Genome-wide analysis in the mouse embryo reveals the importance of dna methylation for transcription integrity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Mouse embryos acquire global DNA methylation of their genome during implantation. However the exact roles of DNA methyltransferases (DNMTs) in embryos have not been studied
comprehensively. Here we systematically analyze the consequences of genetic inactivation of _Dnmt1_, _Dnmt3a_ and _Dnmt3b_ on the methylome and transcriptome of mouse embryos. We find a
strict division of function between DNMT1, responsible for maintenance methylation, and DNMT3A/B, solely responsible for methylation acquisition in development. By analyzing severely
hypomethylated embryos, we uncover multiple functions of DNA methylation that is used as a mechanism of repression for a panel of genes including not only imprinted and germline genes, but
also lineage-committed genes and 2-cell genes. DNA methylation also suppresses multiple retrotransposons and illegitimate transcripts from cryptic promoters in transposons and gene bodies.
Our work provides a thorough analysis of the roles of DNA methyltransferases and the importance of DNA methylation for transcriptome integrity in mammalian embryos. SIMILAR CONTENT BEING
VIEWED BY OTHERS DYNAMICS OF DNA HYDROXYMETHYLATION AND METHYLATION DURING MOUSE EMBRYONIC AND GERMLINE DEVELOPMENT Article 20 December 2022 IDENTIFICATION OF DISTINCT LOCI FOR DE NOVO DNA
METHYLATION BY DNMT3A AND DNMT3B DURING MAMMALIAN DEVELOPMENT Article Open access 24 June 2020 STRAND-SPECIFIC SINGLE-CELL METHYLOMICS REVEALS DISTINCT MODES OF DNA DEMETHYLATION DYNAMICS
DURING EARLY MAMMALIAN DEVELOPMENT Article Open access 24 February 2021 INTRODUCTION DNA methylation in vertebrate genomes has important functions in gene regulation, development, and
diseases1. This modification occurs at CpGs and is abundant genome-wide, except at CpG islands (CGIs) that are refractory to DNA methylation. The mouse genome encodes four active DNA
methyltransferases (DNMT): DNMT1, DNMT3A, DNMT3B, and DNMT3C. Another member of the family, DNMT3L, is catalytically inactive but stimulates the activity of the other DNMT3 enzymes. DNMT3C
and DNMT3L are expressed in germ cells and their inactivation leads to impaired gametic DNA methylation and infertility2,3. In contrast, the inactivation of DNMT1, DNMT3A, or DNMT3B in mice
leads to embryonic or postnatal lethality4,5, which illustrates the essential role of these enzymes in development. Global genome methylation is established after implantation of the embryo
in the mouse and subsequently maintained in most cell lineages. DNMT3A and DNMT3B are thought to perform all de novo methylation of DNA during development4,6, however the consequences of the
double knockout (DKO) of _Dnmt3a_/_b_ on the methylome have not been investigated genome-wide. Moreover, the single inactivation of _Dnmt3a_ or _Dnmt3b_ has only a moderate impact on DNA
methylation levels in mouse embryos4,7, suggesting either strong redundancy or involvement of other enzymes in de novo methylation. In contrast, DNMT1 is thought to be the main enzyme
responsible for maintenance DNA methylation after replication. However, DNMT1 also shows capabilities for de novo DNA methylation in vitro, in mouse embryonic stem (ES) cells, and
oocytes8,9,10,11,12. Conversely, late passage _Dnmt3a/b_ knockout ES cells show reduced methylation genome-wide13,14,15 and at imprinted differentially methylated regions (DMRs)16,
suggesting that DNMT3A/B are also required for the faithful maintenance of CpG methylation in development. Despite these studies suggesting complex functions of DNMTs, the in vivo roles of
these enzymes in embryonic development remain elusive. Previous investigations of the roles of DNMTs in embryos were limited to locus-specific analysis4,6,17,18,19, which highlights the
necessity for complete methylomes of _Dnmt_ mutant embryos to validate models of DNMT functions in vivo. Further work is also needed to illuminate the transcriptional roles of DNA
methylation in development. Previous studies in _Dnmt_ knockout embryos showed that DNA methylation is required to repress imprinted genes20,21, _Rhox_ genes6, germline genes22, and
intracisternal A-particle (IAP) transposons17. However, no genome-wide transcriptome analysis in strongly hypomethylated embryos has been conducted. Moreover, transcriptome profiling in
_Dnmt_ triple knockout (TKO) mouse ES cells devoid of DNA methylation revealed only a minor impact on the expression of genes and transposable elements (TEs)23,24. One explanation is that ES
cells use other mechanisms to compensate for the loss of DNA methylation, mimicking what is happening during epigenetic reprogramming in preimplantation embryos and primordial germ cells25.
Indeed, the repression of endogenous retroviruses (ERVs) in mESCs is primarily mediated by KAP1 and SETDB1 responsible for H3K9me3, rather than DNA methylation23,26,27,28. In contrast, IAP
repression becomes dependent on DNA methylation in differentiated cells29, supporting the model that DNA methylation is not important for initial repression in early embryonic cells but for
the transition to long-term silencing. Here we perform a comprehensive investigation of the role of DNMTs during global genome remethylation in the mouse embryo. We report genome-wide
methylomes in _Dnmt1_ knockout and _Dnmt3a/b_ DKO embryos (embryonic day 8.5), which elucidates the in vivo roles of these enzymes in setting up DNA methylation patterns. We show that
severely hypomethylated embryos overexpress a panel of genes, transposons, and illegitimate transcripts initiating from cryptic promoters, revealing the multiple roles of DNA methylation for
the maintenance of transcriptional integrity in development. RESULTS METHYLOME PROFILING OF _DNMT_ MUTANT EMBRYOS To assess the contribution of DNMTs to DNA methylation in vivo, we
generated base-resolution methylomes in _Dnmt_ mutant embryos. Using a _Dnmt1_-2lox allele30, we created _Dnmt1_ mutant embryos lacking the exons 4 and 5, which creates an out-of-frame
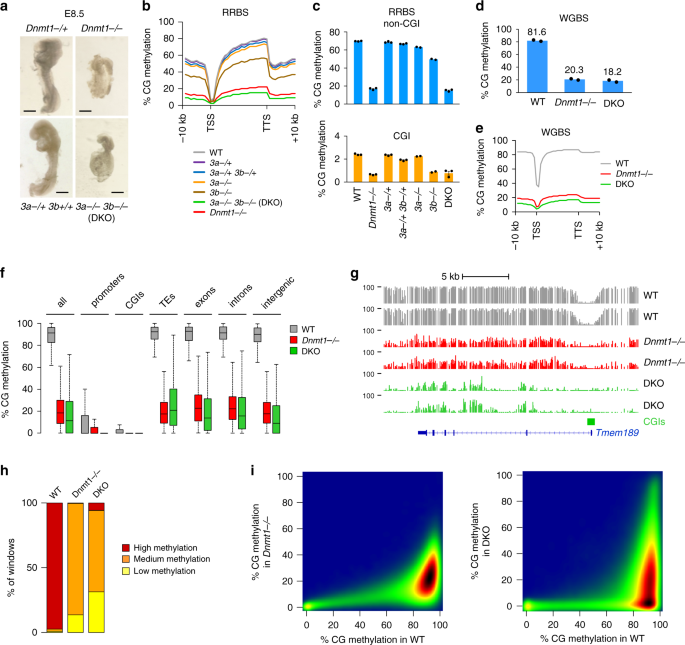
splice and a functional null allele. As shown previously19, the _Dnmt1_−/− embryos showed growth retardation at embryonic day (E) 8.5 (Fig. 1a). For DNMT3A and DNMT3B, we previously showed
that methylation is only partially reduced in single knockouts, suggesting redundancy7. To address this, we generated _Dnmt3a/b_ DKO embryos. Confirming previous observations4, DKO embryos
resembled _Dnmt1_-null embryos and showed growth retardation at E8.5 (Fig. 1a). We performed _Msp_I-based reduced representation bisulfite sequencing (RRBS) in three _Dnmt1_−/− and WT
littermate controls at E8.5, as well as three DKO embryos and controls from the same litters (Supplementary Table 1). The RRBS data were highly reproducible between replicates (Supplementary
Fig. 1a–c). To have a complete view, we also performed whole genome bisulfite sequencing (WGBS) in two independent WT, _Dnmt1_−/− and DKO E8.5 embryos (Supplementary Table 2). The average
sequencing depth after deduplication was 12× and close to 90% of the CpGs were sequenced at least 5× in each dataset (Supplementary Fig. 1d and Supplementary Table 2). The WGBS data were
reproducible between independent embryos (Supplementary Fig. 1e, f), demonstrating the reliability of the datasets. DNMT1 IS REQUIRED TO SUSTAIN DNA METHYLATION GENOME-WIDE We found a strong
reduction of genomic methylation in _Dnmt1__−/−_ embryos (Fig. 1b). The mean CG methylation level measured by RRBS in non-CGI sequences dropped from 69.8% in WT to 16.7% in _Dnmt1_−/−
embryos, whereas CG methylation of CGIs dropped from 2.4% in WT to 0.7% in _Dnmt1_−/− embryos (Fig. 1c). Confirming the RRBS results, the global CG methylation levels measured by WGBS in WT
and _Dnmt1_−/− embryos were 81.6% and 20.3%, respectively (Fig. 1d, e). The loss of methylation upon inactivation of _Dnmt1_ is truly global and occurs across all genomic sequences including
exons, introns, intergenic regions and TEs (Fig. 1f, g). All sequences have low to intermediate methylation and no sequences retain high methylation in _Dnmt1__−/−_ embryos (Fig. 1h, i),
indicating that DNMT1 is universally required to sustain DNA methylation at all genomic sequences in embryonic development. DE NOVO DNA METHYLATION IS ABOLISHED IN _DNMT3A_ −/− _DNMT3B_ −/−
EMBRYOS Next, we analyzed the methylome of _Dnmt3a/b_ DKO embryos. In contrast to the single knockouts7, the double inactivation of _Dnmt3a_/_b_ lead to a strong reduction of DNA
methylation, demonstrating redundancy between DNMT3A and DNMT3B genome-wide (Fig. 1b). The mean CG methylation level measured by RRBS in non-CGI sequences dropped from 69.8% in WT to 63.0%
in _Dnmt3a__−/−_, 49.6% in _Dnmt3b__−/−_, and 15.0% in DKO embryos (Fig. 1c). Accordingly, the CG methylation level measured by WGBS dropped from 81.6% in WT to 18.2% in DKO embryos (Fig.
