Tuning electron delocalization of hydrogen-bonded organic framework cathode for high-performance zinc-organic batteries
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Stable cathodes with multiple redox-active centres affording a high energy density, fast redox kinetics and a long life are continuous pursuits for aqueous zinc-organic batteries.
Here, we achieve a high-performance zinc-organic battery by tuning the electron delocalization within a designed fully conjugated two-dimensional hydrogen-bonded organic framework as a
cathode material. Notably, the intermolecular hydrogen bonds endow this framework with a transverse two-dimensional extended stacking network and structural stability, whereas the multiple C
= O and C = N electroactive centres cooperatively trigger multielectron redox chemistry with super delocalization, thereby sharply boosting the redox potential, intrinsic electronic
conductivity and redox kinetics. Further mechanistic investigations reveal that the fully conjugated molecular configuration enables reversible Zn2+/H+ synergistic storage accompanied by
10-electron transfer. Benefitting from the above synergistic effects, the elaborately tailored organic cathode delivers a reversible capacity of 498.6 mAh g−1 at 0.2 A g−1, good cyclability
and a high energy density (355 Wh kg−1). SIMILAR CONTENT BEING VIEWED BY OTHERS _D_-ORBITAL STEERED ACTIVE SITES THROUGH LIGAND EDITING ON HETEROMETAL IMIDAZOLE FRAMEWORKS FOR RECHARGEABLE
ZINC-AIR BATTERY Article Open access 17 November 2020 COORDINATION MODULATION OF HYDRATED ZINC IONS TO ENHANCE REDOX REVERSIBILITY OF ZINC BATTERIES Article Open access 14 June 2023
ELECTROLYTE DESIGN FOR REVERSIBLE ZINC METAL CHEMISTRY Article Open access 02 January 2025 INTRODUCTION Because of the inherent merits of resource abundance, high theoretical capacity (820
mAh g−1), environmental benignity and safety, rechargeable aqueous zinc-ion batteries are booming for next-generation large-scale energy storage1,2. Accordingly, great efforts have been made
to explore high-performance cathode materials for zinc-ion batteries3,4,5. In particular, organic electrode molecules as cathode materials have received growing attention in recent years
due to their environmental friendliness, sustainability, structural designability, and abundant resources6. However, they still suffer from limited electron conductivity, low energy density,
and low cyclability, resulting from the intrinsic molecular structure, random stacking properties, and inevitable dissolution of functional groups7,8. Consequently, exploring new types of
organic cathodes that can overcome the above barriers is vital for advanced zinc organic batteries. Conjugated organic molecules featuring large π-electron configurations have attracted
considerable attention as organic electrodes owing to their electrical conductivity, structural designability, and material accessibility9,10. Noticeably, the electrochemical activity and
redox chemistry of the conjugated molecules can be systematically manipulated by molecular tailoring and electron modulation strategies11,12,13. Despite the significant progress in
electrochemical reversibility, the reported conjugated systems obtained by molecular modification and conjugate structure extension strategies always involve some functional groups with
insufficient redox activity that inevitably reduce the capacity14,15. In addition, the weak electron delocalization induced by nucleophilic functional groups makes it difficult to obtain a
satisfactory output voltage16. Meanwhile, these organic small molecules have a strong tendency to dissolve into the electrolyte during the redox process, thereby leading to poor
cyclability17. Therefore, to address these challenges, exploiting new conjugated organic cathodes through rational molecule design is highly urgent and significant for high-performance
zinc-organic batteries (ZOBs). Herein, we achieve a super-electron-delocalized fully conjugated two-dimensional (2D) hydrogen-bonded organic framework (F-HOF) by manipulating a strong
nucleophilic functionalization strategy, i.e., the benzo[a]benzo[7,8]quinoxalino[2,3-i]phenazine-5,6,8,14,15,17-hexane (BBQPH) F-HOF featuring synergistic C = O and C = N
electron-withdrawing motifs that alter the intramolecular electron distribution, thus boosting the redox voltage and triggering multielectron storage chemistry for ZOBs. Impressively, the
multiple intermolecular hydrogen bonds (C = N···H/C = O···H) combined with the _π_–_π_ stacking interactions further enhance the structural stability of BBQPH. In addition, the super
electron delocalization simultaneously improves the redox potential, intrinsic electronic conductivity, and redox kinetics. As expected, the BBQPH electrodes deliver a high voltage of 1.2 V,
a significantly improved capacity (498.6 mAh g−1 at 0.2 A g−1), corresponding to an energy density of 355 Wh kg−1, and an exceptional cycling performance of >1000 cycles at 5.0 A g−1 for
aqueous ZOBs. Furthermore, ex situ investigations and theoretical simulations clarify the reversible Zn2+/H+ synergistic storage mechanism accompanied by 10-electron transfer. Our work will
provide new insight for realizing advanced organic materials through rational molecular design towards diverse applications. RESULTS THEORETICAL PREDICTION AND GUIDANCE FOR MOLECULAR DESIGN
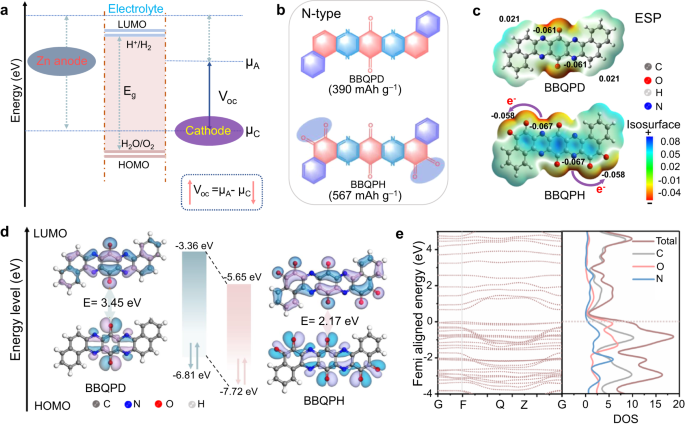
A high output voltage together with a high capacity contributes to a high energy density of batteries, which is highly desirable for their practical application18. On the one hand, the
redox potential of an organic molecule is closely related to its highest occupied molecular orbital (HOMO) energy level, and a lower HOMO energy signifies a higher oxidation capability based
on molecular orbital theory prediction, resulting in a higher open-circuit voltage (_V_oc) in ZOBs (Fig. 1a)19. Generally, the HOMO energy can be lowered by induced electron delocalization
of the electron-withdrawing group20. On the other hand, the delivered capacity of organic molecules mainly depends on the number of electroactive centers per weight21. Increasing the number
of active sites and reducing their relative molecular mass by regulating the π-conjugated building units can realize a high-capacity performance. Currently, rationally designing
low-HOMO-energy conjugated molecules with multiple redox-active centers is supposed to realize high-energy-density ZOBs. Given this, a super-electron-delocalized fully conjugated organic
