Terminal flower 1-fd complex target genes and competition with flowering locus t
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Plants monitor seasonal cues to optimize reproductive success by tuning onset of reproduction and inflorescence architecture. TERMINAL FLOWER 1 (TFL1) and FLOWERING LOCUS T (FT) and
their orthologs antagonistically regulate these life history traits, yet their mechanism of action, antagonism and targets remain poorly understood. Here, we show that TFL1 is recruited to
thousands of loci by the bZIP transcription factor FD. We identify the master regulator of floral fate, _LEAFY_ (_LFY_) as a target under dual opposite regulation by TFL1 and FT and uncover
a pivotal role of FT in promoting flower fate via _LFY_ upregulation. We provide evidence that the antagonism between FT and TFL1 relies on competition for chromatin-bound FD at shared
target loci. Direct TFL1-FD regulated target genes identify this complex as a hub for repressing both master regulators of reproductive development and endogenous signalling pathways. Our
data provide mechanistic insight into how TFL1-FD sculpt inflorescence architecture, a trait important for reproductive success, plant architecture and yield. SIMILAR CONTENT BEING VIEWED BY
OTHERS LEAFY IS A PIONEER TRANSCRIPTION FACTOR AND LICENSES CELL REPROGRAMMING TO FLORAL FATE Article Open access 27 January 2021 TWO FLORIGENS AND A FLORIGEN-LIKE PROTEIN FORM A TRIPLE
REGULATORY MODULE AT THE SHOOT APICAL MERISTEM TO PROMOTE REPRODUCTIVE TRANSITIONS IN RICE Article 27 March 2023 THE F-BOX PROTEIN UFO CONTROLS FLOWER DEVELOPMENT BY REDIRECTING THE MASTER
TRANSCRIPTION FACTOR LEAFY TO NEW _CIS_-ELEMENTS Article 02 February 2023 INTRODUCTION Of particular importance for reproductive success of flowering plants is optimal timing of onset of
reproductive development and of the transition from branch to floral fate in the inflorescence in response to seasonal cues1,2,3,4,5. For example, in plants that flower only once, like
_Arabidopsis_ and most crops, an early switch to flower formation allows rapid completion of the life-cycle and is beneficial in a short growing season5,6,7. At the same time early onset of
flower formation reduces seed set and yield since flowers form in lieu of branches, which support production of more flowers per plant5,6,7,8. By contrast, delaying flower formation
increases branching and total flower number, but prolongs time to seed set5,6,7,8. Key regulators of seasonal control of onset of reproductive development and of the switch from branch to
floral fate in primordia of the inflorescence are members of the phosphatidylethanolamine-binding protein (PEBP) family of proteins5,6,9,10. Among these, FT promotes onset of the
reproductive phase and flower formation (determinacy), while TFL1 promotes vegetative development and branch fate (indeterminacy)9,11,12,13. _Arabidopsis_ flowers in the spring and FT
accumulates when the daylength exceeds a critical threshold, while TFL1 is present in both short-day and long-day conditions2,3,14. FT and TFL1 are small mobile proteins, which have been
implicated in transcriptional regulation but do not have DNA-binding domains14,15,16,17,18. Biochemical and genetic studies showed that FT physically interacts with the bZIP transcription
factor FD via 14-3-3 proteins and similar interactions have recently been described for TFL119,20,21,22. Indeed, despite their antagonistic roles, TFL1 and FT are distinguished by only a
small number of nonconservative amino acid changes11,12,23,24. FT can be converted into TFL1 and vice versa by a single amino acid substitution and such mutations have been selected for
during crop domestication23,24,25,26. Accumulating evidence suggests that FT acts as a transcriptional co-activator, while TFL1 may either prevent FT activity or act as a co-repressor23,27.
However, non-nuclear roles have also been described for both TFL1 and FT28,29. A key unanswered question is how the florigens modulate plant form—what are the downstream processes they set
in motion and what is molecular basis for their antagonism? Here we show that TFL1 is recruited to target loci by the bZIP transcription factor FD. We identify the master regulator of floral
fate, LEAFY, as a target under dual opposite regulation by TFL1 and FT and uncover a prominent role for FT in LFY upregulation. We find that the antagonism between TFL1 and FT relies on
competition for access to chromatin bound FD at the _LFY_ locus and other shared targets. Finally, we identify hundreds of TFL1–FD regulated genes linking this complex not only to repression
of master regulators of floral fate, but also of diverse endogenous signalling pathways. The combined data reveals how TFL1 and FT tune inflorescence architecture in response to seasonal
cues by altering transcriptional programs that direct primordium fate in the inflorescence. RESULT TFL1 IS RECRUITED TO THOUSANDS OF LOCI BY THE BZIP TRANSCRIPTION FACTOR FD Mechanistic
insight into TFL1 activity has been hampered by low protein abundance. To overcome this limitation and to test the role of TFL1 in the nucleus, we first generated a biologically active,
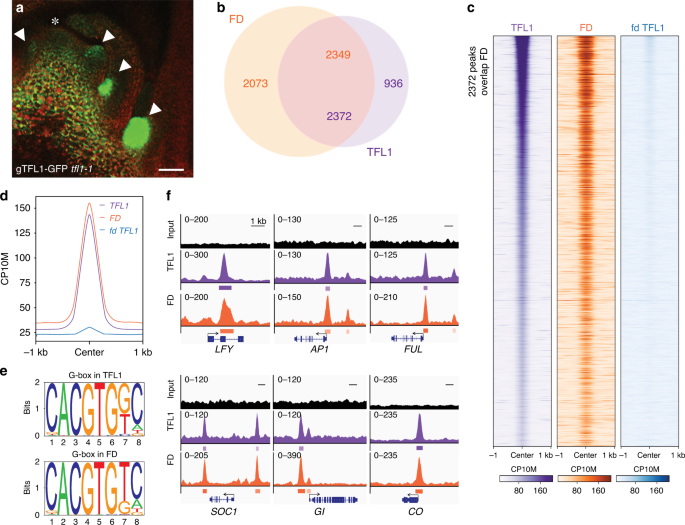
genomic GFP-tagged version of TFL1 (gTFL1-GFP _tfl1-1_) (Supplementary Fig. 1a–c) and identified a developmental stage and tissue where TFL1 accumulates. TFL1 protein strongly accumulated in
branch meristems in the axils of cauline leaves in 42-day-old short-day grown plants just prior to the switch to flower formation (Fig. 1a). To conduct TFL1 chromatin immunoprecipitation
followed by sequencing (ChIP-seq), we next isolated shoot apices at this stage for anti-GFP immunoprecipitation. Because TFL1 is present in very few cells and binds chromatin indirectly, we
combined eight individual ChIP reactions per replicate to enhance detection. We conducted FD ChIP-seq in analogous fashion using a published, biologically active, genomic fusion protein
(gFD-GUS _fd-1_)20 (Supplementary Fig. 1d). This approach yielded high-quality ChIP-seq data in both cases (Supplementary Figs. 2 and 3a). In total, we identified 3308 and 4422 significant
TFL1 and FD peaks (MACS2 summit qval ≤ 10−10), respectively (Fig. 1b). The TFL1 peaks significantly overlapped with the FD peaks (72% overlap, _p_ val < 10−300, hypergeometric test; Fig.