1d, e). The loss of methylation in DKO embryos affects all genome compartments (Fig. 1f). However, in contrast to _Dnmt1__−/−_ embryos, DKO embryos do not display a uniform loss of
methylation (Fig. 1g) but contain a high proportion of sequences fully demethylated or highly methylated (Fig. 1h, i). To determine the origin of high methylation in DKO embryos, we compared
the WGBS methylation patterns of DKO embryos to those of preimplantation inner cell mass (ICM)31. Strikingly, visual inspection of the methylation patterns in ICM and DKO embryos revealed
that they are highly similar (Fig. 2a, Supplementary Fig. 2a). To confirm this, we performed a pairwise comparison of WGBS methylation scores and revealed a strong positive correlation
between methylation in ICM and DKO embryos (Fig. 2b, _r_ = 0.80). We found that the most highly methylated sequences in DKO embryos are enriched for TEs (Supplementary Fig. 2b). Amongst the
most methylated TE families are IAP, RLTR6, and MMERVK10C elements, which carry high methylation in DKO embryos at levels identical to blastocysts (Supplementary Fig. 2c, d). This strongly
suggests that in absence of DNMT3A/B, methylation in E8.5 DKO embryos arises from the maintenance of preexisting DNA methylation from the blastocysts. Finally, to determine if de novo
methylation happens in DKO embryos, we selected all the sequences that are hypomethylated in blastocysts and gain methylation in E8.5 embryos, and found that de novo methylation at these
sequences is completely abolished in DKO embryos (Fig. 2c). Altogether this indicates that DNMT3A/B are responsible for the bulk de novo DNA methylation between the blastocyst and
postimplantation stages and that DNMT1 has a negligible capacity for de novo DNA methylation during embryonic development. ROLE OF DNMT3A/B IN METHYLATION MAINTENANCE Given the proposed
function of DNMT3A/B in maintenance DNA methylation in ES cells, we investigated the role of DNMT3A/B in maintenance DNA methylation in vivo. First, we quantified methylation of 20 known
germline DMRs (gDMRs) of imprinted loci, which arise from the postfertilization maintenance of allelic methylation established in the parental gametes (Supplementary Data 1). The methylation
of all gDMRs is preserved in DKO embryos (Fig. 3a, b), indicating that DNMT3A/B have no significant contribution to the maintenance of gametic-derived methylation imprints in embryos. This
agrees with two previous studies showing that DNMT3A/B are dispensable for maintenance methylation of the _Igf2r_, _H19_, and _Dlk1/Gtl2_ gDMRs in vivo6,18. In contrast, all gDMRs are
demethylated in _Dnmt1__−/_− embryos, confirming that DNMT1 is the main enzyme propagating methylation imprints in embryos (Fig. 3a, b). Moreover, extending previous findings20,21, RNA-seq
in _Dnmt_ mutant embryos (see below) revealed marked changes in expression of imprinted genes in _Dnmt1__−/_− but not DKO embryos, including, as expected, both downregulation (_Grb10_,
_Igf2_, and _Kcnq1_) and ~2-fold upregulation (_Snrpn_, _Peg3_, and _H19_) (Fig. 3c). The exception is _Zdbf2_ that shows reduced expression in DKO embryos (Fig. 3c), validating the model
that embryonic de novo methylation is required for an unusual switch from a maternal to paternal DMR and activate paternal-specific transcription of _Zdbf2_32. In summary, we show that DNMT1
alone mediates maintenance of methylation imprints and provide in vivo validation for the role of DNA methylation at many imprinted loci. To investigate a possible maintenance function of
DNMT3A/B at other genomic loci, it is necessary to perform conditional inactivation after de novo methylation has been completed. To this aim, we derived and immortalized _Dnmt3a__2lox/2lox_
_Dnmt3b__2lox/2lox_ MEFs and generated _Dnmt3a/b_ conditional double knockouts (cDKO) with a tamoxifen-inducible CRE recombinase (Cre-ERT2) (Fig. 3d). Tamoxifen treatment led to efficient
recombination of the _Dnmt3a_ and _Dnmt3b_ alleles (Fig. 3e). Furthermore, RT-qPCR confirmed that the expression of the floxed exons of _Dnmt3a/b_ became undetectable following tamoxifen
treatment in cDKO MEFs while _Dnmt1_ expression is unchanged (Supplementary Fig. 3a, b). The strong reduction of DNMT3A protein in cDKO cells was validated by western blot (Supplementary
Fig. 3c), whereas we could not detect DNMT3B by western blot even in untreated MEFs consistently with the known lack of DNMT3B expression in differentiated cells. The cells were cultivated
for up to 69 days to allow multiple cell divisions. RRBS performed at 23 days and 69 days of culture (Supplementary Table 3) revealed no evidence of decreased methylation genome-wide (Fig.
3f, Supplementary Fig. 3d). After 69 days of culture, we only identified 11 hypomethylated DMRs in _Dnmt3a/b_ cDKO fibroblasts that most likely reflect de novo methylation events that
happened during the course of cell culture (Supplementary Fig. 3e, f). As a control, we also derived and immortalized _Dnmt1__2lox/2lox_ MEFs to generate _Dnmt1_ conditional knockout MEFs
with Cre-ERT2 (Supplementary Fig. 3g–i). Conditional inactivation of _Dnmt1_ led to an immediate and global hypomethylation of genomic DNA as measured by RBBS after 5 and 7 days of tamoxifen
treatment (Supplementary Fig. 3j, k) associated with a block of cellular division, confirming that DNMT1 is the sole maintenance enzyme. Taken together, our data indicate no major role of
DNMT3A/B in maintenance methylation in embryos and differentiated cells. DNA METHYLATION SUPPRESSES THE GERMLINE PROGRAM IN EMBRYOS Next, we used RNA-seq to investigate the consequences of
the absence of DNA methylation on gene expression in embryos. RNA-seq was performed on three _Dnmt1__−/_− and WT littermate embryos, as well as six DKO embryos and six WT and _Dnmt3a__−/+_
littermate controls (Supplementary Table 4). RNA-seq confirmed the knockout of critical exons in the _Dnmt_ genes (Supplementary Fig. 4a, b). This analysis identified 414 upregulated and 68
downregulated genes in _Dnmt1__−/−_ embryos, and 564 upregulated and 47 downregulated genes in DKO embryos (fold change > 3, DESeq2 adjusted _p_ value < 0.001) (Supplementary Fig. 4c,
Supplementary Data 2). Principal component analysis showed high similarity between _Dnmt1__−/_− and DKO samples, which cluster separately from the controls (Fig. 4a). Indeed, there is a good
correlation between the expression changes in _Dnmt1__−/_− and DKO embryos (Supplementary Fig. 4d), and the genes misregulated in _Dnmt1__−/−_ and DKO embryos strongly overlap (Fig. 4b). In
contrast, these genes only weakly overlap with the genes upregulated in TKO mESCs (Supplementary Fig. 4e, f), mostly because many show a strong basal expression in WT ESCs (Supplementary
Fig. 4g). We then focused on the genes upregulated in DKO embryos and classified them in three groups: group 1 includes genes with low CpG promoters (LCP), group 2 includes genes with
unmethylated CpG-rich promoters (intermediate or high CpG promoters, ICP or HCP), and group 3 includes genes with methylated CpG-rich promoters (Fig. 4c). The genes of group 2 show only weak
derepression compared with the other groups and could partly reflect indirect effects (Supplementary Fig. 4h). Whereas no significant ontology terms are associated with the groups 1 and 2,
the group 3 is strongly enriched for ontology terms related to germline functions (Fig. 4c). In total, 137 germline genes acquire dense promoter CpG methylation in WT embryos and are
derepressed in DKO embryos (Supplementary Data 2), with some (e.g., _Tuba3b_, _Sohlh2_, _Tex13_, _Rpl10l_, _Dazl_, _Asz1_, and _Hormad1_) reaching up to ~1000-fold upregulation (Fig. 4d).
