Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Breast cancer is the most common type of cancer worldwide and one of the major causes of cancer death in women. Epidemiological studies have established a link between night-shift
work and increased cancer risk, suggesting that circadian disruption may play a role in carcinogenesis. Here, we aim to shed light on the effect of chronic jetlag (JL) on mammary tumour
development. To do this, we use a mouse model of spontaneous mammary tumourigenesis and subject it to chronic circadian disruption. We observe that circadian disruption significantly
increases cancer-cell dissemination and lung metastasis. It also enhances the stemness and tumour-initiating potential of tumour cells and creates an immunosuppressive shift in the tumour
microenvironment. Finally, our results suggest that the use of a CXCR2 inhibitor could correct the effect of JL on cancer-cell dissemination and metastasis. Altogether, our data provide a
conceptual framework to better understand and manage the effects of chronic circadian disruption on breast cancer progression. SIMILAR CONTENT BEING VIEWED BY OTHERS THE METASTATIC SPREAD OF
BREAST CANCER ACCELERATES DURING SLEEP Article 22 June 2022 CIRCADIAN REGULATION OF CANCER STEM CELLS AND THE TUMOR MICROENVIRONMENT DURING METASTASIS Article 23 April 2024 CIRCADIAN RHYTHM
REGULATES THE FUNCTION OF IMMUNE CELLS AND PARTICIPATES IN THE DEVELOPMENT OF TUMORS Article Open access 27 April 2024 INTRODUCTION Globally, breast cancer (BC) is the most frequent cancer
in women. The cumulative risk that a woman will develop BC is around 5% worldwide, with a 1.4% risk of death. In 2018, there were more than 2 million newly diagnosed cases, representing
almost 25% of all cancer cases in women. BC is the leading cause of death for women in most countries1. Genetic causes account for <10% of BC; instead, the majority of BC development has
been linked with non-hereditary causes. These include nutrition-related factors, alcohol consumption, exogenous hormone intake, reproductive history, and menstruation parameters1.
Environmental factors such as air pollution or altered light/dark cycles, such as those experienced by night-shift workers, can also affect BC incidence2,3,4. Indeed, in 2007 the
International Agency for Research on Cancer (IARC) classified circadian rhythm disruption (CRD) as probably carcinogenic based on eight epidemiological studies and data from animal models4.
Since then, additional and better-documented epidemiological studies, genome-wide association studies (GWAS), and cellular and animal studies have substantiated the link between BC
development and circadian disruption5. A recent population-based case-control study confirmed that factors including night-work duration, length of shifts, and time since last night shift
affect the odd ratios for BC, mostly in premenopausal women6. In this, hormonal receptor status also plays an important role: BC risk associated with night work is only higher for ER+HER2+
cancer. These epidemiological studies are supported by GWAS analyses that have revealed a significant statistical association between genetic variation located in circadian genes (_ARNTL,
CLOCK, CRY1, CRY2, RORA, RORB, RORC, PER1_) and the risk of breast cancer, as well as between circadian clock-gene expression and metastasis-free survival7,8. Experimental studies on mammary
epithelial cells have also provided evidence of an important role for core circadian clock genes in mammary gland formation and function. Specifically, female _Per2__−/−_ mutant mice fail
to form normal terminal mammary ducts, and instead have an excess of basal progenitors9. Female _Arntl__−/−_ mutants have fewer ductal branches, shorter ductal length and more terminal end
buds, while female _Clock__−/−_ mutants present defects in daytime maternal behaviour and milk production10,11. Studies of core circadian genes in human mammary epithelial cells have
confirmed these in vivo observations and have identified a strong effect of these genes on the stemness of mammary epithelial cells, either by decreasing or increasing stemness9,12,13.
However, it is not always clear whether the observed phenotypes result from the non-circadian function of these transcription factors or are instead an indirect consequence of global
dysregulation of the circadian clock in cells and tissues. Experimental results on the effects of CRD on breast cancer onset have already been obtained using an inducible _p53_ mutant mouse
model that was predisposed to developing primary mammary tumours. These mice typically developed mammary tumours in 50 weeks, but onset was 8 weeks earlier when the mice experienced CRD.
This study supported the key role that CRD can have in driving breast cancer development14. Here, we wanted to progress beyond the onset of tumourigenesis and explore the effects of CRD on
tumour progression, cancer-cell dissemination, and immune phenotype. To do this, we use the MMTV:PyMT model of spontaneous murine mammary carcinogenesis15 and test the effects of chronic CRD
applied for 10 weeks at the beginning of puberty-initiated tumourigenesis. We observe that circadian disruption significantly increases cancer-cell dissemination and metastasis by acting on
the stemness and tumour-initiating potential of tumour cells and by creating an immunosuppressive shift in the tumour microenvironment. RESULTS CHRONIC CRD MODERATELY AFFECTS PRIMARY TUMOUR
DEVELOPMENT The original MMTV:PyMT mouse model with FVB background (FVB PyMT) is known to experience rapid and strong metastasis15; instead, the PyMT mouse model with the C57Bl/6J (B6)
background experiences delayed tumourigenesis, with a more gradual but variable tumour growth rate and reduced lung metastasis compared with FVB PyMT mice16. As the aggressive FVB PyMT model
was incompatible with our goal of modelling long-term chronic CRD, and because we were also interested in investigating the earlier/linear phase of tumour growth, we decided to use a mixed
B6*FVB PyMT background. In line with previous observations, we observed a delayed onset of tumour development and slower cancer progression in these mice compared with typical FVB mice, with
a low prevalence (ca. 30%) of lung metastasis at the age of 16 weeks. To maximise the possibility of observing differences between our experimental groups, mice were analysed in the
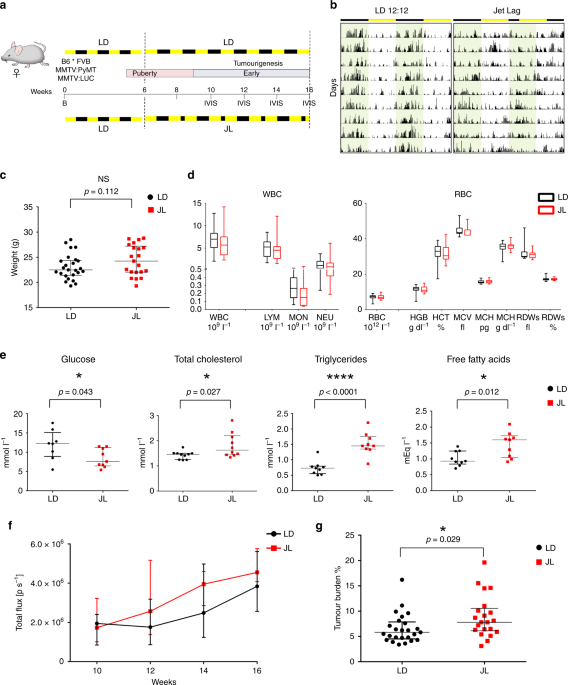
early/mid phase of tumour development, from 6 to 16 weeks (Fig. 1a). At 6 weeks old, mice carrying the MMTV:PyMT and MMTV:LUC transgenes were divided into two lots. One was maintained for 10
weeks in normal conditions of alternating light and dark periods (LD, 12-h light and 12-h dark), while the other group was exposed for 10 weeks to chronic CRD through continuous jet lag,
simulated by a reduction of 8 h in the dark period every other day (JL) (Fig. 1a). This jet-lag protocol was previously shown to totally disrupt the 24-h periodicity of the rhythmic pattern
of rest (12-h light period) and activity (12-h dark period) and is frequently used to mimic the effects of shift work or frequent eastbound transmeridian flights17. By assessing the
locomotor activity and body temperature of these mice, we confirmed that this jet-lag protocol also disrupted circadian activity in our transgenic model (Fig. 1b, Supplementary Fig. 1 and
Supplementary Table 1). From 10 weeks of age to the end of the experiment at 16 weeks, the growth of primary tumours was monitored every 2 weeks by imaging the luciferase activity derived
from the MMTV:LUC transgene. At 16 weeks of age, mice were weighed and sacrificed, and their tissues were processed for further analysis. The median weight of JL mice was slightly but not
significantly higher than that of LD mice, as expected from previous studies (Fig. 1c)18,19. Peripheral blood cell counts were similar between the two experimental conditions (Fig. 1d). In
addition, there were only a few differences in the blood biochemistry parameters of the two groups of mice: glucose level was significantly reduced in JL mice, while total levels of
cholesterol, triglycerides and free fatty acids were significantly increased in the plasma of these mice (Fig. 1e and supplementary Fig. 2). In vivo imaging revealed no significant
difference in the onset of tumourigenesis (Fig. 1f). However, in JL mice, in vivo bioluminescence measurement of tumour growth showed a slight increase with age, and tumour burden was
significantly higher (Fig. 1f, g). We assessed the grade of primary tumours on paraffin-embedded sections stained with HES (hematoxylin, eosin, and saffron). Multiple tumour grades, ranging
from hyperplasia to late carcinoma20, were observed in primary tumours from both LD and JL mice, but, overall, the lesions in JL mice were more malignant (Supplementary Table 2 and
Supplementary Fig. 3). CRD PROMOTES CANCER-CELL DISSEMINATION AND METASTASIS Using these mice, we then explored if chronic CRD affected the dissemination of cancer cells. We quantified the
presence of disseminated cancer cells (DCCs) using flow cytometry and real-time PCR analyses of the expression of the PyMT transgene. With both types of analyses, we observed a significant
elevation of transgene expression in the bone marrow (BM) of JL mice, with an almost two-fold increase in DCCs in the Lin− mononucleated cells of BM (Fig. 2a). Flow analysis also confirmed
an increase in circulating cancer cells (CTCs)in the bloodstream of JL mice (Fig. 2b). Furthermore, we observed DCC cells with H&E staining of bone sections, and µCT analysis also
revealed bone lesions, highlighting the dissemination of cancer cells to bone (Supplementary Fig. 4a). Consistent with these observations, we observed a significant increase in the
prevalence of metastasis in JL compared with LD mice (Fig. 2c). The proportion of mice with lung metastasis increased from 28% in LD to 52% in JL (Fig. 2c) and the number of metastatic foci
was also significantly higher in JL mice (Fig. 2d; Supplementary Fig. 4b). Altogether, our results reveal a significant impact of chronic CRD on cancer-cell dissemination and metastasis. CRD