molecule, benzo[a]benzo[7,8]quinoxalino[2,3-i]phenazine-5,6,8,14,15,17-hexane (BBQPH), possessing multiple electron-withdrawing carbonyl groups (C = O), was designed (Fig. 1b). Similarly,
benzo[a]benzo[7,8]quinoxalino[2,3-i]phenazine-8,17-dione (BBQPD) was also designed as a conceptual reference. Notably, the designed BBQPH intrinsically possesses almost the highest
theoretical capacity level (567 mAh g–1) compared with the other organic cathode materials previously reported16. To precisely verify the electron delocalization of the designed molecules,
the electrostatic potential (ESP) and molecular orbitals were first calculated to evaluate the intrinsic electronic properties. Compared to BBQPD (0.021 a.u.), the ESP value (−0.058 a.u.) of
o‑quinone functional groups for BBQPH is dramatically decreased because of its super electron delocalization and highly conjugated symmetric structure22. Meanwhile, BBQPH shows more
dramatic electron delocalization, which results in smaller molecular dipole moments and robust intramolecular electron migration from the conjugate planar center to the o‑quinone groups
(Fig. 1c). Moreover, the lower HOMO value of the BBQPH molecule (−7.72 eV) compared with BBQPD (−6.81 eV) indicates a higher cation affinity and promisingly leads to an increase in Voc. In
addition, the super-electron-delocalized fully conjugated BBQPH demonstrates a smaller gap (Eg) (2.17 eV) between the LUMO and HOMO, which endows it with higher electrical conductivity for
rapid electron transport as well as fast redox kinetics. Interestingly, there is a significant density of electron states at the Fermi level for the designed BBQPH according to the energy
band and partial density of states (pDOS; Fig. 1e and Supplementary Fig. 1), which is primarily contributed by C=O group, verifying that o‑quinone increases the super electron delocalization
and further underscores the potential for fast charge transfer of BBQPH. Therefore, the elaborately tailored BBQPH would be significantly attractive for ZOBs. SYNTHESIS AND STRUCTURAL
CHARACTERIZATIONS BBQPH featuring multiple carbonyls and pyrazine redox sites was synthesized via a two-step solvothermal reaction (Supplementary Figs. 2 and 3). Meanwhile, BBQPD was also
synthesized as a reference (Supplementary Fig. 4). The chemical compositions of the samples were investigated using a variety of characterization methods. The 1H NMR spectra together with
high-resolution mass spectra verify the successful synthesis of BBQPH (Supplementary Fig. 5). The Fourier transform infrared (FTIR) spectrum of BBQPH shows peaks located at 1590 and 1689
cm−1, which are attributed to the C=N and C=O stretching vibration groups, respectively23. The Raman spectra further confirm that the molecular functional structures of the two products are
as expected (Supplementary Fig. 6)24. Notably, the BBQPH molecule shows a rotationally symmetric molecular configuration consisting of multiple carbonyls and pyrazine groups, which could
easily form a fully conjugated 2D hydrogen-bonded organic framework via intermolecular noncovalent locking. Consequently, powder X-ray diffraction (PXRD) was performed to reveal the
crystallinity and molecular stacking patterns (Fig. 2a). The prominent diffraction peak at 2_θ_ = 27.1° can be assigned to the (002) plane, which conforms to the simulated AA stacking model,
and the interlayer distance between fully conjugated BBQPH layers is 3.3 Å (Fig. 2b). The diffraction peaks at 3.5° and 16.8° correspond to the (100) and (300) crystal facets, respectively.
However, BBQPD only exhibits weak amorphous diffraction peaks due to its random molecular arrangement (Supplementary Fig. 7). To further reveal the stacking orientation of BBQPH, Pawley
refinement was performed with an optimized model to fit the obtained experimental data. The refined crystal structure for BBQPH was assigned to the monoclinic system with the P1 space group
(Supplementary Table 2), and the unit cell parameters were refined to _a_ = 17.204 Å, _b_ = 9.163 Å, _c_ = 3.553 Å, _α_ = 114°, _γ_ = 63.4°, and _β_ = 94.4°. The calculated hydrogen bond
lengths between the hydrogen atom of C-H and the O/N atoms of the carbonyl/phenazine groups are ~2.35–2.40 Å. Specifically, owing to the centrosymmetric fully conjugated structure with
abundant C = O and C = N electron-withdrawing groups, each BBQPH molecule is locked with four adjacent BBQPH molecules by multiple hydrogen bonds between strong hydrogen bond acceptor C =
N/C = O functional groups and weak hydrogen donor C-H groups (C = N···H/C = O···H), forming a 2D hydrogen-bonded organic framework structure (Fig. 1b and Supplementary Fig. 8). The
high-resolution TEM image (Supplementary Fig. 9) visualizes the ordered and robust structure of the BBQPH materials with a lattice fringe of 0.33 nm, which is consistent with the XRD
measurement. Notably, the calculated green peak located at sign(_I__2_)_r_ values of −0.02–0.00 in the reduced density gradient diagram (Fig. 2c) further clarifies the existence of π-π
interactions between adjacent molecular layers, which agrees well with the PXRD result25. It should be noted that the existence of strong intermolecular forces of π-π interactions and
hydrogen bonding renders high thermostability and poor solubility in water solvents (Supplementary Figs. 10 and 11), which is significantly advantageous for suppressing the shuttle effect
and for a favorable cycling stability in aqueous batteries26,27. The porous-honeycomb morphology was revealed by scanning electron microscopy (SEM) (Supplementary Fig. 12) for the prepared
BBQPH, which has a larger surface area of 68.12 m2 g−1 and a micropore size of 0.55 nm (Supplementary Fig. 13), thus facilitating ion diffusion and shortening the transport pathways as an
electrode material. Meanwhile, the modulated BBQPH crystal is verified to have a parallelepiped structure, well matching the simulated molecular model (Fig. 2d). The corresponding
energy-dispersive spectroscopy (EDS) mapping results further show homogeneous distributions of C, O, and N elements in the modulated BBQPH crystal (Supplementary Fig. 14). Noticeably, the
electron paramagnetic resonance spectrum (Fig. 2e) of BBQPH presents three obvious signals with _g_ values of 1.9556, 2.0084 and 2.0237, revealing a typical spin-orbit delocalization
coupling effect and the existence of multiple radicals with an unpaired electron28. The cation radical could generate electron holes for high electron mobility in the fully conjugated