This includes numerous germ-cell specific piRNA pathway factors (_Gtsf1_, _Tex19_, _Topaz1_, _Rnf17_, _Piwil2_, _Mov10l1_, _Asz1_, _Ddx4_, _Mael_, _Fkbp6_, and _Gpat2_). Interestingly, we
previously found some germline genes upregulated in _Dnmt3b__−/_− embryos7,22. As expected, all these genes are also upregulated in DKO embryos, nevertheless many additional germline genes
are derepressed in DKO embryos (Supplementary Fig. 4i). Moreover, the degree of reactivation of germline genes is much higher in DKO embryos, which correlates with the degree of methylation
loss (Supplementary Fig. 4j). This supports a direct relationship between CpG-island promoter methylation and repression of a large panel of germline genes. To firmly demonstrate that local
CpG-island methylation mediates repression of germline genes, we performed dCas9-based targeted demethylation with the TET1 catalytic domain (TET1CD) in MEFs using gRNAs targeting the _Dazl_
and _Asz1_ promoters (Supplementary Fig. 5a, b). We first compared the efficiency of dCas9-TET1CD fusion and the dCas9-SunTag-TET1CD system33 and found that only dCas9-SunTag-TET1CD
achieved robust demethylation of _Dazl_ and _Asz1_ (Supplementary Fig. 5c–e). Targeted demethylation with dCas9-SunTag-TET1CD induced strong derepression of _Dazl_ and _Asz1_ (Fig. 4e),
demonstrating that dense promoter methylation of germline genes plays a causal role in the maintenance of their repressed state. In summary, we reveal an extensive role of DNA methylation in
keeping CpG-rich promoters of the germline program silent in embryos. Given the derepression of germline genes in hypomethylated embryos, we wondered what their expression is in
hypomethylated blastocysts. We analyzed RNA-seq from E3.5 ICM34 and found that, while approximately 1/3 of the most derepressed germline genes in DKO embryos have abundant mRNAs in ICM, the
majority has low or undetectable expression in ICM (Supplementary Fig. 6a). For example, the _Dazl_ and _Slc25a31_ genes show weak expression in E3.5 ICM despite similar hypomethylation than
in E8.5 DKO embryos (Supplementary Fig. 6b). This suggests that either activators of germline genes are absent in blastocysts or that transient repression mechanisms compensate for erased
DNA methylation in preimplantation stages before a switch to DNA methylation-dependent repression in postimplantation embryos. DNA METHYLATION LIMITS EARLY EXPRESSION OF LINEAGE-COMMITTED
GENES We then investigated what other genes are in the overexpressed groups (Fig. 4c). Besides expected targets such as _Rhox_ genes6, we found that several somatic lineage-committed genes
harboring a CpG-dense promoter acquire promoter DNA methylation in WT embryos and are overexpressed in DKO and _Dnmt1__−/−_ embryos. These genes belong in majority to the group 3 and include
genes expressed in hematopoietic cells (_Bin2_, _Arhgap30_, _Ly86_, _Pf4,_ and _Nckap1l_), brain (_A330102I10Rik_), eye (_Rbp3_), or digestive tissues (_Iyd_, _Gstp2_) (Fig. 4f,
Supplementary Data 2). Their overexpression was validated by RT-qPCR in DKO embryos (Fig. 4g). This suggests that DNA methylation of CpG-dense promoters contributes to prevent ectopic
expression of some lineage-committed genes. One prediction of this model is that promoter DNA methylation of these genes should be low in the tissues where they are expressed. To test this
prediction, we explored public WGBS data from mouse tissues35,36,37 and confirmed that the promoters of these genes are specifically hypomethylated in the tissues where they are expressed
(Fig. 4h, Supplementary Fig. 7). Taken together, this suggests a role of DNA methylation in suppressing precocious expression of lineage-committed genes in embryos. DE NOVO DNA
METHYLTRANSFERASES ARE REQUIRED TO REPRESS 2C-SPECIFIC GENES We noticed that many genes specifically expressed in 2-cell embryos and 2C-like ES cells (2C-genes) are strongly derepressed (up
to ~1000-fold) in DKO embryos, such as _Zscan4_ genes, _Tmem92_, _Tcstv1/3_, and _Eif1a_-like genes (_Gm5662_, _Gm2022_, BB287469, _Gm2016_, _Gm21319_, _Gm8300,_ and _Gm5039_) and
_Usp17-like_ genes (Fig. 4i), whose differential expression was validated by RT-qPCR (Fig. 4j). To confirm this finding, we compared the list of genes upregulated in DKO embryos with genes
upregulated in 2C-like ES cells38 and found a significant overlap (Supplementary Fig. 8a). These 2C-genes are frequently organized in clusters (Supplementary Fig. 8b). Intriguingly, the
extent of upregulation of 2C-genes is variable between DKO embryos (Fig. 4i). Moreover, most of these 2C-genes contain CpG-poor promoters and are much less overexpressed in _Dnmt1__−/_−
embryos compared with DKO embryos despite similar hypomethylation (Fig. 4i, Supplementary Fig. 8c), suggesting a possible role of de novo methyltransferases independent of DNA methylation.
Previous studies have established that another feature of 2C embryos is the expression of MERVL retrotransposons and that many 2C-genes initiate from promoters in ERVL LTRs39,40,41.
Accordingly, we found a threefold activation of MERVL-int transposons in DKO embryos but not in _Dnmt1__−/_− embryos (Supplementary Fig. 8d). In addition, several 2C-specific genes
derepressed in DKO embryos (i.e., _Zfp352_, _Tcstv1_, _Tcstv3_, _B020031M17Rik_, AF067061, _Gm20767_, _Ubtfl1_, _Gm2022_, and AA792892) initiate from MERVL LTRs (annotated as MT2_Mm) or
other ERVL transposons (MT2B-C, ORR1B) (Supplementary Fig. 8e). Thus de novo methyltransferases are required for the extinction of the ERVL-driven and 2C-specific transcriptional network in
postimplantation embryos. DNA METHYLATION REPRESSES A HIGH NUMBER OF TES AND CHIMERIC TRANSCRIPTS The contribution of DNA methylation to the regulation of TEs in the embryo has not been
studied comprehensively, which prompted us to analyze the expression of TEs in _Dnmt_ mutant embryos. TE expression was quantified either by counting reads in RepeatMasker annotations or by
mapping reads on Repbase sequences (see “Methods”) (Supplementary Data 3). IAPs showed a dramatic reactivation (50–100 fold) in _Dnmt1__−/−_ embryos (Fig. 5a), confirming previous data by
northern blot and in situ hybridization17. In addition to IAPs, several other retrotransposon families of the LINE-1, ERV1, and ERVK families were significantly upregulated in _Dnmt1__−/−_
embryos (Fig. 5a, Supplementary Fig. 9a). The same set of transposons was upregulated in DKO embryos but with a lower magnitude, which correlates with higher residual methylation of TEs in
DKO embryos (Fig. 5a). The RepBase method yielded similar results (Supplementary Fig. 9b) and revealed that among the LINE-1 elements, only the most recent subfamilies are upregulated
(Supplementary Fig. 9c). Having shown that DNA methylation is required to repress TEs at the family level, we analyzed the expression of individual copies of TEs by using uniquely mapped
reads. It should be mentioned that this method underestimates the counts of upregulated TE copies because very young TEs cannot be uniquely mapped. This analysis identified 4593 activated TE
copies (fold change > 3, DESeq2 adjusted _p_ value < 0.001) in _Dnmt1__−/−_ embryos (Supplementary Fig. 9d–g, Supplementary Data 3). For IAPs, the most active retroelements in the
mouse, some families showed a massive reactivation of up to 30% annotated copies (Fig. 5b), in particular IAPEz-int that represent more than half (2484 out of 4593) of all upregulated TEs in
_Dnmt1__−/_− embryos (Supplementary Fig. 9f). Other ERVs like ERVB4_1B-I_MM-int, MMERGLN-int, MMEtn-int, and MMERVK10C-int show activation of a limited set of copies representing no more
than 4% of all annotated copies (Fig. 5b). Interestingly, these activated copies have a higher size and presumably correspond to full length, potentially active copies (Fig. 5c). Next, we
investigated the impact of the derepression of ERVs on the expression of neighboring genes. We identified 715 genes located close to activated ERVs (<20 kb from the TSS) and found that
they were significantly more upregulated than control genes in _Dnmt1__−/−_ embryos (Fig. 5d, e), indicating that derepressed ERVs alter the expression of proximal genes. Out of the 414
upregulated genes in _Dnmt1__−/−_ embryos, 10% (_n_ = 42 genes) are upregulated in association with derepression of an intragenic or proximal ERV (Supplementary Data 2). In some cases,
intergenic ERVs initiate long RNAs that extend into adjacent genes and produce chimeric transcripts by splicing to an internal exon, as exemplified by the _Cyp2b23_, _Serpinb1c_, and
_Olfr316_ genes (Fig. 5f, Supplementary Fig. 10a, b). We also observed intragenic initiation from intronic IAPEz and its flanking LTR IAPLTR1_Mm inserted in the antisense orientation to the
host gene, as exemplified by the _Capn11_, _Trpm2_, and _Apoh_ genes (Fig. 5g, Supplementary Fig. 10c, d). To determine if antisense transcription from IAPLTR1_Mm elements is frequent, we
counted the RNA signal from all IAPLTR1_Mm elements and found that, while there is a strong activation in the sense orientation, there is also a noticeable increase in antisense
transcription initiation from these elements in _Dnmt1__−/_− embryos (Supplementary Fig. 10e, f). Altogether this shows that derepressed TEs alter the expression of nearby genes, similarly
to previous observations made in _Setdb1_ KO ES cells23 and _Dnmt3L_ mutant spermatocytes42. Interestingly, in contrast to _Dnmt3L_ KO spermatocytes42, we did not see activation of nearby
