Genetic drug target validation using mendelian randomisation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Mendelian randomisation (MR) analysis is an important tool to elucidate the causal relevance of environmental and biological risk factors for disease. However, causal inference is
undermined if genetic variants used to instrument a risk factor also influence alternative disease-pathways (horizontal pleiotropy). Here we report how the ‘no horizontal pleiotropy
assumption’ is strengthened when proteins are the risk factors of interest. Proteins are typically the proximal effectors of biological processes encoded in the genome. Moreover, proteins
are the targets of most medicines, so MR studies of drug targets are becoming a fundamental tool in drug development. To enable such studies, we introduce a mathematical framework that
contrasts MR analysis of proteins with that of risk factors located more distally in the causal chain from gene to disease. We illustrate key model decisions and introduce an analytical
framework for maximising power and evaluating the robustness of analyses. SIMILAR CONTENT BEING VIEWED BY OTHERS ADVANCING THE USE OF GENOME-WIDE ASSOCIATION STUDIES FOR DRUG REPURPOSING
Article 23 July 2021 A ROBUST CIS-MENDELIAN RANDOMIZATION METHOD WITH APPLICATION TO DRUG TARGET DISCOVERY Article Open access 18 July 2024 A TRANSLATIONAL FRAMEWORK OF GENOPROTEOMIC STUDIES
FOR CARDIOVASCULAR DRUG DISCOVERY Article Open access 06 August 2024 INTRODUCTION Mendelian randomisation (MR) studies estimate the causal relationship of a risk factor of interest to
disease outcomes using genetic variants as instruments to index the risk factor. The naturally randomised allocation of genetic variation at conception limits the potential for confounding,
which compromises causal inference drawn from the directly observed association between risk factor and disease1. Risk factors of interest (some of which are amenable to modification by
drugs or behaviour change) can be both exogenous and endogenous, encompassing health-related behaviours (e.g. smoking and alcohol consumption), complex biological traits (e.g. blood pressure
and body mass index) or circulating constituents of the blood e.g. complex analytes such as lipoproteins, metabolites such as uric acid, or proteins such as interleukin-62,3,4 (protein
quantitative trait loci; pQTL). Interest has also emerged in tissue-level mRNA expression as an exposure of interest5 (expression quantitative trait loci; eQTL). Most prior MR analyses
utilise an approach whereby multiple SNPs identified from GWAS are used as instruments to increase precision. SNPs are drawn from throughout the genome, often with a single variant selected
per locus ensuring instruments are independent (i.e. in linkage equilibrium, LD); preventing erroneously statistical significance. This standard approach has been applied regardless of the
position of the exposure of interest in the biological pathway connecting genetic variation to disease risk. As an example, prior work used MR analysis to assess the causal relevance of the
major circulating lipid fractions (LDL-C, HDL-C and triglycerides) for coronary heart disease (CHD), utilising variants from throughout the genome as instruments. However, the approach we
explore in this paper uses genetic instruments restricted to the target of interest (acting in _cis_). The two approaches are both relevant but seek to answer different questions. Whereas
the former addresses the causal relevance of the biomarker for CHD, the latter seeks to address whether modification of a specified drug target will reduce CHD and uses the biomarker as a
proxy of protein concentration and activity. These two approaches may yield different estimates when the protein drug target, for example CETP, affects multiple pathways (e.g. HDL-C and
LDL-C) through so-called post-translational pleiotropy (defined below). Implicitly _cis_-MR analyses of drug targets such as CETP, attempt to understand the involvement of the encoded
protein in a disease. When proteins of interest are potentially druggable (as with CETP) such MR analyses can be referred to as ‘drug target MR’. Recent technological developments enable
measurement of hundreds or thousands of proteins on an –omics scale in a single biological sample4. This opens up the possibility of scaled drug target MR analysis of thousands of proteins
against hundreds of diseases to inform understanding of their causes and improve drug development yield. In the current manuscript we develop a mathematical framework for drug target MR,
showing why these analyses are more robust than MR analyses of more distal traits. We next discuss the choice of exposure variables (mRNA, protein or downstream biomarker), indicating that
proteins may be preferred as exposures but when unavailable that drug target MR analyse weighted by mRNA or downstream biomarkers may provide valid test of a protein’ effect on disease.
Next, we introduce four positive control loci that encode targets of licensed or clinical phase drugs (_HMGCR_, _PCSK9_, _NPC1L1_, and _CETP_), and empirically evaluate instrument selection
strategies, proposing a scalable approach to maximise power while safeguarding against erroneous significance. We further show that tissue-specific drug target MR estimates of eQTL weighted
analyses do not always agree with that of similar blood-based pQTL analyses or with evidence from drug trials. Finally, the scalability of our approach is showcased in a phenome-wide scan of
an additional five targets on 35 therapeutically relevant outcomes. RESULTS A MATHEMATICAL FRAMEWORK FOR _CIS_-MR ANALYSIS MR studies determine the causal effect of a risk factor on a
disease using instrumental variable (IV) methods6, leveraging two estimates: the genetic association with the risk factor (exposure) and the genetic association with the disease (outcome).
For the effect estimate in MR to equate to a causal estimate the following critical assumptions should hold: (i) the genetic instrument is (strongly) associated with the exposure, (ii) the
genetic instrument is independent of observed and unobserved confounders of the exposure-outcome association (which is secure because genetic variants are fixed and allocated at random), and
(iii) conditional on the exposure and confounders, the genetic instrument is independent of the outcome (i.e. there is no instrument—outcome effect other than through the exposure of
interest—the ‘no horizontal pleiotropy’ assumption). The no horizontal pleiotropy assumption is violated when there are additional pathways by which the instrument may be related to the
disease, sidestepping the exposure of interest. In contrast, the association of a genetic instrument with exposures that lie in the causal chain distal to the exposure of interest (vertical
pleiotropy7) does not violate the assumptions underpinning MR analysis. When proteins serve as the exposure of interest in MR analysis, it becomes possible to give biological context to the
concepts of vertical and horizontal pleiotropy. This is because horizontal pleiotropy equates to pathways from gene to disease, which precede translation of the protein of interest, e.g.
through alternative splicing or micro-RNA effects. By contrast, vertical pleiotropy refers to the downstream actions of the translated protein, which should be reproduced by a drug with
specific action on the protein. Therefore, in the context of MR analysis of proteins, vertical and horizontal pleiotropy correspond to ‘pre-’ and ‘post’-translational effects, respectively.