structure. Significantly, the delocalization enhances the spin density of the cation radical in the fully conjugated structure, further confirming its good stability. That is, such a super
electron delocalization provided by o-quinone groups induced along the fully conjugated configuration is favorable for electron implantation into the organic skeleton with a low energy
barrier and ensures high stability of free radicals. ELECTROCHEMICAL PERFORMANCE The unique structure of the 2D hydrogen-bonded organic framework with electron-delocalization-enhanced redox
centers of the BBQPH framework inspired us to further investigate its electrochemical properties in aqueous ZOBs. Cyclic voltammetry (CV) curves were recorded to analyze the
reduction/oxidation behavior of BBQPH and BBQPD at 0.5 mV s–1 (Fig. 3a and Supplementary Fig. 15). The BBQPH electrode displays three distinct pairs of redox peaks at 1.31/1.16, 0.81/0.72,
and 0.58/0.45 V, confirming the three-step 10-electron transfer redox reaction of BBQPH derived from the multiple redox-active centers29. The initial five cycles overlap almost completely,
indicating stable and highly reversible redox behavior16. Impressively, the following galvanostatic charge/discharge test of the two batteries confirms that the introduction of the carbonyl
groups induces super π-electron delocalization, thus significantly elevating the redox potential (1.2 V; Fig. 3b), which is the highest output voltage of an n-type material30. The enhanced
voltage plateau together with multielectron redox significantly contributes to the enhanced capacity (increases by 45%) and energy density (increases by 25% to 355 Wh kg−1) of the ZOBs (Fig.
3c). Specifically, the BBQPH electrode shows good rate performance with energy densities and capacities of 498.6, 455.0, 436.8, 422.9, 406.2, and 393.6 mAh g−1 at 0.2, 0.5, 1.0, 2.0, 5.0,
and 8.0 A g−1, respectively (Fig. 3d and Supplementary Figs. 16 and 17). Moreover, when the current density is returned to 0.2 A g−1, the capacity recovers to 487.2 mAh g−1, revealing the
enhanced rate performance and reversibility of the BBQPH electrode. For the BBQPD electrode, the corresponding capacities monotonically decrease from 305.4 to 208.3 mAh g−1 as the applied
current density increases from 0.2 to 8.0 A g−1. Remarkably, the ZOBs with BBQPH electrodes exhibit a satisfactory performance of up to 1000 cycles under 5.0 A g−1 with a 95% capacity
retention (Fig. 3e). In contrast, the BBQPD cathode demonstrates capacity fading to 152.6 mAh g−1 after 1000 cycles (Supplementary Fig. 18). Notably, BBQPH represents one of the best in
terms of comprehensive performance compared to organic-based electrodes in previous related reports (Fig. 3f and Supplementary Table 3)31. To further confirm the practicability and
superiority of the BBQPH electrode, the cycling stability of ZOBs coin cells was evaluated under various working temperatures (Fig. 3g). The BBQPH electrode delivers satisfactory discharge
capacities of approximately 409 and 418 mAh g−1 and good cycling stability at working temperatures of 25 and 40 °C, respectively. More encouragingly, the battery can still deliver 306 mAh
g−1 even at 0 °C, revealing a preeminent work tolerance under low temperatures. Meanwhile, the ZOBs can power a timer-integrated display, verifying the promising application prospects in
electronic devices (Supplementary Fig. 19). Moreover, the assembled Zn-ion pouch cell outputs a 14.0 mAh capacity at 0.1 A g−1 (Fig. 3h and Supplementary Fig. 20), and the exceptional
electrochemical performance of the battery holds considerable promise in practical applications and highlights the advantages of this molecular design, including the increased energy density
and reduced solubility of organic materials. Overall, combining the abundant electroactive sites and hydrogen-bonding-stabilized network skeleton, the assembled ZOBs show great advantages
in terms of sustainability, cycling stability, and energy density as grid-scale energy storage systems. REDOX KINETICS The galvanostatic intermittent titration technique (GITT) was first
carried out to explore the redox kinetics of the two electrodes (Fig. 4a). The higher ion diffusion coefficient (1.5 × 10−9 cm2 s−1 vs. 6.8 × 10−10 cm2 s−1 for BBQPD) combined with the low
activation energy (1.58 eV) calculated from electrochemical reaction curves evidence the faster reaction kinetics and superior ion transport ability of BBQPH (Supplementary Figs. 21 and
22)32. To further reveal the origin of the superior kinetic performance of BBQPH, CV tests of the two electrodes were performed at different scan rates (0.5–3.0 mV s−1) (Fig. 4b and
Supplementary Fig. 15). The BBQPH electrodes present a small peak shift with increasing scan rate, indicating remarkable electrochemical reversibility33. Of note, the well-linearly fitted
b-value at the redox peaks of the BBQPH electrode varies from 0.69 to 0.78, indicating fast surface-controlled charge-storage kinetics (Fig. 4c)34. Almost 86.2% of the total stored charge is
contributed by the surface redox reaction at 3.0 mV s−1 (Fig. 4d). Considering the surface-controlled charge storage character, the anti-self-discharge and anti-dissolution abilities were
determined, further revealing the good capacity retention and structural stability (Supplementary Fig. 23). Accordingly, the dominant reason for the fast charge storage is attributed to
strong electron delocalization bringing highly electroactive C=O/C=N zincophilic sites and a robust molecular H-bonded organic network, which facilitates faster redox kinetics, enhanced
stability, and elevated conductivity compared to BBQPD (Fig. 4f). A computational fluid dynamics simulation was also employed to evaluate the effect of the porous-honeycomb structure on
electrolyte diffusion35. Here, we established a flower-like structure model according to the SEM results to simulate BBQPH (Fig. 4g). Over the course of the simulation, the volume fraction
distribution of the fluid (which corresponds to Zn2+ in the model) gradually accelerates and eventually reaches 90%, indicating that Zn2+ is capable of completely penetrating the deep
interior of the model. Additionally, according to the structure model, the normalized volume fraction of ions gradually increases from the surface to the center. The nonporous structure
model of BBQPD demonstrates a discontinuous increase in the normalized volume fraction and displays a maximum volume fraction at a radius of ~0.17 from the surface (Supplementary Fig. 24).