genes by L1 retrotransposons, suggesting that the impact of hypomethylated TEs on the integrity of the transcriptome is different between germ cells and somatic cells. In summary, we
conclude that DNMT1 is the main enzyme involved in TE protection and that DNA methylation is required to repress intact, potentially active copies of many retrotransposon families and
prevent them from disturbing expression of nearby genes. DNA METHYLATION SUPPRESSES CRYPTIC INITIATION SITES IN GENE BODIES Upon further exploring the transcriptional changes in mutant
embryos, we noticed that several genes upregulated in DKO embryos initiate from intragenic sequences not associated with TEs. For example, the _Mgl2_, _Mlana_, _C8b_, and _Plekhd1_ genes
produce truncated mRNAs initiating in the gene body (Fig. 6a, Supplementary Fig. 11a–c). To measure if cryptic intragenic transcription initiation is a general phenomenon, we calculated the
ratio of expression of downstream exons versus the first exon for all genes. This revealed no significant increase in DKO compared with WT embryos (Fig. 6b), suggesting that cryptic internal
initiation is limited to a subset of genes. Using a bioinformatic pipeline (see “Methods”), we identified 46 genes in DKO and 25 genes in _Dnmt1__−/−_ embryos upregulated from intragenic
sequences not annotated as transposons or alternative promoters (Supplementary Data 2). Consistent with a primary role of DNA methylation, the genes identified in DKO and _Dnmt1__−/−_
embryos largely overlapped (Supplementary Fig. 11d). The sites of cryptic intragenic initiation in these genes tend to be CpG-rich and, in contrast to canonical promoter sequences, are
strongly methylated in WT embryos (Fig. 6c). To explore the mechanisms of intragenic initiation, we focused on the _Mgl2_ gene. Interestingly, the activation of the cryptic _Mgl2_ promoter
is recapitulated in TKO ES cells (Fig. 6d), making these cells a good model to investigate _Mgl2_ regulation. Analysis of previous datasets generated in TKO ES cells24 revealed that the
absence of DNA methylation is associated with the appearance of a new DNase-I hypersensitive site and binding of the methylation-sensitive transcription factor NRF1 at the site of intragenic
initiation in the intron 6 (Fig. 6d). The sequence of this intron contains three repetitions of the NRF1 binding motif GAGCATGCGC (Supplementary Fig. 11e). This suggests that internal
binding of NRF1 in absence of DNA methylation creates an intragenic initiation site in the _Mgl2_ gene. To validate this hypothesis, we monitored _Mgl2_ expression in TKO ES cells knocked
down for NRF1 and found that _Mgl2_ internal initiation is abolished in these cells (Fig. 6e). Taken together, this reveals that DNA methylation is critical to prevent methylation-sensitive
transcription factors from creating cryptic intragenic initiation sites in embryos. DISCUSSION In this paper, we interrogated the contribution of DNMTs to the establishment of genome-wide
DNA methylation patterns in mouse embryos. Our results support a strict division of function between DNMT1 and DNMT3A/B in vivo. DNMT1 alone mediates the faithful maintenance of DNA
methylation in developing embryos with no contribution of DNMT3A/B, as supported by several lines of evidence: (1) DNMT1 alone is sufficient to maintain preexisting patterns of DNA
methylation from the blastocysts to the E8.5 stage in DKO embryos; (2) All gametic methylation imprints are faithfully maintained in DKO embryos; (3) Global patterns of DNA methylation are
unaffected upon conditional inactivation of _Dnmt3a/b_ over multiple cell divisions in embryonic fibroblasts. Conversely, DNMT3A/B are strongly redundant and responsible for all de novo
methylation in development, confirming what was speculated since the discovery of these enzymes4, and excluding a de novo function of DNMT1 in embryonic development. This however does not
exclude the possibility that DNMT1 catalyzes de novo methylation in other developmental contexts, for instance in oocytes11,12. Furthermore, we cannot exclude the possibility that some de
novo activity of DNMT1 is implicated in the perdurance of DNA methylation in DKO embryos. Indeed, several families of ERVK and LINE-1 retrotransposons are targets of TET enzymes in mouse
embryonic cells43, suggesting that a de novo activity of DNMT1 could be required to counteract TET-mediated demethylation at these sites. The lack of evidence for a role of DNMT3A/B in
maintenance methylation contradicts several studies showing that DNMT3A/B are required for maintenance methylation in ES cells13,14,15,16,44. One possible explanation for this discrepancy is
the discovery that ES cells continuously cycle in and out of a transient hypomethylated state marked by MERVL expression38. Therefore the reduced methylation of _Dnmt3a/b_ knockout ES cells
could reflect a requirement for continuous de novo methylation to exit the MERVL+ state rather than true maintenance methylation. Our results also contradict a previous study that concluded
on a role of DNMT3B in maintenance methylation in MEFs based on rough estimation of DNA methylation with restriction digestion45. In support of our conclusions, combined acute inactivation
of DNMT3A/B does not lead to genome hypomethylation in human embryonic carcinoma cells46. Another key finding of our study is the interrogation of the transcriptional roles of DNA
methylation by performing RNA-seq in severely hypomethylated embryos. We establish that DNA methylation of CpG-island promoters is a primary and causal silencing mechanism for restricting
ectopic expression of a large panel of germline genes. While we and others previously found some germline genes reactivated in partially hypomethylated embryos and cells7,22,47,48,49, our
study reveals that the set of germline genes repressed by CpG-island DNA methylation is larger than anticipated. This set includes many genes involved in the piRNA pathway, reinforcing the
model that this might have evolved as a defense mechanism against transposons to couple genome demethylation with immediate activation of piRNA defense genes47. Future studies should be
aimed at understanding the mechanisms that limit the expression of germline genes in preimplantation stages and subsequently direct de novo CpG-island DNA methylation to germline genes
during development. Our work reveals other functions of DNA methylation for gene regulation in embryos. Notably, we found that a small number of lineage-committed genes acquire promoter DNA
methylation in WT embryos and are derepressed in methylation-deficient embryos. Furthermore the same genes display tissue-specific promoter hypomethylation in differentiated tissues. This
strongly supports a role for DNA methylation in limiting precocious expression of lineage-committed genes in embryos. In addition, we demonstrate that intragenic methylation of CpG-rich
sequences is essential to mask cryptic promoters in gene bodies and prevent the production of truncated gene transcripts. Using the _Mgl2_ gene as a model, we were able to demonstrate that
intragenic DNA methylation directly prevents methylation-sensitive transcription factors such as NRF124 from initiating cryptic intragenic transcripts. Previously, other epigenetic factors
have been shown to limit cryptic intragenic initiation such as KDM5B and SETD250,51. In addition, a recent study suggested that gene body DNA methylation suppresses widespread cryptic
intragenic initiation in mouse ES cells52. In contrast to this report, we found no evidence for widespread intragenic transcription in hypomethylated embryos by quantifying RNA-seq signals
in downstream versus the first exon of expressed genes. Instead, our results suggest that DNA methylation limits cryptic intragenic initiation from defined sequences in a small number of
genes. Another surprising finding is that 2C-genes are derepressed in DKO embryos. This was unexpected because DNA hypomethylation does not drive expression of 2C-genes in ES cells38, which
are instead repressed by CAF1, KDM1a, KAP1, G9a, HP1, and PRC1 in ES cells39,53,54,55,56. Recently it was found that _Dux_, _Dppa2_, and _Dppa4_ activate the 2C program in 2-cell-like ES
cells57,58, although _Dux_ has a minor role in activating genes in 2C embryos59. Interestingly _Dux_, _Dppa2,_ and _Dppa4_ are derepressed in DKO embryos (Supplementary Data 2), which could
provide an explanation for the coordinated derepression of 2C-genes. Adding to the complexity, _Dux_ and 2C-genes are not strongly activated in _Dnmt1__−/−_ embryos, suggesting either an
indirect regulation by DNA methylation or a possible role of non-catalytic functions of DNMT3A/B. Hence, it is possible that DNMT3A/B repress 2C-genes by recruiting silencing complexes
independently of their catalytic activity60,61. The prevailing model for transposon regulation is that they switch from H3K9me3-mediated silencing in preimplantation embryonic cells to a DNA
methylation dominant mechanism in postimplantation embryos. However, the latter aspect of this model lacked experimental evidence in the mouse because, besides IAPs17,29, it was unclear if
other TE families require DNA methylation for repression. Our analysis demonstrates that DNA methylation is universally required to maintain repression of potentially active copies of
numerous ERV and LINE transposons in postimplantation embryos, confirming that DNA methylation becomes a major epigenetic barrier against transposon expression in differentiated cells.
