Multi-ancestry gwas meta-analyses of lung cancer reveal susceptibility loci and elucidate smoking-independent genetic risk
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Lung cancer remains the leading cause of cancer mortality, despite declining smoking rates. Previous lung cancer GWAS have identified numerous loci, but separating the genetic risks
of lung cancer and smoking behavioral susceptibility remains challenging. Here, we perform multi-ancestry GWAS meta-analyses of lung cancer using the Million Veteran Program cohort
(approximately 95% male cases) and a previous study of European-ancestry individuals, jointly comprising 42,102 cases and 181,270 controls, followed by replication in an independent cohort
of 19,404 cases and 17,378 controls. We then carry out conditional meta-analyses on cigarettes per day and identify two novel, replicated loci, including the 19p13.11 pleiotropic cancer
locus in squamous cell lung carcinoma. Overall, we report twelve novel risk loci for overall lung cancer, lung adenocarcinoma, and squamous cell lung carcinoma, nine of which are externally
replicated. Finally, we perform PheWAS on polygenic risk scores for lung cancer, with and without conditioning on smoking. The unconditioned lung cancer polygenic risk score is associated
with smoking status in controls, illustrating a reduced predictive utility in non-smokers. Additionally, our polygenic risk score demonstrates smoking-independent pleiotropy of lung cancer
risk across neoplasms and metabolic traits. SIMILAR CONTENT BEING VIEWED BY OTHERS ASSOCIATION OF SMOKING AND POLYGENIC RISK WITH THE INCIDENCE OF LUNG CANCER: A PROSPECTIVE COHORT STUDY
Article Open access 22 February 2022 CROSS-ANCESTRY GENOME-WIDE META-ANALYSIS OF 61,047 CASES AND 947,237 CONTROLS IDENTIFIES NEW SUSCEPTIBILITY LOCI CONTRIBUTING TO LUNG CANCER Article 01
August 2022 A COMPREHENSIVE META-ANALYSIS AND A CASE–CONTROL STUDY GIVE INSIGHTS INTO GENETIC SUSCEPTIBILITY OF LUNG CANCER AND SUBGROUPS Article Open access 16 July 2021 INTRODUCTION Lung
cancer remains the leading cause of overall cancer mortality, as the most prevalent cancer type in men, and the second highest in women after breast cancer1,2,3. Despite declines in smoking
rates in the US since the 1980s4, tobacco use is currently implicated in upwards of 80% of lung cancer diagnoses1. Even in those who have never smoked, nor had meaningful exposure to
environmental carcinogens1,5, there exists a heritable risk component of lung cancer conferred by genetic factors6,7,8. Differentiating the mutations that directly predispose an individual
to lung cancer from those whose effect is mediated through environmental components remains challenging. Genome-wide association studies (GWAS) have identified lung cancer risk variants
associated with oncogenic processes such as immune response7, cell cycle regulation9, and those affecting DNA damage response and genomic stability8. Several lung cancer GWAS have also
reported strong effects of genes such as _CHRNA_ nicotine receptor genes which putatively increase the risk of lung cancer through behavioral predisposition towards smoking5. Characteristic
molecular markers and genetic risk factors in smokers and never-smokers have been identified10,11, though fewer variants have been found in GWAS performed exclusively in never-smokers12.
Lung cancer has a heterogeneous genetic architecture across ancestral groups13,14. In the two most well-studied ancestries, European (EA) and East Asian (EAS), the majority of genome-wide
significant loci are not shared15,16; this is in agreement with molecular studies showing differences in tumor characteristics between EA and EAS17. Smaller African ancestry (AA) cohorts
have replicated known loci from EA or EAS8,18, though no AA-specific GWAS loci have been reported. In this study, we examined lung cancer genetic variation in EA as well as in the largest AA
cohort to date. Our discovery analysis is performed in an older cohort of mostly male US veterans in the Department of Veterans Affairs Million Veteran Program (MVP)19. Lung cancer
incidence is approximately twice as high in men than in women2, and additionally, MVP contains a large number of cigarette smokers, positioning this biobank as particularly valuable for
these analyses. We performed GWAS in overall cases of lung cancer as well as two non-small cell lung cancer (NSCLC) subtypes, adenocarcinoma (LUAD) and squamous cell lung carcinoma (LUSC).
RESULTS GENOME-WIDE ASSOCIATION STUDIES FOR LUNG CANCER We performed a GWAS on overall lung cancer within EA participants in MVP (10,398 lung cancer cases and 62,708 controls; Supplementary
Data 1), followed by a meta-analysis with the EA International Lung Cancer Consortium OncoArray study (ILCCO)7, for a total of 39,781 cases and 119,158 controls (Supplementary Fig. 1). The
EA meta-analysis for overall lung cancer identified 26 conditionally independent SNPs within 17 genome-wide significant loci (_P_ < 5 × 10−8; Supplementary Fig. 2a; Supplementary Data 2).
All 12 loci reported by ILCCO7 were confirmed, with consistent direction of effect in all single nucleotide polymorphisms (SNPs) with _P_ < 1 × 10−5, as well as high correlation of
effect sizes and allele frequency (Supplementary Fig. 3). Of the 17 genome-wide significant loci for overall lung cancer, four were novel with respect to the broader literature: neuronal
growth regulator _LSAMP_, Wnt signaling regulator _NMUR2_, DNA damage repair protein _XCL2_, and hedgehog signaling regulator _TULP3_, (Table 1; Supplementary Fig. 4a–d). Further association
tests stratified by cancer subtypes LUAD and LUSC in MVP EA (Supplementary Fig. 2bc; Supplementary Data 3, 4) replicated associations reported by ILCCO7 (Supplementary Fig. 3) and
identified additional loci. Two novel EA meta-analysis loci were identified for LUAD, proto-oncogene _MYC_, and Wnt signaling inhibitor _TLE3_ (Table 1; Supplementary Fig. 4e–h). For LUSC,
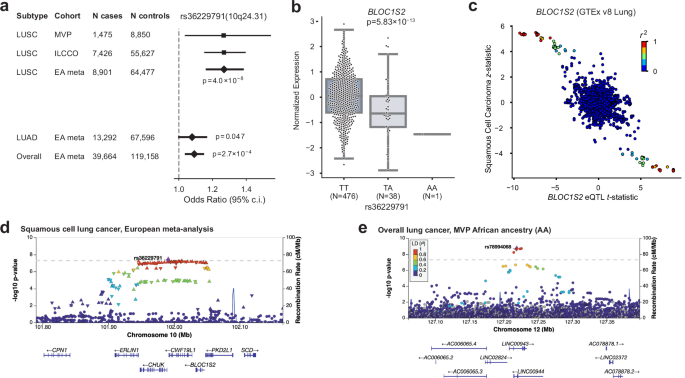
we identified one novel locus at 10q24.31 near NFκB inhibitor _CHUK_ and _BLOC1S2_. Across all subtypes for EA meta-analysis index variants, the MVP cohort had associations with _P_ <
0.05 in all but one in overall lung cancer, five in LUAD, including approximately nominal significance at rs67824503 (_MYC; P_ = 0.057), and one in LUSC (Supplementary Data 2–4). We
investigated expression quantitative trait loci (eQTL) relationships between top SNPs from the EA meta-analysis across all lung cancer GWAS in GTEx v8 Lung20 and the Lung eQTL Consortium21
(Supplementary Data 2–4). This analysis showed that the LUSC index SNP rs36229791 on 10q24.31 was associated with the mRNA expression levels of _BLOC1S2_ (Fig. 1a–d), consistent with
previous TWAS22. _BLOC1S2_ is an oncogene whose gene product is associated with centrosome function; centrosomal abnormalities have previously been observed in vitro in LUSC23,24. We
improved our variant selection by fine-mapping and estimating credible sets of candidate causal variants in the EA meta-analysis using sum of single effects (SuSiE)25,26 modeling. For
overall lung cancer, LUAD, and LUSC, we identified 23, 23, and 9 high-quality credible sets, respectively, containing 370, 246, and 192 total SNPs (Supplementary Data 5). GWAS IN AA We
analyzed overall lung cancer risk in 2438 cases and 62,112 controls of African ancestry (AA), the largest AA GWAS discovery cohort to date (Supplementary Fig. 5a). Two loci reached
genome-wide significance in our discovery scan: 15q25, replicating the association in _CHRNA5_ for AA populations reported by an earlier GWAS18, and a putative novel locus at 12q23 with
index SNP rs78994068 (Table 1; Fig. 1e). We further performed GWAS in AA within LUAD and LUSC subtypes but found no genome-wide significant associations (Supplementary Fig. 5b, c). The
putative AA locus at 12q23 is driven by six SNPs in high linkage disequilibrium (LD; _R__2_ > 0.8) found in long non-coding RNAs _LINC00943_ and _LINC00944_ (Fig. 1e). These imputed SNPs