These simulation results and experimental evidence highlight the tremendous ion diffusion and storage capability of the electron-delocalized fully conjugated H-bonding framework, which
further reveals the structural advantages of BBQPH as a cathode of ZOBs. REDOX CHEMISTRY MECHANISM We deeply delved into the detailed electrochemical mechanism of BBQPH in ZOBs through a
series of characterizations. Note that plentiful protons exist in aqueous Zn2+ electrolytes because of the hydrolysis reaction of Zn salt. Therefore, an in-situ pH test was conducted using a
direct-reading pH meter in a homemade two-electrode reaction device to track the pH changes (Fig. 5a). The pH value shows an increase during discharge (voltage range from 1.0 to 0.2 V) and
a downward trend when charging, revealing the existence of reversible proton storage behavior for BBQPH36. Additionally, in situ EIS was used to monitor the real-time impedance variation,
indicating a significant decrease in the charge storage process, which is mainly attributed to the enhanced ion/electron conductivity (Fig. 5b)37. Following ex situ XRD, FTIR spectroscopy
and X-ray photoelectron spectroscopy (XPS) were further performed to elucidate the chemical structure evolution of BBQPH during the charge storage process. The characteristic peak of
Zn4SO4(OH)6·_x_H2O (8.17°) derived from proton insertion gradually appears and disappears during discharging and charging, respectively (Fig. 5c and Supplementary Figs. 25 and 26). Moreover,
the typical peaks of BBQPH slightly decrease while new peaks appear upon the cation intercalation process, corroborating the Zn2+/H+ reversible costorage behavior. In addition, the two
typical peaks in the FTIR spectra weaken in the reduction process, corresponding to the coordination of cations with redox-active centers (Supplementary Fig. 27). Similarly, the XPS spectra
of _O 1s_ and _N 1s_ reveal the evolution of the C=O and C=N bonds, which are transformed into the C-N bond (400.1 eV) and C-O bond (533.3 eV) upon the discharge process (Fig. 4d),
respectively, suggesting the coordination reaction of the two C=N/C=O redox centers with Zn2+/H+ 38,39. In the subsequent oxidation process, the C=O/C=N peaks reappear and gradually
strengthen, illustrating the release of Zn2+/H+ and the highly reversible feature of the BBQPH redox activity. To reveal the proton storage contributions, we further investigated the
electrochemical properties of the BBQPH electrode using an electrolyte without Zn salt (i.e., 0.05 M H2SO4) and aqueous ZnSO4 electrolytes with different concentrations (Supplementary Figs.
28 and 29). Notably, the BBQPH cathode demonstrates three similar pairs of redox peaks with lower redox potentials in the 0.05 M H2SO4 electrolyte according to the calibrated CV curves (Fig.
5e), indicating that the initial coordination is derived from Zn2+, followed by two continuous successive H+ uptakes. In addition, a nonaqueous Zn2+ electrolyte (0.5 M Zn(OTf)2/ACN) was
adopted to further confirm the priority coordination of Zn2+ and exclude the effect of protons (Supplementary Figs. 30 and 31), and the higher Zn2+-coordination potential further confirms
the previous speculation. Interestingly, the BBQPH electrode still exhibits a moderately reduced capacity (372 mAh g−1) but with lower redox kinetics (Supplementary Fig. 32). In view of the
above analyses, Zn2+ contributes approximately 45% of the capacity in the BBQPH electrode, while the capacity contribution of protons can reach 55%, which is consistent with the EDS mapping
analysis and XPS analysis results (Supplementary Fig. 33 and Supplementary Table 1). Based on the above mechanistic studies, it is clear that the carbonyl and phenazine groups of BBQPH have
highly reversible redox activity, and the stepwise co-intercalation of Zn2+/H+ enables BBQPH to exhibit enhanced redox dynamics. THEORETICAL CALCULATION To clarify the redox path of the
10-electron reduction reactions of BBQPH, we established four cation-coordinated models and quantified the absorption energies of each cation on coordinated structures by theoretical
calculation (Supplementary Fig. 34). According to the calculation results, the stable O···Zn···N coordination (BBQPH(Zn)2) is first formed by bonding Zn cations with one neighboring O of
o-quinone and one N of pyrazine functional groups due to the strongest affinity to the cations23. Then, 2H+ tends to be separately coordinated with a pair of O atoms of the o-quinone group
to form BBQPH(Zn)2(H)2. Finally, H+ inserts into the phenazine site with two H+ bonded by two N atoms, generating BBQPH(Zn)2(H)6 and agreeing with the experimental analysis. Based on the
above results, the optimized total charge storage path of BBQPH coordinated by Zn2+/H+ during the multistep redox processes is summarized in Fig. 6a. Moreover, the reduction potentials
together with the Gibbs free energy (Fig. 6b, c) were calculated according to the adsorption energy diagram. It is worth mentioning that small energy differences in the adjacent redox
process at different steps can lead to overlap of redox peaks. Therefore, at least three main redox potential levels related to the step-by-step 10-electron transfer mechanism were
established, in agreement with the CV curve. The Gibbs free energy (ΔG) of the cation coordination models shows a decreasing trend in the first several stages, indicating stable adsorption
of cations on the active sites29. The increase in ΔG in the final proton intercalation step can be attributed to the deformation of the molecular plane structure. Furthermore, the Zn2+
mobility within the BBQPH framework was also computed using the DFT model to determine the optimal ion migration path. Two representative migration paths (P1 and P2) were evaluated based on
a transition state search, as shown in Fig. 6d and Supplementary Fig. 3540. Specifically, P1 represents the intralayer diffusion of Zn2+ ions, while P2 corresponds to Zn2+ cation migration
along nanochannels. The corresponding diffusion barrier energies for P1 and P2 were calculated to be 10 eV and 1.42 eV, respectively. Accordingly, the lower migration energy barrier within
molecularly constructed nanochannels promotes rapid penetration of Zn2+/H+ to the internal active sites and boosts fast redox dynamics, thus affording an improved rate performance as well as
superb stability during long-term cycling for BBQPH. DISCUSSION In summary, we have demonstrated a super-electron-delocalized fully conjugated 2D hydrogen-bonded organic framework based on
molecular engineering for high-energy-density ZOBs. The super electron delocalization alters the intramolecular electron distribution, thus significantly boosting the redox potential.