Interestingly, SETDB1 is still required to repress some ERV1 transposons (MMVL30-int, RLTR6_Mm, and MULV-int) in mouse differentiated cells62, which we find do not depend on DNA methylation.
In summary, our work provides a detailed description of the multiple functions of DNA methylation in maintaining the transcription integrity of mouse embryos. These results contribute to
our understanding of why DNA methylation is essential for mammalian development. METHODS MOUSE LINES AND EMBRYOS All mouse lines used in the study were maintained on a C57BL/6J genetic
background. All experimental animal procedures were performed following the ethical regulations of the Comité d’Ethique Régional en Expérimentation Animale de Strasbourg (CREMEAS). Mice were
housed with free access to food and water, a 12 h light/dark cycle and controlled temperature (20–24 °C) and humidity (40–70%). We obtained a _Dnmt1_-null allele by crossing _Dnmt1_-2lox
mice30 with an ACTB-Cre deleter line63, which creates a _Dnmt1_ allele lacking the exons 4 and 5. _Dnmt1__−/_− embryos were obtained by natural mating of heterozygous males and females.
_Dnmt3a_ and _Dnmt3b_ knockout alleles were obtained by deleting critical catalytic exons as previously described7. We generated _Dnmt3a__−/−_ _Dnmt3b__−/−_ (DKO) embryos by natural mating
of _Dnmt3a_+_/−_ _Dnmt3b_+_/_− males and females. As controls, we recovered WT, _Dnmt3a_+_/−_ and _Dnmt3a_+_/−_ _Dnmt3b_+_/_− embryos from the same litters. The morning of the vaginal plug
was designated E0.5 and embryos were manually dissected in M2 medium at E8.5. We simultaneously prepared genomic DNA and total RNA from the same embryos with the AllPrep DNA/RNA Mini Kit
(Qiagen). CULTURE OF MEFS AND CONDITIONAL INACTIVATION OF _DNMTS_ Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 embryos and immortalized by serial passages. MEFs were grown in
DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. All cells were tested negative for mycoplasma
contamination. _Dnmt3a_2lox/2lox _Dnmt3b_2lox/2lox MEFs were derived from an embryo obtained by crossing _Dnmt3a_-2lox and _Dnmt3b_-2lox mouse lines45,64 on a C57BL/6J genetic background.
_Dnmt1_2lox/2lox MEFs were derived from an embryo obtained by crossing _Dnmt1_-2lox mice30 on a C57BL/6J genetic background. For conditional inactivation, the MEFs were transduced with a
retrovirus coding for the Tamoxifen-inducible Cre-ERT2 recombinase and selected with puromycin (2 μg ml−1). Recombination was induced by treating MEFs with 2 μM 4-OH-Tamoxifen (Sigma). The
medium containing 4-OH-Tamoxifen was renewed every day during the first 2 weeks and then every 3 days. The efficiency of the recombination was validated by PCR genotyping, RT-qPCR, and
western blotting. The conditional inactivation was performed 3 times independently and the cells were harvested at different time points of culture for genotyping and DNA methylation
analysis by RRBS. The oligo sequences for PCR are provided in the Supplementary Data 4. EPIGENETIC EDITING WITH DCAS9-TET1 FUSION The plasmids coding for the dCas9-TET1 fusion were
constructed based on the pdCas9-DNMT3A-EGFP plasmid (Addgene #71666). The EGFP sequence was substituted with the puromycin sequence from the PX459-V2 plasmid (Addgene #62988). Subsequently,
the plasmid was digested with BamHI and FseI to replace the DNMT3A fragment with the catalytic domain of human TET1 (hTET1-CD). The sequence coding for hTET1-CD was synthesized by Integrated
DNA Technologies (IDT) and amplified by PCR using forward and reverse primers introducing BamHI and FseI restriction sites. Two BbsI restriction sites within the TET1 sequence were removed
by introducing silent mutations by site-directed mutagenesis. The gRNAs targeting _Dazl_ (gRNA4: ACGCACTCCGTGGGCGACGT) and _Asz1_ (gRNA5: GTGAAAGGCCAGCTCGTGGG) were designed using
http://crispr.mit.edu, synthesized as pairs of oligonucleotides, annealed and cloned into the BbsI site. Immortalized MEFs isolated from a C57BL/6J embryo were transfected with the
dCas9-hTET1-CD plasmid using Polyethylenimine (PEI) transfection reagent. In brief, 10 µg of plasmid and 20 µL of PEI were diluted in 250 µl of 150 mM NaCl each, combined and incubated for
30 min at RT. The complexes were added to 70–80% confluent MEF cells in 100 mm dishes. Twenty-four hours after transfection, the cells were selected with 3 µg mL−1 of puromycin (Gibco,
Thermo Fisher Scientific) for 48 h before harvesting the cells for DNA/RNA extraction using the AllPrep DNA/RNA Mini Kit (Qiagen). EPIGENETIC EDITING WITH THE DCAS9-SUNTAG-TET1 SYSTEM The
gRNAs were cloned in the pPlatTet-gRNA2 all-in-one vector33. pPlatTET-gRNA2 was a gift from Izuho Hatada (Addgene # 82559). Briefly, two 60mer oligonucleotides containing the gRNA sequence
were annealed and extended to make a 100 bp double stranded DNA fragment using Q5 high fidelity polymerase (New England Biolabs #M0491S), and incorporated into the linearized pPlatTet-gRNA2
vector by Gibson assembly (New England Biolabs #E2611S). MEFs were transfected with the Neon electroporation system (Thermo Fisher Scientific) and cells expressing GFP were selected 72 h
post-transfection by flow cytometry using a BD FACS Vantage cell Sorter (BD Biosciences) for DNA/RNA extraction using the AllPrep DNA/RNA Mini Kit (Qiagen). GENE EXPRESSION ANALYSIS BY
RT-QPCR RNAs were reverse transcribed with the Maxima first strand cDNA synthesis kit (Thermo Fischer Scientific). qPCR was performed with the KAPA SYBR FAST qPCR kit (KAPA Biosystems) on a
StepOnePlus PCR system (Applied Biosystems) using the standard curve method. We used fast PCR conditions as follows: 95 °C for 20 s, 40 cycles (95 °C for 20 s, 64 °C for 30 s), followed by a
dissociation curve. The expression of target genes was normalized with three housekeeping genes (_Gusb_, _Rpl13a_, _B2m,_ or _Mrpl32)_. qPCR reactions were performed in triplicates with
no-RT controls to rule out the presence of contaminating DNA. The oligo sequences are provided in the Supplementary Data 4. WESTERN BLOT ANALYSIS Nuclear extracts were run on a SDS PAGE gel
and transferred to a 0.2 µm nitrocellulose membrane. The membrane was blocked with TBS, 0.1% Tween-20, 5% milk for 2 h at room temperature and incubated with the primary antibodies overnight
at 4 °C. The membrane was washed three times, incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature, and washed three times. The signal was
detected by chemiluminescence using the ECL detection reagent (Amersham, GE Healthcare). The following primary antibodies were used: DNMT3A (NB120-13888, NovusBio, 1:200 dilution), LAMIN B1
(ab16048, Abcam, 1:2000 dilution). BISULFITE SEQUENCING Hundred nanograms of genomic DNA were bisulfite converted using the EpiTect Bisulfite Kit (Qiagen) according to the manufacturer’s
instructions. The target regions were amplified by PCR with the Platinum Taq DNA Polymerase (Thermo Fisher Scientific) using the following conditions: 20 cycles of 30 s at 95 °C, 30 s at
58–48 °C (with a 0.5 °C decrease per cycle), 50 s at 72 °C followed by 35 cycles of 30 s at 95 °C, 30 s at 52 °C, 50 s at 72 °C. The PCR products were cloned by TA cloning in the pCR2.1
vector (TA Cloning Kit, Invitrogen) and 15–30 clones were sequenced. Sequences were aligned with the BISMA software and filtered to remove clonal biases. The oligo sequences are provided in
the Supplementary Data 4. RRBS RRBS libraries were prepared from single embryos by _Msp_I digestion7. Fifty nanograms genomic DNA was digested for 5 h at 37 °C with _Msp_I (Thermo Fisher
Scientific), end-repaired and A-tailed for 40 min at 37 °C with 5 U Klenow-fragment exo- (Thermo Fisher Scientific) and ligated to methylated adapters overnight at 16 °C with 30 U T4 DNA
ligase (Thermo Fisher Scientific) in Tango 1X buffer. Fragments between 150 and 400 bp were excised from a 3% agarose 0.5X TBE gel, purified with the MinElute gel extraction kit (Qiagen) and
bisulfite converted with the EpiTect bisulfite kit (Qiagen) with two consecutive rounds of conversion. Final libraries were amplified with PfU Turbo Cx hotstart DNA polymerase (Agilent) (2
min at 95 °C; 12–14 cycles of 30 s at 95 °C, 30 s at 65 °C, 45 s at 72 °C; final extension 7 min at 72 °C). Libraries were purified with AMPure magnetic beads (Beckman Coulter) and sequenced
on an Illumina HiSeq4000 (2 × 75 bp) at Integragen SA (Evry, France). Reads were trimmed to remove low quality bases with Trim Galore v0.4.2 and aligned to the mm10 genome with BSMAP v2.74
(parameters -v 2 -w 100 -r 1 -x 400 -m 30 -D C-CGG -n 1). We calculated methylation scores using methratio.py in BSMAP v2.74 (parameters -z -u -g). Only CpGs covered by a minimum of eight
reads were retained for analyses. WGBS WGBS was performed independently on two embryos for each genotype. Fifty nanograms of genomic DNA were fragmented to 350 bp using a Covaris E220
sonicator. DNA was bisulfite converted with the EZ DNA Methylation-Gold kit (Zymo Research) and WGBS libraries were prepared using the Accel-NGS Methyl-Seq DNA Library Kit (Swift
Biosciences) according to the manufacturer’s instructions with seven PCR cycles for the final amplification. The libraries were purified using Ampure XP beads (Beckman Coulter) and sequenced
in paired-end (2 × 100 bp) on an Illumina HiSeq4000 at Integragen SA (Evry, France). Low quality bases as well as the first five bases of reads R1 and ten bases of reads R2 were trimmed