all had odds ratios (ORs) close to 2, with 1.3% frequency in AA and 0% in EA, consistent with gnomAD v3. _LINC00944_ is highly expressed in immune cells and enriched in T cell pathways in
lung tissue and cancer27,28,29,30. We fine-mapped this locus to define a 95% credible set (Supplementary Data 6) and annotated the functional consequence of the variants using the Variant
Effect Predictor (VEP)31. Two variants, rs78994068, and rs115962601, were in a known enhancer regulatory region (ENSR00000974920) and thus may involve regulatory changes. However, this locus
was directionally consistent but not significant in our AA replication cohort (discussed below); therefore, larger-scale AA analyses are needed to confirm this finding. GWAS MULTI-ANCESTRY
META-ANALYSIS We conducted fixed-effect inverse variance-weighted multi-ancestry meta-analyses, combining the EA meta-analysis and the MVP AA GWAS for overall lung cancer, LUAD, and LUSC
(Supplementary Data 7–9; Supplementary Fig. 6a–c). These analyses identified two additional novel genome-wide significant loci in overall lung cancer (Table 1; Supplementary Data 10;
Supplementary Fig. 4i, j): ubiquitin ligase _JADE2_, previously associated with smoking initiation32, and RNA polymerase-associated _RPAP3_. Neither of these novel multi-ancestry
meta-analysis loci were reported in a recent multi-ancestry analysis by Byun et al.8 that included fewer AA and more EAS samples, indicating the value our larger AA sample provided for novel
discovery. All genome-wide significant EA meta-analysis associations reached genome-wide significance in the multi-ancestry meta-analyses except rs11855650 (_TLE3_) in LUAD (_P_ = 6.19 ×
10−8). We additionally performed random-effects meta-analyses using the Han-Eskin method (RE2)33 and observed similar _P_-values to the fixed effect meta-analyses, with all index variants
_P_RE2 < 5 × 10−8 (Supplementary Data 7–9). POLYGENIC RISK SCORING To gain an understanding of the penetrance and pleiotropy of lung cancer risk, we constructed polygenic risk scores
(PRSs) based on the ILCCO summary statistics7 for every EA subject in MVP. As expected, the PRS was highly associated with both lung cancer risk as well as smoking behavior (Supplementary
Fig. 7a, b). Even after removing individuals with any history of lung cancer risk to prevent the enrichment of risk factors and comorbidities, the association with smoking behavior remained,
suggesting that the PRS is partially capturing genetic smoking behavioral risk factors (Supplementary Fig. 7c). In all groups, individuals at the top decile of the PRS were at significantly
higher risk of lung cancer than those in the lowest decile. MULTI-TRAIT CONDITIONAL ANALYSIS FOR SMOKING STATUS Despite adjusting for smoking status, both in MVP EA and ILCCO7, a
significant genetic correlation was observed between all subsets of lung cancer GWAS and a recently published GWAS of smoking behaviors34 (Fig. 2a, Supplementary Data 11). In order to remove
all residual effects of smoking on lung cancer susceptibility, we conducted a multi-trait-based conditional and joint analysis (mtCOJO)35,36, conditioning on a GWAS for cigarettes per
day34, which was the smoking trait most strongly correlated with overall lung cancer and subtype GWAS from the EA meta-analysis. Because lung cancer case selection also preferentially
selects smokers, conventional adjustment for smoking may inadvertently cause selection bias, which functions as a collider to induce biased genetic effects37. mtCOJO is considered more
robust to potential collider bias than conventional covariate adjustment35,36. The total observed-scale SNP-heritability38 of lung cancer risk decreased substantially after conditioning on
cigarettes per day, from 5.4% to 3.1% in overall LC, from 6.7% to 5.5% in LUAD, and from 5.8% to 3.8% in LUSC (Fig. 2b; Supplementary Data 12). Significant loci from the conditional analyses
are shown in Supplementary Figs. 8 and 9 and Supplementary Data 13–15. As expected, the statistical significance of loci harboring smoking-related genes (e.g., _CHRNA5_, _CYP2A6_, _CHRNA4_)
dropped to below genome-wide significance after conditioning (Fig. 3). Conversely, five signals (four loci) became significant only after conditioning, including novel signals at _MMS22L_
in overall lung cancer and 19p13.1 (_ABHD8_) in LUSC. _MMS22L_ is a novel GWAS signal but was previously identified as overexpressed in lung cancer in a genome-wide gene expression scan39.
These may represent biological lung cancer signals partially masked by countervailing genetic effects on smoking behavior. We performed fine-mapping to identify candidate causal variants in
the conditioned EA meta-analysis summary statistics, and for overall lung cancer, LUAD, and LUSC, we identified 11, 15, and 6 high-quality credible sets, respectively, containing a total of
243, 277, and 78 SNPs (Supplementary Data 5). We constructed PRS based on mtCOJO-conditioned ILCCO summary statistics7 to directly compare the predictive performance of PRS derived from the
conditioned and non-conditioned GWAS in MVP EA. While the PRS based on the non-conditioned overall lung cancer GWAS exhibited reduced performance in never-smokers compared to ever-smokers,
the PRS based on the conditional analysis resulted in similar performance across smoking status (Fig. 2c; Supplementary Data 16). REPLICATION OF NOVEL VARIANTS AND COMBINED META-ANALYSIS We
queried the OncoArray Consortium Lung Study (OncoArray)8,40 as an external non-overlapping replication dataset for our significant GWAS signals (Supplementary Data 1, 17, and 18). For GWAS
in the EA meta-analyses for overall lung cancer, LUAD, and LUSC, we replicated five of seven novel loci (_P_ < 0.01) in an OncoArray European ancestry cohort: _XCL2_ and _TLE3_ in overall
lung cancer, _MYC_ and _TLE3_ in LUAD, and _BLOC1S2_ in LUSC. The novel African ancestry association for overall lung cancer at _LINC00944_ was not replicated. We meta-analyzed OncoArray
European and African ancestry participants to replicate our multi-ancestry meta-analysis signals (Supplementary Fig. 10, Supplementary Data 18) for overall lung cancer at _RPAP3_ (_P_ =
0.0044) and _JADE2_ which bordered on nominal significance (rs329122; _P_ = 0.053). For the two novel loci that were identified in the EA meta-analysis conditioned on cigarettes per day, we
included smoking as a covariate for association analysis in the OncoArray European ancestry cohort. These association signals were replicated for overall lung cancer at _MMS22L_ (_P_ =
0.006) and LUSC at _ABHD8_ (_P_ = 0.003). In a variant-level replication of 137 conditionally independent discovery associations that fell within ≤1 Mb of a previously reported lung cancer
GWAS signal, 134 had _P_ < 0.05 in OncoArray, and 42 had _P_ < 5 × 10−8 (Supplementary Data 19). We then performed a combined meta-analysis of our discovery results with OncoArray
replication results (Supplementary Data 19). We considered a conservative threshold of _P_ < 4.17 × 10−9 (_P_ < 5 × 10−8/12 total GWAS analyses) to be significant, which was met by 9
of the 12 loci. Because rs329122 in _JADE2_ achieved the more conservative significance threshold (_P_ = 3.69 × 10−9), and has also been associated with smoking behavior32 and identified as
a splicing-related variant associated with lung cancer41, we considered this locus to be replicated. In the combined meta-analysis we observed similar _P_-values in fixed effects and random
effects (RE2) models. Next, for all previously reported lung cancer and subtype loci in this study, we identified lung cancer associations from the GWAS Catalog which fell within the same
loci as our index variants (Supplementary Data 20). We confirmed two loci that previously had been reported only in a recent genome-wide association by proxy (GWAx) of lung cancer42: _CENPC_
(rs75675343) in overall lung cancer in the EA meta-analysis (_P_ = 2.40 × 10−8) and the multi-ancestry meta-analysis, and _TP53BP1_ in overall lung cancer in the multi-ancestry
meta-analysis (rs9920763; _P_ = 1.63 × 10−8). Our multi-ancestry meta-analysis for overall lung cancer also confirmed a recently reported locus at 4q32.2 (_NAF1_)15 in EAS. MULTI-TRAIT
