Robust leaf trait relationships across species under global environmental changes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Recent studies show coordinated relationships between plant leaf traits and their capacity to predict ecosystem functions. However, how leaf traits will change within species and
whether interspecific trait relationships will shift under future environmental changes both remain unclear. Here, we examine the bivariate correlations between leaf economic traits of 515
species in 210 experiments which mimic climate warming, drought, elevated CO2, and nitrogen deposition. We find divergent directions of changes in trait-pairs between species, and the
directions mostly do not follow the interspecific trait relationships. However, the slopes in the logarithmic transformed interspecific trait relationships hold stable under environmental
changes, while only their elevations vary. The elevation changes of trait relationship are mainly driven by asymmetrically interspecific responses contrary to the direction of the leaf
economic spectrum. These findings suggest robust interspecific trait relationships under global changes, and call for linking within-species responses to interspecific coordination of plant
traits. SIMILAR CONTENT BEING VIEWED BY OTHERS LEAF TRAIT VARIATION IN SPECIES-RICH TROPICAL ANDEAN FORESTS Article Open access 11 May 2021 LEAF-LEVEL COORDINATION PRINCIPLES PROPAGATE TO
THE ECOSYSTEM SCALE Article Open access 04 July 2023 LEAF WATER CONTENT CONTRIBUTES TO GLOBAL LEAF TRAIT RELATIONSHIPS Article Open access 21 September 2022 INTRODUCTION Leaf traits
represent plant functional strategies and have fundamental effects on vegetation properties and ecosystem functions1,2,3. Divergent leaf traits between species present strong trade-offs that
called the leaf economic spectrum (LES)2. For example, plants with low specific leaf area (SLA) and leaf nitrogen content (Nm) have slow photosynthetic returns, while plants with the
opposite traits have fast photosynthetic returns4,5,6. Large trait variations between species typically represent the evolutionary divergences resulting from genotypes or species turnover.
The plant LES integrates trade-offs of leaf traits and thus provides a useful framework for elucidating leaf-to-ecosystem scaling and for modeling vegetation functional diversity and
dynamics in a changing climate7,8,9,10. In addition, correlations among leaf traits can provide significant constraints on the estimates of vegetation–atmosphere carbon exchange11,12,13.
Leaf traits of a given species can also vary widely in response to environmental changes through a diverse array of physiological, behavioral, and ecological mechanisms14,15, which is
defined as leaf trait plasticity16. Understanding leaf trait plasticity is a major challenge for predicting plant responses to global environmental changes17,18. Various empirical
trait–environment relationships9,19,20 and trait–trait correlations21,22 have been presented to incorporate trait variations into mechanistic vegetation models. However, the extant patterns
are the results of historical evolutionary selections from their specific environmental conditions23. Leaf trait plasticity may differ in magnitude and even direction from the existing
trait–environment relationships24,25. Thus, it is still unclear whether the plasticity-caused trait changes would follow the principles derived from current LES analyses. In fact, the
uncertain changes of trait-pairs in response to environmental changes have long been limiting the predictive application of the interspecific trait relationships26. Furthermore, the
projected novel climate may lead to nonanalog plant functional types and/or trait combinations27,28,29. A better understanding of how leaf traits and their relationships in response to
future environmental changes is crucial for predicting vegetation distribution and ecosystem function30. With the advent of global change manipulative experiments which characterize the
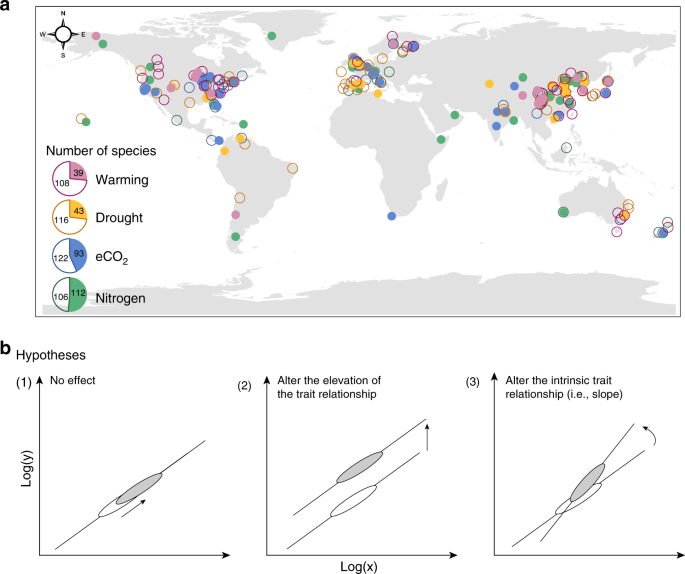
effects of environmental changes on leaf traits, directly quantifying leaf trait plasticity is possible31,32. Here, we combine leaf traits data from 102 field experiments and 108
environmentally controlled experiments (Supplementary Table 1 and Fig. 1a). The field experiments in this database cover extensive climatic regions, with mean annual temperature (MAT)
ranging from −9 to 27 °C, and precipitation from 40 to 4158 mm (Supplementary Fig. 1). The environmental temperature of environmentally controlled experiments ranges from 5 to 33 °C and
relative humidity from 27 to 97%. In total, we compile records of 515 species from 90 families, 60% of which from environmentally controlled experiments and 40% from field experiments (Inset
in Fig. 1a). Based on these data, we quantify the plasticity of net photosynthetic rate (Am), Nm, and SLA to multiple global environmental changes. In addition, we analyze if the changes in
trait-pairs tend to be follow the directions of the interspecific trait relationships. Furthermore, we test whether the intrinsically interspecific trait relationships hold under future