MODULATES EXPRESSION OF PHOTOTRANSDUCTION GENES In order to identify the potential molecular players and pathways that drove the increase in dissemination in JL mice, we performed an
mRNA-seq study on bulk of dissociated primary tumour cells and on bulk of BM mononuclear cells from five JL and five LD mice (Supplementary Fig. 5a). Hierarchical clustering based on global
gene expression profiles clearly separated BM and primary tumour samples, but did not distinguish between LD and JL conditions or the absence/presence of metastasis in lungs (Fig. 3a). We
confirmed this observation through principal components analysis on BM and primary cancer cells (Supplementary Fig. 5b), in which the two first principal components axes did not separate LD
and JL samples. We then performed differential gene expression analysis. As expected from the hierarchical clustering analysis, only a few genes were significantly differentially expressed
between JL and LD conditions (Supplementary Data 1 and Supplementary Data 2). Intriguingly, in mononuclear BM cells, the genes with the largest differences in expression (_Rhodopsin, Gnat1,
Rbp3_ and _Prph2_) were associated with Gene Ontology (GO) terms linked with photoperception and phototransduction (Fig. 3b). Compared with LD controls, these genes were strongly
downregulated in mononuclear BM cells from JL mice: _Rhodopsin_ by 23-fold, _Gnat1_ by 11-fold, _Rbp3_ by 7-fold and _Prph2_ by 6-fold (Supplementary Data 1). Similarly, in primary tumours
these genes showed significant downregulation (3–4-fold), together with other genes associated with light perception and phototransduction, such as _Cngb1_, _Nrl_ (6-fold) or _Bhlhe40_
(Supplementary Data 2). Hierarchical clustering using the genes belonging to the GO term phototransduction clearly separates BM samples from JL and LD mice while a subset of these genes also
clearly separates primary tumour samples from JL and LD (Fig. 3c and Supplementary Fig. 5c). These data suggest a strong effect of CRD on the expression of genes whose function is
associated with light perception and light signal transduction, even in tissues that are not directly exposed to light (due to poor tissue penetration) or not known to respond to light
stimulation. CRD PROMOTES STEMNESS OF PRIMARY TUMOUR CELLS In order to understand how chronic CRD can affect the dissemination and metastatic potential of mammary cancer cells, we further
characterised the cellular composition of primary tumours. We first tried to assess whether the proportion of mammary cancer stem cells was different between LD and JL conditions. To do
this, we quantified the expression of known markers of mammary cancer-cell stemness from primary tumours. Using flow cytometry, we observed a statistically significant increase in the
percentage of cancer cells that were positive for CD24, CD49f, and CD326 in JL mice (Supplementary Fig. 6a). Similarly, we observed an increase in the expression of _Itgb1_ (coding for CD29)
and _Itga6_ (coding for CD49f) (Supplementary Fig. 6b and Supplementary Data 3), together with the upregulation of genes associated with EMT (Supplementary Fig. 6c and Supplementary Data
3), a key biological process also known to modulate the stemness of breast cancer cells21,22. Moreover, the expression of _Inhibin-βA_ (_Inhba_) was four times higher in the primary tumours
of JL mice. _Inhba_ encodes a protein subunit that is necessary to activate TGFβ signalling, a key pathway associated with EMT23. We then evaluated the proportions of cancer stem cells using
the mouse mammary stem-cell (MaSC) signature CD24medCD29hiCD49fhi (Fig. 4a), which has also been described for mouse mammary cancer stem cells24,25,26,27. We observed a significant
enrichment in CD24medCD29hiCD49fhi cancer cells in the primary tumours of JL mice (Fig. 4b). Next, we tested whether this enrichment was associated with an actual increase in the stemness
potential of primary tumours. To test this hypothesis, we purified cancer cells from primary tumours of LD and JL mice (see Methods) and performed mammosphere-formation assays.
Mammosphere-formation efficiency (MFE) was significantly higher in cancer cells from primary tumours of JL mice (Fig. 4c). Previous studies showed that _Per2_ plays an important role in
tumour suppression as its knockdown increases the stemness of mammary epithelial and cancer cells13,28. We also observed a decrease of _Per2_ and _Cry2_ expression in primary tumours of JL
mice (Fig. 4d and Supplementary Data 3) while expression levels of other core clock genes remained similar between treatments (Fig. 4d and Supplementary Data 3). Furthermore, we observed
that circadian oscillations of clock genes modulated the stemness of human mammary epithelial cells. Specifically, we synchronised MCF12A cells with respect to their circadian rhythm and
sampled cells at times that corresponded to peaks in _PER2_ or _BMAL1_ expression (Supplementary Fig. 6d, e)29. We then assessed the stemness of these cells at the different time points. We
observed a diurnal oscillation in mammosphere-formation efficiency, with a negative correlation with the expression peaks of _PER_ genes (Fig. 4e). Finally, to quantify the tumour-initiation
potential of cells from the primary tumours, we performed orthotopic injection of isolated tumour cells into the mammary fat pads of recipient mice (see Methods). We found that grafted
cancer cells that were purified from JL donor mice demonstrated increased tumour-initiating potential in immunocompetent wild-type C57BL/6J mice compared with cells obtained from LD donors
(Fig. 4f). We further confirmed that the tumours used for transplants and mammospheres formation were all of similar subtypes, ruling out the possibility that this may be the cause of the
observed differences in MFE and tumour-initiating potential (Supplementary Table 2). Altogether, these data support the findings of previous studies, but also provide further evidence for
the importance of a functional circadian clock in regulating the stemness of mammary epithelial cells and deepen our understanding of the biological consequences of CRD. CRD PROMOTES AN
IMMUNOSUPPRESSIVE TUMOUR MICROENVIRONMENT The immune system plays a critical role in tumour progression and metastatic dissemination. To investigate whether the increased prevalence of
metastasis in JL mice resulted from changes in the immune microenvironment of tumours, we characterised tumour-infiltrating cells (TICs) using flow cytometry (see the representative gating
strategy30 in the Supplementary Fig. 7). We found reduced numbers of CD45+ immune cells in tumours from JL mice (Fig. 5a), but no significant alteration in the proportional distribution of
different immune cell types (Fig. 5b; Supplementary Fig. 8). However, fine-scale characterisation of subpopulations of macrophages and T cells revealed significant differences between the LD
and JL tumour immune microenvironment. Specifically, the CD64+CD24− macrophages, determined by using the gating strategy described by Yu et al.30, were further dissected based on their MHC
II expression level. Similarly to previous report31, we identified CD11b+MHC IIhi anti-tumour and CD11b+MHC IIlow pro-tumour tumour-associated macrophages (TAMs) (Supplementary Fig. 7a). In
JL tumours, the percentage of tumour-suppressive MHC IIhi TAMs was not significantly altered compared with LD tumours (Fig. 5c). Instead, JL tumours had a significantly higher proportion of
tumour-supporting MHC IIlow TAMs (Fig. 5d). Characterization of the major T-cell populations revealed a decrease in the number of infiltrating CD8+ T cells and an increase in the CD4/CD8
ratio in JL tumours (Fig. 5e). Importantly, we observed a significant enrichment of the immunosuppressive CD4+FoxP3+ Treg population in the primary tumours of JL mice and consequent
elevation of Treg/CD8 ratio (Fig. 5f), which together with CD4/CD8 ratio is a prognostic indicator of therapy responsiveness and survival in breast cancer patients32,33,34. A significant
elevation in the CD4/CD8 ratio was also detected in the peripheral blood of JL mice (Fig. 5g, Supplementary Fig. 8b). This additional use of CD4 or Treg to CD8 ratio also provides cleaner
representation of TIL/lymphocyte values, which in general show high inter-individual variability (Fig. 5e, f, Supplementary Fig. 8b). Our results suggest that chronic CRD weakens the
anti-tumour immune response and creates an immunosuppressive pro-tumour microenvironment. The latter effect, together with the effects of CRD on cancer-cell stemness, may help to facilitate
the dissemination of mammary cancer cells and the formation of lung metastasis. CHRONIC CRD ALTERS THE CYTOKINE–CHEMOKINE NETWORK The importance of circadian regulation in leukocyte
homoeostasis and migration is well-documented35 to better understand how chronic CRD could modify the immune microenvironment of tumours, we decided to investigate the cytokine–chemokine
network in JL mice. Using a magnetic Luminex assay, we first quantified levels of 17 circulating cytokines in plasma from JL and LD mice. No significant differences were observed, with the
exception of IL-4 whose circulating levels were slightly lower in JL mice (Supplementary Table 3). Since plasma cytokine/chemokine levels are not necessarily representing the tumour
microenvironment, we used the data of our transcriptomic study and real-time PCR to assess the expression levels of cytokines/chemokines and their receptors in primary tumours. We observed
that the most downregulated (<−0.8 Log2FC) cytokines/chemokines in JL primary tumours, including _Ifng, Cxcl13, Tnfs18_ and _Cxcl11_, are known to favour an anti-tumour immune response
(Fig. 6a; Supplementary Data 3). While the other hand, the most upregulated (>0.8 Log2FC) ones, including _Cxcl3_, _Cxcl5, Il10_ and _Il1b_, are linked to immunosuppression or tumour
progression (Fig. 6a, Supplementary Data 3; Supplementary Fig. 9b). In BM mononucleated cells, transcriptomic analysis revealed a high degree of similarity between LD and JL conditions.