ANALYSIS WITH BREAST CANCER At 19p13.1, a known pleiotropic cancer locus43,44, the index SNP of LUSC conditioned on smoking (rs61494113) sits in a gene-rich region where a recent
fine-mapping effort of breast cancer risk loci45 proposed two independent associations, one affecting the regulation of _ABHD8_ and _MRPL34_, and another causing a coding mutation in
_ANKLE1_. Here, we used the increased power provided by a multi-trait analysis of GWAS (MTAG)46 of LUSC and estrogen receptor negative (ER−) breast cancer47 to disentangle the complex
relationships between cancer risk and the genes in this locus (Fig. 4a). Overexpression of _ABHD8_ has been shown to significantly reduce cell migration43,44. Similar odds ratios at
rs61494113 were observed across LUSC and breast cancer, and MTAG enhanced the GWAS signal at this locus (Fig. 4b). We used the coloc-SuSiE method48 to assess colocalized associations between
pairs of credible sets in this locus underlying the risk of LUSC and ER− breast cancer, allowing for multiple causal signals. We found evidence for a shared causal signal between credible
sets in the LUSC conditional meta-analysis and ER− breast cancer (97.7% posterior probability; Supplementary Data 21). The index SNPs for the credible sets of LUSC conditioned on smoking and
ER− breast cancer (rs61494113 and rs56069439, respectively) have _r__2_ = 0.99. The eQTL effect of _ABHD8_ was replicated in multiple tissues of GTEx v8, including Lung (Fig. 4c).
Interestingly, the group of SNPs in the LUSC-BC credible set did not have the most significant eQTL effect, suggesting a complex relationship between the multiple causal variants at the
locus and gene expression (Fig. 4d). For instance, a recent splice variant analysis49 implicated splicing of _BABAM1_ (a BRCA1-interacting protein) as a culprit of the associations observed
in 19p13.1. Consistent with previous reports43,44, the cancer risk-increasing haplotype was correlated with increased expression of _ABHD8_ and alternative splicing of _BABAM1_. However,
there was no overlap between the 95% eQTL credible sets of _ABHD8_ and _BABAM1_, and neither of the credible sets included rs61494113. PHENOME-WIDE ASSOCIATION STUDY Finally, to investigate
the pleiotropy of lung cancer genetic risk in the absence of the overwhelming effect of smoking behavior, we performed phenome-wide association studies (PheWAS) in MVP using the PRS scores
constructed from the ILCCO summary statistics7 for overall lung cancer, both based on the standard GWAS (“unconditioned PRS”; Fig. 5a; Supplementary Data 22) and the GWAS conditioned on
cigarettes per day using mtCOJO (“conditioned PRS”; Fig. 5b; Supplementary Data 23). Each PRS was tested for association with 1772 phecode-based phenotypes. Overall, 240 phenotypes were
associated with the unconditioned PRS and 112 were associated with the conditioned PRS at a Bonferroni-corrected significance threshold (_P_ < 0.05/1772). Although lung cancer remained a
top association with the conditioned PRS, the association with tobacco use disorder was greatly reduced, from an OR associated with a standard deviation increase in the PRS of 1.151
[1.142–1.160] (_P_ = 2.32 × 10−237) in the unconditioned PRS to OR = 1.046 [1.038–1.053] (_P_ = 1.05 × 10−32) in the conditioned PRS. However, the effect on alcohol use disorder was only
modestly attenuated between the unconditioned (OR = 1.098 [1.089–1.108]; _P_ = 1.05 × 10−87) and conditioned LC (OR = 1.078 [1.069–1.088], _P_ = 4.41 × 10−60) PRSs. Whether a role for
alcohol in lung cancer exists independently of smoking is controversial50,51; this analysis suggests that may be the case. Other putatively smoking-related associations, such as chronic
obstructive pulmonary disease, pneumonia, and peripheral vascular disease were greatly diminished with the conditioned PRS. Mood disorders, depression, and post-traumatic stress disorder
were also significantly associated with the unconditioned PRS but no longer significantly associated with the conditioned PRS, reflecting neuropsychiatric correlates of smoking behavior.
Intriguingly, a category of metabolic traits that were not associated with the unconditioned PRS was highly associated with the conditioned PRS and in a negative effect direction. We
observed protective associations of the conditioned PRS with metabolic traits such as type 2 diabetes (OR = 0.945 [0.938–0.952], _P_ = 9.46 × 10−52) and obesity (OR = 0.952 [0.945–0.959],
_P_ = 2.48 × 10−41). Neither were associated with the unconditioned PRS (OR = 1.006 [0.999–1.014]; _P_ = 0.092, and OR = 1.005 [0.998–1.012]; _P_ = 0.183, respectively). Other traits in this
category included sleep apnea and hyperlipidemia. These findings are consistent with prior observational findings of an inverse relationship between BMI and lung cancer52 and illustrate the
extent to which smoking may be a major confounder of this relationship. Finally, we observed strong associations of the lung cancer PRS with skin cancer and related traits, such as actinic
keratitis. In basal cell carcinoma, the OR increased from 1.087 [1.072–1.102] (_P_ = 6.06 × 10−32) with the unconditioned PRS to 1.105 [1.090–1.120] (_P_ = 1.82 × 10−47) with the conditioned
PRS. As a sensitivity analysis, we tested the strength of this association after removing the _TERT_ locus, which is prominently associated with both traits. Doing so only modestly reduced
the effect of the conditioned PRS to OR = 1.092 [1.077–1.107] (_P_ = 4.08 × 10−36). Thus, our results are consistent with a genome-wide genetic correlation between lung cancer and basal cell
carcinoma that is strengthened when the effect of smoking is removed. Overall, our results suggest that the biology underlying lung cancer risk may be partially masked by the residual
genetic load of smoking. DISCUSSION We identified novel lung cancer-associated loci in a new cohort of EA and AA participants, including the largest AA cohort analyzed to date. We also show
that, despite studies on the genetic basis of lung cancer risk taking smoking status into account, the effects of smoking continue to obfuscate our understanding of lung cancer genetics. In
particular, we report two novel loci, at _MMS22L_ (overall) and _ABHD8_ (LUSC), which may be partially masked by countervailing genetic effects on smoking. Our replication analysis which
adjusted for smoking pack-years confirmed these loci. Additionally, our analyses demonstrated that PRSs for lung cancer contain large uncorrected genetic loading for smoking behavioral
factors. Our results indicate that controlling for these factors can improve risk assessment models, potentially improving lung cancer screening even for non-smokers. Finally, our phenomic
scans comparing PRSs derived from GWAS with and without genomic conditioning on smoking showed divergent associations across numerous traits, especially metabolic phenotypes. The increased
sample size in this study enabled the interpretation of multiple causal variants underlying the gene-rich _ADHL8_-_BABAM1_ region, synthesizing prior observations into a clearer
understanding of this locus. Our other novel loci strengthen established lung cancer mechanisms. We identify for the first time a susceptibility locus at _MYC_, a well-known oncogene and
master immune regulator. _XCL2_ is involved in cellular response to inflammatory cytokines53. _LSAMP_ is a tumor suppressor gene in osteosarcoma54, and 3q13.31 homozygous deletions have been
implicated in tumorigenesis55. _TLE3_ is a transcriptional corepressor involved in tumorigenesis and immune function56. The transcription factor _TULP3_ has been implicated in pancreatic
ductal adenocarcinoma and colorectal cancer57. _XCL2_, _NMUR2_, and _TULP3_ may also be related to cancer progression via G-protein-coupled receptor (GPCR) signaling pathways58. _JADE2_
expression has been experimentally linked to NSCLC59 and has been identified in GWAS of smoking behavior34. Finally, DNA damage repair genes are implicated, including _RPAP3_, an RNA