with Trim Galore v0.4.2 (parameters -q 20 --clip_R1 5 --clip_R2 10). The reads were aligned to the mm10 genome and cleaned for duplicates using Bismark v0.22.1 with default parameters. Reads
with signs of incomplete conversion were removed using the filter_non_conversion option in Bismark with the parameters --minimum_count 5 and --percentage_cutoff 50. Methylation calls were
extracted using the Bismark methylation extractor. Only CpGs covered by a minimum of five reads were retained for analyses in each replicate. For global methylation analysis, both replicates
of each genotype were combined by adding the number of Cs and Ts at each CpG position (mean sequencing depth WT: 24.81×; _Dnmt1__−/−_: 23.85×; DKO: 25.62×). METHYLATION DATA ANALYSIS The
methylation of genomic features (Fig. 1f) was calculated by intersecting CpG positions with genomic annotations using the IRanges package in R, and averaging methylation of individual CpGs
in each feature. We used the CpG-island annotation, RefSeq gene annotation, and RepeatMasker annotation (only elements > 400 bp) downloaded from the UCSC website, promoters were defined
as −1 kb to +1 kb around RefSeq TSS, and intergenic regions were defined as genomic regions that do not overlap with any of the previous annotations. To identify regions with high residual
methylation in ICM and DKO embryos, methylation was averaged in 1 kb windows containing at least 3 CpGs. To select regions de novo methylated during implantation (Fig. 2c), we selected 1 kb
windows with <5% methylation in ICM and >50% methylation in E8.5 WT embryos. Metaplots of CG methylation in genes were generated by calculating methylation in twenty equal-sized
windows within each RefSeq gene (excluding the X and Y chromosomes) and ten 1 kb windows of flanking sequences. Pairwise correlation plots of methylation scores were generated in 500 bp
windows for RRBS and WGBS. Promoter classification based on CpG density was done as follows. For each promoter (−1 kb to +1 kb around RefSeq TSS), we calculated the CpG ratio and GC content
in 500 bp sliding windows with 20 bp increments. LCP were defined as containing no window with a CpG ratio > 0.45, HCP were defined as containing at least one window with a CpG ratio >
0.65 and a GC content > 55%, and the remaining promoters were defined as ICP. To identify DMRs in _Dnmt3a/b_ cDKO MEFs, we used eDMR from the methylKit R package with the following
criteria: at least three differentially methylated CpGs (DMCs), difference in methylation > 20%, adjusted _p_ value < 0.001. RNA-SEQ AND TRANSCRIPTOME ANALYSIS RNA-seq libraries were
prepared from single embryos with the TruSeq Stranded Total RNA Sample Prep Kit with Ribo-Zero ribosomal RNA reduction (Illumina). We prepared libraries from three _Dnmt1__−/−_ and three WT
littermate embryos, as well as six DKO, two WT, and four _Dnmt3a__−/+_ littermate embryos. The libraries were sequenced in paired-end (2 × 100 bp) on an Illumina HiSeq4000. Reads were mapped
to the mm10 genome using TopHat v2.0.13 with a RefSeq transcriptome index and default parameters, reporting up to 20 alignments for multi-mapped reads. For data visualization, bigwig files
were generated using bam2wig.py from the RSeQC package v2.6.4 (parameters -u -t 5000000000) and visualized in the Integrative Genomics Viewer (IGV). For differential gene expression
analysis, reads mapping to repeats (with the exception of simple repeats and low complexity DNA sequences) were removed from the bam files using bedtools intersect to avoid that genes are
falsely called upregulated because of the presence of TEs in UTRs. Unique reads were counted in RefSeq genes with HTSeq v0.7.2 (parameters –t exon –s reverse), and differentially expressed
genes were determined using DESeq2 v1.16.1 (fold change > 3, adjusted _p_ value < 0.001). For imprinted genes, the GTF annotation file was modified to allow quantification of _Snrpn_,
_Gnas1a_, and _Nesp_. Splice junctions were visualized in IGV using the splice junctions files produced by TopHat. The FPKM values and PCA analysis were generated using DESeq2. Gene ontology
analysis of differentially expressed genes was performed using DAVID 6.8 (https://david.ncifcrf.gov). INTRAGENIC TRANSCRIPTION To study intragenic transcription initiation, reads were
counted in individual exons of all RefSeq gene isoforms using featureCounts from the Rsubread package v1.30.9, and the ratio of FPKM values of downstream exons over the first exon was
plotted for all genes with at least 5 exons and a FPKM score > 1. To identify genes with intragenic initiation, differentially expressed exons were identified using DESeq2 v1.16.1 (fold
change > 3, _p_ value < 0.001) and the percentage of upregulated exon was calculated for each RefSeq isoform. The following criteria were then applied: (i) percentage of upregulated
exons <100 %, (ii) the first exon is not upregulated, (iii) no other isoform of the same gene with 100% upregulated exons, (iv) if <5 upregulated exons they should be consecutive, (v)
if ≥5 upregulated exons a gap of one exon is tolerated, (vi) the fold change of upregulated exons is >3 times higher than for the other exons, (vii) the FPKM values of upregulated exons
is higher than for the other exons. Finally, the list of genes was manually curated to keep only one RefSeq isoform for each gene, eliminate false positives and eliminate genes initiating
from transposons. For each gene, the position of intragenic initiation was defined manually from the bigwig files. Metaplots of CpG density and CpG methylation around cryptic intragenic
initiation sites and RefSeq TSS were calculated in 250 bp windows. TRANSPOSON ANALYSIS Methylation of TE families was estimated by intersecting CpG positions with the UCSC RepeatMasker
annotation using the IRanges package in R, and averaging the CpG methylation scores in each family. For the comparison of TE methylation between DKO embryos and ICM, only LTR and LINE
families covered by at least 100 CpGs were retained. Expression of TEs was analyzed in several ways at the family and copy level. First, unique and multiple-mapping reads were counted in TE
families using featureCounts from the Rsubread package v1.30.9 with a GTF file built from the UCSC RepeatMasker annotation, with the option to weight multi-mapping reads by the number of
mapping sites (parameters countMultiMappingReads = TRUE, fraction = TRUE, useMetaFeatures = TRUE). In parallel, expression of TE families was also analyzed by mapping reads to RepBase
consensus sequences using TopHat v2.0.13 allowing five mismatches, and counting reads with HTSeq v0.7.2. Differentially expressed TE families were identified using DESeq2 v1.16.1 (fold
change > 2, adjusted _p_ value < 0.001). To analyze the expression of individual copies of TEs, only unique reads were counted in individual TEs from the RepeatMasker GTF file using
featureCounts (parameters countMultiMappingReads = FALSE, useMetaFeatures = FALSE). We then identified differentially expressed copies using DESeq2 v1.16.1 (fold change > 3, adjusted _p_
value < 0.001), and calculated the percentage of upregulated copies within each TE family. The age of LINE-1 families was taken from Sookdeo et al.65. To represent sense and antisense
transcription for IAPLTR1_Mm elements, elements were merged if distant from less than 8 kb. The RNA-seq signals were extracted in regions spanning from −5 kb to 5 kb from the start of the
IAPLTR1_Mm elements on the forward and reserve strands using bwtool extract. The signals were averaged in 50 bp windows and plotted as metaplots or heatmaps using pheatmap in R. DATASETS The
following datasets were used: WGBS in gametes and early embryos (GSE56697), WGBS in mouse adult tissues (GSE42836), WGBS in B cells (GSE100262), WGBS in hematopoietic stem cells (GSE52709),
RNA-seq in E3.5 ICM (GSE84234), RNA-seq, DNAse-seq, and NRF1 ChIP-seq in TKO ES cells (GSE67867). STATISTICS AND REPRODUCIBILITY All measurements were biological replicates taken from
individual embryos or independent experiments. Details on the statistical tests and samples sizes are provided in the Figure legends. Statistical significance of differences in gene
expression by RT-qPCR were evaluated by two-tailed unpaired Student’s _t_ test with unequal variances assuming normality of the distributions. REPORTING SUMMARY Further information on
research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The methylome and RNA-seq sequencing data generated in this study are
available in the NCBI Gene Expression Omnibus under the accession number GSE130735. All other data generated in this study are available in the Supplementary Information files or from the
corresponding author upon reasonable request. The Source data for Figs. 1c, d, 3e, f, 4d–g, i, j and Supplementary Figs. 2c, 3a–c, h–j, 5e, and 8d are provided in the Source data file. The
Source data for Figs. 3b, c, 4b, c, 5a, b and Supplementary Figs. 4c, d, h, 9a–f, and 11d are provided in the Supplementary Data files. UCSC genome annotations are available at
http://genome.ucsc.edu. Source data are provided with this paper. REFERENCES * Smith, Z. D. et al. DNA methylation: roles in mammalian development. _Nat. Rev. Genet._ 14, 204–220 (2013).