Meanwhile, the intermolecular multiple hydrogen bonds endow the framework with a transverse 2D extension and longitudinal π-π stacking structure, which extends the electron delocalization
area, narrows the energy gap between the HOMO and LUMO, and boosts the structural stability. Benefiting from these synergetic superiorities, BBQPH delivers an elevated output voltage, a high
reversible capacity of 498.6 mAh g−1 at 0.2 A g−1, good cyclability (capacity retention of 95% after 1000 cycles), and a high energy density (355 Wh kg−1) for ZOBs, indicating that it has
advanced prospects in practical applications. Furthermore, the redox kinetics and charge-storage mechanism of BBQPH were systematically investigated, revealing a boosted reversible Zn2+/H+
synergistic storage behavior accompanied by 10-electron transfer. This work offers new opportunities for molecular design of redox-reversible multielectron organic cathodes and thus bright
practical prospects for high-performance ZOBs. METHODS MATERIALS All commercially available reagents and solvents were purchased from Adamas, Aladdin, or Innochem Co., Ltd. and used directly
without additional purification. SYNTHESIS OF TETRAAMINO-P-BENZOQUINONE Tetraamino-p-benzoquinone (TABQ) was prepared based on a simple dehydration condensation reaction together with an
oxidation reaction, as shown in Supplementary Fig. 21. In detail, tetrachlorobenzoquinone (TCBQ) (10.0 g, 0.04 mol) and potassium phthalimide (30.0 g, 0.16 mol) were placed in a 250 mL round
bottom flask, and 100.0 mL of acetonitrile was slowly added to the above mixture. After stirring at room temperature for 10 min, the solution was further heated and kept at 80 °C for 12 h
under stirring. After cooling to room temperature, the suspension was poured into 1.0 L of boiled water, filtered, and washed successively several times. The resulting brown‒yellow product
was dried at 60 °C overnight to obtain tetra(phthalimido)-benzoquinone (TPBQ; 31.2 g, 78%). The synthesized TPBQ (4.5 g, 0.014 mol) was first dispersed in 100 mL of 80 wt% hydrazine hydrate
in a 250 mL three-necked flask under magnetic stirring at room temperature for 2 h. Then, the mixture was heated at 65 °C for 2 h to facilitate the reduction process. Finally, the reaction
system was cooled to room temperature, filtered, and washed with water and ethanol, and then, the dark purple product of TABQ was obtained in 45% isolated yield. SYNTHESIS OF
BENZO[A]BENZO[7,8]QUINOXALINO[2,3-I]PHENAZINE-8,17-DIONE (BBQPD) AND BBQPH BBQPH was prepared, inspired separately by the literature, based on a two-step reaction, as shown in Supplementary
Fig. 441. Typically, first, 4.5 g (20 mmol) of 2,3-dichloro-1,4-napthoquinone and 1.0 g (10 mmol) of TABQ were added to 150 mL of pyridine solution, and the mixture was stirred and refluxed
for 1.0 h. After cooling to room temperature, the mixture was vacuum filtered and washed to remove unreacted components to generate a solid precipitate in 70% isolated yield. Second, 1.2 g
of the as-synthesized sample and a mixture of 1.0 mL of concentrated nitric acid and 0.5 mL of H2O were added to 10 mL of HAc. The mixture was stirred at 100 °C for 1 h and then poured into
100 mL of H2O after it was cooled. The precipitate was collected, washed, and dried, affording 0.41 g of BBQPH as a brown powder in 35% isolated yield. BBQPD as a reference was prepared
based on a simple reaction as shown in Supplementary Fig. 3. MATERIAL CHARACTERIZATIONS Fourier transform infrared (FT-IR) spectra were recorded with a Nicolet iS50. Raman spectra were
collected through an inVia Reflex Evolution confocal microscope (excitation wavelength of 532 nm). X-ray diffraction (XRD) tests were performed to investigate the crystallinity of BBQPH on
an Empyrean X-ray diffraction system by scanning in the 2_θ_ range of 5–80°. X-ray photoelectron spectroscopy (XPS) characterizations were performed on a scanning X-ray microprobe
(PHI5000Versa Probe ESCALAB 250xi). The calibration of the binding energy was performed at 284.8 eV relative to C1s. The morphology and microstructure were characterized by field-emission
scanning electron microscopy (SEM, Zeiss GeminiSEM450) equipped with energy-dispersive spectroscopy (EDS) for elemental analysis. High-resolution transmission electron microscopy (HRTEM)
observations were carried out on a JEM-2100F. UV‒vis spectrum characterization was performed on a Lambda950 in a range of 200–1200 nm to investigate the structural stability of the
electrodes. Nitrogen adsorption/desorption isotherms were collected on a JW-BK200C surface area analyser operating at −196 °C to obtain the pore size of BBQPH. ELECTRODE PREPARATION AND
MEASUREMENTS The BBQPD- and BBQPH-based electrodes for the general tests were prepared by mixing 70 wt% active materials, 20 wt% conductive carbon black (TIMCAL) and 10 wt% binders
(polytetrafluoroethylene (PTFE emulsion, 10 wt% in water) or poly(1,1-difluoroethylene) (PVDF, 10 wt% in N-methylpyrrolidone (NMP)) (Sinopharm Chemical Reagent Co., Ltd.). The BBQPH/PTFE
electrode was prepared by rolling and pressing it onto nickel foam (Φ = 12 mm), while the BBQPH/PVDF electrode was prepared by spreading the stock on carbon cloth (Φ = 12 mm). Then, the
electrodes were dried at 60 °C for 12 h under vacuum. An active material loading of 1.5 mg cm−2 was achieved. The zinc foil (0.03 mm thick) was sanded to remove the oxide layer on the
surface and then cut into a round sheet with a diameter of 16 mm as the zinc anode. Subsequently, CR-2032 coin cell batteries were assembled with the prepared electrode as the cathode, glass
fiber as the separator and Zn sheet as the counter electrode, and then, 100 μL of 3 M ZnSO4 (0.3 M ZnTFS/ACN) was used as the electrolyte. In the fabrication of the Zn//BBQPH pouch cell,
the prepared BBQPH powder was directly applied as the active cathode material. The active cathode materials were mixed with conductive carbon black and PVDF in an agate mortar at a ratio of
7:2:1 using N-methyl-2-pyrrolidone (NMP) as the solvent. The above slurry was then scratched onto a piece of carbon cloth (3 cm × 4 cm). The mass loading of the active material was 2.54 mg