environmental changes. Leaf traits show allometric relationships with the form of power function:1,2 \(y = cx^k\). In the power law, _c_ is the coefficient and _k_ the dimensionless scaling
exponent33. On the logarithmic axes of trait relationships \(\left( {{\mathrm{log}} \,y = k \, {\mathrm{log}} \,x + b;\,b \equiv {\mathrm{log}} \, c} \right)\), slope (_k_) represents the
fundamental mechanisms of the bivariate relationship and slope elevation (intercept, _b_) indicates the resource-use efficiency34. As illustrated in Fig. 1b, if both the slope and elevation
of the log–log axes have no response to environmental changes, then the empirical interspecific trait relationship stays unchanged. Alternatively, if the slope keeps constant but the
elevation shifts, then the scaling exponent remains robust but the resource-use efficiency varies. Conversely, if the slope changes significantly, then the empirical trait relationships are
shifted. In this study we show that, although the direction of trait change in response to environmental changes varies enormously between species and generally does not follow the LES, the
slopes of interspecific trait relationships hold stable. RESULTS RESPONSE OF LEAF TRAITS TO GLOBAL ENVIRONMENTAL CHANGES The response ratios of leaf traits to global environmental changes
were normally distributed (Fig. 2a). On average, experimental warming had no significant effect on Am and Nm, except for a slightly increase in SLA values by 6.9% (Fig. 2b). In contrast, all
trait values varied significantly when the plants experience drought condition. Experimental drought significantly decreased Am (−38.3%) and SLA (−8.7%), respectively, but increased Nm
(6.5%). The elevated atmospheric CO2 concentration roughly induced 16.1% and 12.6% reductions in Nm and SLA but increased Am by 12.6%. Nitrogen addition significantly increased Nm (34.0%)
and Am (12.8%), respectively. However, the commonly measured morphological trait SLA did not shift significantly under nitrogen addition. Plant growth environment (field or environmentally
controlled) and treatment conditions (treatment strength and duration) were important factors affecting trait response35. Our results showed that field experiments presented larger effect on
leaf photosynthesis, but lower impact on leaf nitrogen and SLA than environmentally controlled experiments (Supplementary Fig. 2). Furthermore, the responses of some leaf traits were
affected by treatment strength of warming, drought, and nitrogen addition as well as the experimental duration of elevated CO2 (Supplementary Table 2, Supplementary Fig. 3). The percentage
changes in Am was negatively correlated with warming strength and temperature conditions, and the warming effects varied from positive to negative with the increase of the temperature
(Supplementary Fig. 3a). The effect of drought on Am increased linearly with enhanced drought strength (_R_2 = 0.21, _P_ < 0.01). Similarly, the positive responses of leaf nitrogen to
nitrogen addition increased linearly with applied dose (_R_2 = 0.14, _P_ < 0.01). Our results also revealed that response of SLA to eCO2 logarithmically decreased with increasing
experimental duration. THE DIRECTION OF CHANGES IN PAIRWISE TRAITS UNDER GLOBAL ENVIRONMENTAL CHANGES Importantly, we also tested the consistency between direction of trait plasticity and
the interspecific trait relationships. We plotted the direction of trait plasticity induced by environmental changes for each species into trait–trait space, and found that the changes in
trait-pairs were divergent between species. In addition, we found that only the plasticity of Nm–SLA relationship under eCO2 and the plasticity of Am–SLA under nitrogen addition tended to be
follow the LES direction (Fig. 3i, j). Most of the trait plasticity was equally abundant in all directions of the trait–trait space under warming and drought (Fig. 3a–f). Furthermore, the
directions of trait plasticity under eCO2 and nitrogen addition were mainly asymmetric distributed on one side of the LES (Fig. 3g–l). PLASTICITY OF THE INTERSPECIFIC TRAIT RELATIONSHIPS
UNDER GLOBAL ENVIRONMENTAL CHANGES As shown by the standardized major axis (SMA) regression, the SMA slopes of the interspecific trait relationships held stable under global environmental
changes, but only the SMA elevations varied. Experimental warming had no significant effect on the elevations of interspecific trait relationships (Fig. 4a–c and Supplementary Table 3). The
elevation for Am–SLA correlation was slightly altered by drought (Fig. 4d and Supplementary Table 3). The relatively lower elevation revealed a reduction in photosynthetic rate at a given
unit of SLA under the drought treatment. Similarly, the increased CO2 treatment altered the Am–Nm and Am–SLA relationships via significant enhancement on their elevations (Fig. 4g, h and
Supplementary Table 3). The increased elevations respectively indicated higher photosynthetic rate for a given leaf nitrogen concentration and higher photosynthetic rate for the equivalent
leaf area. Meanwhile, the nitrogen addition significantly lowered the elevations of Am–Nm correlation, but lifted the elevation of SLA–Nm relationship (Fig. 4k, l and Supplementary Table 3).