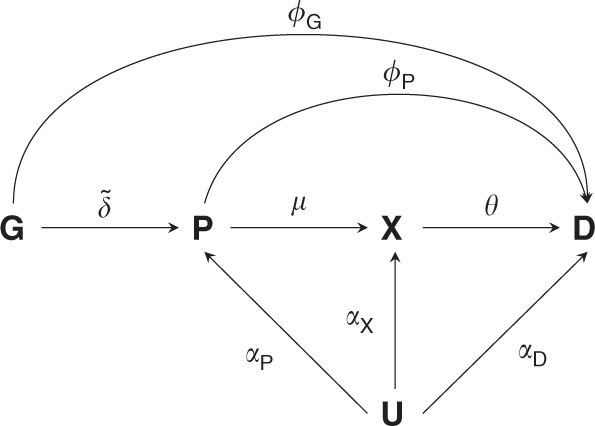
In the methods section we describe how to analytically explore and adjust for horizontal pleiotropy. Figure 1 illustrates the MR considerations, where a genetic variant (_G_) influences the
disease risk (_D_) directly (_ϕ__G_) or through its effect (\(\tilde \delta\)) on a protein (_P_), which exerts its action through a downstream biomarker (_X_), which in turn influences
disease risk. The relevant genetic effects can be resolved as follows: * (1) The genetic effect on the protein \(\tilde \delta\). * (2) The genetic effect on a downstream complex biomarker
\(\tilde \delta \mu\). * (3) The genetic effect on disease \(\phi _{\boldsymbol{G}} + \tilde \delta (\phi _{\boldsymbol{P}} + \mu \theta )\), which comprises: * (a) A _direct effect_ of the
variant on disease _ϕ__G_. * (b) An _indirect effect_: \(\tilde \delta (\phi _{\boldsymbol{P}} + \mu \theta )\), which is a function of the genetic effect on a protein \(\tilde \delta\), the
direct effect of the protein on disease _ϕ__P_, the effect of the protein on a biomarker _μ_, and the biomarker effect on disease _θ_. Depending on the risk factor of interest, an MR
analysis constitutes a simple quotient of the genetic effect on disease by the genetic effect on the risk factor. For example, if we are interested in the causal effect the biomarker _X_ on
disease, i.e. _θ_, we use the following ratio. $$\frac{{\phi _{\boldsymbol{G}} + \tilde \delta \left( {\phi _{\boldsymbol{P}} + \mu \theta } \right)}}{{\tilde \delta \mu }}.$$ (1) For this
expression to equate to the causal effect on disease we need to additionally assume that there is no horizontal pleiotropy, in other words _ϕ__G_ = _ϕ__P_ = 0, which reduces the expression
to: $$\frac{{\phi _{\boldsymbol{G}} + \tilde \delta \left( {\phi _{\boldsymbol{P}} + \mu \theta } \right)}}{{\tilde \delta \mu }} = \frac{{\tilde \delta \mu \theta }}{{\tilde \delta \mu }},
\\ = \theta .$$ (2) In contrast, if we are interested in the causal effect of the protein _P_ on disease _D_, we want to obtain an estimate of _ω_, where _ω_ = _ϕ__P_ + _μθ_, and assuming
_ϕ__G_ = 0 we calculate the ratio: $$\frac{{\tilde \delta \left( {\phi _{\boldsymbol{P}} + \mu \theta } \right)}}{{\tilde \delta }} = \phi _{\boldsymbol{P}} + \mu \theta ,\\ = \omega .$$ (3)
Critically, where the causal effect of the protein is the parameter of interest, we only need to assume that there is no direct effect of the genetic variant on disease, i.e. _ϕ__G_ = 0,
and the protein can have any mixture of direct (_ϕ__P_), and indirect (_μθ_) effects. For this reason, MR analysis of protein-disease relationships is less prone to violation of the ‘no
horizontal pleiotropy’ assumption than MR analysis of downstream exposures. In the methods section we further elaborate on the statistical inference of drug target MR analyses using genetic
associations with up- or downstream proxies of a (protein) drug target. We show that in most cases the estimand is distinct from _ω_. However, under the same no pre-translational pleiotropy
assumption, statistical tests will correctly reject the null-hypothesis _ω_ = 0, without inflated false positive rates. In addition, we prove that in the presence of post-translational
pleiotropy drug target MR remains valid, even when a downstream proxy (such as lipids) does not causally affect disease. SELECTION OF LOCI ENCODING PROTEINS Unlike MR analysis of non-protein
traits, where it has become common to select instruments from throughout the genome, _cis-_MR analysis necessitates the selection of genetic variants from within or near a protein-coding
gene. The Ensembl 97 GRCh38 human genome assembly contains an estimated 20,454 protein-coding genes, encoding an estimated 24,700 protein-coding transcripts (merged Ensembl/Havana
annotation). Of these transcripts, 21,869 have a degree of experimental support for the presence of these transcripts. In addition, UniProt (version 2019_06, combining SwissProt and TrEMBL)
reports 20,416 high quality manually annotated proteins. SELECTION OF LOCI ENCODING DRUGGABLE GENES Not all encoded proteins are amenable to pharmacological action by (small molecule) drugs,
or peptide and monoclonal antibody therapeutics, which currently account for the majority of medicines. _cis-_MR for drug target validation requires the selection of genes encoding
druggable proteins. Progressive efforts to delineate the druggable genome8,9 (available through the DGI database (DGIdb10), have culminated in the latest iteration containing 4,479 genes11
encompassing targets of existing therapeutics, potentially druggable close orthologues and targets accessible by monoclonal antibodies. Of these, ChEMBL v.24 identifies 896 genes as encoding
the target components for existing therapeutics. These include single protein targets, protein complex targets and targets comprising whole protein families. A further 535 genes encode
target components of compounds currently in clinical testing. The druggable genome is not static and will be redefined periodically, reflecting changes in drug targeting mechanisms. However,
currently, to define the druggable genome is to progressively reduce the high-dimensional search space for genetic instruments from the whole genome to around 20,000 protein-coding genes to
fewer than 5000 genes encoding druggable targets. As such, a specific subset of _cis-_MR can inform drug development, which we term ‘drug target MR’. INSTRUMENT SELECTION Drug target MR
focuses on a gene known to encode a protein drug target, and variants within and around a gene are used to characterise the effect(s) of the drug target on a single or multiple outcome(s).
Given the inferential target, it would seem logical to select variants based on the variant to protein level association (\(\tilde \delta\)) in a relevant tissue. Ideally one would only
select causal variants known to affect the drug target, maximising precision (power). However, typically the nature and number of causal variant(s) is unknown, imposing the need for
instrument selection. Similarly, while the first GWAS on the proteome are becoming available, currently most drug target MR analyses utilise biomarkers downstream of the drug target protein.
As such, to evaluate the current modus operandi, we explore instrument selection using biomarker proxies, and compare this to using the actual pQTL effects, and alternatively eQTL effects
(as upstream proxies of a drug target). In such cases, variants are often selected based on (1) a biomarker association (e.g. LDL-C in the case of PCSK9 discussed earlier), (2) predicted
functionality; and (3) low LD, typically including a single12,13 or perhaps a handful of SNPs14,15, out of a multitude of potential candidate SNPs. It is often unclear how well such a small
subset of SNPs characterises the drug target effect, and how influential such strategies are on the final result. To explore this, we mimicked instrument selection by repeatedly (500 times)
sampling four SNPs at random per locus from four known drug target encoding loci _HMGCR_ (statins), _NPC1L1_ (ezetimibe), _PCSK9_ (PCSK9 inhibitors), and _CETP_ (CETP inhibitors). These loci
contain variants that influence LDL-cholesterol (_HMGCR_, _NPC1L1_, _PCSK9_, _CETP_) with variants at the CETP locus additionally influencing HDL-cholesterol and triglycerides as identified
by the Global Lipids Genetics Consortium (GLGC16). We used a generalised least squares (GLS) method17,18 to account for pairwise LD between variants at each locus. Variants were extracted
from within the gene ±2.5 kB, with a minor allele frequency (MAF) above 0.01, and LD <0.80 (Supplementary Tables 1–5, Supplementary Fig. 1). The first and third quartiles (Q) of the CHD
odds ratios (OR) per standard deviation (SD) in LDL-C for _HMGCR_, _NPC1L1_, _PCSK9_ (or HDL-C in the case of _CETP_) indicated modest variability in the point estimate: (Q1 1.61, Q3 1.78)
for _HMGCR_, (Q1 1.42, Q3 1.77) for _PCSK9_, (Q1 1.19, Q3 1.68) for _NPC1L1_, and (Q1 0.87, Q3 0.91) for _CETP_. Between 95 and 99% of the estimates across all four genes were in the
expected direction as inferred from the findings of drugs used in clinical trials to target the corresponding proteins19,20,21,22,23,24,25. We further categorised effect estimates based on
the EnsEMBL Variant Effect Prediction (VEP)26 of each variant (Fig. 2), finding little to no difference between estimates derived using non-coding variants only and those estimates based on
functional variants. The overall stability and agreement between estimates derived with or without functional annotations suggests a strong influence of multivariate LD between the selected
and unselected variants in small _cis-_regions (Supplementary Fig. 1). We did however observe a large degree of variability in the _p_ values which is explored in the subsequent section
(Supplementary Fig. 2). TAKING ADVANTAGE OF LINKAGE DISEQUILIBRIUM WITHIN THE REGION Given the observed influence of LD it seems desirable to leverage this in drug target MR. For example,
after defining a _cis-_genetic region (discussed further below) one can LD-clump highly correlated variants that might destabilise a statistical model (multicollinearity) and account for
remaining pairwise LD using an LD-reference panel to maximize power and decrease variability. Besides increasing power and robustness, this strategy introduces some complexities, e.g. the
choice of LD threshold. The effect of LD thresholds can be readily explored by performing a ‘grid search’, clumping variants at different R-squared thresholds. From modelling theory (and
empirically: Fig. 2), one would expect that when using such a grid search the point estimate stabilises early (at low thresholds), while the standard errors decrease further until, at a
certain point, multicollinearity results in a clear deviation from the overall tendency. Such a grid search was implemented in Fig. 3 showing signs of multicollinearity for the _HMGCR_ and