cm−2. The typical weight of the Zn foil was 13 mg cm−2, and its thickness was ~30 μm. The electrolyte was a 3 M ZnSO4 aqueous solution. Then, the cathode, glass fiber (applied as the
separator, Whatman, GF/A), and anode Zn foil were assembled into a pouch cell for further investigation. The electrochemical performance of BBQPD and BBQPH was evaluated by galvanostatic
charge/discharge (GCD) tests on a LAND CT2001A at 25 °C, and cyclic voltammetry (CV) curves and electrochemical impedance spectroscopy (EIS) spectra were collected with an electrochemical
workstation (CHI 660E) within a potential of 0.2–1.6 V and a frequency range of 106-0.01 Hz, respectively. In the three-electrode system, the counter electrode was a Pt wire, and the
reference electrode was Hg/HgCl. All the current densities and specific capacities of the battery were based on the active mass of the cathode. DENSITY FUNCTIONAL THEORY CALCULATIONS AND
DYNAMICS SIMULATION The molecular orbital levels, including the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) levels, and the electrostatic
potential (ESP) of BBQPH/BBQPD were studied at the B3LYP-D3/TZVP level of theory via the Dmol3 suite of programs. A negative (blue color) ESP suggests electrophilic properties, while a
positive (red color) ESP denotes nucleophilic properties. Reduced density gradient (RDG) simulations were carried out by using the Multiwfn program, where the RDG value provides the
interaction strength, while the sign(_l_2)_r_ value shows the interaction types42. Large and negative values of sign(_l_2)_r_ are suggestive of H-bond interactions, and values near zero
indicate π-π stacking interactions43. The colored gradient isosurface map intuitively exhibits the interaction area and corresponding strength. VMD software was employed to plot the
color-filled isosurface graphs of molecular orbitals. The charge-storage mechanisms of BBQPD and BBQPH were investigated by using VASP. The SDD basis set was used for the Zn/H atom, and 6–31
G(d, p) was chosen for the C, H, O, and N atoms. Frequency calculations with the same methods and basis sets were performed to confirm a true local minimum and obtain the thermochemical
data at P = 1 atm and T = 298.15 K. To obtain theoretical insight into the charge storage process in BBQPH, density functional theory (DFT) calculations were carried out by using the Vienna
ab initio simulation package (VASP)44. The van der Waals (vdW) correction was considered by applying the DFT-D2 approach45. Projector-augmented wave (PAW) pseudopotentials were adopted with
an energy cutoff of 500 eV46. The generalized gradient approximation with the Perdew-Burke-Ernzerhof functional (GGA-PBE) was applied to describe the exchange-correlation functional.
Frequency calculations were performed with the same methods and basis sets to confirm a true local minimum and obtain the thermochemical data at P = 1 atm and T = 298.15 K. The Brillouin
zone was sampled with Gamma (Γ)-centered Monkhorst-Pack mesh sampling (1 × 1 × 1 for all isolated molecular structures, 2 × 2 × 2 for all bulk crystal structures) for geometry relaxation.
The convergence criteria of the residual Hellmann-Feynman force and energy during structure optimization of BBQPH were set to 0.02 eV Å−1 and 10−6 eV, respectively. BBQPH structures were
represented by a supercell with two periodic units. Transition states were searched for by the climbing image nudged-elastic-band (CI-NEB) method, with convergence to 0.03 eV/Å and < 1 ×
10−7 eV47. In addition, the visualization of the structure was performed by the VESTA package. Computational fluid dynamics (CFD) simulation was performed using COMSOL Multiphysics software
to untangle the fundamental mechanisms of the Zn2+ ion transport process in BBQPH. A flower-shaped model was constructed, which denotes the theoretical structure of BBQPH. The geometric
dimensions of BBQPH were obtained from Fig. 1d. The ion diffusion process within BBQPH can be calculated and simulated according to the Poisson‒Nernst‒Planck equations: $$\nabla \cdot
\left(D\nabla {c}_{i}+\frac{{{{{{\rm{D}}}}}}{z}_{i}{{{{{\rm{e}}}}}}}{T{k}_{B}}\nabla {{{{{\rm{V}}}}}}{c}_{i}\right)=0$$ (1) where D, c, z, e, T, and kB represent the diffusion coefficient
(10−9 cm2 s−1), Zn2+ concentration (3 mol/L), Zn2+ valence (2), elementary charge (1.6 × 10−19 C), absolute temperature (300 K), and Boltzmann constant (1.38 × 10−23 J/K), respectively. The
mentioned equations were solved in a time-dependent state to achieve a steady state. In this work, the densest conventional triangular meshes were utilized for all simulations on the surface
to pursue high accuracy of the calculation result. The MUMPS solver was used with a relative tolerance of 0.001. The volume fraction was normalized by the maximum volume fraction in the
center of the BBQPH model. EVALUATION OF THE ENERGY DENSITY According to the simplified pouch-cell configuration shown in Fig. 3h, the gravimetric energy density can be calculated based on
the parameters of the assembled pouch-cell battery using the following equation: $${E}_{g}=\frac{{VC}}{\sum {m}_{i}}$$ (2) where _E__g_ is the full-cell gravimetric energy density (Wh kg−1),
_V_ represents the average output voltage of the Zn//BBQPH battery (1.08 V is assumed), _C_ represents the areal capacity (mAh cm−2), and _m__i_ is the mass per unit square (mg cm−2) of the
pouch-cell components, including the anode (5 × Zn excess is assumed) and cathode. The energy density of the pouch cell can be calculated based on actual measurements. Therefore, the energy
density of the pouch cell based on active materials (BBQPH) reaches 330 Wh kg−1, which is higher than that in most literature reports on organic materials in ZOBs. Moreover, the energy
density of the pouch cell based on whole devices is ~24 Wh kg−1, which is mainly attributed to the high mass ratio of the zinc anode, diaphragm and electrolyte. DATA AVAILABILITY The
experiment data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper. REFERENCES * Lin, Z.
