACHO [2:4:3]) served as positive control. FLIPR ASSAY FOR AGONIST-EVOKED CA2+ INFLUX
High-throughput FLIPR assays were conducted using 384-well BioCoat (Corning) plates. Cells were washed briefly in assay buffer (HyClone™ HEPES-buffered saline [GE Life Sciences] comprising
149 mM NaCl, 4 mM KCl, 10 mM HEPES, and 5 mM glucose at pH 7.4 and 300 mOsm osmolality, supplemented with 2 mM CaCl2 and 1 mM MgCl2) prior to Calcium5 dye (Molecular Devices) loading for 1 h
at RT. Following removal of excess dye, plates were placed in the FLIPR Tetra (Molecular Devices) chamber, and fluorescent Ca2+ signal was captured using ScreenWorks 4.0™ software
(Molecular Devices). To obtain neuronal α7 mediated FLIPR responses, we used 5 μM PNU-12059615—a selective drug that attenuates receptor desensitization. Tetrodotoxin, or TTX (500 nM) was
included in the assay buffer to inhibit spontaneous action potentials. IMMUNOFLUORESCENT STAINING AND IMAGE ANALYSIS HEK293T cells seeded on 384-well BioCoat plates (Corning) were incubated
with primary antibody against HA tag—Dylight 650® (Invitrogen™) in culture media for 1 h at 37 °C. Cells were fixed with 4% PFA (in HyClone™ HEPES-buffered saline) for 60 min, and after
washing, nuclei were stained with DAPI or NucBlue® reagent (Invitrogen™). For neurons, cells were fixed with 4% PFA for 1 h and then stained with fluorescent α-Bgt conjugates (1 μg/mL,
AlexaFluor 647, Invitrogen™, B35450) or primary antibodies for 1 h. Then cells were simultaneous permeabilized and blocked in 0.2% Triton X-100 and 10% normal goat serum for 30 min. To label
intracellular components, neurons were incubated in primary and secondary antibodies sequentially for 1 h each. Nuclei were stained with DAPI or NucBlue® reagent (Invitrogen™) prior to
imaging. Primary antibodies used were: HA (Mouse, 1:500, Invitrogen™ 2-2.2.14) conjugated to DyLight 650, GluA1 N-terminal (Mouse, 1:250, Millipore-Sigma RH95), Cytochrome C (Mouse, 1:100,
Invitrogen™ 7H8.2C12), MAP2 (Chicken, 1:1000, Millipore-Sigma AB15452). Secondary antibody used: goat anti-mouse IgG (H + L) AlexaFluor 488 (Invitrogen™, A-11001), goat anti-chicken IgY (H +
L) AlexaFluor 555 (Invitrogen™, A-21437) at 1:1000 dilution. For quantification, images were acquired using Harmony™ high-content imaging software on an Opera Phenix™ screening instrument
(PerkinElmer) with a ×20 objective. Data were further analyzed with the Columbus™ data storage and analysis system (PerkinElmer). Viable cells were identified from the DAPI signal (area
>30 μm2 for HEK293T; area >10 μm2 for neurons). Cell perimeter was determined from the nuclear staining, and area of the cytoplasm was calculated based on the cytoplasmic region of
interest. Average fluorescence intensity of wells was determined after subtracting the background signal, which was defined as anti-HA labeling of untransfected HEK293T cells or as α-Bgt647
labeling not displaced by 10 μM epibatidine. RADIOLIGAND BINDING ASSAYS HEK293T cells or cortical neurons were harvested in 50 mM ice-cold TrisHCl buffer (pH 7.4). Cells were homogenized for
30 s using the T-25 Ultra-Turrax homogenizer (Ika) and total protein concentration of the homogenate was determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific). Cell
homogenates were incubated with 10 nM [3H]epibatidine (for nAChR) or with 30 nM [3H]flunitrazepam (for GABA) in 96-well plates for 3 h at room temperature. Nonspecific binding was determined
by co-incubation of the cell samples with 10 μM unlabeled epibatidine or 100 μM unlabeled flunitrazepam. Assays were terminated by filtration through polyethylenimine-treated 96-well
Unifilter GF/B plates (PerkinElmer). Filter plates were washed with 500 mL TrisHCL buffer and then desiccated at 65 °C for 30 min. MicroScint-0 scintillant cocktail (50 μL; PerkinElmer) was
added to each well and plates read with a TopCount NXT scintillation counter (PerkinElmer). ELECTROPHYSIOLOGY HEK293T cells were seeded (1 million/well) on uncoated six-well plates and
transfected with cDNA combinations (total 2 μg/well) using FuGENE®6 transfection reagent (Promega Corporation). eGFP plasmid (10% of total cDNA) identified transfected cells. After 24 h,
cells were dissociated using CellStripper™ dissociation reagent (Corning) and re-seeded on 12 mm glass coverslips (40,000/well). Electrophysiological recordings were done 48 h after
transfection using external solution composed of HyClone™ HEPES-buffered saline (149 mM NaCl, 4 mM KCl, 10 mM HEPES, 2 mM CaCl2, 1 mM MgCl2 and 5 mM Glucose at pH 7.4; 300 mOsm osmolality).