_PCSK9_ estimates, but less so for _NPC1L1_. While trends observed for _HMGCR_ and _PCSK9_ are examples of what one would expect on theoretical grounds, this does not occur at the same
threshold, and seemingly not at all for the _CETP_ locus. Given the ongoing debate on whether the beneficial effect of CETP-inhibition depends on HDL-C raising or LDL-C lowering activity, we
repeated these analyses using LDL-C weights (Supplementary Fig. 3) with similar results to those observed using HDL-C weights. LINKAGE DISEQUILIBRIUM MODELLING COMPARED WITH SELECTING
FUNCTIONAL VARIANTS Based on these considerations, we explored the performance of a very limited instrument selection strategy, geared towards characterising a _cis-_genetic region encoding
a drug target as fully as possible by (1) considering all variants with limited LD clumping to prevent multicollinearity; (2) selecting the most significant variant in an ‘LD-block’; (3)
modelling LD using external data such as the 1000 genomes reference panel. This strategy (with _R_2 = 0.60) was applied to our four empirical examples and compared with MR estimates at the
same locus using only variants with strong evidence of function based on VEP (Fig. 4 and Supplementary Tables 6–9). We found general agreement between the effect estimates from both
analytical approaches, with the GLS estimates having higher precision (1/SE) than those based on functional variants alone. For example, the precision of the two _PCSK9_ splice variants used
in MR was 5.72 compared with 13.33 for the GLS estimates (incorporating variants selected based on LD structure irrespective of function). These results confirm that precision/power is
increased by including more correlated variants. To prevent erroneously low _p_ values in such analyses, we accounted (conditioned) for pairwise LD using the European 1000 genomes panel. We
further investigated the influence of different 1000G ancestry reference panels on the effect estimates and found these to be stable for the four examples evaluated (Supplementary Fig. 4);
although significance of the _NPC1L1_ was dependent on the panel used. We did, however, observe that the GLS method often failed because of (small) changes in LD resulting in
multicollinearity. After inspection, this seemed to be related to LD-estimates of low MAF variants varying across ethnicities (Supplementary Fig. 5). Improved behaviour may be expected with
either increased sample size (1000G sample size _n_~100), or with the removal of low MAF variants (at the risk of losing information). DRUG TARGET MR USING PQTLS The analyses to this point
utilised lipid exposures to index the effect of four drug targets. Large scale GWAS studies of the proteome have recently become possible (e.g. the INTERVAL study4), opening up the
possibility of using the genetic effect on protein concentration as a more direct proxy of the drug target effects. Of the four proteins considered thus far, we had access to pQTL estimates
from GWAS of circulating CETP and PCSK9 concentration measured by enzyme-linked immunosorbent assays (ELISA)27,28 in about 4000 and 3000 subjects, respectively. Initially focussing on
variants in the same ±2.5 kB region as before, we found circulating CETP increased CHD risk, consistent with the findings of a recent large-scale clinical trial where CETP inhibition reduces
CHD risk (Fig. 5). Similarly convincing results were observed for the analyses of log(PCSK9) concentration and CHD. In the biomarker weighted analysis, the size of the genetic flanking
region was constrained to prevent erroneously modelling effects from neighbouring genes not encoding the drug target of interest (horizontal pleiotropy). However, pQTL associations provide a
direct estimate of the genetic association with the drug-target and hence may reduce the need to focus on small flaking regions. We therefore compared findings from the ±2.5 kB region to
pQTL MR results using a broader ±1 MB flanking region. To further guard against potential horizontal pleiotropy (for example through LD) we additionally implemented the Egger adjustment.
CETP was (again) robustly causally associated with CHD, with larger R-squared values decreasing variability without any indication of model instability (Fig. 5). Due to the limited number of
additional variants, the ±1 MB PCSK9 pQTL analysis did not differ markedly from the ±2.5 kB, and the MR-Egger estimate was fairly unstable. We further performed eQTL weighted analyses of
the same positive control drug targets, observing persistent directional discordance between the tissue specific CHD association of a single drug target. For example, PCSK9 mRNA expression
in the adrenal gland was associated with an increase in CHD risk: OR 1.09 (95%CI 1.02; 1.16), while PCSK9 expression in the uterus was associated with decreased CHD risk: OR 0.92 (95% 0.88;
0.97). This discordance was not explained by horizontal pleiotropy, region size, or influence of enhancer variants (Supplementary Discussion, and Supplementary Tables 9,10). FURTHER
EXAMPLES: PHENOME-WIDE DRUG TARGET ANALYSIS To further showcase the generalisability of the proposed approach, we conducted drug target analyses focussing on circulating proteins that are
the direct efficacy targets of clinical phase developmental or licensed drugs. Genetic instruments for _cis-_MR analyses were identified from the INTERVAL study which conducted a GWAS of
around 3000 circulating proteins4, measured using the Somalogic aptamer-based proteomics platform. From the reported data we identified five proteins encoded in the druggable genome11 (_F10,
IL-12B, PLG, IL-1R1, MMP-9_) for which sufficient sentinel variants could be identified. We then conducted a phenome-wide drug target MR analysis against 35 clinically relevant disease and
biomarker phenotypes (see ‘Methods’). Circulating factor X (encoded by _F10)_ was associated with a higher risk of any stroke (OR 1.13 95%CI, 1.05,1.21), which is keeping with the use of
direct-acting anticoagulant drugs that inhibit factor X (e.g. apixaban) to prevent stroke in patients with atrial fibrillation (AF)29. Furthermore, we found a possible effect of factor X on
asthma (OR 0.78 95%CI 0.62, 0.99) (Fig. 6). The monoclonal antibody ustekinumab directed against a common subunit of interleukin 12 and interleukin 23 interferes with the binding of these
cytokines with the IL-12 receptor to inhibit inflammatory signalling30. Ustekinumab has European marketing authorisation for the treatment of psoriasis and Crohn’s disease (CD) after
demonstrating efficacy in clinical trials31, and is under evaluation for ulcerative colitis (UC). Consistent with this, genetically instrumented higher interleukin-12 subunit beta (encoded
by _IL12B_) was associated with a higher risk of CD (OR 2.02; 95% CI 1.48, 2.76), UC (OR 1.56; 95% CI, 1.31, 1.87), and inflammatory bowel disease (IBD) (OR 1.56 95%CI 1.31, 1.87) (Fig. 6).
Genetically-instrumented higher circulating concentration of plasminogen (encoded by _PLG_) was associated with a lower ischaemic stroke risk (OR 0.85; 95% CI 0.72, 1.00) (Fig. 6),
consistent with the known efficacy of recombinant tissue plasminogen activator (tPA) for acute ischaemic stroke32. The PLG association with an increased risk of any stroke (OR 1.06; 95%CI
1.01, 1.11) is presumably due to an increase in haemorrhagic events. Increased levels of PLG were furthermore associated with increased risk of AF, IBD, CD, Alzheimer’s disease (Fig. 6) as
well as lipids, and increased SBP (Fig. 6), but these effects may not be observed therapeutically because tPA is given as a single dose in acute MI and ischaemic stroke. Higher circulating
concentration of interleukin-1 receptor 1 (encoded by _IL1R1_) was associated with a lower risk of both CD, IBD and UC. This would be in keeping with the circulating form of the receptor
functioning as decoy to reduce signalling through the membrane-bound form of the receptor by the pro-inflammatory cytokine interleukin 1β. We also found evidence through _cis-_MR of a
causative role for MMP9 in CD and IBD. Recent phase 2 trials failed to demonstrate efficacy of andecaliximab, a monoclonal antibody targeting MMP9 in either UC or CD33,34 (Fig. 6). However,
given the evidence from the MR analysis, further consideration should be given to the type, dose, frequency and duration of ant-MMP9 therapy in Crohn’s disease before this target is
discounted for these diseases. DISCUSSION We have used biological and mathematical arguments to formalise the distinction between locus-specific Mendelian randomisation (MR) analysis for
drug target validation, where the appropriate instruments are variants in or within the vicinity of the encoding genes, and other types of MR analysis, e.g. for risk factor validation, where
instruments are used from throughout the genome. Using algebraic derivations, we show that because drug target MR considers the effects of perturbing a protein drug target on disease, this
type of MR may be applied in settings where traditional MR, focussed on distal traits, could be biased through horizontal pleiotropy. We also illustrate the challenges when undertaking MR
for drug target validation. These include defining the loci of interest, accounting for linkage disequilibrium, and selecting the exposure through which to weight the genetic outcome
association. We discuss resources available for the identification of ‘druggable’ protein-coding genes and show that, because MR for drug target validation is framed as a _cis-_focused
analysis, instrument selection is distinct from that for MR for validating the causal relevance of a non-protein or environmental exposure. We investigated strategies for characterising the
drug target-encoding region through linkage disequilibrium (LD). Grid-search algorithms were introduced aiding researcher in optimising LD-thresholds, as well as genetic regions, with
intuitive sensitivity analyses to estimate robustness to the choices of LD reference panel, the presence of functional variants as well as regulatory enhancers, and outliers using