et al. A high capacity small molecule quinone cathode for rechargeable aqueous zinc-organic batteries. _Nat. Commun._ 12, 4424 (2021). Article ADS CAS PubMed PubMed Central Google
Scholar * Feng, D., Jiao, Y. & Wu, P. Proton-reservoir hydrogel electrolyte for long-term cycling Zn/PANI batteries in wide temperature range. _Angew. Chem. Int. Ed._ 62, e202215060
(2023). Article CAS Google Scholar * Qiu, X. et al. A high-voltage Zn-organic battery using a nonflammable organic electrolyte. _Angew. Chem. Int. Ed._ 133, 21193–21200 (2021). Article
ADS Google Scholar * Wang, N. et al. Zinc-organic battery with a wide operation-temperature window from -70 to 150 oC. _Angew. Chem. Int. Ed._ 59, 14577–14583 (2020). Article CAS Google
Scholar * Zhang, H., Fang, Y., Yang, F., Liu, X. & Lu, X. Aromatic organic molecular crystal with enhanced π–π stacking interaction for ultrafast Zn-ion storage. _Energy Environ. Sci._
13, 2515–2523 (2020). Article CAS Google Scholar * Lu, Y. & Chen, J. Prospects of organic electrode materials for practical lithium batteries. _Nat. Rev. Chem._ 4, 127–142 (2020).
Article CAS PubMed Google Scholar * Ye, Z. et al. High-rate aqueous zinc-organic battery achieved by lowering HOMO/LUMO of organic cathode. _Energy Storage Mater._ 37, 378–386 (2021).
Article Google Scholar * Yang, W. et al. Hydrated eutectic electrolytes with ligand-oriented solvation shells for long-cycling zinc-organic batteries. _Joule_ 4, 1557–1574 (2020). Article
CAS Google Scholar * Peng, C. et al. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. _Nat. Energy_ 2, 1–9 (2017).
Article Google Scholar * Xiang, Z., Li, W., Wan, K., Fu, Z. & Liang, Z. Aggregation of electrochemically active conjugated organic molecules and its impact on aqueous organic redox
flow batteries. _Angew. Chem. Int. Ed._ 62, e202214601 (2023). Article CAS Google Scholar * Peng, X. et al. Heterocyclic conjugated polymer nanoarchitectonics with synergistic
redox-active sites for high-performance aluminium organic battery. _Angew. Chem. Int. Ed._ 25, e202203646 (2022). ADS Google Scholar * Wang, S. et al. A fully conjugated 3D covalent
organic framework exhibiting band-like transport with ultrahigh electron mobility. _Angew. Chem. Int. Ed._ 60, 9321–9325 (2021). Article CAS Google Scholar * Yu, M., Dong, R. & Feng,
X. Two-dimensional carbon-rich conjugated frameworks for electrochemical energy applications. _J. Am. Chem. Soc._ 142, 12903–12915 (2020). Article CAS PubMed Google Scholar * Zhang, C.
et al. Tailoring the linking patterns of polypyrene cathodes for high-performance aqueous Zn dual-ion batteries. _Energy Environ. Sci._ 14, 462–472 (2021). Article CAS Google Scholar *
Nam, K. W. et al. Redox-active phenanthrenequinone triangles in aqueous rechargeable zinc batteries. _J. Am. Chem. Soc._ 142, 2541–2548 (2020). Article CAS PubMed Google Scholar * Wang,
X. et al. Advances and perspectives of cathode storage chemistry in aqueous zinc-ion batteries. _ACS Nano_ 15, 9244–9272 (2021). Article CAS PubMed Google Scholar * Cui, H. et al.
High-voltage organic cathodes for zinc-ion batteries through electron cloud and solvation structure regulation. _Angew. Chem. Int. Ed._ 61, e202203453 (2022). Article ADS CAS Google
Scholar * Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. _Nat. Energy_ 6, 495–505 (2021). Article
ADS CAS Google Scholar * Zhang, L., Wang, H., Zhang, X. & Tang, Y. A review of emerging dual‐ion batteries: fundamentals and recent advances. _Adv. Funct. Mater._ 31, 2010958 (2021).
Article CAS Google Scholar * Jia, C. et al. Redox control of charge transport in vertical ferrocene molecular tunnel junctions. _Chem._ 6, 1172–1182 (2020). Article CAS Google Scholar
* He, Q., Yu, B., Li, Z. & Zhao, Y. Density functional theory for battery materials. _Energy Environ. Mater._ 2, 264–279 (2019). Article CAS Google Scholar * Ma, Q. et al. Single
lithium-ion conducting polymer electrolytes based on a super-delocalized polyanion. _Angew. Chem. Int. Ed._ 55, 2521–2525 (2016). Article CAS Google Scholar * Chen, Y. et al.