Intracellular solution contained (mM): 140 potassium gluconate, 10 HEPES, 4 Mg-ATP, 0.4 Na-GTP, and 0.6 EGTA (pH 7.3). To study most receptors, fast perfusion of compounds was achieved with
the Perfusion Fast-Step system (Warner Instruments). To study α7 nAChRs, ultra-fast perfusion of compounds was achieved with a piezo-driven perfusion system and theta glass (Siskiyou) on to
eGFP-expressing cells. The membrane holding potential was −70 mV. All recordings were performed at room temperature using an Axopatch 200B amplifier (Axon Instruments) and signals were
filtered at 2 kHz and digitized at 10 kHz. For α7 nAChRs, signals were filtered at 10 kHz with a digitization rate of 50 kHz. Data acquisition and subsequent analysis were done with pClamp9
software (Axon Instruments). STATISTICS Results are represented as mean ± SD unless stated otherwise. All FLIPR assay, immunostaining experiments and radioligand binding assay in HEK293T
cells and rat neurons were replicated thrice. Significance analyses between two datasets were performed with nonparametric Mann–Whitney _U_ test, while statistical analyses between three or
more datasets used one-way ANOVA (GraphPad Prism, Carlsbad, CA). Significance level α = 0.05 was set. REPORTING SUMMARY Further information on research design is available in the Nature
Research Reporting Summary linked to this article. DATA AVAILABILITY Data supporting the findings of this manuscript are available from the corresponding author upon reasonable request. A
reporting summary for this Article is available as a Supplementary Information file. The source data underlying Figs. 1b, d; 2b–d; 3b, c, g, i; 5b, c, e; 6b–e; 7c–e and Supplementary Figs.
2b; 3b, c; 4b, c, f; 5d, e; 6b–d are provided in the Source Data File. Source data are provided with this paper. REFERENCES * Role, L. W. & Berg, D. K. Nicotinic receptors in the
development and modulation of CNS synapses. _Neuron_ 16, 1077–1085 (1996). Article CAS PubMed Google Scholar * Gotti, C. & Clementi, F. Neuronal nicotinic receptors: from structure
to pathology. _Prog. Neurobiol._ 74, 363–396 (2004). Article CAS PubMed Google Scholar * Lindstrom, J. Nicotinic acetylcholine receptors in health and disease. _Mol. Neurobiol._ 15,
193–222 (1997). Article CAS PubMed Google Scholar * Hogg, R. C., Raggenbass, M. & Bertrand, D. Nicotinic acetylcholine receptors: from structure to brain function. _Rev. Physiol.,
Biochem. Pharmacol._ 147, 1–46 (2003). Article CAS Google Scholar * Le Novere, N., Corringer, P. J. & Changeux, J. P. The diversity of subunit composition in nAChRs: evolutionary
origins, physiologic and pharmacologic consequences. _J. Neurobiol._ 53, 447–456 (2002). Article PubMed CAS Google Scholar * Picciotto, M. R. Nicotine as a modulator of behavior: beyond
the inverted U. _Trends Pharmacol. Sci._ 24, 493–499 (2003). Article CAS PubMed Google Scholar * Dineley, K. T., Pandya, A. A. & Yakel, J. L. Nicotinic ACh receptors as therapeutic
targets in CNS disorders. _Trends Pharmacol. Sci._ 36, 96–108 (2015). Article CAS PubMed PubMed Central Google Scholar * Hurst, R., Rollema, H. & Bertrand, D. Nicotinic
acetylcholine receptors: from basic science to therapeutics. _Pharmacol. Therapeutics_ 137, 22–54 (2013). Article CAS Google Scholar * Cooper, S. T. & Millar, N. S. Host cell-specific
folding and assembly of the neuronal nicotinic acetylcholine receptor alpha7 subunit. _J. Neurochem._ 68, 2140–2151 (1997). Article CAS PubMed Google Scholar * Kassner, P. D. &
Berg, D. K. Differences in the fate of neuronal acetylcholine receptor protein expressed in neurons and stably transfected cells. _J. Neurobiol._ 33, 968–982 (1997). Article CAS PubMed
Google Scholar * Gu, S. et al. Brain alpha7 nicotinic acetylcholine receptor assembly requires NACHO. _Neuron_ 89, 948–955 (2016). Article CAS PubMed Google Scholar * Matta, J. A. et