However, consistent with the results from primary tumours, we detected significantly elevated levels of _Cxcl5_ in JL BM mononuclear cell samples compared with LD samples (Supplementary Fig.
9e). As CXCL5 has been previously linked to recruitment of suppressive immune cell phenotypes36, metastatic processes37,38,39 and also shown to be under circadian gating35,40 we assessed
its receptor, CXCR2 level in primary tumours. In addition, as a major regulator of tumour cell dissemination we also investigated the expression of CXCR441,42, which is also under circadian
regulation35. In primary tumours, we observed a significant increase in the number of CXCR4+ (Fig. 6b), but not of CXCR2+ (Supplementary Fig. 9c) cancer cells while the number of CXCR2+ TICs
was also significantly increased in JL tumours (Fig. 6c). Collectively, these data support the existence of CRD-driven alterations in the cytokine–chemokine network that promote cancer-cell
dissemination and the immune-suppressive tumour phenotype: potentially a more prominent CXCL12-CXCR4 axis favouring cancer-cell dissemination and specifically, an enhanced role for the
CXCL5-CXCR2 axis that enriches immune-suppressive cell types in the tumour microenvironment. To test this, we explored the effects of CXCR2 on tumour progression in JL mice by treating them
with a CXCR2 inhibitor (SB265610)43,44. Briefly, 10-week-old MMTV:PyMT mice, who had been subjected to chronic jet lag since the age of 6 weeks, were injected with the CXCR2 inhibitor daily
for 5 days then given 2 days’ rest; this treatment continued until they reached 18 weeks of age (Fig. 6d). Mice were then sacrificed and examined as in our previous analyses. In the group
treated with the CXCR2 inhibitor, we observed a significant decrease in the prevalence of lung metastasis (Fig. 6e) and in the amount of PyMT-positive DCCs in the BM (Fig. 6f). While the
percentage of TIC remains similar between conditions (Fig. 6g), the CD4/CD8 ratio was significantly lower in the group treated with the CXCR2 inhibitor, indicating enrichment in cytotoxic
CD8 T cells (Fig. 6h, i, Supplementary Fig. 10a). Finally, in agreement with previous studies37,38,44 we observed that CXCR2 inhibition under LD conditions (Supplementary Fig. 10b–f) also
led to a significant reduction in PyMT-positive DCC and in the CD4/CD8 ratio, without altering the percentage of total TIC, lymphoid and myeloid cells (Supplementary Fig. 10c–f). However,
there was no significant difference in the number of metastases detected in LD mice treated with the CXCR2 inhibitor (Supplementary Fig. 10b). DISCUSSION In addition to epidemiological
studies, there is a growing body of experimental evidence linking circadian disruption to increased breast cancer risk and poorer survival outcome6,8,45,46. Here, we present findings showing
that chronic CRD favours cancer-cell dissemination and metastasis formation, and we shed light on the underlying cellular and molecular alterations. For this purpose we used the MMTV::PyMT
model of spontaneous murine mammary carcinogenesis15,47, which recapitulates many processes involved in human metastatic breast cancer20,48. This model allowed us to work over extended time
periods, representing a physiological context closer to that of human breast cancer, and thus to perform a comprehensive analysis of cancer cells and the tumour microenvironment, with an eye
towards systematic changes and metastatic dissemination. Mice were raised under jet-lag conditions that mimicked the effects of shift work or frequent eastbound transmeridian flights; this
protocol results in severe perturbations in rest-activity cycles, body temperature and clock-gene expression in the CNS and peripheral organs17. It also takes into account the observation
that the circadian rhythm is more disturbed by advances rather than delays in local time49. Our observations on the systemic physiology of these mice confirmed the importance of circadian
rhythm in metabolic processes. In line with previous studies, we found a significant increase in the plasma lipid levels in JL mice compared with controls, supporting the link between CRD
and cardiovascular diseases50,51. Besides lipid metabolism, the feeding-signalling and insulin-glucose axes are also under circadian regulation. Several studies have reported elevated leptin
and insulin resistance in CRD conditions, associated with weight gain, obesity and type-2 diabetes14,52,53. In our study, we detected only fine-scale differences in weight and leptin or
insulin levels, which might be due to the timeframe of our study and to the continuous feeding activity of JL mice, which reduces physiological differences between rest and activity
phases54. Furthermore, we did not examine the ability of carcinogenesis to reprogram hepatic homeostasis and metabolism55,56. For this reason, we cannot exclude that the developing mammary
tumours could have partially rewired hepatic circadian homeostasis and consequently buffered the CRD-induced metabolic changes. Consistent with the slight metabolic changes observed between
conditions, we did not observe major histological differences in primary tumours following 10 weeks of CRD. This supports the hypothesis that the increase in DCCs and the observed enrichment
in malignant lesions in JL mice represent a global speed-up toward carcinogenesis rather than a selective process leading to the development of different tumour subtypes in LD and JL mice.
Previous studies proposed that CRD boosts tumour progression through increased proliferation and metabolic reprogramming17,56,57,58,59. Here we demonstrate that CRD also leads to
significantly enhanced cancer-cell dissemination and metastatic burden. Our results provide experimental evidence that reinforces the findings of previous genetic studies linking cancer
severity, relapse and higher risk of metastasis with the compromised expression of clock genes8,60,61. Specifically, we observed reduced expression of the _Per2_ and _Cry2_ clock genes in
primary tumours of JL mice. Intriguingly, genes involved in phototransduction were among the most significantly altered in both primary tumours and mononuclear BM cells from JL mice. The
expression of several phototransduction genes is under circadian regulation through the NR1D1 (Rev-Erbα)—NRL, CRX and NR2E3 complexes62,63, and their downregulation is certainly related to
the CRD conditions experienced, despite the fact that the analysed tissues were not directly exposed to light stimuli. In mammals, the functionality of phototransduction molecules in
non-visual tissues has only been poorly investigated. Some data have suggested a light-independent mechanism of activation of the phototransduction (transducin/PDE6/Ca2+/cGMP) cascade
through Wnt/Frizzled-2, which might function as an anti-apoptotic mechanism64,65. Two recent studies also showed that peripheral clocks can be synchronised by light independently of a
functional central circadian clock, and suggested that phototransduction players could drive this66,67. Clock genes and circadian oscillation have been shown to regulate the EMT
programme13,68, which is one of the mechanisms behind the spread and colonisation of tumour cells23,69. Our differential gene expression analysis of primary tumours revealed an elevated
expression of genes associated with EMT under CRD conditions. The upregulated EMT-inducers—_Zeb2_, _Foxc2_ and _Inhba_—have also been implicated in promoting cancer stem-cell and metastatic
properties70,71,72. As found in previous studies, our data support the link between the EMT process and the stemness/tumour-initiating ability of cancer cells21,22,73. In particular, we
observed enrichment in the stem-cell population of primary tumours from JL mice. Furthermore, our data reveal an enhanced degree of stemness and tumour-initiation potential for JL cancer
cells in vitro and in vivo. In addition, we demonstrate here that the stemness of mammary epithelial cells oscillates diurnally. This provides further evidence for the role that circadian
rhythm and clock genes play in regulating the stemness of mammary cells. The negative correlation between oscillatory _PER2_ expression and capacity for mammosphere formation confirms the
suppressive effect of _Per2_ on self-renewal and tumour initiation13. Similarly, mammary epithelial cells derived from ClockΔ19 mutant mice showed reduced mammosphere-formation capacity12.
Collectively, these data suggest that CRD promotes metastasis by enhancing the EMT programme and, consequently, the tumour-initiating potential of cancer cells. Circadian regulation of
leukocyte homeostasis, trafficking and immune responses, especially in contexts of infection and inflammation, is well-documented74,75,76. However, little is known about the effect of
circadian rhythms on the tumour microenvironment and tumour immunity. Our results demonstrate that CRD has a profound effect on tumour immunity through modulation of the cytokine–chemokine
network. Our data suggest that CRD attenuates immune infiltration to tumour sites potentially by disrupting the diurnal trafficking of leukocytes and consequently reducing the daily total
number of these cells in the circulation77. Here, we provide evidence that CRD induces a pro-tumourigenic switch of the tumour immune microenvironment, primarily driven by alterations in the
CXCL5-CXCR2 axis. The circadian expression of the CXCL5-CXCR2 axis has been described in previous studies, along with its involvement in inflammatory diseases35,40,78,79. Furthermore, CXCR2
and several of its ligands (CXCL1, CXCL2, CXCL5, CXCL7 and CXCL8) have also been linked to breast cancer progression and metastatic invasion80. Therefore, considering the results of
previous studies, we propose the following inflammatory cascade as a possible mechanism behind CRD-related enhanced tumourigenesis and metastatic spread (Fig. 7): CRD increases the
expression of _Cxcl5_ in tumours, leading to enhanced infiltration of CXCR2+ myeloid cells, e.g. MDSCs. The consequent accumulation of MDSCs, TAMs and TANs promotes an immunosuppressive
microenvironment36. These cells are able to directly suppress T-cell responses and inhibit CD8 T-cell infiltration, resulting in impaired anti-tumour activity36,81,82,83. Collectively, this
autocrine cascade promotes tumour growth and metastasis. In JL mice, the inhibition of CXCR2 significantly reduced lung metastasis and dissemination to bones, which strongly supports our
hypothesized model of CRD-linked tumourigenesis. Two mechanisms have been described that explain how the inhibition of CXCR2 signalling suppresses metastasis. First, CXCR2 signalling has
been shown to play a role in the modulation of the tumour immune microenvironment and the recruitment of MDSCs in distant metastatic sites, along with the consequent development of the
pre-metastatic niche37,38. Second, the CXCL5-CXCR2 axis has also been associated with the process by which circulating tumour cells home to the bone39. In addition, a more recent study
showed its importance in colonization during bone metastasis84. In line with these studies, we observed fewer cancer cells in the BM after inhibition of CXCR2 in both LD and JL conditions,
and a lower degree of metastasis in JL condition (but not in the LD group), but it remains unclear which one of these mechanisms (or both) might be responsible. Here, in line with previous
studies37,38, we suggest the use of CXCR2 inhibition in combination with conventional chemotherapy or immunotherapy to reduce cancer-cell dissemination and improve therapy outcome. This
could be especially beneficial in cases with known CRD (either systemic or localised, tumour specific alteration of the circadian molecular clock) where the CXCR2 driven mechanisms are
accelerated, as our results show. Furthermore, we also describe here upregulation in the CXCL12-CXCR4 axis, which is another key mechanism that promotes metastatic spread in JL mice.