1b–d). De novo motif analysis of ChIP peak summits identified the G-box _cis_ motif, a known FD-binding site30, as most significantly enriched (_p_ val < 10−470) and frequently present
(>84%) under TFL1 bound and TFL1/FD co-bound peaks (Fig. 1e and Supplementary Fig. 2). To test whether TFL1 chromatin occupancy is dependent on the presence of FD, we also performed TFL1
ChIP-seq in the _fd-1_ null mutant. TFL1 chromatin occupancy was strongly reduced in _fd-1_ (Fig. 1c, d). Our data point to a prominent nuclear role for TFL1 and show that FD recruits TFL1
to the chromatin of target loci. Annotating FD and TFL1 peaks to genes identified 2699 joint TFL1 and FD targets. Gene Ontology (GO) term enrichment analysis implicates these targets in
abiotic and endogenous stimulus response and reproductive development (Supplementary Table 1). TFL1 and FD peaks were present at loci that promote onset of the reproductive phase in response
to inductive photoperiod2,3,31 like _GIGANTEA_ (_GI_), _CONSTANS_ (_CO_), and _SUPPRESSOR OF CONSTANS 1_ (_SOC1_) and at loci that promote floral fate31,32 such as _LFY_, _APETALA1_
(_AP1_), and _FRUITFULL_ (_FUL_) (Fig. 1f). Identification of these TFL1 and FD co-bound targets fits with the known biological role of TFL1 as a suppressor of onset of reproduction and of
flower fate and the proposed molecular function of TFL1 in opposing gene activation11,12,13,27. LEAFY IS UNDER DUAL OPPOSITE REGULATION BY TFL1/FD AND FT/FD We selected the _LEAFY_ (_LFY_)
gene, which encodes a master regulator of flower fate33,34, to further probe the molecular mechanism of action of TFL1. While TFL1 promotes branch fate, LFY promotes flower fate in primordia
(Supplementary Fig. 4a–f)13,33,34,35. Using independent biological replicates, we confirmed FD-mediated TFL1 binding to _LFY_ by ChIP-qPCR (Supplementary Fig. 4g, h). To test whether _LFY_
expression is rapidly repressed by the TFL1–FD complex, we generated transgenic plants expressing a steroid inducible version of TFL1 (TFL1ER; Supplementary Fig. 5). A single steroid
treatment reduced _LFY_ levels by 50% after 4 h (Supplementary Fig. 4i). The combined data suggest that the TFL1–FD complex directly represses _LFY_. To better understand TFL1 recruitment to
the _LFY_ locus, we identified the genomic region sufficient and the _cis_ motifs necessary for TFL1 association with the _LFY_ locus. TFL1 and FD peak summits located to the second exon of
_LFY_ (Fig. 1f and Supplementary Fig. 4g, h) and _LFY_ reporters that lacked the second exon were not repressed in response to TFL1 overexpression (Supplementary Fig. 6a, b). Exonic
transcription factor-binding sites, although rare, are found in both animals and plants, and frequently link to developmental regulation36,37. To test whether LFY exon 2 (e2) alone is
sufficient to recruit TFL1–FD, we transformed gTFL1-GFP _tfl1-_1 plants with a T-DNA containing only _LFY_ e2. We detected strong TFL1 recruitment to the introduced copy of e2, using primer
sets that specifically amplify the transgene borne exon (Fig. 2a). Next we identified three putative bZIP-binding sites in the second exon of _LFY;_ These include an evolutionarily conserved
G-box and two partially conserved C-boxes (Fig. 2b). When we transformed gTFL1-GFP _tfl1-_1 with a version of LFY e2 in which the three bZIP binding were mutated (e2m3), we were unable to
detect TFL1 binding to the introduced copy of e2m3 (Fig. 2a). The three bZIP-binding sites in the second exon of _LFY_ are thus necessary for TFL1 recruitment. Similar results were obtained
when we tested TFL1 recruitment to e2 and e2m3 via FD in yeast (Supplementary Fig. 6c). Having identified the _cis_ motifs necessary for TFL1 recruitment to _LFY_, we next probed their
contribution to spatiotemporal _LFY_ accumulation. _LFY_ reporters that contain e2 (pLFYi2-GUS, Supplementary Fig. 6a) and a genomic _LFY_ reporter (gLFY-GUS, Fig. 2b) recapitulated
endogenous LFY expression (Fig. 2c, Supplementary Fig. 6d)34,35. Mutating the three bZIP-binding sites in pLFYi2-GUS or gLFY-GUS caused ectopic reporter expression in the centre of the
inflorescence shoot apex (Fig. 2c, Supplementary Fig. 6d). This is the precise region where TFL1 protein accumulates during reproductive development (Fig. 2c)14. Indeed, _LFY_ is known to be
ectopically expressed in the inflorescence shoot apex of _tfl1_ mutants during reproductive development13. Thus, TFL1–FD binding to the bZIP motifs of e2 of LFY is required to prevent
ectopic _LFY_ accumulation in the centre of the shoot apex. Surprisingly, the bZIP-binding site mutations in the second exon of _LFY_ in addition strongly reduced reporter expression in
incipient and young flower primordia (Fig. 2c, Supplementary Fig. 6d). This suggests that the bZIP motifs may be required for _LFY_ upregulation in these flower primordia, perhaps via FT.
Based on prior studies31,38,39,40,41, _LFY_ was not thought to be an immediate early FT target. Because constitutive mutants that delay onset of the reproductive phase, like _ft_, indirectly
delay the switch to flower formation42 we wished to deplete FT specifically during the reproductive phase to test whether FT promotes _LFY_ expression. Towards this end, we used a minimal
_FT_ promoter (p4kbFT) that is active in parts of leaves and stems in long-day grown plants only after day 1243. We fused p4kbFT to a previously characterized FT-specific artificial
microRNA44. In the resulting conditional _ft_ mutant (p4kbFT:amiRFT), onset of reproduction was not delayed (Fig. 3a, Supplementary Fig. 7). However, p4kbFT:amiRFT plants displayed a
significantly delay in onset of flower formation and failed to upregulate _LFY_ expression (Fig. 3a and Supplementary Fig. 7). In a parallel approach to test the role of _FT_ in LFY
induction, we induced endogenous _FT_ expression by treating 42-day-old short-day grown plants with a single far-red-enriched long-day photoperiod (FRP). Far-red light enhances _FT_
induction by photoperiod (Supplementary Fig. 8a, b)45,46. In addition, FRP triggered significant _LFY_ induction, which was dependent on the presence of FT (Supplementary Fig. 8c, d). A
single FRP treatment also induced the gLFY-GUS reporter, but only if the bZIP-binding sites in e2 were intact (Fig. 3b). Finally, we probed for rapid _LFY_ induction by FT after generating
an estradiol inducible version of FT (35S:FT-HAER) (Supplementary Fig. 9a–c). A single steroid treatment triggered significant _LFY_ induction after 4 h (Supplementary Fig. 9d). After
crossing FT-HAER to LFY reporters containing (pLFYi2:GUS) or lacking (pLFYi2m3:GUS) the bZIP-binding sites in e2, we tested reporter activity in response to steroid activation. GUS
upregulation was similar to that of endogenous _LFY_ when the bZIP-binding sites were present (Supplementary Fig. 9e). By contrast, GUS expression was not upregulated in the pLFYi2m3:GUS
FT-HAER plants after steroid induction (Supplementary Fig. 9e). The combined loss-of-function, photoinduction and gain-of-function data indicate that FT–FD directly activates _LFY_
expression via bZIP-binding sites in the second exon. These findings prompted us to assess the biological importance of the _LFY_ bZIP-binding sites for inflorescence architecture. While a
genomic GFP-tagged _LFY_ construct (gGLFY) fully rescued the _lfy-1_ null mutant (in 24 out of 25 independent transgenic lines), a construct which preserves LFY protein sequence but has
mutated bZIP-binding sites (gGLFYm3) yielded only partial rescue (in 15 out of 15 independent transgenic lines) (Fig. 3c, d and Supplementary Fig. 10). In the gGLFYm3 _lfy-1_ plants onset of
flower formation was significantly delayed, leading to formation of many more branches, and _LFY_ accumulation was strongly reduced (Fig. 3c, d and Supplementary Fig. 10). The dramatic
reduction of _LFY_ accumulation is striking given the many additional positive inputs into _LFY_ upregulation previously identified38,40,47,48. Our combined data uncover a pivotal role of FT
in _LFY_ upregulation and reveal that FT promotes flower formation via _LFY_. We note that _LFY_ accumulation in the centre of the gGLFYm3 _lfy-1_ shoot apex was much lower than that
observed in _tfl1_ mutants and that gGLFYm3 _lfy-1_ did not exhibit the terminal flower phenotype typical of _tfl1_ (Supplementary Fig. 10)13. This is expected since the bZIP mutations at
the _LFY_ locus prevent access of both TFL1 and of activating PEBP family members FT and the closely related TWIN SISTER OF FT (TSF). Indeed, it has been shown that the terminal flower
phenotype of _tfl1_ is suppressed in _tfl1 ft tsf_ triple mutants49. FT COMPETES TFL1 FROM FD BOUND AT SHARED TARGET LOCI Having identified _LFY_ as a target under dual opposite regulation
by TFL1 and FT, we next investigated the mechanism underlying the TFL1–FT antagonism at this locus. To test for possible competition between FT and TFL1 at the chromatin, we conducted
anti-HA ChIP-qPCR in 42-day-old short-day grown FT-HAER gTFL1-GFP plants four hours after mock or steroid application. Estradiol induction led to rapid recruitment of FT-HA to the second
exon of _LFY_, the region occupied by FD and TFL1 (compare Fig. 4a, b to Supplementary Fig. 4h). Anti-GFP ChIP-qPCR performed on the same sample uncovered a concomitant reduction in TFL1
occupancy (Fig. 4a, b). We next asked whether upregulation of endogenous FT also triggers reduced TFL1 occupancy at the _LFY_ locus. Towards this end, we treated plants with a single FRP to
upregulate FT (Supplementary Fig. 8b). The single FRP likewise significantly reduced TFL1 occupancy at the _LFY_ chromatin (Fig. 4c). By contrast, photoinduction of FT did not alter FD
occupancy (Fig. 4d). To probe whether FT is recruited to the second exon of LFY via the bZIP-binding sites, we transformed FT-HAER with either a wild-type version of e2 or a bZIP-binding
site mutated version thereof (e2m3). After steroid induction, we used transgene-specific primers to monitor FT binding to the two versions of _LFY_ e2 by ChIP-qPCR. As described above for
TFL1 (Fig. 2a), FT was recruited to _LFY_ e2 alone (Fig. 4e). In addition, FT recruitment to the introduced copy of _LFY_ e2 was abolished when the three bZIP-binding sites were mutated
(Fig. 4e). Our combined data suggest that FT competes TFL1 from FD bound at exonic bZIP motifs at the _LFY_ locus. The loss of TFL1 from the LFY locus via competition by FT is further
supported by the finding that neither steroid nor FRP induction of FT reduced _TFL1_ mRNA accumulation (Supplementary Figs. 8c and 9d). Competition of TFL1 from FD by FT is not limited to
the _LFY_ locus. We tested whether FT induction by FRP competes TFL1 from the other direct TFL1–FD target loci we identified (Fig. 1f). FRP treatment reduced TFL1 occupancy at all loci
tested (Fig. 4f). To confirm that FT indeed occupies the TFL1–FD bound sites at these loci, we also conducted ChIP-qPCR in FT-HAER after steroid induction. In the estradiol treated samples,
we saw significant FT recruitment to the TFL1–FD bound regions at all loci tested (Fig. 4g). We conclude that the antagonism between FT and TFL119,21,27 relies on competition for FD bound at
the chromatin of shared target loci (Fig. 4h). DIRECT TFL1–FD REPRESSED GENES PROMOTE ONSET OF FLOWER FORMATION AND ENDOGENOUS SIGNALLING Our findings place florigens directly upstream of
LFY, yet prior genetic data suggest that florigens act both upstream of and in parallel with LFY39,50. To gain insight into additional gene expression programs repressed by the TFL1–FD
complex, we next conducted RNA-seq with and without FRP treatment. We isolated inflorescences with associated primordia from 42-day-old short-day-grown _ft_ mutant, wild-type and _tfl1_
mutant plants and identified the significant gene expression changes in each genotype relative to untreated siblings. On the basis of Principle Component Analysis (PCA) and replicate
analysis, RNA-seq quality was high (Supplementary Fig. 11). We next defined genes directly repressed by TFL1–FD. Towards this end, we focussed on TFL1–FD complex bound loci that exhibit
FT-dependent de-repression upon photoinduction. Six-hundred four TFL1–FD bound genes were significantly (DESeq2 adjusted _p_ < 0.005) de-repressed upon FRP treatment in the wild-type or
in _tfl1_ mutants but not in _ft_ mutants (Fig. 5a). GO term enrichment linked the TFL1–FD repressed genes to reproductive development and to response to endogenous and abiotic signals (Fig.