Overall, the elevation of Am–Nm correlation was most sensitive to global environmental changes, with the elevation changes were −0.19 and 0.15 under nitrogen addition and elevated CO2,
respectively, (Fig. 5). The elevation of Nm–SLA relationship was relatively robust, only significantly altered by nitrogen addition. Note that the relationship change of SLA–Nm under warming
was not detected because of their nonsignificant correlation (Supplementary Table 3). Recent studies have emphasized the equivalent importance of mass- and area-based interspecific trait
relationships36,37. Here, we also tested the robustness of area-based interspecific trait relationships under environmental changes. First, we reconfirmed the disparate patterns of mass- and
area-based trait relationships3,37 (Supplementary Fig. 4). The area-based A–SLA and A–N were weakly related, whereas stronger correlations for mass-based traits were presented. In addition,
contrasting relationships were found between the area- and mass-based N–SLA. Then we showed that the slopes of area-based Na–SLA relationships remained unchanged (all _P_ > 0.05,
Supplementary Table 4). In addition, we found different elevation changes for mass- and area-based N–SLA relationship under elevated CO2 (Supplementary Fig. 4o, r). The robust Nm–SLA
correlation under eCO2 resulted from the proportional changes of Nm and SLA along the original axis, while larger eCO2-dependent decrease in SLA than Na was responsible for the significant
reduction in elevation of Na–SLA. It has been confirmed that species from different functional groups and growth environment (field and environmentally controlled) may have different
eco-physiological constraints when experiencing global environmental changes38,39. We found that interspecific trait relationships have consistent slopes between angiosperm and gymnosperm
woody species as well as between dicotyledons and monocotyledons were consistent. Only the slopes of the Nm–SLA relationship were different between C3 and C4 herbs (Supplementary Table 5 and
Supplementary Fig. 5). Moreover, the slope of trait relationship was not significantly affected by environmental factors in any of the functional groups (all _P_ > 0.05, Supplementary
Tables 6–8). The environmental changes altered the elevation of trait relationship in angiosperm woody but not gymnosperm woody species (Supplementary Fig. 6a). Dicotyledons and
monocotyledons showed similar trait adjustment strategies under eCO2 and nitrogen addition (Supplementary Fig. 6b). We also detected a lower sensitivity of the photosynthetic nitrogen use
efficiency in C4 than C3 plants to eCO2 (Supplementary Fig. 6c). Overall, different response among functional groups have implications for how these plant groups are likely to perform in
future climate change. In addition, we found that the slopes of trait relationships from field and environmentally controlled experiments were both unchanged (all _P_ > 0.05,
Supplementary Table 9), and their elevations changed in the same direction (Supplementary Fig. 7a). The impact of treatment strength on the elevation shift of trait relationship was only
detected in Am–SLA under drought (Supplementary Table 10 and Supplementary Fig. 7b). DISCUSSION This study shows the high plasticity of leaf economic traits under global environment changes.
Plants generally have great plasticity in leaf characteristics to optimize their function under the prevailing changes in environmental conditions40. Globally, climate warming showed
zero-sum impact on leaf traits (Fig. 2a). Our results corroborate the dependence of warming effects on temperature conditions41, and show that warming accelerate leaf photosynthesis in cold
environments, but negatively affect leaf photosynthesis in warmer climates35,42 (Supplementary Fig. 3a). The negative effect of warming may result from warming-induced water deficit or the
excessive temperature that beyond its optimum points43. In addition, both SLA and Nm of plants are sensitive to elevated CO2. The opposite responses of leaf photosynthesis and other leaf
traits strongly suggest that leaf traits move in the opposite direction of the common recognized LES under the increased CO2 concentration44,45, as expressed by higher leaf photosynthesis
and higher carbon investment in leaves. The reduced Nm under elevated CO2 has been attributed to the dilution effect due to rapid growth46 or redistribution of ecosystem N stocks47.
Moreover, our results show that plants tend to alter Nm rather than SLA under higher nitrogen availability. SLA has been found to be a reliable indicator of plant resource-use strategy48.
However, our findings corroborate a recent study49 that leaf nutrient, but not SLA, is an appropriate indicator of plant response to increased nutrients inputs. Considering the extremely
high variability of leaf photosynthesis due to stomata closure under drought, Am is not a reliable indicator of functional response under drought stress. As suggested by some previous
studies13,30, the quantification of the leaf traits plasticity could also provide observational constraints on modeled vegetation and ecosystem function. The unchanged slopes support the
robustness of the reported trait relationships in disparate environmental conditions. We further propose a conceptual framework to show why the interspecific LES maintains despite the
divergent trait plasticity (Fig. 3m). There are three response patterns of pairwise traits under environmental changes: (1) the direction of trait plasticity follows the LES; (2) the
direction of trait plasticity is contrary to the LES with asymmetric responses; and (3) the direction of trait plasticity is contrary to the LES with symmetric responses. As shown by Fig. 3,
most of the directions of trait plasticity were contrary to the LES with asymmetric responses, leading to the invariant slopes of trait–trait relationship under global environmental change.