Circadian control of the CXCL12-CXCR4 axis35,77,85 and its role in immunosuppression and breast cancer metastasis are well described41,42,86. The CRD-induced alterations we describe in
chemokine/chemokine-receptor signalling drive the changes in the tumour microenvironment and metastatic capacity. Recent studies have also suggested the use of CXCR2 or CXCR4 inhibitors in
combination with immunotherapy to overcome therapeutic resistance83,87. These therapeutic strategies, together with the use of predictive tools based on clock-gene expression for cancer
prognosis, will lead to precision circadian medicine with improved efficacy and responsiveness to immunotherapy46,88. In conclusion, our study provides, experimental evidence of the link
between CRD and an increase in cancer-cell dissemination and metastasis. Based on our results, we propose two molecular mechanisms by which CRD promotes tumourigenesis: (1) CRD increases the
stemness and tumour-initiating potential of cancer cells, at a minimum by promoting a pro-EMT intra-tumoural context, and (2) CRD reduces anti-tumour immunity through modifications in
chemokine/chemokine-receptor signalling. Finally, our findings draw attention to the potential role of genes involved in phototransduction in peripheral tissues. However, our data do not
confirm whether the observed downregulation of these genes in JL mice is due to their circadian rhythmicity in LD mice or whether it reflects a functional role in peripheral tissues. This
intriguing observation in bone marrow cells and primary tumours during CRD need further investigation. METHODS MOUSE AND TUMOUR MODEL Mouse strains MMTV-PyMT (FVB/N background, JAX #002374)
and MMTV-Luc2 (B6; FVB background, JAX #027827) were purchased from the Jackson Laboratory. Five-week-old female C57BL/6JOlaHsd mice were purchased from Envigo (The Netherlands). To
establish our experimental colony, the FVB PyMT mice were bred to C57Bl/6J (B6) mice for three generations. Mice in the F4 generation of the B6*FVB PyMT cross were then bred to B6*FVB
MMTV-Luc2 mice. The F1 generations of these PyMT and Luc2 crosses were used in our experiment. We observed that these mixed-background mice (B6*FVB PyMT::Luc2) developed palpable mammary
tumours by 10–12 weeks of age and reached the late/exponential phase of tumour growth at 18–20 weeks of age. Pulmonary macro-metastases were detected starting from 16 weeks, with increased
prevalence at 19–20 weeks of age. Based on these observations, we decided to sacrifice mice at 16 weeks of age (Fig. 1a), in the early/mid phase of tumour growth. Details of tumour
evaluation and sample processing can be found below. Mice were crossed, reared and housed in the institutional animal facility SEIVIL (Service D’Experimentation Animale in vivo INSERM
Lavoisier). Mice were housed in groups in standard rectangular cages with unrestricted access to food and water and provided with nesting material. Mean temperature and humidity were 22 °C
and 50%, respectively. Telemetry experiments were performed in the LET (Laboratoire d’Enregistrement Télémétrique, Inserm U776). All mouse experiments were performed in accordance with
DIRECTIVE 2010/63/EU guidelines and were approved by the CEEA26-Paris-Sud Ethics Committee and the French Ministry of Higher Education and Research (APAFIS #8475-2017011015222063, APAFIS
#13183-2018012412194882, APAFIS #22201-2019093016134321). CELL-LINE CULTURE The immortalised non-tumourigenic human epithelial cell line MCF12A (ATCC, CRL-10782)89 was cultured in DMEM/F12
(Gibco) supplemented with 5% horse serum (Sigma), 10 ng ml−1 cholera toxin (Sigma), 10 μg ml−1 insulin (Sigma), 0.5 μg ml−1 hydrocortisone (Sigma), 20 ng ml−1 human recombinant epidermal
growth factor (StemCell Technologies), 1% Penicillin/Streptomycin (Gibco) and 1% L-Glutamine (Lonza). JET-LAG CONDITIONS Mice were kept under normal 12/12 h light/dark (LD) conditions until
6 weeks of age. At this point they were randomly assigned either to remain in LD or to be exposed to jet-lag (JL) conditions, with an 8-h advance in the light/dark cycle every 2 days17,59.
To induce chronic circadian rhythm disruption (CRD), mice were kept in JL conditions for 10 weeks in a specialised chronobiological facility; all mice had free access to food and water.
Zeitgeber time (ZT) 0 corresponded to the onset of light, while ZT12 corresponded to the onset of dark. LD mice were sampled at ZT3-ZT4; in the case of JL mice, ZT was not followed.
REST/ACTIVITY AND TEMPERATURE RECORDING We used the DSI Implantable Telemetry system (Data Sciences International, St. Paul, MN) to assess locomotor activity and core body temperature of
mice kept in either LD or JL conditions. A telemetric transmitter (PhysioTel TA-F10, DSI) was implanted in the peritoneal cavity of each mouse under isoflurane anaesthesia. Dataquest A.R.T.
v4.30 software was used for data collection. Data were recorded every 10 min throughout the experiment. We implanted transmitters in 6 mice at the age of 14 weeks (mice had reached the
required weight of 20 g). Mice were kept 2 weeks in standard light conditions (LD) for recovery/synchronisation, then they were randomly assigned to either the LD or JL protocol for 4 weeks
of recording. Actograms were created using Microsoft Excel following the method described by Oike et al.90. Actogram data were analysed and visualised in ImageJ using the ActogramJ software
package91 and in GraphPad Prism v601. Data from D10-D20 (mice age of 15 to 16 weeks) and D32-D46 (mice age of 18 to 19 weeks) post-implantation were used for periodogram analysis. Period was
determined in ActogramJ software package for activity and core body temperature patterns using Fourier and Lomb-Scargle methods, respectively. To confirm circadian rhythmicity of activity
and temperature patterns we defined the coefficients of determination (R2) by fitting a cosine algorithm in GraphPad Prism v60129. BIOLUMINESCENCE IMAGING For in vivo imaging of tumours,
mice were intraperitoneally (i.p.) injected with 150 mg luciferin kg−1. Luciferin was resuspended in PBS at a concentration of 15 mg/ml and filter-sterilised with a 0.22-µm filter. Following
injection, mice were anaesthetised with isoflurane gas (2% during imaging period). Bioluminescence imaging was performed 15, 20, 25 and 30 mins after injection, as kinetic analysis
determined that peak luciferase activity occurred 20–25 min post-injection (IVIS Spectrum imaging system, PerkinElmer). Images were analysed with Living Image software. To determine tumour
burden, total flux (photons per second) was measured in a fixed region of interest (whole body). SAMPLE COLLECTION Mice were anesthetised with isoflurane inhalation. Blood was collected by
cardiac puncture and placed in EDTA (10% 0.5 M EDTA). Following cervical dislocation, we collected tumours/mammary glands, lungs, and both hind-limb bones and stored them in ice-cold PBS
until further processing. LD mice were sampled at ZT3-ZT4; in the case of JL mice, ZT was not followed. A blood cell count analysis was performed on 60 μl of whole blood using a VETSCAN HM5
haematology analyser (ABAXIS). The remainder of the blood was spun down at 300 × _g_ for 20 min at 4 °C; from this, 150–300 μl plasma were collected and stored at −80 °C until further
analysis. Erythrocytes were lysed using 1X red blood cell (RBC) lysis buffer (15.5 mM ammonium chloride, 1 mM potassium bicarbonate, 10 μM EDTA in distilled water). White blood cells were
frozen in 10% DMSO/FBS and preserved at −150 °C for flow cytometry analysis. Tumours were weighed to calculate tumour burden (tumour burden % = [tumour weight (g) per body weight (g)] ×
100). A small portion of the tumours was fixed in 4% paraformaldehyde (PFA) for histological analyses. The rest of the tumours were dissociated (see details below). Lungs were incubated in 1
μg ml−1 heparin in PBS for 1 h at 4 °C, then fixed in 4% PFA for 2–3 days. Bone marrow (BM) was isolated from femurs and tibias of either one or both hind limbs. BM cells were washed two
times with PBS. Erythrocytes were removed by red blood cell lysis. BM cells were frozen in 10% DMSO/FBS and preserved at −150 °C for flow cytometry analysis. In the cases in which only one
hindlimb was flushed out, the other was fixed in 4% PFA for histological examination. TUMOUR DISSOCIATION AND CELL ISOLATION Tumours were minced finely with scalpels. Samples were placed
into 50-ml tubes with 10–15 ml of pre-warmed dissociation media (1 mg ml−1 collagenase type I, 0.5 mg ml−1 Dnase I and 1% Penicillin-Streptomycin in DMEM F12) and incubated at 37 °C on a
rotator shaker for 1 h. Samples were gently vortexed every 20 min to avoid clumping. Following incubation, samples were filtered through a 70-μm nylon strainer and topped up to 40 ml with
PBS/EDTA (2 mM). Cells were spun down at 450 × _g_ for 10 min. The top fatty layer was collected, topped up to 40 ml with PBS/EDTA, gently vortexed, and centrifuged. Tumour cells were
resuspended in 2 ml 1x RBC lysis buffer and incubated for 5 min to remove red blood cells. The cell suspension was filtered through a 40-μm nylon strainer. Single cells were frozen in 10%
DMSO/FBS and preserved at −150 °C for flow cytometry analysis and tumour-initiation study. DETECTION AND QUANTIFICATION OF LUNG METASTASIS Lungs fixed in 4% PFA were washed in PBS then
permeabilised with 0.1% Triton X/PBS for 2 days. Lungs were incubated in 3% hydrogen peroxide (H2O2) for 30 min, then the solution was diluted to 1% H2O2 with PBS and incubated overnight.