heterogeneity statistics. We further introduce an exploratory analysis to determine the influence of horizontal pleiotropy, pruning variants that associate with other genes around a locus
encoding a drug target (see Methods section and Supplementary Information). Finally, we illustrate the generalisability of our approach by presenting findings from a pQTL drug target
phenome-wide scan across 35 clinically relevant outcomes. Due to our focus on four positive control examples, we were able to perform exhaustive analyses on the robustness of drug target MR
findings based on regulatory versus coding variants showing that robust causal inferences could be drawn from regulatory variants despite a widely held view that functional variants should
be naturally preferred in (drug target) MR. Instead we showed that applying a straightforward clumping algorithm, agnostic of the type of variants, resulted in the same OR, compared with
selecting functional variants, with greater precision (increased power) by including larger numbers of (partially dependent) variants. Similarly, we showed the limited influence of
LD-reference panels used in LD-modelling with non-European ancestry panels resulting in comparable estimates. While promising, these findings should be replicated and above all extended to a
larger set of drug targets, for example to analyse targets outside of the lipids-cardiovascular domain presented here. While there are an impressive number of estimator functions (see
methods), there has been limited advice on instrument selection for drug target MR, with sparse attention given to empirically exploring the influence on MR estimates. This manuscript
addresses both issues and introduces a generic framework (Fig. 7) for obtaining robust inference of a drug target’ effect on disease, irrespective of the type of MR estimator method
preferred. In this framework we suggest that for each exposure outcome pair grid-searches are employed to select the optimal LD threshold and genetic region, while at the same time exploring
the robustness of MR estimates to LD-reference panel, the influence of functional and regulatory variants, as well as assessing the influence of outlying instruments. MR of protein exposure
have been conducted before, sometimes selecting both _cis_ and _trans_ and sometimes selecting only _trans_ variants. However, the use of _cis_ instruments for MR analysis of proteins is
less prone to violation of the horizontal pleiotropy assumption than the use of _trans_ instruments (see Supplementary Methods) and is amply illustrated by the example of C-reactive protein
(CRP). Circulating CRP concentration is associated with CHD risk in nonrandomized observational studies. In 2011, in a paradigm example, a _cis-_MR analysis of CRP in CHD35 showed the
nonrandomized association is non-causal. Consistent findings were obtained by others36. Subsequent GWAS of CRP have been conducted37 and have identified variants in genes outside CRP (acting
in _trans_) that associate with CRP concentration, including in genes encoding the receptor for the inflammatory cytokine IL-6 and _APOC1_, involved in lipid metabolism. Variants in _IL6R_
that are associated with lower CRP concentration are associated with lower risk of CHD2,3 and variants in _APOC1_ that are associated with higher concentration of CRP are associated with
increased risk of CHD38. However, it would be erroneous to suppose that a _trans-_MR analysis of CRP instrumented using _APOC1_ variants provides evidence of a causal role of CRP in CHD38
since the same variants are also associated with LDL-C. Clearly, leveraging variants in _APOC1_ (or _IL6R_) acting in _trans_ to probe the causal relevance of CRP for CHD would introduce
bias through horizontal pleiotropy. The same argument applies to the use of variants in _IL6R_ for the same purpose. Signalling through the interleukin-6 receptor encoded by _IL6R_
influences many inflammatory molecules beyond CRP that are the likely mediators of its effect on CHD. Over 90% of drug targets are proteins, therefore weighting by protein expression in a
disease-relevant tissue would provide the most informative _cis-_MR analysis for drug target validation. Many of the circulating proteins measured by the currently available proteomics
platforms (e.g. from Somalogic [2036 druggable proteins] or O-link [973 druggable proteins]) are the actual biological efficacy targets of many licensed or developmental therapeutics. Thus,
the available data on the circulating proteome already provide a step change in the ability to apply genetic target validation. We have illustrated the potential of this approach by
conducting a _cis-_MR and PheWAS for five such targets. Where drugs bind membrane bound or intracellular proteins, which subsequently affect non-protein constituents of the circulating
blood, drug target MR studies might fruitfully employ genetic associations with downstream proxies of protein level and concentration. We illustrated this with reference to four drug targets
for lipid-lowering (HMGCR, NPC1L1, PCSK9 and CETP). For two of these targets (PCSK9 and CETP) it was also possible to compare the findings of _cis-_MR analyses weighted by the level of the
circulating protein with _cis-_MR analyses weighted by the relevant lipid fraction. However, for many drugs or drug targets, for example, those used in, or being developed for, the treatment
of neurological, myocardial, or musculoskeletal conditions, a circulating biomarker may be unavailable or may not represent a strong proxy for the drug target. Tissue-specific pQTL data
have yet to be generated at any scale and, until such data become available, we investigated tissue-specific eQTLs as a potentially relevant alternative exposure that might closely proxy
pQTL effects. We found that eQTL-based MR estimates may differ both in magnitude as well as direction across tissues, as demonstrated by exhaustive analyses of the _HMGCR_, _NPC1L1_, _PCSK9_
and _CETP_ loci. This tissue-dependent heterogeneity was independently reported by the GTEx consortium for _PCSK9_39. We extend those observations to demonstrate their potential to
undermine reliable causal inference when using mRNA expression as a weighting variable in MR analysis. Possibly, the observed heterogeneity may relate to the inclusion of non-European
ancestries in the GTEx database, or due to the post-mortem collection of samples40. For example, GTEx previously reported that gene expression changed post-mortem in a tissue-specific
manner, which they attempted to ameliorate through multiple regression40. This tissue-specific heterogeneity likely reflects actual biology which might also extend to tissue-specific pQTL
data. A key uncertainty is identifying the ‘relevant’ tissue for a drug target validation MR analysis. This would be informed by greater knowledge of the protein expression profiles of all
human drug targets, an area that has so far received limited attention. When these data become available, it will become important to evaluate tissue dependency and the underlying mechanisms
in more detail. Where it is relevant, and possible, to use circulating proteins or biomarkers such as lipids as exposure variables in two-stage drug target MR analysis this may help
mitigate the complexity of weighting based on tissue-specific eQTL or pQTL data. In this current manuscript we have exclusively focussed on MR as a tool for drug target validation, however
many complimentary methods exist, often utilising non-genetic cell, tissue and animal experiments. A key challenge to further improve (early) drug development will be to incorporate these
different sources of evidence to accurately predict in-human efficacy. In conclusion, we expect that combining the discussed concepts with the ever-increasing magnitude of genetic and other
‘omics’ data will move drug target MR from manually curated, often proof of concept-like analyses, to more automated and scalable projects able to systematically guide and enrich the entire
drug development process. METHODS MENDELIAN RANDOMISATION FOR PROTEIN EXPOSURES There are reasons for considering MR analysis of a protein drug target to be a distinct category of MR
analysis. First, an analysis of this type induces a natural dichotomy in the genetic instruments that might be used: those that are located in and around the encoding gene (‘_cis-_MR’) vs
those located elsewhere in the genome (_trans-_MR)41. Second, aside from mRNA expression, differences in protein expression or function are the most proximal consequence of natural genetic
variation. This has two consequences: frequently, variants located in and around the encoding gene can be identified with a very substantial effect on protein expression in comparison to
other traits; moreover such instruments may also be less prone to violating the ‘no horizontal pleiotropy’ assumption than variants located elsewhere in the genome (discussed below and in
ref. 41). Third, in the case of MR analysis of proteins, Crick’s ‘Central Dogma’42 imposes an order on the direction of information flow from gene to mRNA to encoded protein, which does not
extend beyond this to other biological traits that lie more distally in the causal chain that connects genetic variation to disease risk. Finally, _cis-_MR of a protein risk factor greatly
reduces the risk of reverse causation, because Crick’s dogma indicates that the pathway gene → encoded protein → disease would always be favoured over the pathway gene → disease → encoded
protein, especially given that the gene → encoded protein association is typically derived from population-based (disease-free) samples. Thus, from an MR perspective, proteins are in a
privileged position compared with other categories of risk factor and the use of _cis-_MR represents an optimal approach to instrument their causal effect for disease (See Supplementary
Methods and Supplementary Figs. 7, 8). The key to realising this potential is the development of a robust conceptual and mathematical framework for _cis-_MR analysis of proteins. Since