polymerase that may be involved in DNA damage repair regulation60, and _MMS22L_ which repairs double-strand breaks61. Although smoking is the major risk factor for lung cancer, it is
important to clearly disentangle the effect of smoking to fully understand the complex genetic and environmental causes of the disease. Our approach enables the development of new polygenic
scores, which can improve precision medicine applications for lung cancer in both smokers and nonsmokers. METHODS ETHICS/STUDY APPROVAL The VA Central Institutional Review Board (IRB)
approved the MVP000 study protocol. Informed consent was obtained from all participants, and all studies were performed with approval from the IRBs at participating centers, in accordance
with the Declaration of Helsinki. Only previously generated data were analyzed in this study. COHORT DEFINITION Patients were identified from MVP participants19 utilizing clinical
information available through the United States Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) with ICD codes for primary lung cancer. Occurrences of the ICD-9 codes
162.3, 162.4, 162.5, 162.8, and 162.9 or the ICD-10 codes C34.10, C34.11, C34.12, C34.2, C34.30, C34.31, C34.32, C34.80, C34.81, C34.82, C34.90, C34.91, and C34.92 were used in case
identification. Patients with secondary lung cancer were excluded from the cohort using ICD-9/10 codes 197.x, C78.00, C78.01, and C78.02. Additional patients were identified in the VA Cancer
Registry using the ICD-O site, including lung/bronchus, other respiratory system or intrathoracic organs, or trachea. The Cancer Registry was also used to determine the lung cancer subtypes
LUAD and LUSC among cases. Preliminary totals of 18,633 and 10,845 patients with MVP participation were identified from the VA CDW and Cancer Registry, respectively. A combined cohort of
20,631 unique patients was generated for further analysis. The cohort was predominantly male (~95%) with a median age of 64–68 for sub-cohorts, depending on ancestry assignments and cancer
subtypes. The cohort was curated further to remove any participant with missing data. The final cohorts are described in Supplementary Data 1. Once patients were identified from VA’s CDW and
Cancer Registry, cases were used to gather records related to age, sex, smoking status, and ancestry. Smoking status included former, current, and never, based on the MVP survey at the time
of enrollment and on electronic medical records. Ancestry was defined using a machine learning algorithm that harmonizes self-reported ethnicity and genetic ancestry (HARE)62. All analyses
described here were performed on patients of EA or AA ancestry in ancestry-stratified cohorts. Additionally, the cohorts were further stratified by lung cancer subtypes for analysis. Matched
controls were selected based on age, gender, smoking status, and HARE assignments. Age was binned into 5-year intervals for this purpose. GENOTYPING AND PRINCIPAL COMPONENT ANALYSIS
Genotyping and quality control were conducted as described previously63. Briefly, we removed all samples with excess heterozygosity (F statistic < −0.1), excess relatedness (kinship
coefficient ≥ 0.1 with 7 or more MVP samples), and samples with call rates <98.5%. Additional samples with a mismatch between self-reported sex and genetic sex were removed. Principal
component (PC) analysis was conducted as described previously63. Briefly, PCs were generated with PLINK 2.064 (v2.00a3LM) using a pruned set of SNPs (window size 1 Mb, step size 80, _r__2_
< 0.1, minor allele frequency (MAF) < 0.01, Hardy–Weinberg equilibrium _P_ < 1 × 10−10, missingness rate < 10%) within unrelated European ancestry (EA) and African ancestry (AA)
individuals. (Unrelated individuals were defined as greater than third-degree relatives.) PCs were then projected onto related individuals. IMPUTATION Prior to imputation, a within-cohort
pre-phasing procedure was applied across the whole cohort by chromosome using Eagle265. Imputation was then conducted on pre-phased genotypes using Minimac466 and the 1000 Genomes Phase 3
(v5) reference panel67 in 20 Mb chunks and 3 Mb flanking regions. The quality of imputation was then re-computed in EA and AA separately to be used as filters for respective GWAS (Minimac
Rsq or INFO > 0.3). An MAF cutoff of >0.001 was applied for all analyses. Imputed loci reaching genome-wide significance were tested for deviation from Hardy–Weinberg equilibrium (HWE)
in 61,538 EA controls (Supplementary Data 24). Of the 93 conditionally independent SNPs across the GWAS analyses, 6 SNPs had a significant (_P_ < 1 × 10−6) HWE signal; unsurprisingly,
the strongest HWE signal was from SNPs in the Major Histocompatibility Complex region. However, none of the 12 novel loci reported in Table 1 significantly deviated from HWE. ASSOCIATION
ANALYSES For the EA lung cancer overall and subtype GWAS, we performed standard logistic regression using PLINK 2.0 (v2.00a2LM)64 with a matched control design. EA GWAS was performed in
unrelated individuals, defined as greater than third-degree relatives. For the AA lung cancer overall and subtype analyses, because the case numbers were smaller, we performed a mixed-model
logistic regression using REGENIE (v1.0.6.7)68; REGENIE applies a whole-genome regression model to control for relatedness and population structure and includes a Firth correction to control
for bias in rare SNPs as well as case-control imbalance. GWAS covariates for each ancestry included age, age-squared, sex, smoking status as a categorical variable (current, former, never),
and the first ten principal components. Participants with missing smoking status (_n_ = 786) were removed. Pearson’s _r_ was calculated for effect size concordance between MVP EA and ILCCO7
cohorts. EA META-ANALYSIS We performed inverse-variance weighted meta-analyses of MVP-EA summary statistics and summary statistics previously reported by ILCCO7 using METAL (v20100505)69
with scheme STDERR. Significant inflation across GWAS and meta-analyses was not observed (all genomic control values (λ) for GWAS in this study ≤1.15). Only variants present in both studies
were meta-analyzed. We further performed a sensitivity analysis using the Han-Eskin random effects model (RE2) in METASOFT v2.0.133. LUNG EQTL CONSORTIUM The lung tissues used for eQTL
analyses were from human subjects who underwent lung surgery at three academic sites: Laval University, the University of British Columbia (UBC), and the University of Groningen. Genotyping
was carried out using the Illumina Human1M-Duo BeadChip. Expression profiling was performed using an Affymetrix custom array (see GEO platform GPL10379). Only samples that passed genotyping
and gene expression quality controls were considered for eQTL analysis, leaving sample sizes of 409 for Laval, 287 for UBC, and 342 for Groningen. Within each set, genotypes were imputed in
each cohort with the Michigan Imputation Server66 using the Haplotype Reference Consortium70 version 1 (HRC.r1-1) data as a reference set, and gene expression values were adjusted for age,
sex, and smoking status. Normalized gene expression values from each set were then combined with ComBat71. eQTLs were calculated using a linear regression model and additive genotype effects
as implemented in the Matrix eQTL package in R72. Cis-eQTLs were defined by a 2 Mb window, i.e., 1 Mb distance on either side of lung cancer-associated SNPs. Pre-computed lung eQTLs were
also obtained from the Genotype-Tissue Expression (GTEx) Portal20. Lung eQTLs in GTEx (version 8) are based on 515 individuals and calculated using FastQTL73. FINE-MAPPING We performed
Bayesian fine-mapping of the genome-wide significant loci from EA meta-analysis and AA using the FinnGen fine-mapping pipeline74 (https://github.com/FINNGEN/finemapping-pipeline) and the
SuSiE R package (v0.9.1.0)25,26. Pairwise SNP correlations were calculated directly from imputed dosages on European-ancestry MVP samples from this analysis using LDSTORE 2.074. The maximum
number of allowed causal SNPs at each locus was set to 10. Fine-mapping regions which overlapped the major histocompatibility complex (MHC; chr6:25,000,000–34,000,000) were excluded.