Blocking was performed with 5% FBS/1% BSA/PBS for 24 h. Lungs were again rinsed with PBS, then incubated with anti-PyMT antibody (sc-53481, Santa Cruz, 1:100 dilution) for 48 h. Following
overnight washing, lungs were incubated with rabbit anti-rat IgG AP (Thermo Fisher, 1:1000 dilution) secondary antibody for 24 h. To reveal antibody labelling, lungs were placed into
NBT/BCIP working solution. After colour developed, lungs were washed, each lobe was imaged using a stereomicroscope, and metastatic foci were counted. All incubations and washing were
performed at 4 °C. BONE TISSUE PROCESSING AND ANALYSIS Tissues samples were fixed in 4% paraformaldehyde overnight at 4 °C. Micro-CT imaging and analyses were performed with a SkyScan 1172
apparatus (Bruker micro-CT), using a voxel size of 12 μm, a voltage of 50 kV, an intensity of 200 μA and an exposure of 900 ms. NRecon reconstruction and DataViewer software were used for 3D
reconstruction. Analysis of structural parameters was performed using CTVox. After the micro-CT scan, hind limbs were processed to obtain paraffin sections for histological analysis as
follows: Hind limbs were decalcified in 20% EDTA (pH 7.5) at 4 °C with constant shaking for 15 days; the EDTA solution was replaced every 2–3 days. Decalcified bones were embedded in
paraffin and sectioned at 5 μm using a Leica microtome. Sections were then stained with hematoxylin and eosin. FLOW CYTOMETRY We characterised tumour cells using the following antibodies:
CD45 PB (1:200), CD31 PB (1:500), TER-119 PB (1:200), CD140A BV421 (1:100), CD24 BV510 (1:100), CD29 FITC (1:100), CD49f PE (1:100), CD44 Pe/Cy7 (1:100), CD90.1 APC (1:100), CD326 APC/Vio770
(1:100), CD45 PE/Dazzle594 (1:100), CXCR1(CD181) AF750 (1:50), CXCR2(CD182) PE/Vio770 (1:50), and CXCR4(CD184) APC (1:50). Tumour cells were identified as Lin− (CD45−CD31−CD140a−Ter119−).
To phenotype tumour-infiltrating immune cells we used the antibodies CD45 PB (1:200), CD24 BV510 (1:200), CD11b FITC (1:100), CD64 PE (1:200), CD11c Pe/Cy7(1:100), MHC II (IA/AE) APC
(1:1500) and Ly6G APC/Vio770 (1:100), and a gating strategy as described by Yu et al.30 and shown in Supplementary Fig. 7A. Major phenotypes of tumour-infiltrating lymphocytes (TILs) and
peripheral blood T cells were identified using the antibodies CD45 PE/Dazzle594 (1:50), CD3 BV510 (1:20), CD8a APC/Fire750 (1:50) and CD4 AF488 (1:50). To detect CD4+FoxP3+ regulatory T
cells we used the True-Nuclear Mouse Treg Flow kit (FoxP3 AF488 (1:20), CD4 APC/CD25 PE (1:10), Biolegend) in combination with CD45 PE/Dazzle594 (1:50), CD3 BV510 (1:20) and CD8a APC/Fire750
(1:50). Gating strategy shown in Supplementary Fig. 7b. To detect disseminated tumour cells, mononuclear cells from blood or bone marrow were, respectively, stained for CD45 (1:100) or CD45
(1:100), CD31 (1:500), TER-119 (1:200) and CD140A (1:100). Following cell-surface staining, cells were fixed with 1% PFA/PBS for 30 min then permeabilised with 0.1% Triton X/PBS for 20 min.
Cells were blocked with 5% FBS/1% BSA/PBS for 30 min. Following a PBS wash, cells were incubated with anti-PyMT AF488 antibody (1:10) for 1 h. All incubations and staining were performed at
4 °C. To distinguish between live/dead cells, either propidium iodide (PI, 1:1000) or Zombie Violet (1:500) fixable viability stain was used. Isotype controls were used, respectively, at
corresponding concentration. Detailed antibody list provided as Supplementary Table 4. FlowJo Software (version 10, Tree Star Inc) was used for flow cytometry data analysis. RNA ISOLATION
AND RT-PCR Total RNA was extracted from bulk of dissociated cells from primary tumours and from bulk of bone marrow mononuclear cells using the PureLink RNA kit (Invitrogen) according to the
manufacturer’s instructions. cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) with Oligo(dT) primers (ThermoScientific). RT-PCR was
performed with QuantStudio 5 (384-well format, Thermo Fisher) using FastStart Universal SYBR Green Master mix (Roche). Samples were analysed in replicates and melting curve analysis was
performed for each run. The geometric mean of Ct values for _Ctbp1_, _Prdx1_ and _Tbp_ or _36B4_, _RPL4_, _HSPCB_ and _TBP_ were, respectively, used for normalisation of mice and human
samples. Relative fold change (2−ΔCt) and gene expression were represented in arbitrary units. Primer sequences were: _PyMT_ fw: CTCCAACAGATACACCCGCACATACT, rv: GCTGGTCTTGGTCGCTTTCTGGATAC
_Cxcl5_ fw: GGGAAACCATTGTCCCTGA, rv: TCCGATAGTGTGACAGATAGGAAA _Cxcl3_ fw: CAGCCACACTCCAGCCTA, rv: CACAACAGCCCCTGTAGC _Il1b_ fw: GCTTCCTTGTGCAAGTGTCT, rv: GGTGGCATTTCACAGTTGAG _Ctbp1_ fw:
GTGCCCTGATGTACCATACCA, rv: GCCAATTCGGACGATGATTCTA _Prdx1_ fw: AATGCAAAAATTGGGTATCCTGC, rv: CGTGGGACACACAAAAGTAAAGT _Tbp_ fw: AGAACAATCCAGACTAGCAGCA, rv: GGGAACTTCACATCACAGCTC _BMAL1_ fw:
TGGAGAAGGTGGCCCAAAGA, rv: TCCTCAGCAATCATTCGGCCTA _PER2_ fw: GTCCACCTCCCTGCAGACAA, rv: CTGGTAATACTCTTCATTGGCTTTCA _36B4_ fw: GTGATGTGCAGCTGATCAAGACT, rv: GAAGACCAGCCCAAAGGAGA _RPL4_
fw:GCCAGGAATCACAAGCTCCG, rv: CCGCCGCCTTCTCATCTGAT _HSPCB_ fw: AGAAGGTTGAGAAGGTGACAATC, rv: AGTTGTCCCGAAGTGCCTG _TBP_ fw: TGCTGCGGTAATCATGAGGA, rv: GTCTGGACTGTTCTTCACTCTT. MRNA-SEQ ANALYSIS
mRNA-seq was performed by BGI (Hong Kong) using their standard procedures. mRNAs were purified using oligo(dT)-attached magnetic beads. cDNA was synthesised from fragmented mRNAs using
random hexamer-primers, then the synthesised cDNA was subjected to end-repair and 3′ adenylation. Adapters were ligated to the ends of these 3′ adenylated cDNA fragments, and cDNA fragments
were then amplified by PCR and purified with Ampure XP Beads (AGENCOURT). The size and quantity of libraries were validated on an Agilent 2100 Bioanalyzer. The double-stranded PCR products
were heat-denaturated and circularised using a splint oligo sequence. The single-strand circular DNA (ssCirDNA) was formatted as the final library. Each library was amplified with phi29 to
make a DNA nanoball (DNB) which contained more than 300 copies of the given molecule. The DNBs were loaded into the patterned nanoarray and single-end 50-base reads were generated on a
BGISEQ-500 sequencing platform. For each library a minimum of 23 million single-end reads were produced. All raw sequencing reads were filtered using SOAPnuke software
(https://github.com/BGI-flexlab/SOAPnuke) to remove reads with adaptors, reads made up of more than 10% unknown bases, and low-quality reads. The resulting clean reads were then stored in
FASTQ format. Reads were mapped using Bowtie292 on the mouse reference genome mm10 (GRCm38); gene expression levels were calculated with RSEM93 and normalised using the value of fragments
per kilobase of exon per million fragments mapped (FPKM) in each sample. Normalised gene expression values were used to produce heatmaps using the ComplexHeatmap package of R94, using
Euclidean distances and the agglomeration method Ward.D2 of the function hclust. Principal Components Analyses were performed using the pca function of the mixOmics package95. Differentially
expressed genes were detected using DESeq2, with un-normalised counts as input96. DEGs were then used to assess pathway functional enrichment with the KEGG annotation database, using the R
package hypeR97. DEGs were ranked in a list according to their _p_-values, and Gene Set Enrichment Analysis (GSEA) was performed using GSEA software from the Broad Institute98.
IMMUNOHISTOCHEMISTRY Primary tumours (fixed in 4% PFA) were washed in PBS and transferred to 70% EtOH. They were then embedded in paraffin wax according to standard histological protocols.