Two-dimensional organic supramolecule via hydrogen bonding and pi-pi stacking for ultrahigh capacity and long-life aqueous zinc-organic batteries. _Angew. Chem. Int. Ed._ 61, e202116289
(2022). Article ADS CAS Google Scholar * Shi, R. et al. Nitrogen-rich covalent organic frameworks with multiple carbonyls for high-performance sodium batteries. _Nat. Commun._ 11, 178
(2020). Article ADS CAS PubMed PubMed Central Google Scholar * Song, Z. et al. Anionic co-insertion charge storage in dinitrobenzene cathodes for high-performance aqueous zinc-organic
batteries. _Angew. Chem. Int. Ed._ 61, e202208821 (2022). Article CAS Google Scholar * Li, W. et al. Multistage self‐assembly engineered mesoporous conjugated polymer with isomeric
nanoarchitecture towards superior performance Li-S batteries. _Energy Storage Mater._ 53, 183–191 (2022). Article Google Scholar * Wu, Y. et al. 2D Molecular sheets of hydrogen-bonded
organic frameworks for ultrastable sodium-ion storage. _Adv. Mater._ 33, e2106079 (2021). Article PubMed Google Scholar * Lin, L. et al. A semi-conductive organic cathode material enabled
by extended conjugation for rechargeable aqueous zinc batteries. _Energy Environ. Sci._ 16, 89–96 (2023). Article CAS Google Scholar * Yang, X. et al. Mesoporous polyimide-linked
covalent organic framework with multiple redox-active sites for high-performance cathodic Li storage. _Angew. Chem. Int. Ed._ 61, e202207043 (2022). Article ADS CAS Google Scholar * Sun,
T. et al. Poly(2,5‐dihydroxy‐1,4‐benzoquinonyl sulfide) as an efficient cathode for high‐performance aqueous zinc-organic batteries. _Adv. Funct. Mater._ 31, 2010049 (2021). Article CAS
Google Scholar * Deng, X. et al. Proton storage chemistry in aqueous zinc‐organic batteries: a review. _InfoMat_ 5, e12382 (2022). Article Google Scholar * Yue, F. et al. An ultralow
temperature aqueous battery with proton chemistry. _Angew. Chem. Int. Ed._ 60, 13882–13886 (2021). Article CAS Google Scholar * Yang, Q. et al. Activating C-coordinated iron of iron
hexacyanoferrate for Zn hybrid-ion batteries with 10 000-cycle lifespan and superior rate capability. _Adv. Mater._ 31, e1901521 (2019). Article PubMed Google Scholar * Gao, H. et al. A
pyrene-4,5,9,10-tetraone-based covalent organic framework delivers high specific capacity as a li-ion positive electrode. _J. Am. Chem. Soc._ 144, 9434–9442 (2022). Article CAS PubMed
PubMed Central Google Scholar * Xiang, L. et al. Porous polymer cubosomes with ordered single primitive bicontinuous architecture and their sodium-iodine batteries. _J. Am. Chem. Soc._
144, 15497–15508 (2022). Article CAS PubMed Google Scholar * Peng, H. et al. N-heterocycles extended π-conjugation enables ultrahigh capacity, long-lived, and fast-charging organic
cathodes for aqueous zinc batteries. _CCS Chem._ 5, 1789–1801 (2023). * Ma, S. et al. Iodine-doped sulfurized polyacrylonitrile with enhanced electrochemical performance for lithium sulfur
batteries in carbonate electrolyte. _Chem. Eng. J._ 418, 129410 (2021). Article CAS Google Scholar * Tie, Z., Liu, L., Deng, S., Zhao, D. & Niu, Z. Proton insertion chemistry of a
zinc-organic battery. _Angew. Chem. Int. Ed._ 59, 4920–4924 (2020). Article CAS Google Scholar * Wan, F. et al. An aqueous rechargeable zinc-organic battery with hybrid mechanism. _Adv.
Funct. Mater._ 28, 1804975 (2018). Article Google Scholar * Schutt, K. T., Sauceda, H. E., Kindermans, P. J., Tkatchenko, A. & Muller, K. R. Schnet-a deep learning architecture for
molecules and materials. _J. Chem. Phys._ 148, 241722 (2018). Article ADS CAS PubMed Google Scholar * Zeglis, B. M. & Barton, J. K. DNA base mismatch detection with bulky rhodium
intercalators: synthesis and applications. _Nat. Protoc._ 2, 357–371 (2007). Article CAS PubMed PubMed Central Google Scholar * Lu, T. & Chen, F. Multiwfn: a multifunctional
wavefunction analyzer. _J. Comput. Chem._ 33, 580–592 (2012). Article PubMed Google Scholar * Song, Z. et al. Proton-conductive supramolecular hydrogen-bonded organic superstructures for
high-performance zinc-organic batteries. _Angew. Chem. Int. Ed._ 135, 202219136 (2023). Article Google Scholar * Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy
calculations for metals and semiconductors using a plane-wave basis set. _Comput. Mater. Sci._ 6, 15–50 (1996). Article CAS Google Scholar * Grimme, S., Antony, J., Ehrlich, S. &
Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. _J. Chem. Phys._ 132, 154104 (2010). Article ADS
PubMed Google Scholar * Blöchl, P. E. Projector augmented-wave method. _Phys. Rev. B_ 50, 17953 (1994). Article ADS Google Scholar * Henkelman, G., Uberuaga, B. P. & Jónsson, H. A
climbing image nudged elastic band method for finding saddle points and minimum energy paths. _J. Chem. Phys._ 113, 9901–9904 (2020). Article ADS Google Scholar Download references
ACKNOWLEDGEMENTS W.L. and H.X. contributed equally to this work. This work was financially supported by the National Natural Science Foundation of China (Grant No. 51773062 and 61831021).
The authors also thank ECNU Multifunctional Platform for Innovation (004) for material characterizations. We also thank Xinyi Zhang for supporting us in testing and analysis. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Precision Spectroscopy; Engineering Research Center of Nanophotonics & Advanced Instrument (Ministry of Education), School
of Physics and Electronic Science, East China Normal University, Shanghai, 200241, P.R. China Wenda Li, Hongyi Zhang, Facai Wei, Lingyan Huang, Shanzhe Ke, Chengbin Jing & Shaohua Liu *
Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, 518055, P.R. China Hengyue Xu * School of Materials Science and Engineering, Zhengzhou University, 75 Daxue
Road, Zhengzhou, 450052, P. R. China Jianwei Fu * State Key Lab of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai,
200050, P. R. China Jiangong Cheng Authors * Wenda Li View author publications You can also search for this author inPubMed Google Scholar * Hengyue Xu View author publications You can also
search for this author inPubMed Google Scholar * Hongyi Zhang View author publications You can also search for this author inPubMed Google Scholar * Facai Wei View author publications You
can also search for this author inPubMed Google Scholar * Lingyan Huang View author publications You can also search for this author inPubMed Google Scholar * Shanzhe Ke View author
publications You can also search for this author inPubMed Google Scholar * Jianwei Fu View author publications You can also search for this author inPubMed Google Scholar * Chengbin Jing
View author publications You can also search for this author inPubMed Google Scholar * Jiangong Cheng View author publications You can also search for this author inPubMed Google Scholar *
Shaohua Liu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.L. and W.L. proposed and designed the idea. W.L. performed the experimental
synthesis and conducted the electrochemical measurements. H.X. performed the DFT calculation. H.Z., L.H., and S.K. assisted with the data analysis and characterization. W.L. wrote the paper.
F.W. contributed to the discussion and electrochemical measurements. J.F., C.J., and J.C. contributed to the discussion. All the authors analyzed the results and commented on the
manuscript. CORRESPONDING AUTHOR Correspondence to Shaohua Liu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION
_Nature Communications_ thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE
SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Li, W., Xu, H., Zhang, H. _et al._ Tuning electron delocalization of hydrogen-bonded organic framework cathode for high-performance zinc-organic batteries. _Nat Commun_ 14,
5235 (2023). https://doi.org/10.1038/s41467-023-40969-5 Download citation * Received: 17 April 2023 * Accepted: 18 August 2023 * Published: 28 August 2023 * DOI:
https://doi.org/10.1038/s41467-023-40969-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