5b). K-means clustering of the 604 genes identified three main patterns of gene expression. Genes encoding promoters of floral fate32 (_LFY_, _AP1_, _FUL_ and _LMI2_) clustered together
(cluster III in Fig. 5c) and displayed stronger upregulation in _tfl1_ mutants than in the wild type. This pattern of de-repression was confirmed for all four loci using independent
biological samples and qRT-PCR (Supplementary Fig. 12a). _SOC1_ clusters with these genes, but was not included in further analyses because it was weakly, but significantly, de-repressed in
_ft_ mutants (Fig. 5c, Supplementary Data 1 and Supplementary Fig. 12b). By contrast, _CO_ and _GI_, which promote cessation of vegetative development2,3, were more strongly upregulated in
the wild-type than in _tfl1_ mutants (cluster I in Fig. 5c), perhaps because these genes are already partially de-repressed in _tfl1_ mutants in the absence of FRP treatment. Indeed, like
RNA-seq, qRT-PCR of independent biological replicates revealed higher accumulation of _GI_ and _CO_ in untreated _tfl1_ mutant compared to wild-type plants (Fig. 5c, Supplementary Data 1 and
Supplementary Fig. 12b). Cluster II genes are only upregulated in the wild type and may represent genes that are transiently de-repressed. Our data identify the TFL-FD complex as a hub for
repression of key regulators of the onset of reproductive development and of the switch to flower fate (Fig. 5c) Consistent with the GO-term enrichment analysis (Fig. 5b), combined ChIP-seq
and RNA-seq analysis additionally identified components of endogenous stimulus response. We identified genes linked to sugar signalling (trehalose-6-phosphate) and hormonal signalling and
response (abscisic acid, cytokinin, brassinosteroid, auxin and strigolactone) as direct TFL1–FD complex repressed targets (Fig. 5c). Using qRT-PCR and independent biological samples, we
confirmed FT-dependent de-repression of members of these pathways by FRP photoinduction (Fig. 5c). Several of the identified pathways link to repression of branching or to promotion of onset
of flower formation in the inflorescence. For example, we identified four trehalose-6-phosphate phosphatases (_TPPH_, _TPPJ_, _TPPG_ and _TPPE_) as direct TFL1–FD complex repressed targets;
TPPs were recently shown to repress branching in the maize inflorescence51. In addition, auxin and the auxin-activated transcription factor _MONOPTEROS_ (_MP_) were direct TFL1–FD complex
repressed targets (Fig. 5c). MP promotes the switch to floral fate in _Arabidopsis_52 and the tomato ortholog of TFL1 executes its role in inflorescence architecture at least in part by
modulating auxin flux and response53. Finally, we identified key components of the brassinosteroid pathway including the _ BRI1_ receptor and the bHLH transcription factor _BIM1_54,55 as
direct TFL1–FD complex repressed targets. Brassinosteroid signalling represses inflorescence branching in Setaria56. The combined data implicate TFL1–FD in direct repression of genes that
promote floral fate or repress branch fate, consistent with the role of TFL1 in promoting branch formation. We also identified the cytokinin activating enzyme _LOG5_57, abscisic acid
biosynthesis (_ABA1_) and response regulators (_ABI5_, _ABF4_, _APF2_)58,59, and components of strigolactone signalling, _SMXL6_ and _SMXL8_60,61, as direct TFL1–FD complex repressed
targets. To gain further insight into the role of the TFL1–FD complex in hormone signalling, we next assessed indirect, downstream, gene expression changes triggered by FRP treatment. In
particular, we identified genes not bound by TFL1 or FD that were significantly differentially expressed (DESeq2 adjusted _p_ < 0.005) in the wild type and in _tfl1_, but not in _ft_. The
identified indirect targets provide a ‘molecular phenotype’ that is consistent with de-repression of the auxin, brassinosteroid and cytokinin hormone pathways upon FRP treatment in the
wild-type and in _tfl1_ mutants (Fig. 5c). By contrast, the abscisic acid signalling pathway signature was more complex (Fig. 5c). Our combined data uncover a prominent role for the TFL1–FD
complex in regulation of endogenous signalling. It is conceivable that components of some of the identified TFL1–FD dependant pathways (strigolactone, cytokinin, auxin, abscisic acid as well
as sugar signalling) may modulate additional aspects of the inflorescence architecture, such as branch outgrowth62,63,64. Support for this hypothesis comes from our phenotypic analyses. We
examined the effect of a single FRP on inflorescence architecture in the _ft_ mutant, the wild-type and the _tfl1_ mutant. Photoperiod induction triggered a reduction in the number of
branches, but not cauline leaves, formed in the wild type and more strongly, in _tfl1_ (Supplementary Fig. 13a–j). This suggests that branch meristems adopt floral fate upon stimulus
perception65. _tfl1_ mutants also formed fewer branches than the wild type in the absence of photoperiod. These phenotypes are consistent with the observed gene expression changes (Fig. 5c).
In addition, FRP triggered a significant increase in inflorescence branch outgrowth in both wild-type and _tfl1_ plants (Supplementary Fig. 13k). FRP had no phenotypic effect in _ft_
mutants. Our combined data suggest that florigens tune plant form to the environment by controlling expression of master developmental regulators and endogenous signalling pathway
components. These developmental changes likely require large-scale transcriptional reprograming in the context of chromatin. Consistently, we identified transcriptional co-regulators and
chromatin regulators among the direct TFL1–FD repressed targets (Supplementary Fig. 14a, b). DISCUSSION Here we identify _LFY_, a master regulator of flower fate33,34, as a target under dual
opposite transcriptional regulation by TFL1 and FT and demonstrate that FT activation of _LFY_ expression is critical to promote floral fate. We provide a molecular framework for the
antagonistic roles5,27 of FT and TFL1 that relies on competition for bZIP transcription factor mediated access to binding sites at regulatory regions of shared target loci. Additional
support for this mechanism comes from recent in vitro studies21. Our data suggest that TFL1 may not simply prevent access of the FT co-activator to the chromatin23 but may be an active
repressor, as mutating bZIP-binding sites results in _LFY_ de-repression specifically in the TFL1 expression domain. The identity of the transcription factors that activate _LFY_ in the
centre of the inflorescence shoot apex in the absence of PEBP/FD binding to _LFY_ is not known. Our identification of FT recruiting motifs in the second exon of _LFY_ fits with prior data
demonstrating that the 2.3 kb upstream intergenic ‘_LFY_ promoter’ is unresponsive to FT38. This upstream regulatory region drives reporter expression in similar domains as endogenous
_LFY_66. The requirement of the bZIP motifs for _LFY_ upregulation in the context of the genomic construct, which contains the 2.3 kb ‘_LFY_ promoter’, suggests the presence of repressive
regulatory elements in the genic region of _LFY_. We identify hundreds of TFL1–FD repressed genes many of which, based on our computational analyses of recently published FD and TFL1
ChIP-seq datasets17,30, are also immediate early gTFL1 or gFD targets in long-day conditions. Eighty two percent of our 604 high-confidence TFL1–FD repressed genes are present in at least
one of the long-day ChIP-seq datasets (Supplementary Fig. 3b). The 604 direct TFL1–FD repressed genes include key regulators of onset of the reproductive phase and of floral fate. Of note,
TFL1 opposes not only LFY, but also LFY targets, such as _LMI2_ and _AP1_67,68. This is consistent with prior genetic investigations that place TFL1 both upstream of LFY and as a modulator
of plant response to LFY50. Finally, we link the TFL1–FD complex to repression of diverse endogenous signalling pathways including sugar and hormonal signals. Several of these pathways have
been shown to impact the switch from branch or flower fate in other plant species51,53,56. The combined data point to an important role of the hormonal environment for the switch from branch
to flower fate in primordia of the inflorescence. Our findings also set the stage for elucidating communalities as well as differences between florigen regulated cell fate reprogramming
during flower initiation and other developmental pathways under seasonal control by florigens such as tuberization, bulb formation and seed dormancy69,70,71,72. Changes in the relative
balance of activating and repressive PEBP family members occurred during domestication of diverse crop species to give rise to desirable traits like everbearing and compact growth
habits5,8,69,73. Thus, mechanistic insight into the antagonism and identification of the targets of PEBPs will benefit traditional or genome editing-based crop improvement. It should further
facilitate elucidation that how PEBP protein act as co-activators or co-repressors in the nucleus. METHODS PLANT MATERIALS _Arabidopsis_ ecotype Columbia plants were grown in soil at 22 °C
in long-day photoperiod (LD, 16 h light/8 h dark, 100 µmol/m2 s) or short-day photoperiod (SD, 8 h light/16 h dark, 120 µmol/m2 s). gFD-GUS20, _fd-1_ null mutants74, _tfl1-14_ hypomorph
mutant27,75, _tfl1-1_ severe mutant27,75,76, _lfy-1_ null mutant33,77,78, _ft-10_ null mutant79, 35S:LFY68 and 35S:TFL1 (ref. 35) were previously described. 35S:LFY (Landsberg _erecta)_ was
introgressed into the Columbia background through backcrossing. _gFD-GUS_80 was crossed into the _fd-1_ null mutant background. CONSTRUCTS FOR TRANSGENIC PLANTS For _gTFL1-GFP_, GFP followed
by a peptide linker (GGGLQ) was fused to an 8.4 kb BamHI (NEB, R0136S) genomic fragment from lambda TFG4 (ref. 81). This fragment was introduced into the binary vector _pCGN1547_ (ref. 82).