High-quality credible sets were defined as those with minimum _r__2_ < 0.5 between variants. The functional consequences of the AA credible set variants were annotated using the Variant
Effect Predictor (VEP)31. REPLICATION ANALYSIS External replication was performed for all genome-wide significant associations in overall lung cancer, LUAD, and LUSC in OncoArray Consortium
Lung Study (OncoArray)8,40. Replication for genome-wide significant multi-ancestry associations was performed in a fixed-effects meta-analysis of OncoArray CEU Europeans for significant EA
meta-analysis associations, and in a YRI AA meta-analysis composed of 5 studies8 for significant MVP AA associations. Meta-analysis associations from this study were replicated against a
meta-analysis of these OncoArray groups; Pearson’s _r_ was calculated for effect size concordance between these groups. To replicate significant variants from EA analysis conditioned on
smoking, pack-years was additionally included as a covariate in replication cohorts. There was no participant overlap between the replication cohorts and the ILCCO study7 used in the
discovery scan. Covariates included the first five genetic principal components and participant study sites. Proxy SNPs were used to replicate known associations at rs75675343
(rs2318539/4:67831628:C:A; _R_2EUR = 1) and rs4586884 (rs4435699/4:164019500:C:G; _R_2EUR = 0.999). MULTI-ANCESTRY META-ANALYSIS A multi-ancestry meta-analysis of MVP EA and AA cohorts with
summary statistics previously reported by ILCCO7 was conducted in METAL69 using an inverse variance-weighted fixed effects scheme. Only variants present in two or more cohorts were
meta-analyzed. Index variants were defined using the two-stage “clumping” procedure implemented in the Functional Mapping and Annotation (FUMA) platform75. In this process, genome-wide
significant variants are collapsed into LD blocks (_r_2 > 0.6) and subsequently re-clumped to yield approximately independent (_r_2 < 0.1) signals; adjacent signals separated by
<250 kb are ligated to form independent loci. Novel variants are defined as meta-analysis index variants located >1 Mb from previously reported lung cancer associations. We
additionally performed a sensitivity analysis using the random effects model (RE2) in METASOFT v2.0.133. POLYGENIC RISK SCORE (PRS) CALCULATION We constructed PRSs based on the ILCCO summary
statistics7 and the conditional meta-analysis of ILCCO adjusted for cigarettes per day34 for every EA subject in MVP. We used PRS-CS76 to generate effect size estimates under a Bayesian
shrinkage framework and then used PLINK 2.0 (v2.00a3LM)64 to linearly combine weights into a risk score using a global shrinkage prior of 1 × 10−4, which is recommended for less polygenic
traits. Finally, scores were normalized to a mean of 0 and a standard deviation of 1. MULTI-TRAIT ANALYSES In order to remove all residual effects of smoking on lung cancer susceptibility,
we conducted a multi-trait meta-analysis35 conditioned on cigarettes per day, which was shown to be most significantly correlated with all lung cancer GWAS34. The meta-analysis was performed
on the EA meta-analysis summary statistics using mtCOJO, part of the GCTA software package77. An LD reference was constructed from 50,000 MVP EA samples. Multi-trait analysis of GWAS
(MTAG)46 (v0.9.0) was applied using genome-wide LUSC summary statistics after conditioning on cigarettes per day, and estrogen receptor negative (ER−) breast cancer summary statistics47
(21,468 ER− cases and 100,594 controls) which were munged using LDSC (v1.01)38. Colocalization between LUSC conditioned on cigarettes per day and ER− breast cancer allowing for multiple
causal signals was performed using the coloc-SuSiE method48 of coloc (R; v5.2.1)78 for variants at _ABHD8_ (chr19: 17,350,000 to 17,475,000). A posterior probability >0.9 for Hypothesis 4
was used as the criteria for colocalization. HERITABILITY AND GENETIC CORRELATIONS Linkage Disequilibrium score regression (LDSC) v1.0.1 was used to calculate observed-scale
SNP-heritability38 using lung cancer and subtypes summary statistics, before and after conditioning on cigarettes per day. Pairwise genetic correlations were estimated between lung cancer
and subtypes from MVP, ILCCO7, and EA meta-analysis, and four smoking traits (smoking initiation, cigarettes per day, smoking cessation, and age of initiation)34. CONDITIONAL AND JOINT SNP
ANALYSIS To find independently associated genome-wide significant SNPs at each locus in a stepwise fashion, we used GCTA-COJO77 using the --cojo-slct option. An LD reference was constructed
from 50,000 MVP EA samples. Variants with MAF < 0.01 in the COJO reference panel were not included in the identification of independent signals. LDTrait79 was queried to identify
previously published significant GWAS variants within 1 Mb of our index variants in all populations. Novel loci were defined as those at which the index variant was located >1 Mb from
previously reported genome-wide significant lead SNPs for lung cancer or its subtypes in any ancestry. PHENOME-WIDE ASSOCIATION STUDY (PHEWAS) We conducted a PheWAS of electronic health
record-derived phenotypes and lab results in EA subjects using either the normalized PRS as the predictor or independently associated genome-wide significant SNPs. Comparison of
unconditioned PRS PheWAS and conditioned PRS PheWAS were based on ILCCO summary statistics7 and used MVP EA as the out-of-sample test set. Associations were tested using the R PheWAS
package80 (v0.1) with QC procedures described previously81. Control and sex-based exclusion criteria were applied. STATISTICS AND REPRODUCIBILITY Samples sizes for the case-control status of
overall lung cancer, LUAD, and LUSC in MVP participants are provided in Supplementary Data 1. EA GWAS meta-analysis was performed across lung cancer and its subtypes using MVP and an
external cohort, ILCCO7, comprised of up to 39,664 cases and 119,158 controls (Supplementary Data 1). GWAS replication and combined meta-analysis were performed using an external
OncoArray8,40 cohort made up of 19,404 cases and 17,378 controls (Supplementary Data 1); these participants had no overlap with ILCCO7. All statistical tests were two-tailed linear or
logistic regressions unless otherwise noted. Nominal significance was defined as _P_ < 0.05. In hypothesis-free scans, we applied strict significance thresholds to account for multiple
hypothesis testing. For GWAS analyses, the standard genome-wide significance threshold (_P_ < 5 × 10−8) was used. In PheWAS analyses, we applied Bonferroni-corrected significance
thresholds. All _P_-values are presented without adjustment for multiple hypotheses. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting
Summary linked to this article. DATA AVAILABILITY The full summary level association data from the individual population analyses in MVP are available via the dbGaP study accession number
phs001672. ILCCO7 summary statistics can be found in GWAS Catalog accession numbers GCST004748, GCST004744, and GCST004750. OncoArray Consortium8,40 summary statistics used for replication
can be found in dbGaP study accession number phs001273. GTEx v8 lung eQTL summary data were accessed on the GTEx portal [https://gtexportal.org]; full data are available via the dbGaP study
accession number phs000424.v8.p2. Source data are provided with this paper. CODE AVAILABILITY This study did not use any custom computer code or algorithms to generate results. All software
tools used in this analysis were open source. REFERENCES * Schabath, M. B. & Cote, M. L. Cancer progress and priorities: lung cancer. _Cancer Epidemiol. Biomark. Prev._ 28, 1563–1579