Paraffin-embedded-sections (5-µm-thick) were mounted on adhesive slides (Klinipath-KP-PRINTER ADHESIVES), then deparaffinised and stained with HES (hematoxylin, eosin and saffron) for
morphological observation of tumours. All slides were scanned with a Pannoramic Scan 150 (3D Histech) and analysed with a CaseCenter 2.9 viewer (3D Histech). TUMOUR-INITIATION STUDY Cancer
cells were enriched by magnetic bead-based negative selection using a cocktail of CD45, CD140a, CD31 and Ter119 biotinylated antibodies (Miltenyi Biotec), followed by labelling with
streptavidin microbeads. Flow-through cells were collected, counted, and kept on ice until further use. One-hundred thousand live tumour cells from each donor were injected into the right
4th mammary fat pad of 10-week-old wild-type C57BL/6J mice. In preparation for injection, tumour cells were resuspended at a concentration of 5 × 106 cells/ml in PBS. Twenty microliters of
cell suspension was mixed with 20 μl of Geltrex (Gibco) and immediately injected into the mammary fat pad using a U-100 insulin syringe. Host mice were kept in LD conditions from 6 weeks of
age until sacrifice at 18 weeks. CXCR2 INHIBITION Mice were kept under JL conditions starting from 6 weeks of age; inhibition treatment took place from 10 to 18 weeks of age. The CXCR2
antagonist SB265610 was resuspended in 5% DMSO–8% Tween80 in 0.9% NaCl and injected into mice daily (5 days i.p. injection + 2 days resting) at a concentration of 2 mg kg−1. The same
experiment was performed on LD mice, for which the injection time corresponded to ZT6-ZT7, when the highest number of CD45+ cells was detected in the peripheral blood82. Under jet-lag
conditions no specific ZT was kept, but injections were always performed at the same time of the day (between 1 and 2 pm, at the same time as the LD group). A subset of each group was
injected only with vehicle and used as control. JL mice were sacrificed at 18 weeks of age. In LD conditions, however, this experiment was performed on PyMT- FVB* Luc2-B6 F1 hybrid mice,
which exhibited faster tumour progression. For this reason, LD mice were sacrificed at the age of 14 weeks. PLASMA CHEMISTRY AND ENDOCRINE ANALYSIS All measurements were performed by the
Clinical Chemistry and Haematology platform of Phenomin-ICS (Institut Clinique de la Souris, Strasbourg). Blood chemistry (glucose, albumin, CK, LDH, ASAT, ALAT, ALP, a-amylase, total
cholesterol, HDL and LDL cholesterol, triglycerides and creatinine) was analysed on an OLYMPUS AU-480 automated laboratory work station (Beckmann Coulter, USA) with the kits and controls
supplied by Beckmann Coulter. Amounts of free fatty acids were measured on the AU-480 using a kit from Wako (Wako Chemical Inc, Richmond, USA). Internal quality control materials (Olympus)
were analysed on a daily basis to ensure precision throughout the experiment. Insulin levels were measured on a BioPlex analyser (BioRad) using the Mouse Metabolic Magnetic bead panel kit
(Reference: MMHMAG-44K—Milliplex map by Millipore). Corticosterone was measured by RIA using the Corticosterone 125I RIA kit for rats & mice (MP biomedical: 07-120102) LUMINEX ASSAY A
mouse magnetic multiplex Luminex assay was purchased from R&D Systems-biotechne for 18 analytes: MCP-1/CCL2, KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5, SDF1/CXCL12, G-CSF, GM-CSF, IFNγ, IL-1β,
IL-2, IL-4, IL-6, IL-10, IL-12p70, Leptin, M-CSF, TNFα and VEGF. Measurements were performed by the Cochin Cytometry and Immunobiology Facility (CYBIO, Institut Cochin, Paris) using a
Bioplex 200 apparatus (Luminex). CIRCADIAN SYNCHRONISATION OF CELLS MCF12A cell were entrained by serum shock. Cells were seeded into 60-mm dishes at a density of 200,000 cells per dish.
Following 4 days of culture in complete medium, cells were washed with PBS and starved for 12 h in basal DMEM/F12 medium. Cells were then stimulated with serum shock (complete growth medium
supplemented with 50% of horse serum) for 2 h. At the end of synchronisation (Zeitgeber Time ZT0), cells were washed with pre-warmed PBS and placed in pre-warmed DMEM/F12 that was
supplemented with 20 ng ml−1 hrEGF. Cells were collected at ZT24, ZT36, ZT48 and ZT60 for the mammosphere-formation assay. MAMMOSPHERE FORMATION To examine mammosphere formation, we plated
either 500 primary murine tumour cells or 1000 MCF12A cells per well into ultra-low-attachment 24-well plates. Cells were cultured in DMEM/F12 that was supplemented with 5 µg ml−1 insulin, 4
µg ml−1 heparin, 5 µg ml−1 hydrocortisone and 20 ng ml−1 recombinant murine or human EGF, respectively. Mammosphere formation was assessed in 2–4 replicates. Spheres were imaged after 20
days for primary tumour cells and 8 days for MCF12A. Measurements were performed in ImageJ software; the threshold was 50 μm for primary tumourspheres and 40 μm for normal human spheres.
Mean values of replicates were used for data analysis. Mammosphere-formation efficiency (MFE) was calculated and presented as a percentage. STATISTICAL ANALYSIS GraphPad Prism v6.01
(GraphPad Software, USA) was used to prepare all graphs and perform statistics, unless stated otherwise. Results were analysed using _t_-tests (unpaired, two-sided), binomial two-sided test
or one-way ANOVA, as appropriate. DATA AVAILABILITY The RNA sequencing data are available at the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under the study accession number
PRJEB33802 . KEGG annotation database is available online at https://www.genome.jp/kegg/annotation/. All other relevant data are available in the Article, Supplementary Information or from
the corresponding authors upon reasonable request. The source data underlying Figs. 1b–f, 2a–d, 3b–f, 4a–d, f, 5a–g and 6a–c and e–i, and Supplementary Figs. 1, 2, 6, 8–10 are provided as a
Source data file. A reporting summary for this article is available as a Supplementary Information file. REFERENCES * Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of
incidence and mortality worldwide for 36 cancers in 185 countries. _Ca. Cancer J. Clin._ 68, 394–424 (2018). Article PubMed Google Scholar * Tagliabue, G. et al. Atmospheric fine
particulate matter and breast cancer mortality: a population-based cohort study. _BMJ Open_ 6, e012580 (2016). Article PubMed PubMed Central Google Scholar * Schernhammer, E. S. et al.
Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. _JNCI J. Natl Cancer Inst._ 93, 1563–1568 (2001). Article CAS PubMed Google Scholar *
Hansen, J. Night shift work and risk of breast cancer. _Curr. Environ. Health Rep._ 4, 325–339 (2017). Article PubMed Google Scholar * Samuelsson, L. B., Bovbjerg, D. H., Roecklein, K. A.
& Hall, M. H. Sleep and circadian disruption and incident breast cancer risk: An evidence-based and theoretical review. _Neurosci. Biobehav. Rev._ 84, 35–48 (2018). Article PubMed
Google Scholar * Cordina-Duverger, E. et al. Night shift work and breast cancer: a pooled analysis of population-based case–control studies with complete work history. _Eur. J. Epidemiol._
33, 369–379 (2018). Article PubMed Google Scholar * Mocellin, S., Tropea, S., Benna, C. & Rossi, C. R. Circadian pathway genetic variation and cancer risk: evidence from genome-wide
association studies. _BMC Med._ 16, 20 (2018). Article PubMed PubMed Central CAS Google Scholar * Cadenas, C. et al. Loss of circadian clock gene expression is associated with tumor
progression in breast cancer. _Cell Cycle_ 13, 3282–3291 (2014). Article CAS PubMed PubMed Central Google Scholar * McQueen, C. M. et al. PER2 regulation of mammary gland development.
_Development_ 145, dev157966 (2018). Article PubMed PubMed Central CAS Google Scholar * Boden, M. J., Varcoe, T. J., Voultsios, A. & Kennaway, D. J. Reproductive biology of female
Bmal1 null mice. _Reproduction_ 139, 1077–1090 (2010). Article CAS PubMed Google Scholar * Hoshino, K., Wakatsuki, Y., Iigo, M. & Shibata, S. Circadian clock mutation in dams
disrupts nursing behavior and growth of pups. _Endocrinology_ 147, 1916–1923 (2006). Article CAS PubMed Google Scholar * Yang, N. et al. Cellular mechano-environment regulates the
mammary circadian clock. _Nat. Commun._ 8, 14287 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Hwang-Verslues, W. W. et al. Loss of corepressor PER2 under hypoxia
up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. _Proc. Natl Acad. Sci. USA_ 110, 12331–12336 (2013). Article ADS CAS PubMed PubMed Central Google Scholar
* Van Dycke, K. C. G. et al. Chronically alternating light cycles increase breast cancer risk in mice. _Curr. Biol._ 25, 1932–1937 (2015). Article PubMed CAS Google Scholar * Guy, C. T.,
Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. _Mol. Cell. Biol._ 12,
954–961 (1992). Article CAS PubMed PubMed Central Google Scholar * Davie, S. A. et al. Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric
oxide synthase deficient mice. _Transgenic Res._ 16, 193–201 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Filipski, E. et al. Effects of chronic jet lag on tumor
progression in mice. _Cancer Res._ 64, 7879–7885 (2004). Article CAS PubMed Google Scholar * Oike, H., Sakurai, M., Ippoushi, K. & Kobori, M. Time-fixed feeding prevents obesity
induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. _Biochem. Biophys. Res. Commun._ 465, 556–561 (2015). Article CAS PubMed Google Scholar *
Casiraghi, L. P., Alzamendi, A., Giovambattista, A., Chiesa, J. J. & Golombek, D. A. Effects of chronic forced circadian desynchronization on body weight and metabolism in male mice.