For _TFL1__ER_ and _FT_-_HA__ER_, _TFL1_ and _FT_ were PCR amplified from cDNA; in the case of FT, the 3′ primer contained three times Hemagglutinin (HA) plus a stop codon. PCR products
were cloned into _pENTRD-TOPO_ (Invitrogen, K243520) and shuffled into _pMDC7_ (ref. 83) by LR reaction (Invitrogen, 11791-020). For _LFY-GUS_ reporters, the bacterial beta-glucuronidase
(_GUS_) gene from the _pGWB3_ (ref. 84) binary vector was fused with _pENTRD_-_TOPO_ vector containing the 2290-bp _LFY_ promoter66 alone (pLFY:_GUS_), the _LFY_ promoter and _LFY_ genic
region up to and including the first intron (pLFYi1:GUS), or the _LFY_ promoter and _LFY_ genic region up to and including the second _LFY_ intron (_pLFYi2_:GUS) by LR reaction. bZIP-binding
site mutations in _LFY_ e2 (_pLFYi2m3_:GUS_)_ were generated by Ω-PCR85. _gGLFY_ was constructed by PCR amplifying a 4929-bp genomic _LFY_ fragment (_gLFY_), including the 2290-bp _LFY_
promoter, from genomic DNA followed by cloning into the KpnI-HF (NEB, R3124S) and NotI-HF (NEB, R3189S) digested _pENTR3C_ vector by Gibson Assembly. Next, GFP was inserted at position + 94
bp, as previously described for _pLFY:GLFY_42,80, by Ω-PCR85. bZIP-binding site mutations were introduced into _pENTR3C_-_gGLFY_ by Ω-PCR to generate _gGLFYm3_. Both constructs were shuffled
into _pMCS:GW_86 using LR reaction. To create _gLFY_:_GUS_, the 4929-bp genomic _LFY_ clone minus the stop codon and the GUS fragment were PCR amplified and inserted into linearized
_pENTR3C_ by Gibson Assembly. For _gLFYm3_:_GUS_, bZIP-binding site mutations were introduced into _pENTR3C-gLFY:GUS_ by Ω-PCR. To test recruitment of TFL1 and FT to e2 of _LFY_, wild-type
(e2) or bZIP-binding site mutated exon 2 (e2m3) were PCR amplified and cloned into _pGWB3_ (ref. 84). For test of recruitment, LFY e2 and e2m3 were amplified by forward
(5′-caccAACAGCAGCAGAGACGGAGAAAGAA-3′) and reverse (5′-TCGTACAAGTGGAACAGATAATC-3′) primers and cloned into pGWB3 binary vectors, which were transformed into gTFL1-GFP and 35S:FT-HAER. For
_pFT4kb:amiRFT_, a 3994-bp truncated _FT_ promoter87 was PCR amplified from genomic DNA as was the published _amiRFT_44 from _pRS300_ (ref. 88). The _amiRFT_ fragment was introduced into
EcoRI-HF (NEB, R3101S) digested _pENTR3C_ (Thermo Fisher Scientific, A10464) by Gibson Assembly (NEB, E5510S) and shuffled into binary vector _pMCS:GW_86 using LR reaction, which resulted in
pMCS:amiRFT. The previously described 3994-bp _FT_ promoter was inserted to XhoI (NEB, R0146S) digested pMCS:amiRFT by Gibson Assembly. Genomic DNA was extracted using the GenElute Plant
Genomic DNA Miniprep Kit (Sigma-Aldrich, G2N70). Primer sequences are listed in Supplementary Table 2. All constructs were sequence verified prior to transformation into plants with
Agrobacterium strain GV3101 by floral dip89. Plant lines generated are listed in Supplementary Table 3. IMAGING Images were taken with a Canon EOS Rebel T5 camera for plant phenotypes and
yeast one-hybrid assays, or with a stereo microscope (Olympus SZX12) equipped with a colour camera (Olympus LC30) for GUS images and inflorescence phenotypes. For GFP images, a Leica TCS SP8
Multiphoton Confocal with a 20× objective was used with a 488 nm excitation laser and emission spectrum between 520 and 550 nm (GFP) or 650–700 nm (chlorophyll autofluorescence) using
standard imaging techniques90,91. For _tfl1-1_ gTFL1-GFP in short-day photoperiod, shoot apices were sectioned longitudinally on an oscillating tissue slicer (Electron Microscopy Sciences,
OTS-4000) after embedding in 5% Agar (Fisher Scientific, DF0812-07-1). PLANT TREATMENT AND GENE EXPRESSION ANALYSIS For test of gene expression, 16-day-old TFL1ER or 12-day-old FT-HAER
plants grown in LD were induced by a single spray application of 10 μmol beta-estradiol (Sigma-Aldrich, 8875-250MG) dissolved in DMSO (Fisher Scientific, BP231-1L) and 0.015% Silwet L-77
(PlantMedia, 30630216-3). Mock solution consisted of 0.1% DMSO and 0.015% Silwet. To probe FT recruitment to and TFL1 occupancy at the _LFY_ locus, 42-day-old FT-HAER gTFL1-GFP plants grown
in SD were treated by a single spray application of 10 μmol beta-estradiol or mock solution. In all cases, tissues were harvested 4 h after treatment. To test for gain-of-function phenotypes
in long-day photoperiod, FT-HAER, TFL1ER and FT-HAER gTFL1-GFP plants were treated with 10 μmol beta-estradiol or mock solution from 5-day onwards every other day until bolting. FRP was
applied at the end of the short day (ZT8) for 24 h using a Percival Scientific E30LED45 with red (660 nm) to far-red (730 nm) ratio = 0.5 and light intensity 80 µmol/m2 s. Control plants
were kept in regular short-day conditions (16 h dark and 8 h light, red to far-red ratio = 12 and 120 µmol/m2 s light intensity) for 24 h. Light intensity and spectral composition were
measured by an Analytical Spectral Devices FieldSpec Pro spectrophotometer. For qRT-PCR analysis, total RNA was extracted from leaves or shoot apices using TRIzol (Thermo Fisher Scientific)
and purified with the RNeasy Mini Kit (Qiagen, 74104). cDNA was synthesized using SuperScript III First-Strand Synthesis (Invitrogen, 18080051) from 1 μg of RNA. Real time PCR was conducted
using a cDNA standard curve. Normalized expression levels were calculated using the 2ˆ(−delta delta CT) method with the housekeeping gene _UBQ10_ (AT4G05320) as the control. Where expression
of multiple different genes was compared, normalized gene expression is shown relative to the control treatment. Primer sequences are listed in Supplementary Table 2. YEAST ONE-HYBRID ASSAY
LFY e2 and the bZIP-binding site mutated version (e2m3) were cloned into the KpnI-HF and XhoI linearized pAbAi vector (Takara) by Gibson Assembly and integrated into the yeast genome
following the Matchmaker Gold Yeast One-Hybrid protocol (Takara) and the Y1Golden strain (Takara). Coding sequences of FD and TFL1 were cloned into the pENTRD-TOPO vector. After sequencing,
constructs were shuffled into either pDEST32 or pDEST22 (Takara) by LR reaction and transformed into the DNA-binding region containing yeast strain. Empty pDEST32 and pDEST22 served as
negative controls. Growth was assayed after serial dilution on growth media with or without 60 ng/ml Aureobasidin A (Clontech, 630499). Primer sequences are listed in Supplementary Table 2.