(2019). Article Google Scholar * Leiter, A., Veluswamy, R. R. & Wisnivesky, J. P. The global burden of lung cancer: current status and future trends. _Nat. Rev. Clin. Oncol._ 20,
624–639 (2023). Article PubMed Google Scholar * Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. _CA
Cancer J. Clin._ 71, 209–249 (2021). Article PubMed Google Scholar * Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. _CA Cancer J. Clin._ 72, 7–33
(2022). Article PubMed Google Scholar * Bossé, Y. & Amos, C. I. A decade of GWAS results in lung cancer. _Cancer Epidemiol. Biomark. Prev._ 27, 363–379 (2018). Article Google Scholar
* Timofeeva, M. N. et al. Influence of common genetic variation on lung cancer risk: meta-analysis of 14 900 cases and 29 485 controls. _Hum. Mol. Genet._ 21, 4980–4995 (2012). Article
CAS PubMed PubMed Central Google Scholar * McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility
across histological subtypes. _Nat. Genet._ 49, 1126–1132 (2017). Article CAS PubMed PubMed Central Google Scholar * Byun, J. et al. Cross-ancestry genome-wide meta-analysis of 61,047
cases and 947,237 controls identifies new susceptibility loci contributing to lung cancer. _Nat. Genet._ 54, 1167–1177 (2022). Article CAS PubMed PubMed Central Google Scholar * Wang,
Y. et al. SNP rs17079281 decreases lung cancer risk through creating an YY1-binding site to suppress DCBLD1 expression. _Oncogene_ 39, 4092–4102 (2020). Article CAS PubMed PubMed Central
Google Scholar * Zhang, T. et al. Genomic and evolutionary classification of lung cancer in never smokers. _Nat. Genet._ 53, 1348–1359 (2021). Article CAS PubMed PubMed Central Google
Scholar * Govindan, R. et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. _Cell_ 150, 1121–1134 (2012). Article CAS PubMed PubMed Central Google
Scholar * Wang, Z. et al. Meta-analysis of genome-wide association studies identifies multiple lung cancer susceptibility loci in never-smoking Asian women. _Hum. Mol. Genet._ 25, 620–629
(2016). Article CAS PubMed PubMed Central Google Scholar * Schabath, M. B., Cress, D. & Munoz-Antonia, T. Racial and ethnic differences in the epidemiology and genomics of lung
cancer. _Cancer Control_ 23, 338–346 (2016). Article PubMed Google Scholar * Long, E., Patel, H., Byun, J., Amos, C. I. & Choi, J. Functional studies of lung cancer GWAS beyond
association. _Hum. Mol. Genet._ 31, R22–R36 (2022). Article PubMed PubMed Central Google Scholar * Shi, J. et al. Genome-wide association study of lung adenocarcinoma in East Asia and
comparison with a European population. _Nat. Commun._ 14, 3043 (2023). Article ADS CAS PubMed PubMed Central Google Scholar * Dai, J. et al. Identification of risk loci and a polygenic
risk score for lung cancer: a large-scale prospective cohort study in Chinese populations. _Lancet Respir. Med._ 7, 881–891 (2019). Article PubMed PubMed Central Google Scholar * Nahar,
R. et al. Elucidating the genomic architecture of Asian EGFR-mutant lung adenocarcinoma through multi-region exome sequencing. _Nat. Commun._ 9, 216 (2018). Article ADS PubMed PubMed
Central Google Scholar * Zanetti, K. A. et al. Genome-wide association study confirms lung cancer susceptibility loci on chromosomes 5p15 and 15q25 in an African-American population. _Lung
Cancer_ 98, 33–42 (2016). Article PubMed Google Scholar * Gaziano, J. M. et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. _J. Clin.
Epidemiol._ 70, 214–223 (2016). Article PubMed Google Scholar * GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. _Science_ 369, 1318–1330
(2020). Article Google Scholar * Hao, K. et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. _PLoS Genet._ 8, e1003029 (2012). Article CAS PubMed PubMed Central
Google Scholar * Bossé, Y. et al. Transcriptome-wide association study reveals candidate causal genes for lung cancer. _Int. J. Cancer_ 146, 1862–1878 (2020). Article PubMed Google
Scholar * Koutsami, M. K. et al. Centrosome abnormalities are frequently observed in non-small-cell lung cancer and are associated with aneuploidy and cyclin E overexpression. _J. Pathol._
209, 512–521 (2006). Article CAS PubMed Google Scholar * Chan, J. Y. A clinical overview of centrosome amplification in human cancers. _Int. J. Biol. Sci._ 7, 1122–1144 (2011). Article
CAS PubMed PubMed Central Google Scholar * Wang, G., Sarkar, A., Carbonetto, P. & Stephens, M. A simple new approach to variable selection in regression, with application to genetic
fine mapping. _J. R. Stat. Soc. Ser. B Stat. Methodol._ 82, 1273–1300 (2020). Article MathSciNet Google Scholar * Zou, Y., Carbonetto, P., Wang, G. & Stephens, M. Fine-mapping from
summary data with the ‘sum of single effects’ model. _PLoS Genet._ 18, e1010299 (2022). Article CAS PubMed PubMed Central Google Scholar * de Goede, O. M. et al. Population-scale tissue
transcriptomics maps long non-coding RNAs to complex disease. _Cell_ 184, 2633–2648.e19 (2021). Article PubMed PubMed Central Google Scholar * Li, Y. et al. Pan-cancer characterization
of immune-related lncRNAs identifies potential oncogenic biomarkers. _Nat. Commun._ 11, 1000 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * de Santiago, P. R. et al.
Immune-related IncRNA LINC00944 responds to variations in ADAR1 levels and it is associated with breast cancer prognosis. _Life Sci._ 268, 118956 (2021). Article PubMed Google Scholar *
Chen, D. et al. Genome-wide analysis of long noncoding RNA (lncRNA) expression in colorectal cancer tissues from patients with liver metastasis. _Cancer Med._ 5, 1629–1639 (2016). Article
ADS CAS PubMed PubMed Central Google Scholar * McLaren, W. et al. The ensembl variant effect predictor. _Genome Biol._ 17, 122 (2016). Article PubMed PubMed Central Google Scholar *
Saunders, G. R. B. et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. _Nature_ 612, 720–724 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Han,
B. & Eskin, E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. _Am. J. Hum. Genet._ 88, 586–598 (2011). Article CAS PubMed
PubMed Central Google Scholar * Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. _Nat. Genet._
51, 237–244 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhu, Z. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data.
_Nat. Commun._ 9, 1–12 (2018). ADS Google Scholar * Xue, A. et al. Genome-wide analyses of behavioural traits are subject to bias by misreports and longitudinal changes. _Nat. Commun._ 12,
20211 (2021). Article ADS CAS PubMed Google Scholar * Munafò, M. R., Tilling, K., Taylor, A. E., Evans, D. M. & Davey Smith, G. Collider scope: when selection bias can
substantially influence observed associations. _Int. J. Epidemiol._ 47, 226–235 (2018). Article PubMed Google Scholar * Bulik-Sullivan, B. K. et al. LD Score regression distinguishes
confounding from polygenicity in genome-wide association studies. _Nat. Genet._ 47, 291–295 (2015). Article CAS PubMed PubMed Central Google Scholar * Nguyen, M.-H., Ueda, K., Nakamura,
Y. & Daigo, Y. Identification of a novel oncogene, MMS22L, involved in lung and esophageal carcinogenesis. _Int. J. Oncol._ 41, 1285–1296 (2012). Article CAS PubMed Google Scholar *
Amos, C. I. et al. The OncoArray Consortium: a network for understanding the genetic architecture of common cancers. _Cancer Epidemiol. Biomark. Prev._ 26, 126–135 (2017). Article Google
Scholar * Yang, W. et al. Deciphering associations between three RNA splicing-related genetic variants and lung cancer risk. _NPJ Precis. Oncol._ 6, 48 (2022). Article CAS PubMed PubMed
Central Google Scholar * Gabriel, A. A. G. et al. Genetic analysis of lung cancer and the germline impact on somatic mutation burden. _J. Natl Cancer Inst._ 114, 1159–1166 (2022). Article
PubMed PubMed Central Google Scholar * Lawrenson, K. et al. Functional mechanisms underlying pleiotropic risk alleles at the 19p13.1 breast-ovarian cancer susceptibility locus. _Nat.