The observed elevation changes are mainly driven by the asymmetric responses which are contrary to the direction of LES (Fig. 3d, g, h, k, l). The across-species LES could shift when the
trait plasticity is symmetric between upward and downward directions of the LES. The interspecific trait relationships are mostly consistent across experimental types (field and
environmentally controlled) and functional groups (Supplementary Note 1). However, the elevation of trait relationship varies in some cases. For example, the elevation changes are higher in
the environmentally controlled than the field experiments (Supplementary Table 9), which might result from the faster growth rates of plants than that in the field39. The elevations of trait
relationship show larger variations in angiosperms than gymnosperms (Supplementary Fig. 6a). This result suggests a more conserved trait adjustment strategy in gymnosperms than angiosperms,
which has also been found in the root traits50. Due to the higher nitrogen use efficiency in C4 than C3 herbs51, it is expected that the elevation of Am–Nm relationship is greater in C4
than C3 herbs (Supplementary Fig. 6c). However, eCO2 treatment significantly increased the elevation of Am–Nm relationship for C3 but not C4 herbs. This finding suggests that the Am–Nm
relationship could be more stable in C4 than C3 herbs under the higher atmospheric CO2 concentration. It should be noted that the sample size of our database is not large enough to compare
all trait relationships between the control and treatment groups for all functional groups. However, the findings of some differences in the elevation of trait relationship between
functional groups imply the need to explore trait variations across phylogenetically distant species. In fact, some recent global analyses have shown the importance of phylogenetic factors
in explaining plant response to global changes52,53. The elevation of Am–SLA correlation represents the photosynthetic efficiency per-unit leaf mass, which is affected by the investment in
photosynthetic mass per-unit leaf area54,55. Similarly, the elevation of Am–Nm correlation indicates photosynthetic N-use efficiency, shaped by nitrogen allocation to Rubisco or Rubisco-use
efficiency56. Plants are expected to increase investment in structural mass and therefore confer higher physical strength and greater resistance to drought stress57, which could decrease
photosynthetic efficiency per-unit leaf area (Fig. 4e). Without evidence of significant increase in photosynthetic mass per-unit area or Rubisco nitrogen fraction47,58, the uplifted
elevations for Am–SLA and Am–Nm correlations under eCO2 (Fig. 4h) could be attributed to the increased Rubisco-use efficiency. The reduced photosynthetic N-use efficiency under nitrogen
addition has been reported due to the decreased fraction of nitrogen to Rubisco59,60 (Fig. 4j). In addition, the elevation changes in the Nm–SLA correlation result from the variation of leaf
nitrogen per-unit area, which is only significantly enhanced by nitrogen addition (Fig. 4l). Further researches are still needed for a deeper understanding of the physiological mechanism
underlying the elevation change under future environmental changes. The robust slopes of leaf trait relationship across species implie that the traits coordination could be used to predict
ecological consequence of global environmental changes. In fact, plant traits are important parameters in regulating vegetation processes in the framework of Earth system models11,61.
However, an increasing body of evidence has indicated the insufficient realism of current models for their ignorance of leaf traits variability and plasticity28,62. The widely demonstrated
leaf traits plasticity can alter leaf structure and function, thus, plant productivity and land–atmosphere fluxes. Recent advances have incorporated leaf trait plasticity into models for a
more realistic presentation of the responses of terrestrial ecosystem to climate change25,30. Currently, connecting trait relationships across plants, micro-organisms and animals remains a
big challenge for ecology and biogeochemical modeling63. Our study suggests that scaling trait relationships from intra- to interspecies and even up to the community or ecosystem level64 is
an important next step to implement trait-based approaches into modeling future dynamics of the Earth system. In summary, the various patterns of the leaf trait relationships along climate
gradient have long been limiting the predictive utility of such empirical correlations15,34. Such a weak predictive ability could largely stem from the divergent directions of the shifts in
leaf traits under environmental changes among species. It is interesting that the divergent changes in pairwise traits have no impact on the slope of interspecific trait relationships at the
global scale. Overall, this study indicates that the direction of changes in pairwise traits in response to global change varies enormously between species, and the within-species changes
generally do not follow the direction of interspecific trait relationships. However, the slopes of interspecific trait relationships across species are stable under environmental changes,
which indicate the fundamental mechanism of the bivariate relationship does not change. These findings underscore the importance of identifying the key ecological processes which link the
changes of different traits within and between species. METHODS DATA COMPILATION We searched for peer-reviewed journal articles using ISI Web of Science and Google Scholar in March 2018 with
no restriction on publication year. We focused on manipulative studies which included key global environmental changes: (1) warming, (2) eCO2, (3) drought, and (4) nitrogen addition.
Drought here refers to rainfall reduction (field experiment) or irrigation reduction (environmentally controlled experiment). More than 4000 published articles reported changes of leaf
traits (SLA, leaf nitrogen content (N), and/or net photosynthetic rate (A)) under experimental manipulations. To minimize publication bias caused by different data sources, we considered
only articles with at least two of the three leaf traits in this study. After systematic screening, a total of 404 published articles were included in our database (Supplementary Table 1 and
Supplementary Fig. 8). Overall, the experimental approaches included greenhouse, growth chamber, pot, garden, and field habitat. In this study, the experiments conducted in garden and field
habitat were defined as field experiments. The experiments conducted in greenhouse, growth chamber, and pot were classified as environmentally controlled experiments, in which the
disturbances of the other variables were minimized. As a result, this study compiled a trait plasticity database from 102 field experiments and 108 environmentally controlled experiments