_cis-_MR analysis restricts selection of genetic instruments to those located in, or near the encoding gene, new questions emerge as how to optimise the selection of such variants. These
include how best to select and define the loci of interest, the physical distance around each gene from which instruments might be drawn; how to select genetic variants as instruments with
options including ‘no selection’, ‘selection by strength of association’, or ‘according to functional annotation’. Regulatory, non-coding variants act through the level of the encoded
protein which is what high-throughput assays detect. Coding-variants might influence protein activity but may also alter the detected rather than actual protein level by protein epitope
changes, resulting in a technical artefact. Further questions include whether to weight such instruments in an MR analysis by the level of protein _expression_ or _activity_, where the
relevant assays are available; or, where they are not, by the level of mRNA expression (and, if so, in which tissue), or by some downstream consequence of protein action, e.g. differences in
the level of a metabolite known to be influenced by the protein such as LDL-C for a lipid-lowering drug target. ALTERNATIVE EXPOSURES IN _CIS_-MR ANALYSIS OF PROTEINS Drug target MR is
concerned with obtaining inference on the effect direction of perturbation of a (protein) drug target, where the effect estimate can be defined as _ω_ = _ϕ__P_ + _μθ_ (Fig. 1 and
Supplementary Fig. 7). It is important to note that a protein can remain the inferential target in an MR analysis even if it is not measured directly. For example, in cardiovascular disease
large sample size GWAS are available on lipids which are often intermediate biomarkers, positioned downstream between the drug target _P_ and disease, _D_ (Fig. 1). In our recent drug target
MR analysis of PCSK914,43 we used instruments selected from the encoding locus and divided the variant to coronary heart disease (CHD) estimates, not by the effect on PCSK9 level (which was
unavailable), but by LDL-C, a variable known to be altered by perturbation of the PCSK9 protein. Thus, using the same notation as above, and assuming _ϕ__G_ = 0: $$\omega _{bw} =
\frac{{\tilde \delta \left( {\phi _{\boldsymbol{P}} + \mu \theta } \right)}}{{\tilde \delta \mu }} = \frac{{\phi _{\boldsymbol{P}} + \mu \theta }}{\mu }, \\ = \frac{1}{\mu } \times \omega
,$$ with _bw_ indicating ‘biomarker weighted’. Clearly because the denominator contains \(\tilde \delta \mu\), instead of \(\tilde \delta ,\omega _{bw}\) does not equal _ω_. However, _ω__bw_
may still provide a valid null-hypothesis test of _ω_ = 0, because _ω__bw_ ≠ 0 implies _ω_ ≠ 0. Notice that if the protein has a direct effect on the disease, that is not mediated by the
downstream biomarker, _ω__bw_ ≠ 0 does not provide evidence for the biomarker itself to causally effect disease; i.e, ω_bw_ ≠ 0 does not imply _θ_≠0. The only additional requirement for
using a ‘biomarker-weighted’ MR for drug target validation is that the protein is strongly correlated with the downstream biomarker, for example when _μ_ ≠ 0; which is a (slightly) different
version of IV assumption (i). In the absence of available measures of the protein of interest, a similar argument can be made for using mRNA expression (this time as an upstream variable)
that proxies the effect of genetic variation on the level of the encoded protein again within the framework of a _cis-_MR analysis (see Supplementary Fig. 7): $$\omega _{ew} = \frac{{\tilde
\delta _{{\boldsymbol{GE}}}\tilde \delta _{{\boldsymbol{EP}}}\left( {\phi _{\boldsymbol{P}} + \mu \theta } \right)}}{{\tilde \delta _{{\boldsymbol{GE}}}}} = \tilde \delta
_{{\boldsymbol{EP}}}\left( {\phi _{\boldsymbol{P}} + \mu \theta } \right),\\ = \tilde \delta _{{\boldsymbol{EP}}} \times \omega.$$ Here the weighting is done by the association with of mRNA
expression and the \(\tilde \delta\) effect has been decomposed into the variant effect on expression \(\tilde \delta _{{\boldsymbol{GE}}}\) and the expression effect on protein level
\(\tilde \delta _{{\boldsymbol{EP}}}\). Similar as for _ω__bw_, the expression weighted (‘_ew_’) drug target effect provides a valid test of _ω_ = 0 conditional on the absence of any
pre-translational pleiotropy; that is, a necessary assumption _ϕ__G_ = _ϕ__E_ = 0 (with index _E_ for expression). It should be noted that, similar as for protein expression, mRNA expression
level (eQTL) is tissue-specific and utilising eQTLs for drug target MRs necessitates a decision on the tissue(s) relevant for (de novo) drug development. EXPLORING HORIZONTAL PLEIOTROPY To
address the possibility of horizontal pleiotropy in genome-wide MR analyses of risk factors, it has been common to select independent instruments from multiple locations across the genome.
In doing so, the average horizontal pleiotropy may reduce to zero (so-called _balanced_ pleiotropy_)_. As noted above, drug target MR is often performed using _cis_ variants, selected form
within and around a drug target encoding locus. While this diminishes the likelihood of horizontal pleiotropy (Supplementary Methods), should horizontal pleiotropy nevertheless occur, due to
the proximity of variants, it is more likely to be directional in nature. In such cases estimators such as MR-Egger44 might recover the causal effect contingent on the Instrument Strength
is Independent of the Direct Effect (INSIDE) assumption44; i.e. that the strength of the genetic association with the risk factor does not correlated with the magnitude of horizontal
pleiotropy. Additionally, an individual variants’ contribution to the degree of horizontal pleiotropy (and excluded from analysis) can be explored using straightforward metrics for outlier
detection such as the Q-statistic (the squared distance from the average estimate), or through leverage statistics (the estimate change after removing a variant). Due to the _cis_ focus of
most drug target MRs it is essential to ensure that pre-translational horizontal pleiotropy is absent. For example, a variant associated with PCSK9 expression may also associate (e.g.
through LD) with the expression of other genes. Here we introduce a novel empirical method to exclude LD-based horizontal pleiotropy by sequentially pruning eQTL data for an association with
the expression of a ‘non-target’ gene within a certain flanking region (here ±1 MB) of the encoding locus (using _p_ value threshold of {10−8,…,1 × 10−3}; Supplementary Fig. 9). This
screening step is showcased using the eQTL weighted MR analyses discussed in detail in the Supplementary Data section. We note that the type of screening can however be applied irrespective
of the intended exposure, for example it could also be used in drug target MR analyses using pQTL exposures. In general, across the four positive control loci we did not see much influence
of LD-based horizontal pleiotropy; and within a single tissue we did not observe much directional discordance. For each drug-target we did observe a few tissue-specific associations that
only obtain significance after pruning potential pleiotropic variants to a very low _p_ value threshold. For example, _CETP_ expression in the colon is only associated with CHD after
removing variants that had a _p_ value < 1 × 10−3 with neighbouring genes. NULL-VARIANTS AND WEAK INSTRUMENT BIAS In the current manuscript we propose to implement drug target MR through
LD clumping of variants and modelling residual correlation using external reference data45. A perceived drawback of this approach is the possible inclusion of ‘null variants’, that do not
affect the intermediate risk factor. In a conservative attempt at excluding null-variants researchers often focus on genome-wide significance (e.g. a _p_ value < 5 × 10−8). Dudbridge46
and many others have shown, however, that such an approach excludes many useful variants, harming power/precision, and lower thresholds (e.g. 10−5) often result in improved performance.
Clearly such lower threshold could result in the inclusion of (many) null-variants. However, by employing the two-sample MR paradigm (using genetic risk factor and outcome estimates from
different samples), any possible weak-instrument bias will attenuate results towards the null47. FALSE POSITIVE RATE Throughout this manuscript we use a type I of error rate of 0.05 (or 95%
confidence interval) and do not correct for multiple testing. While appropriate multiplicity protection is important, by focussing on four thoroughly studied drug targets (NPC1L1, HMGCR,
PCSK9, and CETP) there is an abundance of prior evidence on the expected CHD effect, making analytical control of the false positive rate less relevant. In other settings, for example
gene-based MR analysis of all druggable genes, appropriate control of false discovery rates is clearly essential. It could be argued that applying a genome-wide association _p_ value
threshold (e.g. 5 × 10−8) would be needlessly conservative. Instead one could control for the number of druggable proteins (about 5000; resulting in a 1 × 10−5 threshold). However, (early)
drug development is not performed in isolation, and genetic evidence will be evaluated alongside evidence from cells, tissues, and animal experiments. As such, appropriate false discovery
control will depend on the position of drug target MR within this pre-existing evidence framework. A _p_ value threshold of 1 × 10−5 might be applied when drug target MR is used as a
screening tool, before validating promising leads in further experiments. Positioning drug target MR after successful in vivo experimentation, for example, to check for possible unknown side
effects in human subjects, will likely call for a less-stringent multiplicity correction considering the more extensive prior knowledge and the aim of early detection of possible safety
concerns. We also emphasise that the range of druggable proteins is not fixed. Indeed, our own previous paper expanded the druggable genome from around 2000 to over 4000 proteins11.