al. NACHO mediates nicotinic acetylcholine receptor function throughout the brain. _Cell Rep._ 19, 688–696 (2017). Article CAS PubMed Google Scholar * Gu, S. et al. alpha6-Containing
nicotinic acetylcholine receptor reconstitution involves mechanistically distinct accessory components. _Cell Rep._ 26, 866–874 e863 (2019). Article CAS PubMed Google Scholar * Millar,
N. RIC‐3: a nicotinic acetylcholine receptor chaperone. _Br. J. Pharmacol._ 153, S177–S183 (2008). Article CAS PubMed PubMed Central Google Scholar * Dawe, G. B. et al. alpha7 nicotinic
acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins. _Nat. Commun._ 10, 2746 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Changeux, J. P. Nicotine
addiction and nicotinic receptors: lessons from genetically modified mice. _Nat. Rev. Neurosci._ 11, 389–401 (2010). Article CAS PubMed Google Scholar * Miller-Fleming, L.,
Olin-Sandoval, V., Campbell, K. & Ralser, M. Remaining mysteries of molecular biology: the role of polyamines in the cell. _J. Mol. Biol._ 427, 3389–3406 (2015). Article CAS PubMed
Google Scholar * Pegg, A. E. & Casero, R. A. Jr. Current status of the polyamine research field. _Methods Mol. Biol._ 720, 3–35 (2011). Article CAS PubMed PubMed Central Google
Scholar * Turecki, G. The molecular bases of the suicidal brain. _Nat. Rev. Neurosci._ 15, 802–816 (2014). Article CAS PubMed PubMed Central Google Scholar * Nichols, C. G. & Lee,
S. J. Polyamines and potassium channels: a 25-year romance. _J. Biol. Chem._ 293, 18779–18788 (2018). Article CAS PubMed PubMed Central Google Scholar * Williams, K. Interactions of
polyamines with ion channels. _Biochemical J._ 325, 289–297 (1997). Article CAS Google Scholar * Haghighi, A. P. & Cooper, E. A molecular link between inward rectification and calcium
permeability of neuronal nicotinic acetylcholine alpha3beta4 and alpha4beta2 receptors. _J. Neurosci._ 20, 529–541 (2000). Article CAS PubMed PubMed Central Google Scholar * Muszynski,
C. A., Robertson, C. S., Goodman, J. C. & Henley, C. M. DFMO reduces cortical infarct volume after middle cerebral artery occlusion in the rat. _J. Cereb. Blood Flow. Metab._ 13,
1033–1037 (1993). Article CAS PubMed Google Scholar * Yang, X. et al. A public genome-scale lentiviral expression library of human ORFs. _Nat. methods_ 8, 659–661 (2011). Article CAS
PubMed PubMed Central Google Scholar * Kachel, H. S., Patel, R. N., Franzyk, H. & Mellor, I. R. Block of nicotinic acetylcholine receptors by philanthotoxins is strongly dependent on
their subunit composition. _Sci. Rep._ 6, 38116 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Bewley, M. C. et al. Structures of wild-type and mutant human
spermidine/spermine N1-acetyltransferase, a potential therapeutic drug target. _Proc. Natl Acad. Sci. USA_ 103, 2063–2068 (2006). Article ADS CAS PubMed PubMed Central Google Scholar *
Gerner, E. W. & Meyskens, F. L. Jr. Polyamines and cancer: old molecules, new understanding. _Nat. Rev. Cancer_ 4, 781–792 (2004). Article CAS PubMed Google Scholar * Prince, R. J.
& Sine, S. M. Epibatidine binds with unique site and state selectivity to muscle nicotinic acetylcholine receptors. _J. Biol. Chem._ 273, 7843–7849 (1998). Article CAS PubMed Google
Scholar * Mandal, S., Mandal, A., Johansson, H. E., Orjalo, A. V. & Park, M. H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and
growth in mammalian cells. _Proc. Natl Acad. Sci. USA_ 110, 2169–2174 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Morales-Perez, C. L., Noviello, C. M. & Hibbs,
R. E. X-ray structure of the human alpha4beta2 nicotinic receptor. _Nature_ 538, 411–415 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Stokes, C., Treinin, M. &
Papke, R. L. Looking below the surface of nicotinic acetylcholine receptors. _Trends Pharmacol. Sci._ 36, 514–523 (2015). Article CAS PubMed PubMed Central Google Scholar * Tikhonov, D.