CHIP-QPCR, CHIP-SEQ AND DATA ANALYSIS Forty two-day-old short-day grown plants were trimmed and 1.6 g of non-bolted inflorescences were harvested from 36 plants. Chromatin
immunoprecipitation was conducted following a published protocol92 for ChIP-qPCR. For ChIP-seq, each biological replicate consisted of eight individual IP reactions pooled into one MinElute
PCR (Qiagen, 28004) purification column. For ChIP-seq and ChIP-qPCR, anti-GFP antibody (Thermo Fisher Scientific, A-11122; 1:200 dilution) and anti-GUS antibody (Abcam, ab50148; 1:200
dilution) were used. The antibodies were validated by the manufacturers. ChIP-qPCR was performed using Platinum Taq DNA Polymerase (Invitrogen, 10966034) and EvaGreen dye (Biotium, 31000).
For ChIP-qPCR, the value of the ChIP samples was normalized over that of input DNA as previously described92. Non-transgenic wild-type plants were used as the negative genetic control for
anti-GFP and anti-GUS antibody ChIP. The _TA3_ retrotransposon (AT1G37110) was used as the negative control region for ChIP-qPCR. Primer sequences are listed in Supplementary Table 2.
Anti-GFP ChIP-seq was performed for gTFL1-GFP (A), gTFL1-GFP (B), _fd-1_ gTFL1-GFP and control samples (non-epitope containing plants). Anti-GUS ChIP-seq was performed for _gFD-GUS_. Two
biological replicates were sequenced in each case. Dual index libraries were prepared for the ten ChIP samples listed above and for four input samples using the SMARTer ThruPLEX DNA-Seq Kit
(Takara Bio, R400406). Library quantification was performed with the NEBNext Library Quant Kit for Illumina (NEB, E7630). Single-end sequencing was conducted using High Output Kit v2.5
(Illumina, TG-160-2005) on the NextSeq 500 platform (Illumina). FastQC v0.11.5 was performed on both the raw and trimmed93 reads using TRIMMOMATIC v0.3693
(ILLUMINACLIP:adaptors.fasta:2:30:10 LEADING:3 TRAILING:3 MINLEN:50) to confirm sequencing quality94. Reads with MAPQ ≥ 30 (SAMtools v1.7)95 uniquely mapping to the Arabidopsis Information
Resource version 10 (TAIR 10)96 that were not flagged as PCR or optical duplicates by Bowtie2 v2.3.1(ref. 97,98) were analyzed further by principal component analysis (PCA) using the plotPCA
function of deepTools99. Reads were further processed following ENCODE guidelines100, followed by cross-correlation analysis with the predict function of MACS2 (ref. 101) to empirically
determine the fragment length. Significant ChIP peaks and summits (summit _q_ value ≤ 10−10) were identified in MACS2 for the pooled ChIP relative to the pooled negative controls (ChIP in
non-transgenic wild type). Peak overlap (≥1 bp) was computed using BEDTools intersect v2.26.0 (ref. 102) and statistical significance was computed using the hypergeometric test assuming a
‘universe’ of 10,000 possible peaks103. Heatmaps were generated using deepTools v3.1.2 (ref. 99). The 3308 TFL1 and 4422 FD peaks were mapped to 3699 and 4493 Araport11 (ref. 104) annotated
genes, respectively, if the peak summit was intragenic or located ≤4 kb upstream of the transcription start site. Recently published datasets were analyzed in identical fashion. Genomic
distribution of peak summits was called using the ChIPpeakAnno library104,105. De novo motif analysis was conducted using MEME-ChIP v5.0.2 (Discriminative Mode)106 and HOMER v4.10 (ref. 107)
for MACS2 _q_ value ≤ 10−10 peak summits (±250 base pairs) compared to genome-matched background (unbound regions from similar genomic locations as the peak summits) as previously
described108,109. GO term enrichment analyses were performed using GOSlim in agriGO v2.0 (ref. 110) and significantly enriched GO terms with _q_ value < 0.0001 (FDR, Benjamini and
Yekutieli method111) were identified. CORRELATION ANALYSES Public ChIP-seq datasets for FD30, for TFL117, LFY112 and an unpublished LFY ChIP-seq dataset from our lab (GEO accession
GSE141706) were analyzed as described above for TFL1 and FD ChIP-seq. To compare the relationship between all ChIP-seq datasets we calculated Pearson correlation coefficients for reads in
regions of interest using deeptools v3.1.299. Regions of interest were comprised of the combined significant peak regions (MACS2 ≤ _q_ value 10−10) of all ChIP-seq datasets and read signal
was derived from the sequencing-depth normalized bigwig file for each sample. RNA-SEQ AND DATA ANALYSIS A single 24 h FRP was applied to 42-day-old short-day grown _ft-10_ mutants, wild-type
and _tfl1-1_ mutants, starting at the end of the day (ZT8). After the treatment, 0.1 g of inflorescence shoots were harvested for each biological replicate after removing all leaves and
roots. Three biological replicates were prepared for each experiment. RNA quantity and quality were analyzed by Qubit BR assay (Thermo Fisher Scientific, Q10210) and Agilent RNA 6000 Nano
Kit (Agilent, 5067-1511) on an Agilent 2100 bioanalyzer, respectively. Libraries were constructed from 1 µg total RNA using the TruSeq RNA Sample Prep Kit (Illumina, RS-122-2001). After
library quantification with the NEBNext Library Quant Kit for Illumina (NEB, E7630), single-end sequencing was conducted using the NextSeq 500 platform (Illumina). RNA-seq analysis was
conducted using FastQC v0.11.5 (ref. 94) on raw sequences before and after trimming using Trimmomatic v0.36 (ref. 93) (ILLUMINACLIP:adapters.fasta:2:30:10 LEADING:3 TRAILING:3 MINLEN:50) to
confirm sequencing quality94. Reads were mapped using the STAR mapping algorithm113 (–sjdbOverhang 100 --outSAMprimaryFlag AllBestScore --outSJfilterCountTotalMin 10 5 5 5
--outSAMstrandField intronMotif --outFilterIntronMotifs RemoveNoncanonical --alignIntronMin 60 --alignIntronMax 6000 --outFilterMismatchNmax 2), to the TAIR 10 Arabidopsis genome-assembly96,
and Araport11 Arabidopsis genome-annotation104. Specific read coverage was assessed with HT-Seq114 (--stranded = ‘no’ -minaqual = 30). For PCA, raw read counts were subjected to variance
stabilizing transformation and projected into two principal components with the highest variance115,116,117. In parallel, raw reads were adjusted for library size by DESeq2 v1.24.0 (ref.
118). After PCA, two biological replicates per genotype and treatment were selected for further analysis. Gene normalized z-scores were used for k-means (MacQueen) clustering119. Pairwise
differential expression analyses were performed by comparing FRP and untreated pooled normalized read counts in each genotype using default DESeq2 parameters with no shrinkage (ref. 118) and
an adjusted _p_ value cut-off ≤ 0.005. PHOTOPERIOD SHIFT PHENOTYPE ANALYSIS A single 24-h far-red light enriched photoperiod shift (FRP) was applied to 42-day-old short-day grown _ft-10_
mutants, wild-type and _tfl1-1_ mutants as for RNA-seq, followed by further growth in short-day conditions. To asses onset of reproductive development, the number of rosette leaves formed
were counted at bolting. To analyze the inflorescence architecture, the number of sessile buds, outgrowing branches, flower branches, and single flowers subtended by a cauline leaf were
counted weekly after bolting until the first normal flower (not subtended by a cauline leaf) formed. STATISTICAL ANALYSES The Kolmogorov–Smirnov (K–S) test120 was used to assess whether the
data were normally distributed. All ChIP and qRT-PCR data were normally distributed. An unpaired one-tailed _t_-test was used to test for changes in one direction. Error bars represent the
standard error of the mean (SEM). Two to three independent biological replicates were analyzed. For multiple-group comparisons (phenotypes) the non-parametric Kruskal–Wallis test121 followed
by the Dunn’s _post hoc_ test122 were employed. Box and whisker plots display minima and maxima (whiskers), lower and upper quartile (box) and median (red vertical line). REPORTING SUMMARY
Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The authors declare that the data supporting the
findings of this study are available within the paper, its Supplementary information files and public data repositories. Source data are provided with this paper. The ChIP-seq and RNA-seq
datasets were deposited to the GEO database (GSE141894). Individual replicates and _P_ values for all figures are provided as a source data file. Source data are provided with this paper.