Article CAS PubMed Google Scholar * Bourc’his, D. et al. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. _Nature_ 431, 96–99 (2004). Article ADS
PubMed CAS Google Scholar * Barau, J. et al. The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. _Science_ 354, 909–912 (2016). Article ADS CAS PubMed
Google Scholar * Okano, M. et al. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. _Cell_ 99, 247–257 (1999). Article CAS PubMed
Google Scholar * Li, E. et al. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. _Cell_ 69, 915–926 (1992). Article CAS PubMed Google Scholar * Oda,
M. et al. DNA methylation regulates long-range gene silencing of an X-linked homeobox gene cluster in a lineage-specific manner. _Genes Dev._ 20, 3382–3394 (2006). Article CAS PubMed
PubMed Central Google Scholar * Auclair, G. et al. Ontogeny of CpG island methylation and specificity of DNMT3 methyltransferases during embryonic development in the mouse. _Genome Biol._
15, 545 (2014). Article PubMed PubMed Central CAS Google Scholar * Fatemi, M. et al. The activity of the murine DNA methyltransferase Dnmt1 is controlled by interaction of the catalytic
domain with the N-terminal part of the enzyme leading to an allosteric activation of the enzyme after binding to methylated DNA. _J. Mol. Biol._ 309, 1189–1199 (2001). Article CAS PubMed
Google Scholar * Gowher, H. et al. De novo methylation of nucleosomal DNA by the mammalian Dnmt1 and Dnmt3A DNA methyltransferases. _Biochemistry_ 44, 9899–9904 (2005). Article CAS
PubMed Google Scholar * Arand, J. et al. In vivo control of CpG and non-CpG DNA methylation by DNA methyltransferases. _PLoS Genet._ 8, e1002750 (2012). Article CAS PubMed PubMed
Central Google Scholar * Shirane, K. et al. Mouse oocyte methylomes at base resolution reveal genome-wide accumulation of non-CpG methylation and role of DNA methyltransferases. _PLoS
Genet._ 9, e1003439 (2013). Article CAS PubMed PubMed Central Google Scholar * Li, Y. et al. Stella safeguards the oocyte methylome by preventing de novo methylation mediated by DNMT1.
_Nature_ 564, 136–140 (2018). Article ADS CAS PubMed Google Scholar * Li, Z. et al. Distinct roles of DNMT1-dependent and DNMT1-independent methylation patterns in the genome of mouse
embryonic stem cells. _Genome Biol._ 16, 115 (2015). Article PubMed PubMed Central CAS Google Scholar * Leung, D. et al. Regulation of DNA methylation turnover at LTR retrotransposons
and imprinted loci by the histone methyltransferase Setdb1. _Proc. Natl Acad. Sci. USA_ 111, 6690–6695 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Chen, T. et al.
Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. _Mol. Cell. Biol._ 23, 5594–5605 (2003). Article CAS PubMed PubMed
Central Google Scholar * Thakur, A. et al. Widespread recovery of methylation at gametic imprints in hypomethylated mouse stem cells following rescue with DNMT3A2. _Epigenetics Chromatin_
9, 53 (2016). Article PubMed PubMed Central CAS Google Scholar * Walsh, C. P. et al. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. _Nat. Genet._
20, 116–117 (1998). Article CAS PubMed Google Scholar * Hirasawa, R. et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during
preimplantation development. _Genes Dev._ 22, 1607–1616 (2008). Article CAS PubMed PubMed Central Google Scholar * Lei, H. et al. De novo DNA cytosine methyltransferase activities in
mouse embryonic stem cells. _Development_ 122, 3195–3205 (1996). CAS PubMed Google Scholar * Li, E. et al. Role for DNA methylation in genomic imprinting. _Nature_ 366, 362–365 (1993).
Article ADS CAS PubMed Google Scholar * Weaver, J. R. et al. Domain-specific response of imprinted genes to reduced DNMT1. _Mol. Cell. Biol._ 30, 3916–3928 (2010). Article CAS PubMed
PubMed Central Google Scholar * Borgel, J. et al. Targets and dynamics of promoter DNA methylation during early mouse development. _Nat. Genet._ 42, 1093–1100 (2010). Article CAS
PubMed Google Scholar * Karimi, M. M. et al. DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. _Cell Stem
Cell_ 8, 676–687 (2011). Article CAS PubMed PubMed Central Google Scholar * Domcke, S. et al. Competition between DNA methylation and transcription factors determines binding of NRF1.
_Nature_ 528, 575–579 (2015). Article ADS CAS PubMed Google Scholar * Walter, M. et al. An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in
embryonic stem cells. _eLife_ 5, e11418 (2016). Article PubMed PubMed Central Google Scholar * Matsui, T. et al. Proviral silencing in embryonic stem cells requires the histone
methyltransferase ESET. _Nature_ 464, 927–931 (2010). Article ADS CAS PubMed Google Scholar * Rowe, H. M. et al. KAP1 controls endogenous retroviruses in embryonic stem cells. _Nature_
463, 237–240 (2010). Article ADS CAS PubMed Google Scholar * Robbez-Masson, L. et al. The HUSH complex cooperates with TRIM28 to repress young retrotransposons and new genes. _Genome
Res._ 28, 836–845 (2018). Article CAS PubMed PubMed Central Google Scholar * Hutnick, L. K. et al. Repression of retrotransposal elements in mouse embryonic stem cells is primarily
mediated by a DNA methylation-independent mechanism. _J. Biol. Chem._ 285, 21082–21091 (2010). Article CAS PubMed PubMed Central Google Scholar * Jackson-Grusby, L. et al. Loss of
genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. _Nat. Genet._ 27, 31–39 (2001). Article CAS PubMed Google Scholar * Wang, L. et al. Programming and
inheritance of parental DNA methylomes in mammals. _Cell_ 157, 979–991 (2014). Article CAS PubMed PubMed Central Google Scholar * Duffie, R. et al. The Gpr1/Zdbf2 locus provides new
paradigms for transient and dynamic genomic imprinting in mammals. _Genes Dev._ 28, 463–478 (2014). Article CAS PubMed PubMed Central Google Scholar * Morita, S. et al. Targeted DNA
demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. _Nat. Biotechnol._ 34, 1060–1065 (2016). Article CAS PubMed Google Scholar * Smith, Z. D. et al.
Epigenetic restriction of extraembryonic lineages mirrors the somatic transition to cancer. _Nature_ 549, 543–547 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Hon, G.
C. et al. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. _Nat. Genet._ 45, 1198–1206 (2013). Article CAS PubMed PubMed Central
Google Scholar * Cabezas-Wallscheid, N. et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis.