Moreover, new therapeutic modalities e.g. RNA silencing, are extending the range of therapeutic targets from those that are currently amenable to the action of small molecules, peptides and
monoclonal antibody therapeutics that target proteins, and which remain the mainstay of drug development. MR ESTIMATORS In the current manuscript we pursued drug target MR by applying a
generalised least squares (GLS) solution45, modelling residual LD, to genetic _cis-_regions known to encode protein drug targets. This GLS method is by no means the only relevant estimator
function, and one can ‘repurpose’ many general MR methods for use in drug target MR. For example, the MR-base platform clumps variants to such a low level (e.g. R-squared of 0.001) that one
can apply weighted regression solutions (e.g. IVW), foregoing LD correction. Generalised Summary-data-based Mendelian Randomisation (GSMR)48 provides similar LD-modelling MR functionality as
the GLS method applied here, which GSMR extents by allowing for automated outlier removal through HEIDI, as well as providing a solid integration with the Genome-wide Complex Trait Analysis
suite. Similar outlier removal steps can be readily implemented using the Q-statistic, and standard leverage or Cook’s statistics. Automated outlier removal does however make an implicit
assumption that the outlying observations are incorrect, and not the statistical model; which is unlikely to be generally true. Nevertheless, outlier removal is an important step in
assessing the robustness of results. Clearly, if for any given locus one could perfectly distinguish the causal variants from null-variants, simply selecting the causal set of variants for
MR will result in the most precise/powerful analysis. However, such ‘oracle’ selection is unlikely in practice and difficult to scale. As such the proposed LD modelling approach will not in
general select the perfect (i.e. the causal) set of variants, but instead it suggests a robust set, which uses variants in LD with (unknown) causal variants as sentinels. Combining LD
modelling with clumping requires limited human input and is therefore highly scalable. Finally, we note, the analyses presented in Figs. 2 and 5 are intended as an illustration of LD
modelling, not as proof. The proof follows from straightforward statistical argument and simulation studies conducted by Burgess et al.45, and the seminal work from Yang et al.49 on COJO.
STATISTICAL ANALYSIS MR was conducted using the ‘Inverse Variance Weighted’ (IVW) and ‘MR-Egger’ methods for correlated variants as detailed in Burges et al.18. Here we note that these
methods are specific parametrizations of GLS technique and simply refer to IVW as GLS, and MR-Egger as GLS with Egger correction. In the context of MR, a GLS without an Egger correct,
regresses the genetic association with an outcome (CHD in our case) on the genetic association with an exposure (here lipids, protein level or expression level), forcing the intercept
through zero; reflecting the no-pleiotropy assumption when a zero-exposure effect should be matched by a zero outcome effect. Here the slope estimate equates to a causal estimate of the
exposure to outcome effect. GLS with Egger correction refers to a similar linear model without forcing the intercept through the origin. Here the intercept estimate reflects the amount of
horizontal pleiotropy, while the slope estimates reflects the causal estimate of the exposure on the outcome corrected for (potential) horizontal pleiotropy. Estimates are presented as fixed
effects (with a regression standard error of unity), or as random effects (where the regression standard error is equal or larger than 1). The drug target phenome-wide analysis was
conducted by mapping the INTERVAL pQTL GWAS to the druggable genome11 and selecting the five most significant proteins with sufficient _cis_ variants to conduct further analyses. Variants
were selected from a 2 kB window around the gene, excluding variants with a MAF of 0.05 or lower. The final set of instruments were selected based a LD-clumping algorithm where the
LD-threshold is selected through comparison of the point estimates of threshold _l_ to _l_ − 1; overinfluential (high leverage) or outlying variants were removed. Due to INTERVAL’s modest
sample size the number of available variants were often limited, forcing us to use the IVW estimator and forgoing any eQTL screening for possible LD-related horizontal pleiotropy. REPORTING
SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY All data are publicly available. Information on
the four positive control loci (_HMGCR, PCSK9, CETP_, and _NPC1L1)_ were sourced from the druggable genome (defined in ref. 11). Specifically, for the current analyses we identified
variants within a megabase upstream or downstream from each of the four loci. Outcome data were extracted from CARDIOGRAMplusC4D50 including the genetic association (log odds ratio) with
CHD, as well as their standard errors. Exposure data were leveraged from GLGC16 (lipids), Pott et al.28 (PCSK9 protein level), Blauw et al.27 (CETP protein level), and GTEx51 version 7
(expression level). The 1000 genomes52 data were used as a source of LD. Enhancer data were derived from the Human ACtive Enhancer to interpret Regular variants (HACER53) resource. All
information was curated and normalised to genetic build 37 as described in detail in Finan et al.11 A ±2.5 kB subset of the data is provided in Supplementary Tables 1–4, with the remainder
easily extracted from cited publicly available sources. The phenome-wide scan utilised INTERVAL pQTL4 data from 5 drug targets and evaluated the protein effects on 35 outcomes using
following publicly available resources: UK biobank data (nealelab.is/uk-biobank) were used for lipids (LDL-C, HDL-C, triglycerides [TG], lipoprotein A [LPa], Apolipoprotein B [ApoB],
Apolipoprotein A1 [ApoA1]), glucose and HbA1c, leucocytes, lymphocytes, monocytes, and neutrophils counts. Blood pressure (systolic and diastolic [SBP, DBP]) data were used from Evangelou et
al.54, which includes the UKB as well. CKDGen consortium data provided information on blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), and chronic kidney disease
(CKD)55. Bone mineral density (BMD)56 and fracture57 data were obtained from GEFOS Consortium. Genetic associations with ‘general cognitive function’ were obtained from a meta-analysis of
CHARGE, COGENT and UK biobank58. Data on CHD were available from CardiogramplusC4D50, any stroke, large artery stroke, cardioembolic stroke, and small vessel stroke from the MEGASTROKE
consortium59, Heart Failure (HF) from the HERMES60, atrial fibrillation (AF) from the AFgen consortium61, and finally non-ischaemic cardiomyopathy (CM) from GRADE investigators62. Additional
non-CVD phenotype data was extracted for type 2 diabetes (T2DM)63, Asthma64, inflammatory bowel disease (IBD)65, Chron’s disease (CD)66, ulcerative colitis (UC)67, multiple sclerosis (MS)68
and Alzheimer’s disease69. CODE AVAILABILITY All analyses were conducted using the R programming language70, with packages dplyr71, ggplot272, gridExtra73, openxlsx74, and wesanderson75.
Diagrams were programmed in TikZ76, and the Supplementary written in LaTeX and knitr77. The grid search can be readily implemented with a generic statistical programming language and
irrespective of the desired MR estimator. REFERENCES * Hingorani, A. & Humphries, S. Nature’s randomised trials. _Lancet_ 366, 1906–1908 (2005). PubMed Google Scholar * Swerdlow, D. I.
et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. _Lancet_ 379, 1214–1224 (2012). PubMed Google Scholar * Sarwar,
N. & Butterworth, A. S. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. _Lancet_ 379, 1205–1213 (2012). PubMed Google Scholar *
Sun, B. B. et al. Genomic atlas of the human plasma proteome. _Nature_ (2018) https://doi.org/10.1038/s41586-018-0175-2. * Xu, X. et al. Molecular insights into genome-wide association
studies of chronic kidney disease-defining traits. _Nat. Commun._ 9, 4800 (2018). ADS PubMed PubMed Central Google Scholar * Boef, A. G. C., Dekkers, O. M. & Le Cessie, S. Mendelian
randomization studies: a review of the approaches used and the quality of reporting. _Int. J. Epidemiol._ 44, 496–511 (2015). PubMed Google Scholar * Hemani, G., Bowden, J. & Davey
Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. _Hum. Mol. Genet_ 27, R195–R208 (2018). CAS PubMed PubMed Central Google Scholar * Hopkins, A.