CODE AVAILABILITY Scripts for peak to gene annotation can be found at https://github.com/sklasfeld/ChIP_Annotation. REFERENCES * Song, J., Irwin, J. & Dean, C. Remembering the prolonged
cold of winter. _Curr. Biol._ 23, R807–R811 (2013). Article CAS PubMed Google Scholar * Song, Y. H., Shim, J. S., Kinmonth-Schultz, H. A. & Imaizumi, T. Photoperiodic flowering: time
measurement mechanisms in leaves. _Annu. Rev. Plant Biol._ 66, 441–464 (2015). Article CAS PubMed Google Scholar * Andrés, F. & Coupland, G. The genetic basis of flowering responses
to seasonal cues. _Nat. Rev. Genet._ 13, 627 (2012). Article PubMed CAS Google Scholar * Teo, Z. W., Song, S., Wang, Y. Q., Liu, J. & Yu, H. New insights into the regulation of
inflorescence architecture. _Trends Plant Sci._ 19, 158–165 (2014). Article CAS PubMed Google Scholar * Périlleux, C., Bouché, F., Randoux, M. & Orman-Ligeza, B. Turning meristems
into fortresses. _Trends Plant Sci_. 24, 431–442 (2019). Article PubMed CAS Google Scholar * Prusinkiewicz, P., Erasmus, Y., Lane, B., Harder, L. D. & Coen, E. Evolution and
development of inflorescence architectures. _Science_ 316, 1452–1456 (2007). Article ADS CAS PubMed Google Scholar * Park, S. J. et al. Optimization of crop productivity in tomato using
induced mutations in the florigen pathway. _Nat. Genet._ 46, 1337–1342 (2014). Article CAS PubMed Google Scholar * Eshed, Y. & Lippman, Z. B. Revolutions in agriculture chart a
course for targeted breeding of old and new crops. _Science_ 366, eaax0025 (2019). * Lifschitz, E., Ayre, B. G. & Eshed, Y. Florigen and anti-florigen—a systemic mechanism for
coordinating growth and termination in flowering plants. _Front. Plant Sci._ 5, 465 (2014). Article PubMed PubMed Central Google Scholar * Karlgren, A. et al. Evolution of the PEBP gene
family in plants: functional diversification in seed plant evolution. _Plant Physiol._ 156, 1967–1977 (2011). Article CAS PubMed PubMed Central Google Scholar * Kardailsky, I. et al.
Activation tagging of the floral inducer FT. _Science_ 286, 1962–1965 (1999). Article CAS PubMed Google Scholar * Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. & Araki, T. A pair
of related genes with antagonistic roles in mediating flowering signals. _Science_ 286, 1960–1962 (1999). Article CAS PubMed Google Scholar * Bradley, D., Ratcliffe, O., Vincent, C.,
Carpenter, R. & Coen, E. Inflorescence Commitment and Architecture in _Arabidopsis_. _Science_ 275, 80–83 (1997). Article CAS PubMed Google Scholar * Conti, L. & Bradley, D.
TERMINAL FLOWER1 is a mobile signal controlling _Arabidopsis_ architecture. _Plant Cell_ 19, 767–778 (2007). Article CAS PubMed PubMed Central Google Scholar * Mathieu, J., Warthmann,
N., Kuttner, F. & Schmid, M. Export of FT protein from phloem companion cells is sufficient for floral induction in _Arabidopsis_. _Curr. Biol._ 17, 1055–1060 (2007). Article CAS
PubMed Google Scholar * Jaeger, K. E. & Wigge, P. A. FT protein acts as a long-range signal in _Arabidopsis_. _Curr. Biol._ 17, 1050–1054 (2007). Article CAS PubMed Google Scholar
* Goretti, D. et al. TERMINAL FLOWER1 functions as a mobile transcriptional cofactor in the shoot apical meristem. _Plant Physiol._ 182, 2081–2095 (2020). Article CAS PubMed PubMed
Central Google Scholar * Corbesier, L. et al. FT protein movement contributes to long-distance signaling in floral induction of _Arabidopsis_. _Science_ 316, 1030–1033 (2007). Article ADS
CAS PubMed Google Scholar * Taoka, K. et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. _Nature_ 476, 332–335 (2011). Article ADS CAS PubMed Google
Scholar * Abe, M. et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. _Science_ 309, 1052–1056 (2005). Article ADS CAS PubMed Google
Scholar * Kaneko-Suzuki, M. et al. TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. _Plant
Cell Physiol._ 59, 458–468 (2018). Article CAS PubMed Google Scholar * Wigge, P. A. et al. Integration of spatial and temporal information during floral induction in _Arabidopsis_.
_Science_ 309, 1056–1059 (2005). Article ADS CAS PubMed Google Scholar * Ho, W. W. & Weigel, D. Structural features determining flower-promoting activity of _Arabidopsis_ FLOWERING
LOCUS T. _Plant Cell_ 26, 552–564 (2014). Article CAS PubMed PubMed Central Google Scholar * Ahn, J. H. et al. A divergent external loop confers antagonistic activity on floral
regulators FT and TFL1. _EMBO J._ 25, 605–614 (2006). Article CAS PubMed PubMed Central Google Scholar * Hanzawa, Y., Money, T. & Bradley, D. A single amino acid converts a
repressor to an activator of flowering. _Proc. Natl Acad. Sci. USA_ 102, 7748–7753 (2005). Article ADS CAS PubMed PubMed Central Google Scholar * Pin, P. A. et al. An antagonistic pair
of FT homologs mediates the control of flowering time in sugar beet. _Science_ 330, 1397–1400 (2010). Article ADS CAS PubMed Google Scholar * Hanano, S. & Goto, K. Arabidopsis
TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. _Plant Cell_ 23, 3172–3184 (2011). Article CAS PubMed
PubMed Central Google Scholar * Sohn, E. J. et al. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. _Proc. Natl Acad.
Sci. USA_ 104, 18801–18806 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Abelenda, J. A. et al. Source-sink regulation is mediated by interaction of an FT homolog
with a SWEET protein in potato. _Curr. Biol._ 29, 1178–1186 e6 (2019). Article CAS PubMed Google Scholar * Collani, S., Neumann, M., Yant, L. & Schmid, M. FT modulates genome-wide
DNA-binding of the bZIP transcription factor FD. _Plant Physiol._ 180, 367–380 (2019). Article CAS PubMed PubMed Central Google Scholar * Fornara, F., de Montaigu, A. & Coupland, G.
SnapShot: control of flowering in _Arabidopsis_. _Cell_ 141, 550, 550.e1–e2 (2010). * Wagner, D. Key developmental transitions during flower morphogenesis and their regulation. _Curr. Opin.
Genet Dev._ 45, 44–50 (2017). Article CAS PubMed Google Scholar * Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. LEAFY controls floral meristem identity
in _Arabidopsis_. _Cell_ 69, 843–859 (1992). Article CAS PubMed Google Scholar * Weigel, D. & Nilsson, O. A developmental switch sufficient for flower initiation in diverse plants.
_Nature_ 377, 495–500 (1995). Article ADS CAS PubMed Google Scholar * Ratcliffe, O. J. et al. A common mechanism controls the life cycle and architecture of plants. _Development_ 125,
1609–1615 (1998). CAS PubMed Google Scholar * Stergachis, A. B. et al. Exonic transcription factor binding directs codon choice and affects protein evolution. _Science_ 342, 1367–1372
(2013). Article ADS CAS PubMed PubMed Central Google Scholar * Reyna-Llorens, I. et al. Ancient duons may underpin spatial patterning of gene expression in C4 leaves. _Proc. Natl Acad.
Sci. USA_ 115, 1931–1936 (2018). Article CAS PubMed PubMed Central Google Scholar * Blazquez, M. A. & Weigel, D. Integration of floral inductive signals in _Arabidopsis_. _Nature_
404, 889–892 (2000). Article ADS CAS PubMed Google Scholar * Ruiz-Garcia, L. et al. Different roles of flowering-time genes in the activation of floral initiation genes in
_Arabidopsis_. _Plant Cell_ 9, 1921–1934 (1997). CAS PubMed PubMed Central Google Scholar * Moon, J., Lee, H., Kim, M. & Lee, I. Analysis of flowering pathway integrators in
_Arabidopsis_. _Plant Cell Physiol._ 46, 292–299 (2005). Article CAS PubMed Google Scholar * Lee, J., Oh, M., Park, H. & Lee, I. SOC1 translocated to the nucleus by interaction with
AGL24 directly regulates LEAFY. _Plant J._ 55, 832–843 (2008). Article CAS PubMed Google Scholar * Yamaguchi, N. et al. Gibberellin acts positively then negatively to control onset of
flower formation in _Arabidopsis_. _Science_ 344, 638–641 (2014). Article ADS CAS PubMed Google Scholar * Liu, L., Farrona, S., Klemme, S. & Turck, F. K. Post-fertilization
expression of FLOWERING LOCUS T suppresses reproductive reversion. _Front. Plant Sci._ 5, 164 (2014). PubMed PubMed Central Google Scholar * Schwab, R., Ossowski, S., Riester, M.,
Warthmann, N. & Weigel, D. Highly specific gene silencing by artificial MicroRNAs in _Arabidopsis_. _Plant Cell_ 18, 1121–1133 (2006). Article CAS PubMed PubMed Central Google
Scholar * Hempel, F. D. et al. Floral determination and expression of floral regulatory genes in _Arabidopsis_. _Development_ 124, 3845–3853 (1997). CAS PubMed Google Scholar * Valverde,
F. et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. _Science_ 303, 1003–1006 (2004). Article ADS CAS PubMed Google Scholar * Yamaguchi, N. et al. A
molecular framework for auxin-mediated initiation of flower primordia. _Dev. Cell_ 24, 271–282 (2013). Article CAS PubMed Google Scholar * Yamaguchi, A. et al. The MicroRNA-regulated
SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. _Developmental Cell_ 17, 268–278 (2009). Article CAS PubMed PubMed Central Google
Scholar * Lee, C. et al. Genetic interactions reveal the antagonistic roles of FT/TSF and TFL1 in the determination of inflorescence meristem identity in _Arabidopsis_. _Plant J._ 99,
452–464 (2019). Article CAS PubMed Google Scholar * Ratcliffe, O. J., Bradley, D. J. & Coen, E. S. Separation of shoot and floral identity in _Arabidopsis_. _Development_ 126,
1109–1120 (1999). CAS PubMed Google Scholar * Claeys, H. et al. Control of meristem determinacy by trehalose 6-phosphate phosphatases is uncoupled from enzymatic activity. _Nat. Plants_
5, 352–357 (2019). Article CAS PubMed PubMed Central Google Scholar * Yamaguchi, N., Wu, M. F., Winter, C. & Wagner, D. LEAFY together with polar auxin transport coordinates
_Arabidopsis_ flower development. _Plants_ 3, 251–265 (2014). Article PubMed PubMed Central CAS Google Scholar * Silva, W. B. et al. SELF-PRUNING acts synergistically with DIAGEOTROPICA
to guide auxin responses and proper growth form. _Plant Physiol._ 176, 2904–2916 (2018). Article CAS PubMed PubMed Central Google Scholar * Lozano-Elena, F. & Cano-Delgado, A. I.