(Fig. 1a and Source Data). The consistency of trait relationships between field and environmentally controlled experiments has been tested before using the whole dataset in global analyses
(Supplementary Note 1). The experimental condition of field experiment was characterized by MAT and mean annual precipitation, while the environmentally controlled experiment was described
by experimental temperature (_T_) and relative humidity (%). All original data were extracted from the text, tables, figures, and appendices of the publications. When data were graphically
presented, GetData Graph Digitizer v2.26 was used to obtain numeric data (http://getdata-graph-digitizer.com/). Area- and mass-based measures of any traits were converted through the
following relationship, $$T_m = T_a \times {\rm{SLA}}$$ (1) where _T__m_ and _T__a_ are the values of trait _T_ expressed on per-unit mass and per-unit area, respectively; SLA is the
specific leaf area (in units of cm2 g−1). Error propagation through the conversion is calculated using the following equations: Standard deviation (SD) of _T__m_ equal to, $${\rm{SD}}_{T_m}
= T_m\sqrt {\left( {\frac{{{\rm{SD}}_{T_a}}}{{T_a}}} \right)^2 + \left( {\frac{{{\rm{SD}}_{\rm{{SLA}}}}}{{\rm{{SLA}}}}} \right)^2}$$ (2) where \({\rm{SD}}_{T_m}\), \({\rm{SD}}_{T_a}\) and
\({\rm{SD}}_{\rm{{SLA}}}\) are the SD of trait _T__m_, _T__a_, and SLA, respectively. DATA MODIFICATIONS Considering the statistical assumption of independence among observations, we used
only one measurement of each species from the same study. For all the variables, if more than one observation were reported during the same experiment for the same species, a weighted
average value was calculated by, $$T = \mathop {\sum }\limits_{i = 1}^j \frac{{T_i}}{j}$$ (3) with standard deviation $${\rm{SD}} = \sqrt {\frac{{\mathop {\sum }\nolimits_{i = 1}^j
{\rm{SD}}_i^2\left( {n_i - 1} \right)n_i}}{{\left( {\mathop {\sum }\nolimits_{i = 1}^j n_i - 1} \right)\mathop {\sum }\nolimits_{i = 1}^j n_i}}}$$ (4) where _j_ is the number of
observations, _T__i_, SD_i_, and _n__i_ are the mean, SD, and sample size of the ith sampling data, respectively65. For the potential nonindependence of the same species from different
studies, we created another version of the dataset purged of intraspecific variation by replacing multiple observations per species with the mean of those values3. However, the two datasets
showed same response patterns of trait relationships to different environmental changes (Supplementary Fig. 9 and Supplementary Table 11). Therefore, considering huge experimental difference
(field or environmentally controlled, different treatment strength, different genotypes, etc.) of the same species from different studies, we presented the results with the larger sampling
size (i.e., keeping the same species from different studies) in the main text. We refer to any observation as a species observation in the subsequent analysis. STATISTICAL ANALYSIS The
effects of the global environmental changes on plant functional traits were quantified following the methods described by Hedges et al.66 using Metawin 2.0. The natural logarithm-transformed
response ratio (RR) was used to evaluate the environmental changes effects on leaf traits: $${\mathrm{{ln}RR}} = {\mathrm{ln}}\left( {\frac{{T_t}}{{T_c}}} \right)$$ (5) where _T__t_ and
_T__c_ are the experimental treatment mean and control mean, respectively. The variance (ν) is estimated by: $$v = \frac{{{\rm{SD}}_t^2}}{{n_tT_t^2}} + \frac{{{\rm{SD}}_c^2}}{{n_cT_c^2}}$$
(6) where _n__t_ and _n__c_ represent the sample size, and SD_t_ and SD_c_ are the SD for the treatment and control variables, respectively. The weight of each RR is the reciprocal of its
variance \(\left( {w = \frac{1}{V}} \right)\). Then the weighted response ratio (RR++) is calculated as below (_m_ is the number of groups and _n_ is the number of comparison): $${\rm{RR}}_{
+ + } = \frac{{\mathop {\sum }\nolimits_{i = 1}^m \mathop {\sum }\nolimits_{j = 1}^n W_{ij}{\rm{RR}}_{ij}}}{{\mathop {\sum }\nolimits_{i = 1}^m \mathop {\sum }\nolimits_{j = 1}^n W_{ij}}}$$
(7) with the standard error (SE) is calculated as $${\mathrm{S}}\left( {\rm{{RR}}_{ + + }} \right) = \sqrt {\frac{1}{{\mathop {\sum }\nolimits_{i = 1}^m \mathop {\sum }\nolimits_{j = 1}^n
W_{ij}}}}$$ (8) Then the 95% confidence interval (95% CI) is \({\rm{RR}}_{ + + } \pm 1.96\,{\rm{S}}\left( {{\rm{RR}}_{ + + }} \right)\). The percentage changes of a variable were calculated
as: $${\mathrm{A}} = \left[ {\exp \left( {{\rm{RR}}_{ + + }} \right) - 1} \right] \times 100\%$$ (9) The effects of environmental changes were evaluated as significant, if the 95% CI does
not overlap zero. We also used _Q_-statistic67 to test the heterogeneity of the effect sizes between different functional groups (angiosperm woody vs. gymnosperm woody, dicotyledons vs.
monocotyledons, and C3 herb vs. C4 herb) and growth environment (field vs environmentally controlled conditions, low strength vs high strength, and treatment duration). If _Q__b_ is larger
than a critical value, there would be significant difference between the categories. Statistical significance was tested at the _P_ < 0.05 level. SMA REGRESSION Considering the concurrent
errors in both axes, we used SMA regressions to quantify allometric relationships of pairwise traits under control and treatment conditions. The DOS-based SMATR package used for SMA
regressions allows testing both for homogeneity among SMA slopes via a permutation test and for differences in SMA elevation via the SMA analog of standard ANCOVA68. When homogeneity was
demonstrated (_P_ > 0.05), a common slope was estimated. Elevation homogeneity comparisons were performed only when slopes were homogeneous. Where noted in the results, log10
transformations were carried out on the original data and SMA regression then fitted between leaf functional traits. COVARIANCE ERROR ELLIPSE Covariance error ellipses were created to
summarize the distribution characteristics of leaf traits along the common axis: central tendency, dispersion, and directional trends. These measures define the axes of an ellipse
encompassing the distribution of features. The covariance error ellipse was drawn with Matlab69. REPORTING SUMMARY Further information on research design is available in the Nature Research
Reporting Summary linked to this article. DATA AVAILABILITY The authors declare that the data supporting the findings of this study are available within the paper and its supplementary
information files. Source Data are provided with this paper. CODE AVAILABILITY No custom code or mathematical algorithms were developed for this study. Only existing packages and software
were used for the analysis, which can be found in the “Methods.” REFERENCES * Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: global convergence in plant
functioning. _Proc. Natl Acad. Sci. USA_ 94, 13730–13734 (1997). Article ADS CAS PubMed PubMed Central Google Scholar * Wright, I. J. et al. The worldwide leaf economics spectrum.