B., Mellor, I. R. & Usherwood, P. N. Modeling noncompetitive antagonism of a nicotinic acetylcholine receptor. _Biophys. J._ 87, 159–170 (2004). Article ADS CAS PubMed PubMed
Central Google Scholar * Kawai, H. & Berg, D. K. Nicotinic acetylcholine receptors containing alpha 7 subunits on rat cortical neurons do not undergo long-lasting inactivation even
when up-regulated by chronic nicotine exposure. _J. Neurochem._ 78, 1367–1378 (2001). Article CAS PubMed Google Scholar * Hurst, R. S. et al. A novel positive allosteric modulator of the
alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. _J. Neurosci._ 25, 4396–4405 (2005). Article CAS PubMed PubMed Central Google Scholar *
Marubio, L. M. et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. _Nature_ 398, 805–810 (1999). Article ADS CAS PubMed Google Scholar * Borlongan, C.
V. et al. (–)-nicotine protects against systemic kainic acid-induced excitotoxic effects. _Exp. Neurol._ 136, 261–265 (1995). Article CAS PubMed Google Scholar * Akaike, A., Tamura, Y.,
Yokota, T., Shimohama, S. & Kimura, J. Nicotine-induced protection of cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. _Brain Res._ 644,
181–187 (1994). Article CAS PubMed Google Scholar * Marin, P., Maus, M., Desagher, S., Glowinski, J. & Premont, J. Nicotine protects cultured striatal neurones against
N-methyl-D-aspartate receptor-mediated neurotoxicity. _Neuroreport_ 5, 1977–1980 (1994). Article CAS PubMed Google Scholar * Dajas-Bailador, F. A., Lima, P. A. & Wonnacott, S. The
alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism.
_Neuropharmacology_ 39, 2799–2807 (2000). Article CAS PubMed Google Scholar * Hejmadi, M. V., Dajas-Bailador, F., Barns, S. M., Jones, B. & Wonnacott, S. Neuroprotection by nicotine
against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. _Mol. Cell. Neurosci._ 24, 779–786 (2003). Article CAS
PubMed Google Scholar * Kharbanda, S. et al. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. _Proc. Natl Acad. Sci. USA_ 94,
6939–6942 (1997). Article ADS CAS PubMed PubMed Central Google Scholar * Orrenius, S. & Zhivotovsky, B. Cardiolipin oxidation sets cytochrome c free. _Nat. Chem. Biol._ 1, 188–189
(2005). Article CAS PubMed Google Scholar * Green, W. N. & Millar, N. S. Ion-channel assembly. _Trends Neurosci._ 18, 280–287 (1995). Article CAS PubMed Google Scholar *
Schwartz, R. D. & Kellar, K. J. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. _Science_ 220, 214–216 (1983). Article ADS CAS PubMed Google Scholar *
Lester, H. A. et al. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. _Implic. Drug Discov. AAPS J._ 11, 167–177 (2009). CAS Google
Scholar * Halevi, S. et al. The C. elegans ric‐3 gene is required for maturation of nicotinic acetylcholine receptors. _EMBO J._ 21, 1012–1020 (2002). Article CAS PubMed PubMed Central
Google Scholar * Tran, P. V., Georgieff, M. K. & Engeland, W. C. Sodium depletion increases sympathetic neurite outgrowth and expression of a novel TMEM35 gene-derived protein (TUF1) in
the rat adrenal zona glomerulosa. _Endocrinology_ 151, 4852–4860 (2010). Article CAS PubMed PubMed Central Google Scholar * Youle, R. J. & Strasser, A. The BCL-2 protein family:
opposing activities that mediate cell death. _Nat. Rev. Mol. Cell Biol._ 9, 47–59 (2008). Article CAS PubMed Google Scholar * Tiveron, M. C. et al. LAMP5 fine-tunes GABAergic synaptic
transmission in defined circuits of the mouse brain. _PLoS ONE_ 11, e0157052 (2016). Article PubMed PubMed Central CAS Google Scholar * Stromgaard, K. et al. Solid-phase synthesis and
biological evaluation of a combinatorial library of philanthotoxin analogues. _J. Med. Chem._ 43, 4526–4533 (2000). Article CAS PubMed Google Scholar * Aizenman, C. D., Munoz-Elias, G.
& Cline, H. T. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. _Neuron_ 34, 623–634 (2002). Article CAS
PubMed Google Scholar * Fleidervish, I. A., Libman, L., Katz, E. & Gutnick, M. J. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels.