Emerging roles of vascular brassinosteroid receptors of the BRI1-like family. _Curr. Opin. Plant Biol._ 51, 105–113 (2019). Article CAS PubMed Google Scholar * Yin, Y. et al. A new class
of transcription factors mediates brassinosteroid-regulated gene expression in _Arabidopsis_. _Cell_ 120, 249–259 (2005). Article CAS PubMed Google Scholar * Yang, J. et al.
Brassinosteroids modulate meristem fate and differentiation of unique inflorescence morphology in _Setaria viridis_. _Plant Cell_ 30, 48–66 (2018). Article CAS PubMed Google Scholar *
Kuroha, T. et al. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in _Arabidopsis_. _Plant Cell_ 21, 3152–3169 (2009).
Article CAS PubMed PubMed Central Google Scholar * Qin, F., Shinozaki, K. & Yamaguchi-Shinozaki, K. Achievements and challenges in understanding plant abiotic stress responses and
tolerance. _Plant Cell Physiol._ 52, 1569–1582 (2011). Article CAS PubMed Google Scholar * Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R. & Abrams, S. R. Abscisic acid:
emergence of a core signaling network. _Annu. Rev. Plant Biol._ 61, 651–679 (2010). Article CAS PubMed Google Scholar * Wang, L. et al. Strigolactone signaling in _Arabidopsis_ regulates
shoot development by targeting D53-Like SMXL repressor proteins for ubiquitination and degradation. _Plant Cell_ 27, 3128–3142 (2015). Article CAS PubMed PubMed Central Google Scholar
* Soundappan, I. et al. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in _Arabidopsi_s. _Plant Cell_ 27, 3143–3159 (2015). Article
CAS PubMed PubMed Central Google Scholar * Zhu, Y. & Wagner, D. Plant inflorescence architecture: the formation, activity, and fate of axillary meristems. _Cold Spring Harb.
Perspect. Biol_. 12, a034652 (2019). * Barbier, F. F., Dun, E. A., Kerr, S. C., Chabikwa, T. G. & Beveridge, C. A. An update on the signals controlling shoot branching. _Trends Plant
Sci._ 24, 220–236 (2019). Article CAS PubMed Google Scholar * Hiraoka, K., Yamaguchi, A., Abe, M. & Araki, T. The florigen genes FT and TSF modulate lateral shoot outgrowth in
_Arabidopsis thaliana_. _Plant Cell Physiol._ 54, 352–368 (2013). Article CAS PubMed Google Scholar * Hempel, F. D., Zambryski, P. C. & Feldman, L. J. Photoinduction of flower
identity in vegetatively biased primordia. _Plant Cell_ 10, 1663–1676 (1998). Article CAS PubMed PubMed Central Google Scholar * Blazquez, M. A., Soowal, L. N., Lee, I. & Weigel, D.
LEAFY expression and flower initiation in _Arabidopsis_. _Development_ 124, 3835–3844 (1997). CAS PubMed Google Scholar * Pastore, J. J. et al. LATE MERISTEM IDENTITY2 acts together with
LEAFY to activate APETALA1. _Development_ 138, 3189–3198 (2011). Article CAS PubMed PubMed Central Google Scholar * Wagner, D., Sablowski, R. W. M. & Meyerowitz, E. M.
Transcriptional activation of APETALA1 by LEAFY. _Science_ 285, 582–584 (1999). Article CAS PubMed Google Scholar * McGarry, R. C. & Ayre, B. G. Manipulating plant architecture with
members of the CETS gene family. _Plant Sci._ 188-189, 71–81 (2012). Article CAS PubMed Google Scholar * Lee, R., Baldwin, S., Kenel, F., McCallum, J. & Macknight, R. FLOWERING LOCUS
T genes control onion bulb formation and flowering. _Nat. Commun._ 4, 2884 (2013). Article ADS PubMed CAS Google Scholar * Navarro, C. et al. Control of flowering and storage organ
formation in potato by FLOWERING LOCUS T. _Nature_ 478, 119–122 (2011). Article ADS CAS PubMed Google Scholar * Chen, M. & Penfield, S. Feedback regulation of COOLAIR expression
controls seed dormancy and flowering time. _Science_ 360, 1014–1017 (2018). Article ADS CAS PubMed Google Scholar * Blackman, B. K., Strasburg, J. L., Raduski, A. R., Michaels, S. D.
& Rieseberg, L. H. The role of recently derived FT paralogs in sunflower domestication. _Curr. Biol._ 20, 629–635 (2010). Article CAS PubMed PubMed Central Google Scholar *
Koornneef, M. & Hanhart, C. & Van der Veen, J. A genetic and physiological analysis of late flowering mutants in _Arabidopsis thaliana_. _Mol. Gen. Genet._ 229, 57–66 (1991). Article
CAS PubMed Google Scholar * Bradley, D., Ratcliffe, O., Vincent, C., Carpenter, R. & Coen, E. Inflorescence commitment and architecture in _Arabidopsis_. _Science_ 275, 80–83
(1997). Article CAS PubMed Google Scholar * Shannon, S. & Meeks-Wagner, D. R. A mutation in the _Arabidopsis_ TFL1 gene affects inflorescence meristem development. _Plant Cell_ 3,
877–892 (1991). Article CAS PubMed PubMed Central Google Scholar * Schultz, E. A. & Haughn, G. W. LEAFY, a homeotic gene that regulates inflorescence development in _Arabidopsis_.
_Plant Cell_ 3, 771–781 (1991). Article PubMed PubMed Central Google Scholar * Haughn, G. W. & Somerville, C. R. Genetic control of morphogenesis in _Arabidopsis_. _Developmental
Genet._ 9, 73–89 (1988). Article Google Scholar * Yoo, S. K. et al. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in
_Arabidopsis_. _Plant Physiol._ 139, 770–778 (2005). Article CAS PubMed PubMed Central Google Scholar * Wu, X. et al. Modes of intercellular transcription factor movement in the
_Arabidopsis_ apex. _Development_ 130, 3735–3745 (2003). Article CAS PubMed Google Scholar * Ohshima, S., Murata, M., Sakamoto, W., Ogura, Y. & Motoyoshi, F. Cloning and molecular
analysis of the _Arabidopsis_ gene terminal flower 1. _Mol. Gen. Genet._ 254, 186–194 (1997). Article CAS PubMed Google Scholar * McBride, K. E. & Summerfelt, K. R. Improved binary
vectors for Agrobacterium-mediated plant transformation. _Plant Mol. Biol._ 14, 269–276 (1990). Article CAS PubMed Google Scholar * Curtis, M. D. & Grossniklaus, U. A gateway cloning
vector set for high-throughput functional analysis of genes in planta. _Plant Physiol._ 133, 462–469 (2003). Article CAS PubMed PubMed Central Google Scholar * Nakagawa, T., Ishiguro,
S. & Kimura, T. Gateway vectors for plant transformation. _Plant Biotechnol._ 26, 275–284 (2009). Article CAS Google Scholar * Liu, Y.-G., Wang, X., Wang, F. & Chen, L. Robust
one-Tube Ω-PCR strategy accelerates precise sequence modification of plasmids for functional genomics. _Plant Cell Physiol._ 54, 634–642 (2013). Article PubMed PubMed Central CAS Google
Scholar * Michniewicz, M., Frick, E. M. & Strader, L. C. Gateway-compatible tissue-specific vectors for plant transformation. _BMC Res. Notes_ 8, 63 (2015). Article PubMed PubMed
Central CAS Google Scholar * Adrian, J. et al. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in _Arabidopsis_.