_Nature_ 428, 821–827 (2004). Article ADS CAS PubMed Google Scholar * Osnas, J. L., Lichstein, J. W., Reich, P. B. & Pacala, S. W. Global leaf trait relationships: mass, area, and
the leaf economics spectrum. _Science_ 340, 741–744 (2013). Article ADS CAS PubMed Google Scholar * Wright, I. J. et al. Modulation of leaf economic traits and trait relationships by
climate. _Glob. Ecol. Biogeogr._ 14, 411–421 (2005). Article Google Scholar * Reich, P. B. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. _J. Ecol._ 102, 275–301
(2014). Article Google Scholar * Reich, P. B. & Flores-Moreno, H. Peeking beneath the hood of the leaf economics spectrum. _New Phytol._ 214, 1395–1397 (2017). Article PubMed Google
Scholar * Reich, P. B. Key canopy traits drive forest productivity. _Proc. R. Soc. B_ 279, 2128–2134 (2012). Article PubMed PubMed Central Google Scholar * Sakschewski, B. et al. Leaf
and stem economics spectra drive diversity of functional plant traits in a dynamic global vegetation model. _Glob. Chang Biol._ 21, 2711–2725 (2015). Article ADS PubMed Google Scholar *
van Bodegom, P. M., Douma, J. C. & Verheijen, L. M. A fully traits-based approach to modeling global vegetation distribution. _Proc. Natl Acad. Sci. USA_ 111, 13733–13738 (2014). Article
ADS PubMed CAS PubMed Central Google Scholar * Butler, E. E. et al. Mapping local and global variability in plant trait distributions. _Proc. Natl Acad. Sci. USA_ 114, E10937–E10946
(2017). Article ADS CAS PubMed PubMed Central Google Scholar * Sakschewski, B. et al. Resilience of Amazon forests emerges from plant trait diversity. _Nat. Clim. Change_ 6, 1032–1036
(2016). Article ADS Google Scholar * Wang, H. et al. Towards a universal model for carbon dioxide uptake by plants. _Nat. Plants_ 3, 734–741 (2017). Article CAS PubMed Google Scholar
* Wang, Y. P. et al. Correlations among leaf traits provide a significant constraint on the estimate of global gross primary production. _Geophys. Res. Lett._ 39, L19405 (2012). ADS Google
Scholar * Reichstein, M., Bahn, M., Mahecha, M. D., Kattge, J. & Baldocchi, D. D. Linking plant and ecosystem functional biogeography. _Proc. Natl Acad. Sci. USA_ 111, 13697–13702
(2014). Article ADS CAS PubMed PubMed Central Google Scholar * Anderegg, L. et al. Within-species patterns challenge our understanding of the leaf economics spectrum. _Ecol. Lett._ 21,
734–744 (2018). Article PubMed Google Scholar * Rozendaal, D. M. A., Hurtado, V. H. & Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light;
relationships with light demand and adult stature. _Funct. Ecol._ 20, 207–216 (2006). Article Google Scholar * Doughty, C. E. et al. Tropical forest leaves may darken in response to
climate change. _Nat. Ecol. Evol._ 2, 1918–1924 (2018). Article PubMed Google Scholar * Yang, Y. et al. Quantifying leaf-trait covariation and its controls across climates and biomes.
_New Phytol._ 221, 155–168 (2019). Article CAS PubMed Google Scholar * Reich, P. B., Wright, I. J. & Lusk, C. H. Predicting leaf physiology from simple plant and climate attributes:
a global GLOPNET analysis. _Ecol. Appl._ 17, 1982–1988 (2007). Article PubMed Google Scholar * Meng, T. T. et al. Responses of leaf traits to climatic gradients: adaptive variation versus
compositional shifts. _Biogeosciences_ 12, 5339–5352 (2015). Article ADS Google Scholar * Fyllas, N. M. et al. Analysing Amazonian forest productivity using a new individual and
trait-based model (TFS v. 1). _Geosci. Model Dev._ 7, 1251–1269 (2014). Article ADS Google Scholar * Weng, E., Farrior, C. E., Dybzinski, R. & Pacala, S. W. Predicting vegetation type
through physiological and environmental interactions with leaf traits: evergreen and deciduous forests in an earth system modeling framework. _Glob. Change Biol._ 23, 2482–2498 (2017).