_Proc. Natl Acad. Sci. USA_ 105, 18994–18999 (2008). Article ADS CAS PubMed PubMed Central Google Scholar * Herberg, L. J., Rose, I. C., de Belleroche, J. S. & Mintz, M. Ornithine
decarboxylase induction and polyamine synthesis in the kindling of seizures: the effect of alpha-difluoromethylornithine. _Epilepsy Res._ 11, 3–7 (1992). Article CAS PubMed Google Scholar
* Takechi, K., Suemaru, K., Kiyoi, T., Tanaka, A. & Araki, H. The alpha4beta2 nicotinic acetylcholine receptor modulates autism-like behavioral and motor abnormalities in
pentylenetetrazol-kindled mice. _Eur. J. Pharmacol._ 775, 57–66 (2016). Article CAS PubMed Google Scholar * Limon, A., Mamdani, F., Hjelm, B. E., Vawter, M. P. & Sequeira, A. Targets
of polyamine dysregulation in major depression and suicide: Activity-dependent feedback, excitability, and neurotransmission. _Neurosci. Biobehav. Rev._ 66, 80–91 (2016). Article CAS
PubMed PubMed Central Google Scholar * Sequeira, A. et al. Implication of SSAT by gene expression and genetic variation in suicide and major depression. _Arch. Gen. Psychiatry_ 63, 35–48
(2006). Article CAS PubMed Google Scholar * Gandelman, J. A., Newhouse, P. & Taylor, W. D. Nicotine and networks: Potential for enhancement of mood and cognition in late-life
depression. _Neurosci. Biobehav. Rev._ 84, 289–298 (2018). Article CAS PubMed Google Scholar * Peron, A. et al. Snyder-Robinson syndrome: a novel nonsense mutation in spermine synthase
and expansion of the phenotype. _Am. J. Med. Genet. A_ 161A, 2316–2320 (2013). Article PubMed CAS Google Scholar * Ghasemi, M. & Hadipour-Niktarash, A. Pathologic role of neuronal
nicotinic acetylcholine receptors in epileptic disorders: implication for pharmacological interventions. _Rev. Neurosci._ 26, 199–223 (2015). Article CAS PubMed Google Scholar *
Kaasinen, K., Koistinaho, J., Alhonen, L. & Janne, J. Overexpression of spermidine/spermine N-acetyltransferase in transgenic mice protects the animals from kainate-induced toxicity.
_Eur. J. Neurosci._ 12, 540–548 (2000). Article CAS PubMed Google Scholar * Nordberg, A. Nicotinic receptor abnormalities of Alzheimer’s disease: therapeutic implications. _Biol.
Psychiatry_ 49, 200–210 (2001). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors are grateful to Wes Davini for his valuable inputs. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Neuroscience Discovery, Janssen Pharmaceutical Companies of Johnson and Johnson, 3210 Merryfield Row, San Diego, CA, 92121, USA Madhurima Dhara, Jose A. Matta, Min
Lei, Daniel Knowland, Hong Yu, Shenyan Gu & David S. Bredt Authors * Madhurima Dhara View author publications You can also search for this author inPubMed Google Scholar * Jose A. Matta
View author publications You can also search for this author inPubMed Google Scholar * Min Lei View author publications You can also search for this author inPubMed Google Scholar * Daniel
Knowland View author publications You can also search for this author inPubMed Google Scholar * Hong Yu View author publications You can also search for this author inPubMed Google Scholar *
Shenyan Gu View author publications You can also search for this author inPubMed Google Scholar * David S. Bredt View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS M.D. and M.L. performed cDNA library screening. J.A.M. conducted electrophysiology experiments. M.D. and H.Y. performed immunocytochemistry assays. M.D. conducted
radioligand binding assays. D.K. and S.G. provided chimeric cDNA constructs. M.D. and D.S.B. wrote the manuscript. All authors contributed to the discussion and editing of the paper. D.S.B.
supervised the project. CORRESPONDING AUTHOR Correspondence to David S. Bredt. ETHICS DECLARATIONS COMPETING INTERESTS All contributing authors are full-time employees in Johnson and
Johnson. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports
are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dhara, M., Matta, J.A., Lei, M. _et al._ Polyamine regulation of ion channel
assembly and implications for nicotinic acetylcholine receptor pharmacology. _Nat Commun_ 11, 2799 (2020). https://doi.org/10.1038/s41467-020-16629-3 Download citation * Received: 31 January
2020 * Accepted: 15 May 2020 * Published: 03 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16629-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