Commun._ 7, 12675 (2016). Article ADS PubMed PubMed Central Google Scholar * Lesseur, C. et al. Genome-wide association meta-analysis identifies pleiotropic risk loci for aerodigestive
squamous cell cancers. _PLoS Genet._ 17, e1009254 (2021). Article CAS PubMed PubMed Central Google Scholar * Fachal, L. et al. Fine-mapping of 150 breast cancer risk regions identifies
191 likely target genes. _Nat. Genet._ 52, 56–73 (2020). Article CAS PubMed PubMed Central Google Scholar * Turley, P. et al. Multi-trait analysis of genome-wide association summary
statistics using MTAG. _Nat. Genet._ 50, 229–237 (2018). Article CAS PubMed PubMed Central Google Scholar * Michailidou, K. et al. Association analysis identifies 65 new breast cancer
risk loci. _Nature_ 551, 92–94 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Wallace, C. A more accurate method for colocalisation analysis allowing for multiple
causal variants. _PLoS Genet._ 17, e1009440 (2021). Article CAS PubMed PubMed Central Google Scholar * Gusev, A. et al. A transcriptome-wide association study of high-grade serous
epithelial ovarian cancer identifies new susceptibility genes and splice variants. _Nat. Genet._ 51, 815–823 (2019). Article CAS PubMed PubMed Central Google Scholar * Brenner, D. R. et
al. Alcohol consumption and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium and the SYNERGY study. _Cancer Epidemiol._ 58, 25–32 (2019). Article PubMed
Google Scholar * Larsson, S. C. et al. Smoking, alcohol consumption, and cancer: a mendelian randomisation study in UK Biobank and international genetic consortia participants. _PLoS Med._
17, e1003178 (2020). Article PubMed PubMed Central Google Scholar * Petrelli, F. et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and
meta-analysis. _JAMA Netw. Open_ 4, e213520 (2021). Article PubMed PubMed Central Google Scholar * Lan, T., Chen, L. & Wei, X. Inflammatory cytokines in cancer: comprehensive
understanding and clinical progress in gene therapy. _Cells_ 10, 100 (2021). Article CAS PubMed PubMed Central Google Scholar * Kresse, S. H. et al. LSAMP, a novel candidate tumor
suppressor gene in human osteosarcomas, identified by array comparative genomic hybridization. _Genes Chromosomes Cancer_ 48, 679–693 (2009). Article CAS PubMed Google Scholar * Xie, J.
et al. Copy number analysis identifies tumor suppressive lncRNAs in human osteosarcoma. _Int. J. Oncol._ 50, 863–872 (2017). Article CAS PubMed Google Scholar * Yu, G. et al. Roles of
transducin-like enhancer of split (TLE) family proteins in tumorigenesis and immune regulation. _Front. Cell Dev. Biol._ 10, 1010639 (2022). Article PubMed PubMed Central Google Scholar
* Sartor, I. T. S., Recamonde-Mendoza, M. & Ashton-Prolla, P. TULP3: a potential biomarker in colorectal cancer? _PLoS ONE_ 14, e0210762 (2019). Article CAS PubMed PubMed Central
Google Scholar * Chaudhary, P. K. & Kim, S. An insight into GPCR and G-proteins as cancer drivers. _Cells_ 10, 3288 (2021). Article CAS PubMed PubMed Central Google Scholar *
Murphy, C. et al. An analysis of JADE2 in non-small cell lung cancer (NSCLC). _Biomedicines_ 11, 2576 (2023). Article CAS PubMed PubMed Central Google Scholar * Ni, L. et al. RPAP3
interacts with Reptin to regulate UV-induced phosphorylation of H2AX and DNA damage. _J. Cell. Biochem._ 106, 920–928 (2009). Article CAS PubMed Google Scholar * Saredi, G. et al.
H4K20me0 marks post-replicative chromatin and recruits the TONSL–MMS22L DNA repair complex. _Nature_ 534, 714–718 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Fang,
H. et al. Harmonizing genetic ancestry and self-identified race/ethnicity in genome-wide association studies. _Am. J. Hum. Genet._ 105, 763–772 (2019). Article CAS PubMed PubMed Central
Google Scholar * Hunter-Zinck, H. et al. Genotyping array design and data quality control in the million veteran program. _Am. J. Hum. Genet._ 106, 535–548 (2020). Article CAS PubMed
PubMed Central Google Scholar * Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. _Gigascience_ 4, 7 (2015). Article PubMed PubMed
Central Google Scholar * Loh, P.-R. et al. Reference-based phasing using the Haplotype Reference Consortium panel. _Nat. Genet._ 48, 1443–1448 (2016). Article CAS PubMed PubMed Central
Google Scholar * Das, S. et al. Next-generation genotype imputation service and methods. _Nat. Genet._ 48, 1284–1287 (2016). Article CAS PubMed PubMed Central Google Scholar * 1000
Genomes Project Consortium. et al. A global reference for human genetic variation. _Nature_ 526, 68–74 (2015). Article Google Scholar * Mbatchou, J. et al. Computationally efficient
whole-genome regression for quantitative and binary traits. _Nat. Genet._ 53, 1097–1103 (2021). Article CAS PubMed Google Scholar * Willer, C. J., Li, Y. & Abecasis, G. R. METAL:
fast and efficient meta-analysis of genomewide association scans. _Bioinformatics_ 26, 2190–2191 (2010). Article CAS PubMed PubMed Central Google Scholar * McCarthy, S. et al. A
reference panel of 64,976 haplotypes for genotype imputation. _Nat. Genet._ 48, 1279–1283 (2016). Article CAS PubMed PubMed Central Google Scholar * Johnson, W. E., Li, C. &
Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. _Biostatistics_ 8, 118–127 (2007). Article PubMed Google Scholar * Shabalin, A. A.
Matrix eQTL: ultra fast eQTL analysis via large matrix operations. _Bioinformatics_ 28, 1353–1358 (2012). Article CAS PubMed PubMed Central Google Scholar * Ongen, H., Buil, A., Brown,
A. A., Dermitzakis, E. T. & Delaneau, O. Fast and efficient QTL mapper for thousands of molecular phenotypes. _Bioinformatics_ 32, 1479–1485 (2016). Article CAS PubMed Google Scholar
* Benner, C. et al. FINEMAP: efficient variable selection using summary data from genome-wide association studies. _Bioinformatics_ 32, 1493–1501 (2016). Article CAS PubMed PubMed
Central Google Scholar * Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. _Nat. Commun._ 8, 1826 (2017).
Article ADS PubMed PubMed Central Google Scholar * Ge, T., Chen, C.-Y., Ni, Y., Feng, Y.-C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous
shrinkage priors. _Nat. Commun._ 10, 1776 (2019). Article ADS PubMed PubMed Central Google Scholar * Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for
genome-wide complex trait analysis. _Am. J. Hum. Genet._ 88, 76–82 (2011). Article CAS PubMed PubMed Central Google Scholar * Giambartolomei, C. et al. Bayesian test for colocalisation
between pairs of genetic association studies using summary statistics. _PLoS Genet._ 10, e1004383 (2014). Article PubMed PubMed Central Google Scholar * Machiela, M. J. & Chanock, S.
J. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. _Bioinformatics_ 31, 3555–3557
(2015). Article CAS PubMed PubMed Central Google Scholar * Carroll, R. J., Bastarache, L. & Denny, J. C. R. PheWAS: data analysis and plotting tools for phenome-wide association
studies in the R environment. _Bioinformatics_ 30, 2375–2376 (2014). Article CAS PubMed PubMed Central Google Scholar * Klarin, D. et al. Genetics of blood lipids among ~300,000
multi-ethnic participants of the Million Veteran Program. _Nat. Genet._ 50, 1514–1523 (2018). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This
research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by award #MVP000. This publication does not
represent the views of the Department of Veterans Affairs or the United States Government. R.J.H., J.D.M., J.B., Y.H., X.X., and C.I.A. were supported by the National Institutes of Health
(NIH) for Integrative Analysis of Lung Cancer Etiology and Risk (U19CA203654). C.I.A. was supported by Sequencing Familial Lung Cancer (R01CA243483). Where authors are identified as
personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article, and they do not necessarily
represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization. Full consortium acknowledgements for MVP and ILCCO7 are provided in
Supplementary Information. AUTHOR INFORMATION Author notes * Sun-Gou Ji Present address: BridgeBio Pharma, Palo Alto, CA, USA * Anoop K. Sendamarai Present address: Carbone Cancer Center,
University of Wisconsin, Madison, WI, USA * A full list of members and their affiliations appears in the Supplementary Information. AUTHORS AND AFFILIATIONS * Center for Data and
Computational Sciences (C-DACS), VA Boston Healthcare System, Boston, MA, USA Bryan R. Gorman, Sun-Gou Ji, Michael Francis, Anoop K. Sendamarai, Yunling Shi, Poornima Devineni, Uma Saxena,
Elizabeth Partan, Andrea K. DeVito & Saiju Pyarajan * Booz Allen Hamilton, McLean, VA, USA Bryan R. Gorman, Michael Francis & Andrea K. DeVito * Institute for Clinical and
Translational Research, Baylor College of Medicine, Houston, TX, USA Jinyoung Byun, Younghun Han, Xiangjun Xiao & Christopher I. Amos * Department of Medicine, Section of Epidemiology
and Population Sciences, Baylor College of Medicine, Houston, TX, USA Jinyoung Byun, Younghun Han, Xiangjun Xiao & Christopher I. Amos * The University of British Columbia Centre for
Heart Lung Innovation, St Paul’s Hospital, Vancouver, BC, Canada Don D. Sin * University Medical Centre Groningen, GRIAC (Groningen Research Institute for Asthma and COPD), University of
Groningen, Groningen, Netherlands Wim Timens * Department of Pathology & Medical Biology, University Medical Centre Groningen, University of Groningen, Groningen, Netherlands Wim Timens
* Office of Research and Development, Department of Veterans Affairs, Washington, DC, USA Jennifer Moser, Sumitra Muralidhar & Rachel Ramoni * Lunenfeld-Tanenbaum Research Institute,
Sinai Health System, University of Toronto, Toronto, ON, Canada Rayjean J. Hung * Section of Genetics, International Agency for Research on Cancer, World Health Organization, Lyon, France
James D. McKay * Institut universitaire de cardiologie et de pneumologie de Québec, Department of Molecular Medicine, Laval University, Quebec City, QC, Canada Yohan Bossé * Department of
Biostatistics, University of Texas MD Anderson Cancer Center, Houston, TX, USA Ryan Sun * Dan L Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, TX, USA Christopher
I. Amos * Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA Saiju Pyarajan Authors * Bryan R. Gorman View author publications You can also search
for this author inPubMed Google Scholar * Sun-Gou Ji View author publications You can also search for this author inPubMed Google Scholar * Michael Francis View author publications You can
also search for this author inPubMed Google Scholar * Anoop K. Sendamarai View author publications You can also search for this author inPubMed Google Scholar * Yunling Shi View author
publications You can also search for this author inPubMed Google Scholar * Poornima Devineni View author publications You can also search for this author inPubMed Google Scholar * Uma Saxena
View author publications You can also search for this author inPubMed Google Scholar * Elizabeth Partan View author publications You can also search for this author inPubMed Google Scholar
* Andrea K. DeVito View author publications You can also search for this author inPubMed Google Scholar * Jinyoung Byun View author publications You can also search for this author inPubMed
Google Scholar * Younghun Han View author publications You can also search for this author inPubMed Google Scholar * Xiangjun Xiao View author publications You can also search for this
author inPubMed Google Scholar * Don D. Sin View author publications You can also search for this author inPubMed Google Scholar * Wim Timens View author publications You can also search for
this author inPubMed Google Scholar * Jennifer Moser View author publications You can also search for this author inPubMed Google Scholar * Sumitra Muralidhar View author publications You
can also search for this author inPubMed Google Scholar * Rachel Ramoni View author publications You can also search for this author inPubMed Google Scholar * Rayjean J. Hung View author
publications You can also search for this author inPubMed Google Scholar * James D. McKay View author publications You can also search for this author inPubMed Google Scholar * Yohan Bossé
View author publications You can also search for this author inPubMed Google Scholar * Ryan Sun View author publications You can also search for this author inPubMed Google Scholar *
Christopher I. Amos View author publications You can also search for this author inPubMed Google Scholar * Saiju Pyarajan View author publications You can also search for this author
inPubMed Google Scholar CONSORTIA VA MILLION VETERAN PROGRAM * Jennifer Moser * , Sumitra Muralidhar * , Rachel Ramoni * & Saiju Pyarajan CONTRIBUTIONS Drafted the manuscript: B.R.G.,
M.F., S.-G.J., A.K.S., E.P., A.K.D., and S.P. Acquired the data: B.R.G., S.-G. J., A.K.S., Y.S., P.D., U.S., J.B., Y.H., X.X., D.D.S., W.T., J.M., S.M., R.R., R.J.H., J.D.M., Y.B., C.I.A.,
VA MVP, and S.P. Analyzed the data: B.R.G., S.-G.J., M.F., A.K.S., Y.S., P.D., X.X., U.S., Y.B., and R.S. Critically revised the manuscript for important intellectual content: B.R.G., M.F.,
S.-G.J., A.K.S., Y.S., P.D., U.S., E.P., A.K.D., J.B., Y.H., X.X., D.D.S., W.T., J.M., S.M., R.R., R.J.H., J.D.M., Y.B., R.S., C.I.A., and S.P. CORRESPONDING AUTHOR Correspondence to Saiju
Pyarajan. ETHICS DECLARATIONS COMPETING INTERESTS S.-G.J. is an employee and shareholder of BridgeBio Pharma, unrelated to the present work. The other authors declare no competing interests.
PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SUPPLEMENTARY
DATA 5 SUPPLEMENTARY DATA 6 SUPPLEMENTARY DATA 7 SUPPLEMENTARY DATA 8 SUPPLEMENTARY DATA 9 SUPPLEMENTARY DATA 10 SUPPLEMENTARY DATA 11 SUPPLEMENTARY DATA 12 SUPPLEMENTARY DATA 13
SUPPLEMENTARY DATA 14 SUPPLEMENTARY DATA 15 SUPPLEMENTARY DATA 16 SUPPLEMENTARY DATA 17 SUPPLEMENTARY DATA 18 SUPPLEMENTARY DATA 19 SUPPLEMENTARY DATA 20 SUPPLEMENTARY DATA 21 SUPPLEMENTARY
DATA 22 SUPPLEMENTARY DATA 23 SUPPLEMENTARY DATA 24 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gorman, B.R., Ji, SG., Francis, M. _et al._ Multi-ancestry GWAS meta-analyses
of lung cancer reveal susceptibility loci and elucidate smoking-independent genetic risk. _Nat Commun_ 15, 8629 (2024). https://doi.org/10.1038/s41467-024-52129-4 Download citation *
Received: 08 April 2024 * Accepted: 27 August 2024 * Published: 04 October 2024 * DOI: https://doi.org/10.1038/s41467-024-52129-4 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