Article ADS Google Scholar * Lusk, C. H., Reich, P. B., Montgomery, R. A., Ackerly, D. D. & Cavender-Bares, J. Why are evergreen leaves so contrary about shade? _Trends Ecol. Evol._
23, 299–303 (2008). Article PubMed Google Scholar * Fisher, R. A. et al. Taking off the training wheels: the properties of a dynamic vegetation model without climate envelopes, CLM4.5
(ED). _Geosci. Model Dev._ 8, 3593–3619 (2015). Article ADS Google Scholar * Verheijen, L. M. et al. Inclusion of ecologically based trait variation in plant functional types reduces the
projected land carbon sink in an earth system model. _Glob. Change Biol._ 21, 3074–3086 (2015). Article ADS Google Scholar * Yang, Y., Zhu, Q., Peng, C., Wang, H. & Chen, H. From
plant functional types to plant functional traits: a new paradigm in modelling global vegetation dynamics. _Prog. Phys. Geog._ 39, 514–535 (2015). Article Google Scholar * Williams, J. W.,
Jackson, S. T. & Kutzbach, J. E. Projected distributions of novel and disappearing climates by 2100 AD. _Proc. Natl Acad. Sci. USA_ 104, 5738–5742 (2007). Article ADS CAS PubMed
PubMed Central Google Scholar * van Bodegom, P. M. et al. Going beyond limitations of plant functional types when predicting global ecosystem–atmosphere fluxes: exploring the merits of
traits-based approaches. _Glob. Ecol. Biogeogr._ 21, 625–636 (2012). Article Google Scholar * Keenan, T. F. & Niinemets, Ülo Global leaf trait estimates biased due to plasticity in the
shade. _Nat. Plants_ 3, 16201 (2016). Article PubMed Google Scholar * Kovenock, M. & Swann, A. L. Leaf trait acclimation amplifies simulated climate warming in response to elevated
carbon dioxide. _Glob. Biogeochem. Cycles._ 32, 1437–1448 (2018). Article ADS CAS Google Scholar * Poorter, H., Niinemets, Ü., Poorter, L., Wright, I. J. & Villar, R. Causes and
consequences of variation in leaf mass per area (LMA): a meta-analysis. _New Phytol._ 182, 565–588 (2009). Article PubMed Google Scholar * De Frenne, P. et al. Light accelerates plant
responses to warming. _Nat. Plants_ 1, 15110 (2015). Article PubMed CAS Google Scholar * Marquet, P. A. et al. Scaling and power-laws in ecological systems. _J. Exp. Biol._ 208,
1749–1769 (2005). Article PubMed Google Scholar * Wright, I. J., Reich, P. B. & Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high-
and low-rainfall and high- and low-nutrient habitats. _Funct. Ecol._ 15, 423–434 (2001). Article Google Scholar * Reich, P. B. et al. Effects of climate warming on photosynthesis in
boreal tree species depend on soil moisture. _Nature_ 562, 263–267 (2018). Article ADS CAS PubMed Google Scholar * Lloyd, J., Bloomfield, K., Domingues, T. F. & Farquhar, G. D.
Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand?
_New Phytol._ 199, 311–321 (2013). Article CAS PubMed Google Scholar * Westoby, M., Reich, P. B. & Wright, I. J. Understanding ecological variation across species: area-based vs
mass-based expression of leaf traits. _New Phytol._ 199, 322–323 (2013). Article PubMed Google Scholar * Díaz, S. et al. The global spectrum of plant form and function. _Nature_ 529,
167–171 (2016). Article ADS PubMed CAS Google Scholar * Poorter, H. et al. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled
conditions and in the field. _New Phytol._ 212, 838–855 (2016). Article CAS PubMed Google Scholar * Huang, M. et al. Air temperature optima of vegetation productivity across global
biomes. _Nat. Ecol. Evol._ 3, 772–779 (2019). Article PubMed PubMed Central Google Scholar * Rustad, L. et al. A meta-analysis of the response of soil respiration, net nitrogen
mineralization, and aboveground plant growth to experimental ecosystem warming. _Oecologia_ 126, 543–562 (2001). Article ADS CAS PubMed Google Scholar * Wu, Z., Dijkstra, P., Koch, G.
W., Peñuelas, J. & Hungate, B. A. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. _Glob. Change Biol._ 17,
927–942 (2011). Article ADS Google Scholar * León-Sánchez, L. et al. Altered leaf elemental composition with climate change is linked to reductions in photosynthesis, growth and survival
in a semi-arid shrubland. _J. Ecol._ 108, 47–60 (2020). Article CAS Google Scholar * Temme, A. A. et al. Increases in CO2 from past low to future high levels result in “slower” strategies
on the leaf economic spectrum. _Perspect. Plant Ecol._ 29, 41–50 (2017). Article Google Scholar * Salguero-Gómez, R. Applications of the fast-slow continuum and reproductive strategy
framework of plant life histories. _New Phytol._ 213, 1618–1624 (2017). Article PubMed Google Scholar * Ainsworth, E. A. & Long, S. P. What have we learned from 15 years of free-air
CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. _New Phytol._ 165, 351–371 (2005). Article PubMed
Google Scholar * Luo, Y. et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. _Bioscience_ 54, 731–739 (2004). Article Google Scholar *
Dwyer, J. M., Hobbs, R. J. & Mayfield, M. M. Specific leaf area responses to environmental gradients through space and time. _Ecology_ 95, 399–410 (2014). Article PubMed Google Scholar
* Firn, J. et al. Leaf nutrients, not specific leaf area, are consistent indicators of elevated nutrient inputs. _Nat. Ecol. Evol._ 3, 400–406 (2019). Article PubMed Google Scholar *
Wang, C., McCormack, M. L., Guo, D. & Li, J. Global meta-analysis reveals different patterns of root tip adjustments by angiosperm and gymnosperm trees in response to environmental
gradients. _J. biogeogr._ 46, 123–133 (2019). Article Google Scholar * Taylor, S. H. et al. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening
experiment. _New Phytol._ 185, 780–791 (2010). Article CAS PubMed Google Scholar * Galmes, J., Kapralov, M. V., Copolovici, L. O., Hermida-Carrera, C. & Niinemets, Ü. Temperature
responses of the Rubisco maximum carboxylase activity across domains of life: phylogenetic signals, trade-offs, and importance for carbon gain. _Photosynth. Res._ 123, 183–201 (2015).