_Plant Cell_ 22, 1425–1440 (2010). Article CAS PubMed PubMed Central Google Scholar * Ossowski, S., Schwab, R. & Weigel, D. Gene silencing in plants using artificial microRNAs and
other small RNAs. _Plant J._ 53, 674–690 (2008). Article CAS PubMed Google Scholar * Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium -mediated
transformation of _Arabidopsis thaliana_. _Plant J._ 16, 735–743 (1998). Article CAS PubMed Google Scholar * Vitha, S., Beneš, K., Phillips, J. P. & Gartland, K. M. A. Histochemical
GUS analysis. in _Agrobacterium Protocols_ (eds. Gartland, K. M. A. & Davey, M. R.) 185–193 (Springer New York, Totowa, NJ, 1995). * Prunet, N., Jack, T. P. & Meyerowitz, E. M. Live
confocal imaging of _Arabidopsis_ flower buds. _Developmental Biol._ 419, 114–120 (2016). Article CAS Google Scholar * Yamaguchi, N. et al. PROTOCOLS: Chromatin immunoprecipitation from
_Arabidopsis_ tissues. _Arabidopsis Book_ 12, e0170 (2014). * Bolger, A. M., Usadel, B. & Lohse, M. Trimmomatic: a flexible trimmer for Illumina sequence data. _Bioinformatics_ 30,
2114–2120 (2014). Article CAS PubMed PubMed Central Google Scholar * Andrews, S. _FastQC: A Quality Control Tool for High Throughput Sequence Data_ (Babraham Bioinformatics, Babraham
Institute, Cambridge, 2010). * Li, H. et al. The sequence alignment/map format and SAMtools. _Bioinformatics_ 25, 2078–2079 (2009). Article PubMed PubMed Central CAS Google Scholar *
Lamesch, P. et al. The _Arabidopsis_ Information Resource (TAIR): improved gene annotation and new tools. _Nucleic Acids Res._ 40, D1202–D1210 (2011). Article PubMed PubMed Central CAS
Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357 (2012). Article CAS PubMed PubMed Central Google Scholar *
Berardini, T. Z. et al. The _Arabidopsis_ Information Resource: Making and mining the “gold standard” annotated reference plant genome. _Genesis_ 53, 474–485 (2015). Article CAS PubMed
PubMed Central Google Scholar * Richter, A. S. et al. deepTools2: a next generation web server for deep-sequencing data analysis. _Nucleic Acids Res._ 44, W160–W165 (2016). Article PubMed
PubMed Central CAS Google Scholar * Landt, S. G. et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. _Genome Res._ 22, 1813–1831 (2012). Article CAS PubMed
PubMed Central Google Scholar * Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). _Genome Biol._ 9, R137 (2008). Article PubMed PubMed Central CAS Google Scholar * Quinlan,
A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. _Bioinformatics_ 26, 841–842 (2010). Article CAS PubMed PubMed Central Google Scholar *
Song, L. et al. A transcription factor hierarchy defines an environmental stress response network. _Science_ 354, aag1550 (2016). Article PubMed PubMed Central CAS Google Scholar *
Cheng, C.-Y. et al. Araport11: a complete reannotation of the _Arabidopsis_ thaliana reference genome. _Plant J._ 89, 789–804 (2017). Article CAS PubMed Google Scholar * Zhu, L. J. et
al. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. _BMC Bioinform._ 11, 237 (2010). Article CAS Google Scholar * Machanick, P. & Bailey, T. L.
MEME-ChIP: motif analysis of large DNA datasets. _Bioinformatics_ 27, 1696–1697 (2011). Article CAS PubMed PubMed Central Google Scholar * Heinz, S. et al. Simple combinations of
lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589 (2010). Article CAS PubMed PubMed Central
Google Scholar * Xiao, J. et al. Cis and trans determinants of epigenetic silencing by polycomb repressive complex 2 in _Arabidopsis_. _Nat. Genet._ 49, 1546–1552 (2017). Article CAS
PubMed Google Scholar * Winter, Cara M. et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. _Developmental Cell_ 20, 430–443
(2011). Article CAS PubMed Google Scholar * Yan, H. et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. _Nucleic Acids Res._ 45, W122–W129 (2017).
Article PubMed PubMed Central CAS Google Scholar * Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. _Ann. Stat._ 29,
1165–1188 (2001). Article MathSciNet MATH Google Scholar * Sayou, C. et al. A SAM oligomerization domain shapes the genomic binding landscape of the LEAFY transcription factor. _Nat.
Commun._ 7, 11222 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2012). Article
PubMed PubMed Central CAS Google Scholar * Anders, S., Pyl, P. T. & Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. _Bioinformatics_ 31, 166–169
(2014). Article PubMed PubMed Central CAS Google Scholar * Anders, S. & Huber, W. Differential expression analysis for sequence count data. _Genome Biol._ 11, R106 (2010). Article
CAS PubMed PubMed Central Google Scholar * Huber, W., von Heydebreck, A., Sültmann, H., Poustka, A. & Vingron, M. Parameter estimation for the calibration and variance stabilization
of microarray data. _Stat. Appl. Genet. Mol. Biol._ 2, 1544–6115 (2003). * Tibshirani, R. Estimating transformations for regression via additivity and variance stabilization. _J. Am. Stat.
Assoc._ 83, 394–405 (1988). Article MathSciNet Google Scholar * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2.
_Genome Biol._ 15, 550 (2014). Article PubMed PubMed Central CAS Google Scholar * MacQueen, J. Some methods for classification and analysis of multivariate observations. In _Proc. 5th
Berkeley Symposium on Mathematical Statistics and Probability_, Vol. 1, 281–297 (Oakland, CA, 1967). * Massey, F. J. The Kolmogorov-Smirnov test for goodness of fit. _J. Am. Stat. Assoc._
46, 68–78 (1951). Article MATH Google Scholar * Kruskal, W. H. & Wallis, W. A. Use of ranks in one-criterion variance analysis. _J. Am. Stat. Assoc._ 47, 583–621 (1952). Article MATH
Google Scholar * Dunn, O. J. Multiple comparisons using rank sums. _Technometrics_ 6, 241–252 (1964). Article Google Scholar * Ackerman-Lavert, M. & Savaldi-Goldstein, S. Growth
models from a brassinosteroid perspective. _Curr. Opin. Plant Biol._ 53, 90–97 (2019). Article PubMed CAS Google Scholar * Muller, B. & Sheen, J. _Arabidopsis_ Cytokinin signaling
pathway. _Sci STKE_ 407, cm5 (2007). * Weijers, D. & Wagner, D. Transcriptional responses to the auxin hormone. _Annu. Rev. Plant Biol._ 67, 539–574 (2016). Article CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS We thank undergraduate students Gabriela M. Blandino, Xindi Chen and Zubaida Salman and Dietrich James Nigh for help with the experiments, Dr.
John D. Wagner for input on the manuscript and the Plant Biology group as well as Wagner lab members for feedback on this project. This research was funded by National Science Foundation IOS
grant 1557529 and 1905062 to D.W. AUTHOR INFORMATION Author notes * Cheol Woong Jeong Present address: LG Economic Research Institute, LG Twin tower, Seoul, 07336, Korea * Nobutoshi
Yamaguchi Present address: Division of Biological Science, Graduate School of Science and Technology, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara, 630-0192, Japan
* These authors contributed equally: Cheol Woong Jeong, Nobutoshi Yamaguchi. AUTHORS AND AFFILIATIONS * Department of Biology, University of Pennsylvania, 415S. University Ave, Philadelphia,
PA, 19104, USA Yang Zhu, Samantha Klasfeld, Cheol Woong Jeong, Run Jin, Nobutoshi Yamaguchi & Doris Wagner * Research Institute for Biological Sciences, Okayaka Prefecture, 7549-1,
Kibichuoh-cho, Kaga-gun, Okayama, 716-1241, Japan Koji Goto Authors * Yang Zhu View author publications You can also search for this author inPubMed Google Scholar * Samantha Klasfeld View
author publications You can also search for this author inPubMed Google Scholar * Cheol Woong Jeong View author publications You can also search for this author inPubMed Google Scholar * Run
Jin View author publications You can also search for this author inPubMed Google Scholar * Koji Goto View author publications You can also search for this author inPubMed Google Scholar *
Nobutoshi Yamaguchi View author publications You can also search for this author inPubMed Google Scholar * Doris Wagner View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS D.W. and Y.Z. conceived of the study and Y.Z. conducted the majority of the experiments. S.K. conducted the bioinformatic analyses. N.Y. and C.W.J. identified
the optimal stage to study primordium fate regulation by TFL1, conducted initial TFL1 ChIP analyses and mapped the FD-binding sites in LFY. R.J. and Y.Z. constructed and sequenced ChIP-seq
libraries, K.G generated the biologically active genomic GFP-TFL1 construct. D.W. wrote the manuscript with the help of Y.Z. and input from all other authors. CORRESPONDING AUTHOR
Correspondence to Doris Wagner. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_
thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTIONS OF ADDITIONAL SUPPLEMENTARY FILES
SUPPLEMENTARY DATA 1 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link
to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhu, Y., Klasfeld, S., Jeong, C.W. _et al._ TERMINAL FLOWER 1-FD complex target genes and competition with FLOWERING LOCUS T. _Nat
Commun_ 11, 5118 (2020). https://doi.org/10.1038/s41467-020-18782-1 Download citation * Received: 07 February 2020 * Accepted: 01 September 2020 * Published: 12 October 2020 * DOI:
https://doi.org/10.1038/s41467-020-18782-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