_Physiol. Rep._ 4, e12743 (2016). Article PubMed PubMed Central Google Scholar * Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model
provides a reliable model for human diseases. _Am. J. Pathol._ 163, 2113–2126 (2003). Article PubMed PubMed Central Google Scholar * Mani, S. A. et al. The epithelial-mesenchymal
transition generates cells with properties of stem cells. _Cell_ 133, 704–715 (2008). Article CAS PubMed PubMed Central Google Scholar * Morel, A.-P. et al. Generation of breast cancer
stem cells through epithelial-mesenchymal transition. _PLoS ONE_ 3, e2888 (2008). Article ADS PubMed PubMed Central CAS Google Scholar * Acloque, H., Adams, M. S., Fishwick, K.,
Bronner-Fraser, M. & Nieto, M. A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. _J. Clin. Invest._ 119, 1438–1449 (2009). Article
CAS PubMed PubMed Central Google Scholar * Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. _Nature_ 439, 84–88 (2006). Article ADS CAS PubMed
Google Scholar * Stingl, J. et al. Purification and unique properties of mammary epithelial stem cells. _Nature_ 439, 993–997 (2006). Article ADS CAS PubMed Google Scholar *
Visvader, J. E. & Stingl, J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. _Genes Dev._ 28, 1143–1158 (2014). Article CAS PubMed PubMed
Central Google Scholar * Vassilopoulos, A., Chisholm, C., Lahusen, T., Zheng, H. & Deng, C.-X. A critical role of CD29 and CD49f in mediating metastasis for cancer-initiating cells
isolated from a Brca1-associated mouse model of breast cancer. _Oncogene_ 33, 5477–5482 (2014). Article CAS PubMed Google Scholar * Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C.
C. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. _Cell_ 111, 41–50 (2002). Article CAS PubMed Google Scholar * Hadadi, E.,
Souza, L. E. B., de, Bennaceur-Griscelli, A. & Acloque, H. Identification of valid reference genes for circadian gene-expression studies in human mammary epithelial cells. _Chronobiol.
Int._ 35, 1689–1701 (2018). Article CAS PubMed Google Scholar * Yu, Y.-R. A. et al. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed
murine non-lymphoid tissues. _PLoS ONE_ 11, e0150606 (2016). Article PubMed PubMed Central CAS Google Scholar * Movahedi, K. et al. Different tumor microenvironments contain
functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. _Cancer Res._ 70, 5728–5739 (2010). Article CAS PubMed Google Scholar * Wang, K., Shen, T., Siegal, G. P.
& Wei, S. The CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. _Hum. Pathol._ 69, 110–117 (2017).
Article CAS PubMed Google Scholar * Asano, Y. et al. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast
cancer: tumour-infiltrating lymphocytes as predictors of response to chemotherapy in breast cancer. _Br. J. Surg._ 103, 845–854 (2016). Article CAS PubMed Google Scholar * Shou, J.,
Zhang, Z., Lai, Y., Chen, Z. & Huang, J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: a systematic review and meta-analysis. _BMC Cancer_ 16, 687 (2016).
Article PubMed PubMed Central CAS Google Scholar * He, W. et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte
subsets to tissues. _Immunity_ 49, 1175–1190.e7 (2018). Article CAS PubMed PubMed Central Google Scholar * Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as
regulators of the immune system. _Nat. Rev. Immunol._ 9, 162–174 (2009). Article CAS PubMed PubMed Central Google Scholar * Steele, C. W. et al. CXCR2 inhibition profoundly suppresses
metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. _Cancer Cell_ 29, 832–845 (2016). Article CAS PubMed PubMed Central Google Scholar * Sano, M. et al. Blocking
CXCLs–CXCR2 axis in tumor–stromal interactions contributes to survival in a mouse model of pancreatic ductal adenocarcinoma through reduced cell invasion/migration and a shift of
immune-inflammatory microenvironment. _Oncogenesis_ 8, 8 (2019). Article CAS PubMed PubMed Central Google Scholar * Halpern, J. L., Kilbarger, A. & Lynch, C. C. Mesenchymal stem
cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. _Cancer Lett._ 308, 91–99 (2011). Article CAS PubMed PubMed Central Google Scholar * Gibbs, J. et al. An
epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. _Nat. Med._ 20, 919–926 (2014). Article CAS PubMed PubMed Central Google Scholar * Müller, A. et
al. Involvement of chemokine receptors in breast cancer metastasis. _Nature_ 410, 50–56 (2001). Article ADS PubMed Google Scholar * Helbig, G. et al. NF-κB promotes breast cancer cell
migration and metastasis by inducing the expression of the chemokine receptor CXCR4. _J. Biol. Chem._ 278, 21631–21638 (2003). Article CAS PubMed Google Scholar * Bradley, M., Bond, M.,
Manini, J., Brown, Z. & Charlton, S. SB265610 is an allosteric, inverse agonist at the human CXCR2 receptor. _Br. J. Pharm._ 158, 328–338 (2009). Article CAS Google Scholar *
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. _Cell_ 150, 165–178 (2012). Article CAS PubMed PubMed Central Google Scholar * Sephton, S. E.
et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. _Brain. Behav. Immun._ 30, S163–S170 (2013). Article CAS PubMed Google Scholar * Vlachou, D., Bjarnason, G. A.,
Giacchetti, S., Levi, F. & Rand, D. A. TimeTeller: a new tool for precision circadian medicine and cancer prognosis. _bioRxiv_ https://doi.org/10.1101/622050 (2019). * Ye, X. et al.
Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. _Nature_ 525, 256–260 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Hollern, D.
P., Swiatnicki, M. R. & Andrechek, E. R. Histological subtypes of mouse mammary tumors reveal conserved relationships to human cancers. _PLOS Genet._ 14, e1007135 (2018). Article PubMed
PubMed Central CAS Google Scholar * Delaunay, F. & Laudet, V. Circadian clock and microarrays: mammalian genome gets rhythm. _Trends Genet._ 18, 595–597 (2002). Article CAS PubMed
Google Scholar * Pan, X., Jiang, X.-C. & Hussain, M. M. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. _Circulation_ 128, 1758–1769 (2013). Article
CAS PubMed PubMed Central Google Scholar * McAlpine, C. S. & Swirski, F. K. Circadian influence on metabolism and inflammation in atherosclerosis. _Circ. Res._ 119, 131–141 (2016).
Article CAS PubMed PubMed Central Google Scholar * Shi, S., Ansari, T. S., McGuinness, O. P., Wasserman, D. H. & Johnson, C. H. Circadian disruption leads to insulin resistance and
obesity. _Curr. Biol._ 23, 372–381 (2013). Article CAS PubMed PubMed Central Google Scholar * Kettner, N. M. et al. Circadian dysfunction induces leptin resistance in mice. _Cell
Metab._ 22, 448–459 (2015). Article CAS PubMed PubMed Central Google Scholar * Rudic, R. D. et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in
glucose homeostasis. _PLoS Biol._ 2, e377 (2004). Article PubMed PubMed Central CAS Google Scholar * Hojo, H. et al. Remote reprogramming of hepatic circadian transcriptome by breast
cancer. _Oncotarget_ 8, 34128–34140 (2017). Article PubMed PubMed Central Google Scholar * Masri, S. et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. _Cell_
165, 896–909 (2016). Article CAS PubMed PubMed Central Google Scholar * Filipski, E. et al. Effects of light and food schedules on liver and tumor molecular clocks in mice. _JNCI J.
Natl Cancer Inst._ 97, 507–517 (2005). Article CAS PubMed Google Scholar * Li, X. M. et al. Cancer inhibition through circadian reprogramming of tumor transcriptome with meal timing.
_Cancer Res._ 70, 3351–3360 (2010). Article CAS PubMed Google Scholar * Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. _Cell Metab._ 24, 324–331
(2016). Article CAS PubMed PubMed Central Google Scholar * Climent, J. et al. Deletion of the PER3 gene on chromosome 1p36 in recurrent ER-positive breast cancer. _J. Clin. Oncol._ 28,
3770–3778 (2010). Article CAS PubMed PubMed Central Google Scholar * Oshima, T. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. _Oncol.
Rep._ 25, 1439–1446 (2011). Article CAS PubMed Google Scholar * Cheng, H. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors.
_Hum. Mol. Genet._ 13, 1563–1575 (2004). Article CAS PubMed Google Scholar * Mollema, N. J. et al. Nuclear receptor rev-erb alpha (_Nr1d1_) functions in concert with _Nr2e3_ to regulate
transcriptional networks in the retina. _PLoS ONE_ 6, e17494 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Ahumada, A. Signaling of rat Frizzled-2 through
phosphodiesterase and cyclic GMP. _Science_ 298, 2006–2010 (2002). Article ADS CAS PubMed Google Scholar * Wang, H., Lee, Y. & Malbon, C. C. PDE6 is an effector for the
Wnt/Ca2+/cGMP-signalling pathway in development. _Biochem. Soc. Trans._ 32, 792–796 (2004). Article CAS PubMed Google Scholar * Welz, P.-S. et al. BMAL1-driven tissue clocks respond
independently to light to maintain homeostasis. _Cell_ 177, 1436–1447.e12 (2019). Article CAS PubMed PubMed Central Google Scholar * Koronowski, K. B. et al. Defining the independence
of the liver circadian clock. _Cell_ 177, 1448–1462.e14 (2019). Article CAS PubMed PubMed Central Google Scholar * Mao, L. et al. Circadian gating of epithelial-to-mesenchymal
transition in breast cancer cells via melatonin-regulation of GSK3β. _Mol. Endocrinol._ 26, 1808–1820 (2012). Article CAS PubMed PubMed Central Google Scholar * Lambert, A. W.,
Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. _Cell_ 168, 670–691 (2017). Article CAS PubMed PubMed Central Google Scholar * Vandewalle, C.
SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. _Nucleic Acids Res._ 33, 6566–6578 (2005). Article CAS PubMed PubMed Central Google Scholar *
Mani, S. A. et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. _Proc. Natl Acad. Sci. USA_ 104, 10069–10074
(2007). Article ADS CAS PubMed PubMed Central Google Scholar * Bashir, M., Damineni, S., Mukherjee, G. & Kondaiah, P. Activin-A signaling promotes epithelial–mesenchymal
transition, invasion, and metastatic growth of breast cancer. _Npj Breast Cancer_ 1, 15007 (2015). Article CAS PubMed PubMed Central Google Scholar * Ocaña, O. H. et al. Metastatic
colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. _Cancer Cell_ 22, 709–724 (2012). Article PubMed CAS Google Scholar * Cermakian, N. et al.
Crosstalk between the circadian clock circuitry and the immune system. _Chronobiol. Int._ 30, 870–888 (2013). Article CAS PubMed PubMed Central Google Scholar * Carter, S. J. et al. A
matter of time: study of circadian clocks and their role in inflammation. _J. Leukoc. Biol._ 99, 549–560 (2016). Article CAS PubMed Google Scholar * Scheiermann, C., Gibbs, J., Ince, L.
& Loudon, A. Clocking in to immunity. _Nat. Rev. Immunol._ 18, 423–437 (2018). Article CAS PubMed Google Scholar * Zhao, Y. et al. Uncovering the mystery of opposite circadian
rhythms between mouse and human leukocytes in humanized mice. _Blood_ 130, 1995–2005 (2017). Article CAS PubMed Google Scholar * Sukumaran, S., Jusko, W. J., DuBois, D. C. & Almon,
R. R. Light-dark oscillations in the lung transcriptome: implications for lung homeostasis, repair, metabolism, disease, and drug action. _J. Appl. Physiol._ 110, 1732–1747 (2011). Article
PubMed PubMed Central Google Scholar * Pluquet, O. et al. Posttranscriptional regulation of PER1 underlies the oncogenic function of IRE. _Cancer Res._ 73, 4732–4743 (2013). Article CAS
PubMed PubMed Central Google Scholar * Cheng, Y., Ma, X., Wei, Y. & Wei, X.-W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases.
_Biochim. Biophys. Acta BBA - Rev. Cancer_ 1871, 289–312 (2019). Article CAS Google Scholar * Michaeli, J. et al. Tumor-associated neutrophils induce apoptosis of non-activated CD8
T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. _OncoImmunology_ 6, e1356965 (2017). Article PubMed PubMed Central Google Scholar * Feng, S. et
al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. _Proc. Natl Acad. Sci. USA_ 115, 10094–10099 (2018). Article CAS PubMed PubMed
Central Google Scholar * Peranzoni, E. et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. _Proc. Natl Acad. Sci. USA_ 115,
E4041–E4050 (2018). Article CAS PubMed PubMed Central Google Scholar * Romero-Moreno, R. et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone
metastasis. _Nat. Commun._ 10, 4404 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Méndez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P. S. Haematopoietic stem
cell release is regulated by circadian oscillations. _Nature_ 452, 442–447 (2008). Article ADS PubMed CAS Google Scholar * Nagarsheth, N., Wicha, M. S. & Zou, W. Chemokines in the
cancer microenvironment and their relevance in cancer immunotherapy. _Nat. Rev. Immunol._ 17, 559–572 (2017). Article CAS PubMed PubMed Central Google Scholar * Chen, I. X. et al.
Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. _Proc. Natl Acad. Sci. USA_ 116, 4558–4566 (2019). Article
CAS PubMed PubMed Central Google Scholar * de Assis, L. V. M. et al. Expression of the circadian clock gene BMAL1 positively correlates with antitumor immunity and patient survival in
metastatic melanoma. _Front. Oncol._ 8, 185 (2018). Article PubMed PubMed Central Google Scholar * Paine, T. M., Soule, H. D., Pauley, R. J. & Dawson, P. J. Characterization of
epithelial phenotypes in mortal and immortal human breast cells. _Int. J. Cancer_ 50, 463–473 (1992). Article CAS PubMed Google Scholar * Oike, H., Ogawa, Y. & Oishi, K. Simple and
quick visualization of periodical data using microsoft excel. _Methods Protoc._ 2, 81 (2019). Article PubMed Central Google Scholar * Schmid, B., Helfrich-Förster, C. & Yoshii, T. A
new ImageJ plug-in “ActogramJ” for chronobiological analyses. _J. Biol. Rhythms_ 26, 464–467 (2011). Article PubMed Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read
alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar * Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from
RNA-seq data with or without a reference genome. _BMC Bioinforma._ 12, 323 (2011). Article CAS Google Scholar * Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and
correlations in multidimensional genomic data. _Bioinformatics_ 32, 2847–2849 (2016). Article CAS PubMed Google Scholar * Rohart, F., Gautier, B., Singh, A. & Lê Cao, K.-A. mixOmics:
an R package for ‘omics feature selection and multiple data integration. _PLoS Comput. Biol._ 13, e1005752 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Love, M. I.,
Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). Article PubMed PubMed Central CAS Google
Scholar * Federico, A. & Monti, S. hypeR: an R package for geneset enrichment workflows. _Bioinformatics_ 36, 1307–1308 (2020). PubMed Google Scholar * Subramanian, A. et al. Gene set
enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. _Proc. Natl Acad. Sci. USA_ 102, 15545–15550 (2005). Article ADS CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS We thank all members of UMRS935 for their technical assistance and daily scientific exchanges. We are grateful to Ibrahim Casals
and Benoit Peuteman from the INSERM UMS33 Animal Facility (SEIVIL). We appreciate the assistance of Marie-France Champy, Aurélie Auburtin, Tania Sorg and Yann Herault from Phenomin
(Institut Clinique de la souris, 1 rue Laurent Fries, 67404 ILLKIRCH cedex 2; CNRS, UMR7104, Illkirch, France; INSERM, U964, Illkirch, France; Université de Strasbourg, France) with blood
analyses. We thank Karine Bailly from the Cochin Cytometry and Immunobiology Facility (CYBIO, Institut Cochin, Paris) for performing the Luminex assay. We also thank Lindsay Higgins for her
English editing services. We are grateful to Francis Levi and Angela Nieto for their helpful comments on the manuscript. This work was funded by Inserm, University Paris Sud, INRA,
Association Institut de Cancérologie et d’Immunogénétique (ICIG), Vaincre le Cancer-NRB, Fond Avenir MASFIP, and GEFLUC – Les Entreprises contre le cancer. The post-doctoral fellowship of
E.H. was granted by Vaincre le Cancer-NRB and the University Paris Saclay (Project BioTherAlliance). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Inserm, U935, Université Paris Sud,
Villejuif, France Eva Hadadi, William Taylor, Xiao-Mei Li, Sandrine Dulong, Annelise Bennaceur-Griscelli & Hervé Acloque * Université Paris Sud, Université Paris Saclay, UFR de Médecine
Kremlin Bicêtre, Le Kremlin-Bicêtre, France Xiao-Mei Li, Sandrine Dulong & Annelise Bennaceur-Griscelli * Inserm, U1132, Université Paris Diderot, Hôpital Lariboisière - Centre Viggo
Petersen, 75010, Paris, France Yetki Aslan & Sylvain Provot * GABI, INRA, AgroParisTech, Université Paris-Saclay, 78352, Jouy-en-Josas, France Marthe Villote, Julie Rivière & Hervé
Acloque * Inserm, UMS33, Villejuif, France Gaelle Duvallet & Charlotte Auriau * Département des Sciences Biologiques et Fonctionnelles, Laboratoire d’HistoPathologie Expérimentale et
Comparée (LabHPEC), ENVT, Université de Toulouse, Toulouse, France Isabelle Raymond-Letron * STROMALab, CNRS ERL5311, EFS, ENVT, Inserm U1031, Université de Toulouse, Toulouse, France
Isabelle Raymond-Letron * Service d’hématologie, APHP, GHU Paris Sud, Paris, France Annelise Bennaceur-Griscelli Authors * Eva Hadadi View author publications You can also search for this
author inPubMed Google Scholar * William Taylor View author publications You can also search for this author inPubMed Google Scholar * Xiao-Mei Li View author publications You can also
search for this author inPubMed Google Scholar * Yetki Aslan View author publications You can also search for this author inPubMed Google Scholar * Marthe Villote View author publications
You can also search for this author inPubMed Google Scholar * Julie Rivière View author publications You can also search for this author inPubMed Google Scholar * Gaelle Duvallet View author
publications You can also search for this author inPubMed Google Scholar * Charlotte Auriau View author publications You can also search for this author inPubMed Google Scholar * Sandrine
Dulong View author publications You can also search for this author inPubMed Google Scholar * Isabelle Raymond-Letron View author publications You can also search for this author inPubMed
Google Scholar * Sylvain Provot View author publications You can also search for this author inPubMed Google Scholar * Annelise Bennaceur-Griscelli View author publications You can also
search for this author inPubMed Google Scholar * Hervé Acloque View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.H., A.B.G. and H.A.
designed the experiments. E.H. performed most of the in vitro and in vivo experiments with the support of W.T. Y.A. and S.P. performed the analysis of bone phenotypes. M.V., J.L., H.A. and
I.R.L. performed the analysis of primary tumour histology. G.D. and C.A. were in charge of animal housing, breeding and manipulation. X.L. and S.D. provided all the protocols, equipment and
support for chronobiology experiments. H.A. performed secondary analysis of mRNA-seq data. E.H., S.P., and H.A. wrote the paper. A.B.G. and H.A. supervised the research and funded the
project. CORRESPONDING AUTHORS Correspondence to Eva Hadadi or Hervé Acloque. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER
REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE
DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hadadi, E., Taylor, W., Li, XM. _et al._ Chronic
circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. _Nat Commun_ 11, 3193 (2020). https://doi.org/10.1038/s41467-020-16890-6
Download citation * Received: 14 August 2019 * Accepted: 29 May 2020 * Published: 24 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16890-6 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