Article CAS PubMed Google Scholar * Shao, J. et al. Plant evolutionary history mainly explains the variance in biomass responses to climate warming at a global scale. _New Phytol._ 222,
1338–1351 (2019). Article PubMed Google Scholar * Osnas, J. L. et al. Divergent drivers of leaf trait variation within species, among species, and among functional groups. _Proc. Natl
Acad. Sci. USA_ 115, 5480–5485 (2018). Article CAS PubMed PubMed Central Google Scholar * Onoda, Y. et al. Physiological and structural tradeoffs underlying the leaf economics spectrum.
_New Phytol._ 214, 1447–1463 (2017). Article CAS PubMed Google Scholar * Feng, Y. L. et al. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an
invasive plant. _Proc. Natl Acad. Sci. USA_ 106, 1853–1856 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Onoda, Y. et al. Global patterns of leaf mechanical
properties. _Ecol. Lett._ 14, 301–312 (2011). Article PubMed Google Scholar * Leakey, A. D. et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important
lessons from FACE. _J. Exp. Bot._ 60, 2859–2876 (2009). Article CAS PubMed Google Scholar * Bauer, G. A., Berntson, G. M. & Bazzaz, F. A. Regenerating temperate forests under
elevated CO2 and nitrogen deposition: comparing biochemical and stomatal limitation of photosynthesis. _New Phytol._ 152, 249–266 (2001). Article CAS Google Scholar * Martin, K. C. et al.
Nitrogen fertilization enhances water-use efficiency in a saline environment. _Plant Cell Environ._ 33, 344–357 (2010). Article CAS PubMed Google Scholar * Oleson, K. W. et al.
_Technical Description of Version 4.5 of the Community Land Model (CLM)_. Ncar Technical Note NCAR/TN-503+STR, National Center for Atmospheric Research, Boulder, CO, 422 pp (2013).
https://doi.org/10.5065/D6RR1W7M. * Cui, E. et al. Vegetation functional properties determine uncertainty of simulated ecosystem productivity: a traceability analysis in the East Asian
monsoon region. _Glob. Biogeochem. Cycles_ 33, 668–689 (2019). Article ADS CAS Google Scholar * Fry, E. L. et al. Using plant, microbe, and soil fauna traits to improve the predictive
power of biogeochemical models. _Methods Ecol. Evol._ 10, 146–157 (2019). Article Google Scholar * He, N. et al. Ecosystem traits linking functional traits to macroecology. _Trends Ecol.
Evol._ 34, 200–210 (2019). Article PubMed Google Scholar * Liang, J., Qi, X., Souza, L. & Luo, Y. Processes regulating progressive nitrogen limitation under elevated carbon dioxide: a
meta-analysis. _Biogeosciences_ 13, 2689–2699 (2016). Article ADS CAS Google Scholar * Hedges, L. V., Gurevitch, J. & Curtis, P. S. The meta-analysis of response ratios in
experimental ecology. _Ecology_ 80, 1150–1156 (1999). Article Google Scholar * Gurevitch. J. & Hedges, L. V. Meta-analysis: combining the results of independent experiments. In:
Scheiner, S. M. & Gurevitch, J. (eds) _Design and Analysis of Ecological Experiments_. (Chapman and Hall, New York, 1993). pp 378–425. * Wright, I. J. et al. Assessing the generality of
global leaf trait relationships. _New Phytol._ 166, 485–496 (2005). Article PubMed Google Scholar * Spruyt, V. How to Draw an Error Ellipse Representing the Covariance Matrix? _Computer
Vision for Dummies_, 2014. https://www.visiondummy.com/2014/04/draw-error-ellipse-representing-covariance-matrix/. Download references ACKNOWLEDGEMENTS This work was financially supported by
the National Key R&D Program of China (2017YFA0604600), National Natural Science Foundation of China (31722009 and 41630528) and National 1000 Young Talents Program of China. We also
thank Xiaoni Xu for assistance with meta-analysis, and Chenyu Bian for assistance with statistics. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Zhejiang Tiantong Forest Ecosystem National
Observation and Research Station, State Key Laboratory of Estuarine and Coastal Research, Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration, School of Ecological and
Environmental Sciences, East China Normal University, Shanghai, 200241, China Erqian Cui, Enrong Yan & Jianyang Xia * Research Center for Global Change and Ecological Forecasting, East
China Normal University, Shanghai, 200241, China Erqian Cui & Jianyang Xia * Center for Climate Systems Research, Columbia University, New York, NY, 10025, USA Ensheng Weng * Forest
Ecosystem Research and Observation Station in Putuo Island, East China Normal University, Shanghai, 200241, China Enrong Yan * Institute of Eco-Chongming (IEC), Shanghai, China Jianyang Xia
Authors * Erqian Cui View author publications You can also search for this author inPubMed Google Scholar * Ensheng Weng View author publications You can also search for this author inPubMed
Google Scholar * Enrong Yan View author publications You can also search for this author inPubMed Google Scholar * Jianyang Xia View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS E.C. and J.X. devised and conducted the analysis. E.W. and E.Y. provided critical feedback on the method and results. All authors contributed to
discussion of results and writing the paper. CORRESPONDING AUTHOR Correspondence to Jianyang Xia. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Iván Prieto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cui, E., Weng, E., Yan, E. _et al._ Robust leaf trait relationships across species under global environmental changes. _Nat Commun_ 11, 2999
(2020). https://doi.org/10.1038/s41467-020-16839-9 Download citation * Received: 11 July 2019 * Accepted: 26 May 2020 * Published: 12 June 2020 * DOI:
https://doi.org/10.1038/s41467-020-16839-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