_Cell Stem Cell_ 15, 507–522 (2014). Article CAS PubMed Google Scholar * Duncan, C. G. et al. Base-resolution analysis of DNA methylation patterns downstream of Dnmt3a in mouse naive B
cells. _G3 (Bethesda)_ 8, 805–813 (2018). Article CAS Google Scholar * Eckersley-Maslin, M. A. et al. MERVL/Zscan4 network activation results in transient genome-wide DNA demethylation of
mESCs. _Cell Rep._ 17, 179–192 (2016). Article CAS PubMed PubMed Central Google Scholar * Macfarlan, T. S. et al. Endogenous retroviruses and neighboring genes are coordinately
repressed by LSD1/KDM1A. _Genes Dev._ 25, 594–607 (2011). Article CAS PubMed PubMed Central Google Scholar * Macfarlan, T. S. et al. Embryonic stem cell potency fluctuates with
endogenous retrovirus activity. _Nature_ 487, 57–63 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Peaston, A. E. et al. Retrotransposons regulate host genes in mouse
oocytes and preimplantation embryos. _Dev. Cell_ 7, 597–606 (2004). Article CAS PubMed Google Scholar * Inoue, K. et al. Switching of dominant retrotransposon silencing strategies from
posttranscriptional to transcriptional mechanisms during male germ-cell development in mice. _PLoS Genet._ 13, e1006926 (2017). Article PubMed PubMed Central CAS Google Scholar * de la
Rica, L. et al. TET-dependent regulation of retrotransposable elements in mouse embryonic stem cells. _Genome Biol._ 17, 234 (2016). Article PubMed PubMed Central CAS Google Scholar *
Liang, G. et al. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. _Mol. Cell. Biol._ 22, 480–491 (2002). Article CAS PubMed PubMed
Central Google Scholar * Dodge, J. E. et al. Inactivation of Dnmt3b in mouse embryonic fibroblasts results in DNA hypomethylation, chromosomal instability, and spontaneous immortalization.
_J. Biol. Chem._ 280, 17986–17991 (2005). Article CAS PubMed Google Scholar * Tiedemann, R. L. et al. Acute depletion redefines the division of labor among DNA methyltransferases in
methylating the human genome. _Cell Rep._ 9, 1554–1566 (2014). Article CAS PubMed PubMed Central Google Scholar * Hackett, J. A. et al. Promoter DNA methylation couples genome-defence
mechanisms to epigenetic reprogramming in the mouse germline. _Development_ 139, 3623–3632 (2012). Article CAS PubMed PubMed Central Google Scholar * Velasco, G. et al. Dnmt3b
recruitment through E2F6 transcriptional repressor mediates germ-line gene silencing in murine somatic tissues. _Proc. Natl Acad. Sci. USA_ 107, 9281–9286 (2010). Article ADS PubMed
PubMed Central Google Scholar * Maatouk, D. M. et al. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell
lineages. _Development_ 133, 3411–3418 (2006). Article CAS PubMed Google Scholar * Carvalho, S. et al. Histone methyltransferase SETD2 coordinates FACT recruitment with nucleosome
dynamics during transcription. _Nucleic Acids Res._ 41, 2881–2893 (2013). Article CAS PubMed PubMed Central Google Scholar * Xie, L. et al. KDM5B regulates embryonic stem cell
self-renewal and represses cryptic intragenic transcription. _EMBO J._ 30, 1473–1484 (2011). Article CAS PubMed PubMed Central Google Scholar * Neri, F. et al. Intragenic DNA
methylation prevents spurious transcription initiation. _Nature_ 543, 72–77 (2017). Article ADS CAS PubMed Google Scholar * Ishiuchi, T. et al. Early embryonic-like cells are induced by
downregulating replication-dependent chromatin assembly. _Nat. Struct. Mol. Biol._ 22, 662–671 (2015). Article CAS PubMed Google Scholar * Hisada, K. et al. RYBP represses endogenous
retroviruses and preimplantation- and germ line-specific genes in mouse embryonic stem cells. _Mol. Cell. Biol._ 32, 1139–1149 (2012). Article CAS PubMed PubMed Central Google Scholar *
Maksakova, I. A. et al. Distinct roles of KAP1, HP1 and G9a/GLP in silencing of the two-cell-specific retrotransposon MERVL in mouse ES cells. _Epigenetics Chromatin_ 6, 15 (2013). Article
CAS PubMed PubMed Central Google Scholar * Rodriguez-Terrones, D. et al. A molecular roadmap for the emergence of early-embryonic-like cells in culture. _Nat. Genet._ 50, 106–119
(2018). Article CAS PubMed Google Scholar * De Iaco, A. et al. DPPA2 and DPPA4 are necessary to establish a 2C-like state in mouse embryonic stem cells. _EMBO Rep._ 20, e47382 (2019).
Article PubMed PubMed Central CAS Google Scholar * Eckersley-Maslin, M. et al. Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. _Genes Dev._ 33, 194–208
(2019). Article CAS PubMed PubMed Central Google Scholar * Chen, Z. et al. Loss of DUX causes minor defects in zygotic genome activation and is compatible with mouse development. _Nat.
Genet._ 51, 947–951 (2019). Article CAS PubMed PubMed Central Google Scholar * Nowialis, P. et al. Catalytically inactive Dnmt3b rescues mouse embryonic development by accessory and
repressive functions. _Nat. Commun._ 10, 4374 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Geiman, T. M. et al. DNMT3B interacts with hSNF2H chromatin remodeling
enzyme, HDACs 1 and 2, and components of the histone methylation system. _Biochem. Biophys. Res. Commun._ 318, 544–555 (2004). Article CAS PubMed Google Scholar * Kato, M. et al. A
somatic role for the histone methyltransferase Setdb1 in endogenous retrovirus silencing. _Nat. Commun._ 9, 1683 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Birling,
M. C. et al. Highly-efficient, fluorescent, locus directed cre and FlpO deleter mice on a pure C57BL/6N genetic background. _Genesis_ 50, 482–489 (2012). Article CAS PubMed Google
Scholar * Kaneda, M. et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. _Nature_ 429, 900–903 (2004). Article ADS CAS PubMed Google
Scholar * Sookdeo, A. et al. Revisiting the evolution of mouse LINE-1 in the genomic era. _Mob. DNA_ 4, 3 (2013). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS We thank the staff of the IGBMC GenomEast sequencing platform and Annie Varrault for advices with the dCas9-Suntag-TET1 experiments. This work was funded by the European
Research Council (ERC Consolidator grant no. 615371) and the Institut National du Cancer (INCa). T.D. was recipient of a Doctoral fellowship from the French Ministry for Higher Education and
Research. A.A.L. was supported by the Fondation pour la Recherche Médicale (FRM). AUTHOR INFORMATION Author notes * Richard P. Ngondo Present address: IBMP, CNRS UPR2357, 67084, Strasbourg,
France AUTHORS AND AFFILIATIONS * University of Strasbourg, Strasbourg, France Thomas Dahlet, Andrea Argüeso Lleida, Hala Al Adhami, Michael Dumas, Ambre Bender, Richard P. Ngondo, Manon
Tanguy, Judith Vallet, Ghislain Auclair, Anaïs F. Bardet & Michael Weber * Biotechnology and Cell Signaling, CNRS UMR7242, 300 Bd Sébastien Brant, 67412, Illkirch, Cedex, France Thomas
Dahlet, Andrea Argüeso Lleida, Hala Al Adhami, Michael Dumas, Ambre Bender, Richard P. Ngondo, Manon Tanguy, Judith Vallet, Ghislain Auclair, Anaïs F. Bardet & Michael Weber Authors *
Thomas Dahlet View author publications You can also search for this author inPubMed Google Scholar * Andrea Argüeso Lleida View author publications You can also search for this author
inPubMed Google Scholar * Hala Al Adhami View author publications You can also search for this author inPubMed Google Scholar * Michael Dumas View author publications You can also search for
this author inPubMed Google Scholar * Ambre Bender View author publications You can also search for this author inPubMed Google Scholar * Richard P. Ngondo View author publications You can
also search for this author inPubMed Google Scholar * Manon Tanguy View author publications You can also search for this author inPubMed Google Scholar * Judith Vallet View author
publications You can also search for this author inPubMed Google Scholar * Ghislain Auclair View author publications You can also search for this author inPubMed Google Scholar * Anaïs F.
Bardet View author publications You can also search for this author inPubMed Google Scholar * Michael Weber View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS T.D. performed embryo dissection, conducted RRBS, WGBS, RNA-seq, and gene expression analysis and performed conditional inactivation experiments in MEFs. A.A.L. and
H.A.A. performed dCas9-based methylation editing experiments. M.D. and A.F.B. conducted bioinformatic analysis of the sequencing data. A.B. performed embryo dissection and RRBS experiments.
J.V. and R.P.N. contributed to conditional inactivation experiments in MEFs. M.T. and G.A. participated in the generation of mouse lines and embryo dissection. M.W. conceived and supervised
the project and performed data analysis. M.W. and T.D. wrote the paper with contributions from the other authors. CORRESPONDING AUTHOR Correspondence to Michael Weber. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Kenji Ichiyanagi, Louis Lefebvre and Jörn Walter
for their contribution to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1
SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dahlet, T., Argüeso Lleida, A., Al Adhami, H. _et al._ Genome-wide analysis in the
mouse embryo reveals the importance of DNA methylation for transcription integrity. _Nat Commun_ 11, 3153 (2020). https://doi.org/10.1038/s41467-020-16919-w Download citation * Received: 18
June 2019 * Accepted: 29 May 2020 * Published: 19 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16919-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