L. & Groom, C. R. The druggable genome. _Nat. Rev. Drug Discov._ 1, 727–730 (2002). CAS PubMed Google Scholar * Russ, A. P. & Lampel, S. The druggable genome: an update. _Drug
Discov. Today_ https://doi.org/10.1016/S1359-6446(05)03666-4 (2005). Article PubMed Google Scholar * Cotto, K. C. et al. DGIdb 3.0: a redesign and expansion of the drug-gene interaction
database. _Nucleic Acids Res_ 46, D1068–D1073 (2018). CAS PubMed Google Scholar * Finan, C. et al. The druggable genome and support for target identification and validation in drug
development. _Sci. Transl. Med._ 9, 1–15 (2017). Google Scholar * Swerdlow, D. I. et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis
and randomised trials. _Lancet_ 385, 351–361 (2015). CAS PubMed PubMed Central Google Scholar * Sofat, R. et al. Separating the mechanism-based and off-target actions of cholesteryl
ester transfer protein inhibitors with CETP gene polymorphisms. _Circulation_ 121, 52–62 (2010). CAS PubMed Google Scholar * Schmidt, A. F. et al. PCSK9 genetic variants and risk of type
2 diabetes: a mendelian randomisation study. _Lancet Diabetes Endocrinol._ 0, 735–742 (2016). CAS Google Scholar * Ference, B. A. et al. Variation in PCSK9 and HMGCR and risk of
cardiovascular disease and diabetes. _N. Engl. J. Med._ 375, 2144–2153 (2016). CAS PubMed Google Scholar * Willer, C. J. et al. Newly identified loci that influence lipid concentrations
and risk of coronary artery disease. 40 (2008). * Burgess, S., Dudbridge, F. & Thompson, S. G. Combining information on multiple instrumental variables in Mendelian randomization:
Comparison of allele score and summarized data methods. _Stat. Med._ 35, 1880–1906 (2016). MathSciNet PubMed Google Scholar * Burgess, S., Zuber, V., Valdes-Marquez, E., Sun, B. B. &
Hopewell, J. C. Mendelian randomization with fine-mapped genetic data: choosing from large numbers of correlated instrumental variables. _Genet. Epidemiol._ 41, 714–725 (2017). PubMed
PubMed Central Google Scholar * Schmidt, A. F. et al. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. _Cochrane Datab. Syst. Rev._ 2017,
1–107 (2017). Google Scholar * Cholesterol Treatment Trialists’ (CTT) Collaborators, C. T. T. (CTT). et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk
of vascular disease: meta-analysis of individual data from 27 randomised trials. _J. Vasc. Surg._ 57, 284 (2013). Google Scholar * Collins, R. et al. Interpretation of the evidence for the
effi cacy and safety of statin therapy. _Lancet_ 388, 2532–2561 (2016). CAS PubMed Google Scholar * Keene, D., Price, C., Shun-Shin, M. J. & Francis, D. P. Effect on cardiovascular
risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients._Br. Med. J._ 349,
g4379- (2014). Google Scholar * Bohula, E. A. et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT.
_Circulation_ CIRCULATIONAHA.117.029095 (2017) https://doi.org/10.1161/CIRCULATIONAHA.117.029095. * Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. _N.
Engl. J. Med_ 372, 2387–2397 (2015). CAS PubMed Google Scholar * Schmidt, A. F., Pearce, L. S., Wilkins, J. T., Casas, J. P. & Hingorani, A. D. Cochrane corner: PCSK9 monoclonal
antibodies for the primary and secondary prevention of cardiovascular disease. _Heart_ 104, 1053 LP–1051055 (2018). Google Scholar * McLaren, W. et al. The ensemble variant effect
predictor. _Genome Biol._ 17, 122 (2016). PubMed PubMed Central Google Scholar * Blauw, L. L. et al. CETP (cholesteryl ester transfer protein) concentration: a genome-wide association
study followed by Mendelian randomization on coronary artery disease. _Circ. Genom. Precis. Med._ 11, e002034 (2018). CAS PubMed Google Scholar * Pott, J. et al. Genetic regulation of
PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) plasma levels and its impact on atherosclerotic vascular disease phenotypes. _Circ. Genom. Precis. Med_. 11, 1–11 (2018). Google Scholar
* López-López, J. A. et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. _BMJ_ 359, j5058
(2017). PubMed PubMed Central Google Scholar * Aggeletopoulou, I., Assimakopoulos, S. F., Konstantakis, C. & Triantos, C. Interleukin 12/interleukin 23 pathway: biological basis and
therapeutic effect in patients with Crohn’s disease. _World J. Gastroenterol._ 24, 4093–4103 (2018). CAS PubMed PubMed Central Google Scholar * Sandborn, W. J. et al. Ustekinumab
induction and maintenance therapy in refractory Crohn’s disease. _N. Engl. J. Med._ 367, 1519–1528 (2012). CAS PubMed Google Scholar * IST-3 collaborative group. et al.The benefits and
harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled
trial. _Lancet_ 379, 2352–2363 (2012). Google Scholar * Schreiber, S. et al. A phase 2, randomized, placebo-controlled study evaluating matrix metalloproteinase-9 inhibitor, andecaliximab,
in patients with moderately to severely active Crohn’s disease. _J. Crohns Colitis_ 12, 1014–1020 (2018). PubMed PubMed Central Google Scholar * Sandborn, W. J. et al. Andecaliximab
[anti-matrix metalloproteinase-9] induction therapy for ulcerative colitis: a randomised, double-blind, placebo-controlled, phase 2/3 study in patients with moderate to severe disease. _J
Crohn's Colitis_ 12, 1012–1029 (2018). Google Scholar * Wensley, F. et al. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on
individual participant data. _BMJ_ 342, d548 (2011). PubMed Google Scholar * Genetic loci associated with C-reactive protein levels and risk of coronary heart disease | Genetics and
Genomics | JAMA | JAMA Network. https://jamanetwork.com/journals/jama/fullarticle/184182. * Ligthart, S. et al. Genome analyses of >200,000 individuals identify 58 loci for chronic
inflammation and highlight pathways that link inflammation and complex disorders. _Am. J. Hum. Genet._ 103, 691–706 (2018). CAS PubMed PubMed Central Google Scholar * Genome‐wide mapping
of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun. https://www.nature.com/articles/s41467-018-05512-x. * Barbeira, A. N. et al.
Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. _Nat. Commun._ (2018) https://doi.org/10.1038/s41467-018-03621-1. *
Ferreira, P. G. et al. The effects of death and post-mortem cold ischemia on human tissue transcriptomes. _Nat. Commun._ https://doi.org/10.1038/s41467-017-02772-x (2018). Article PubMed
PubMed Central Google Scholar * Swerdlow, D. I. et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. _Int. J. Epidemiol._ 45, 1600–1616
(2016). PubMed PubMed Central Google Scholar * Crick, F. Central dogma of molecular biology. _Nature_ 227, 561–563 (1970). ADS CAS PubMed Google Scholar * Schmidt, A. F. et al.
Phenome-wide association analysis of LDL-cholesterol lowering genetic variants in PCSK9. _BMC Cardiovasc. Disord._ 240, 1–10 (2019). Google Scholar * Bowden, J., Smith, G. D. & Burgess,
S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. _Int. J. Epidemiol._ 44, 512–525 (2015). PubMed PubMed Central Google
Scholar * Burgess, S., Zuber, V., Valdes-Marquez, E., Sun, B. B. & Hopewell, J. C. Mendelian randomization with fine-mapped genetic data: choosing from large numbers of correlated
instrumental variables. _Genet. Epidemiol._ 41, 714–725 (2017). PubMed PubMed Central Google Scholar * Dudbridge, F. Power and predictive accuracy of polygenic risk scores. _PLoS Genet._
9, 1–17 (2013). Google Scholar * Burgess, S. & Thompson, S. G. Avoiding bias from weak instruments in mendelian randomization studies. _Int. J. Epidemiol._ 40, 755–764 (2011). PubMed
Google Scholar * Zhu, Z. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. _Nat. Commun._ 9, 1–12 (2018). ADS Google Scholar * Yang, J.
et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. _Nat. Genet_ 44, 1–22 (2013). CAS Google Scholar *
Nikpay, M. et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. _Nat. Genet._ 47, 1121–1130 (2015). CAS PubMed PubMed Central Google
Scholar * Aguet, F. et al. Genetic effects on gene expression across human tissues. _Nature_ (2017) https://doi.org/10.1038/nature24277. * The 1000 Genomes Project Consortium. A global
reference for human genetic variation. _Nature_ 526, 68–74 (2015). Google Scholar * Liu, Y., Sarkar, A., Kheradpour, P., Ernst, J. & Kellis, M. Evidence of reduced recombination rate in
human regulatory domains. _Genome Biol._ 18, 193 (2017). PubMed PubMed Central Google Scholar * Evangelou, E. et al. Genetic analysis of over 1 million people identifies 535 new loci
associated with blood pressure traits. _Nat. Genet._ 50, 1412–1425 (2018). CAS PubMed PubMed Central Google Scholar * Wuttke, M. et al. A catalog of genetic loci associated with kidney
function from analyses of a million individuals. _Nat. Genet._ 51, 957–972 (2019). CAS PubMed PubMed Central Google Scholar * Morris, J. A. et al. An atlas of genetic influences on
osteoporosis in humans and mice. _Nat. Genet._ 51, 258–266 (2019). CAS PubMed Google Scholar * Trajanoska, K. et al. Assessment of the genetic and clinical determinants of fracture risk:
genome wide association and mendelian randomisation study. _BMJ_ 362, k3225 (2018). PubMed PubMed Central Google Scholar * Davies, G. et al. Study of 300,486 individuals identifies 148
independent genetic loci influencing general cognitive function. _Nat. Commun._ 9, 1–16 (2018). Google Scholar * Malik, R. et al. Multiancestry genome-wide association study of 520,000
subjects identifies 32 loci associated with stroke and stroke subtypes. _ Nat. Genet_. 50, 524–537 (2018). CAS PubMed PubMed Central Google Scholar * Shah, S. et al. Genome-wide
association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. _Nat. Commun._ 11, 1–12 (2020). Google Scholar * Nielsen, J. B. et al.
Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. _Nat. Genet._ 50, 1234–1239 (2018). CAS PubMed PubMed Central Google Scholar * Aragam Krishna, G. et
al. Phenotypic refinement of heart failure in a national biobank facilitates genetic discovery. _Circulation_ 139, 489–501 (2019). Google Scholar * Mahajan, A. et al. Refining the accuracy
of validated target identification through coding variant fine-mapping in type 2 diabetes. _Nat. Genet._ 50, 559–571 (2018). CAS PubMed PubMed Central Google Scholar * Shrine, N. et al.
Moderate-to-severe asthma in individuals of European ancestry: a genome-wide association study. _Lancet Respir. Med._ 7, 20–34 (2019). PubMed PubMed Central Google Scholar * Jostins, L.
et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. _Nature_ 491, 119–124 (2012). CAS PubMed PubMed Central Google Scholar * Franke, A.
et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. _Nat. Genet._ 42, 1118–1125 (2010). CAS PubMed PubMed Central Google Scholar
* Anderson, C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. _Nat. Genet_ 43, 246–252 (2011). CAS
PubMed PubMed Central Google Scholar * International Multiple Sclerosis Genetics Consortium*†, Multiple sclerosis genomic map implicates peripheral immune cells and microglia in
susceptibility. _Science_ 365, 1–10 (2019). * Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. _Nat. Genet._
51, 404–413 (2019). CAS PubMed PubMed Central Google Scholar * R Core Team. _R: A language and environment for statistical computing_. (R Foundation for Statistical Computing, 2017). *
Wickham, H., Henry, L., Müller, K. & François, R. _dplyr: a grammar of data manipulation_. (2019). * Wickham, H. _ggplot2: elegant graphics for data analysis_. (Springer, 2016). *
Auguie, B. _gridExtra: Miscellaneous Functions for ‘Grid’ Graphics_. (2017). * Walker, A. _openxlsx: Read, Write and Edit XLSX Files_. (2019). * Ram, K. & Wickham, H. _wesanderson: A Wes
Anderson Palette Generator_. (2018). * T Tantau. _The TikZ and PGF Packages_. (2013). * Xie, Y. _Dynamic documents with R and Knitr._ (CRC Press, Taylor & Francis, 2015). Google Scholar
Download references ACKNOWLEDGEMENTS AFS is supported by BHF grant PG/18/5033837 and the UCL BHF Research Accelerator AA/18/6/34223. CF and AFS received additional support from the
National Institute for Health Research University College London Hospitals Biomedical Research Centre. MGM is supported by a BHF Fellowship FS/17/70/33482. RF is supported by UK Medical
Research Council (FC001002, MR/N013867/1). MZ contributed to this work as part of her PhD, which was funded by BenevolentAI, where she was an employee. ADH is an NIHR Senior Investigator. We
further acknowledge support from the Rosetrees and Stoneygate Trusts. FWA is supported by UCL Hospitals NIHR Biomedical Research Centre. RSP is supported by a BHF Fellowship FS/14/76/30933.
This work has received support from the EU/EFPIA Innovative Medicines Initiative [2] Joint Undertaking BigData@Heart grant n° 116074. This research has been conducted using the UK Biobank
Resource under Application Number 12113. The authors are grateful to UK Biobank participants. UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council,
Department of Health, Scottish Government, and the Northwest Regional Development Agency. AUTHOR INFORMATION Author notes * These authors contributed equally: Amand F Schmidt, Chris Finan.
AUTHORS AND AFFILIATIONS * Institute of Cardiovascular Science, Faculty of Population Health, University College London, London, WC1E 6BT, UK Amand F. Schmidt, Chris Finan, Maria
Gordillo-Marañón, Folkert W. Asselbergs, Riyaz S. Patel, Sandesh Chopade, Rupert Faraway, Magdalena Zwierzyna & Aroon D. Hingorani * UCL BHF Research Accelerator Centre, London, UK Amand
F. Schmidt, Chris Finan, Riyaz S. Patel, Sandesh Chopade, Rupert Faraway, Magdalena Zwierzyna & Aroon D. Hingorani * Department of Cardiology, Division Heart and Lungs, University
Medical Center Utrecht, Heidelberglaan 100, 3584 CX, Utrecht, The Netherlands Amand F. Schmidt & Folkert W. Asselbergs * Health Data Research UK, 222 Euston Road, London, UK Folkert W.
Asselbergs & Aroon D. Hingorani * Bayer AG Pharmaceuticals, Open Innovation & Digital Technologies, Aprather Weg 18a, Wuppertal, 42096, Germany Daniel F. Freitag * Center for
Therapeutic Innovation, Cardiovascular and Metabolic Disease, Institut de Recherches Internationales Servier, 50 Rue Carnot, 92284, Suresnes Cedex, France Benoît Tyl * The Francis Crick
Institute, 1 Midland Rd, London, NW1 1AT, UK Rupert Faraway Authors * Amand F. Schmidt View author publications You can also search for this author inPubMed Google Scholar * Chris Finan View
author publications You can also search for this author inPubMed Google Scholar * Maria Gordillo-Marañón View author publications You can also search for this author inPubMed Google Scholar
* Folkert W. Asselbergs View author publications You can also search for this author inPubMed Google Scholar * Daniel F. Freitag View author publications You can also search for this author
inPubMed Google Scholar * Riyaz S. Patel View author publications You can also search for this author inPubMed Google Scholar * Benoît Tyl View author publications You can also search for
this author inPubMed Google Scholar * Sandesh Chopade View author publications You can also search for this author inPubMed Google Scholar * Rupert Faraway View author publications You can
also search for this author inPubMed Google Scholar * Magdalena Zwierzyna View author publications You can also search for this author inPubMed Google Scholar * Aroon D. Hingorani View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS AFS, CF, MGM, RF, MZ and ADH contributed to the idea, and design of the study, AFS, CF, SC and
MGM performed the analyses. AFS, CF and ADH drafted the manuscript. AFS, CF and SC curated, extracted and normalised these data. MG, RP, DFF, BT, FWA, SC, RF and MZ provided critical input
on the analyses and the drafted manuscript. CORRESPONDING AUTHOR Correspondence to Amand F. Schmidt. ETHICS DECLARATIONS COMPETING INTERESTS DFF is a full-time employee of Bayer AG, Germany.
BT is a full-time employee of Servier. RSP has received honoraria from Sanofi, Bayer and Amgen. MZ is a full-time employee of GSK. AFS and FWA have received Servier funding for unrelated
work. MZ conducted this research as an employee of BenevolentAI. Since completing the work MZ is now a full-time employee of GlaxoSmithKline. None of the remaining authors have a competing
interest to declare. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Eric Fauman and Heiko Runz for their contribution to the peer review of this manuscript.
Peer review reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schmidt, A.F., Finan, C., Gordillo-Marañón, M. _et al._ Genetic drug target
validation using Mendelian randomisation. _Nat Commun_ 11, 3255 (2020). https://doi.org/10.1038/s41467-020-16969-0 Download citation * Received: 16 October 2019 * Accepted: 02 June 2020 *
Published: 26 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16969-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
