Tumor draining lymph nodes connected to cold triple-negative breast cancers are characterized by th2-associated microenvironment
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Tumor draining lymph nodes (TDLN) represent a key component of the tumor-immunity cycle. There are few studies describing how TDLNs impact lymphocyte infiltration into tumors. Here
we directly compare tumor-free TDLNs draining “cold” and “hot” human triple negative breast cancers (TDLNCold and TDLNHot). Using machine-learning-based self-correlation analysis of immune
gene expression, we find unbalanced intranodal regulations within TDLNCold. Two gene pairs (_TBX21_/_GATA3_-_CXCR1_) with opposite correlations suggest preferential priming of T helper 2
(Th2) cells by mature dendritic cells (DC) within TDLNCold. This is validated by multiplex immunofluorescent staining, identifying more mature-DC-Th2 spatial clusters within TDLNCold versus
TDLNHot. Associated with this Th2 priming preference, more IL4 producing mast cells (MC) are found within sinus regions of TDLNCold. Downstream, Th2-associated fibrotic TME is found in
paired cold tumors with increased Th2/T-helper-1-cell (Th1) ratio, upregulated fibrosis growth factors, and stromal enrichment of cancer associated fibroblasts. These findings are further
confirmed in a validation cohort and public genomic data. Our results reveal a potential role of IL4+ MCs within TDLNs, associated with Th2 polarization and reduced immune infiltration into
tumors. SIMILAR CONTENT BEING VIEWED BY OTHERS SPATIAL IMMUNOPHENOTYPES PREDICT RESPONSE TO ANTI-PD1 TREATMENT AND CAPTURE DISTINCT PATHS OF T CELL EVASION IN TRIPLE NEGATIVE BREAST CANCER
Article Open access 27 September 2021 ENHANCED IMMUNE RESPONSE OUTPERFORM AGGRESSIVE CANCER BIOLOGY AND IS ASSOCIATED WITH BETTER SURVIVAL IN TRIPLE-NEGATIVE BREAST CANCER Article Open
access 09 August 2022 TUMOR INVASION IN DRAINING LYMPH NODES IS ASSOCIATED WITH TREG ACCUMULATION IN BREAST CANCER PATIENTS Article Open access 29 June 2020 INTRODUCTION Tumor infiltrating
lymphocytes (TIL) are now widely associated with prognosis and response to immune checkpoint inhibitors (ICIs)1. Why some tumors have high levels of TILs (“hot tumors”), but others have low
TILs (“cold tumors”) remains a key open question in oncology. Tremendous efforts have been made to identify hurdles of lymphocyte infiltrations into tumor beds in context of the
cancer-immunity cycle (CIC), which has been considered as a framework of how the immune system surveys and kills cancer cells2. Multiple mechanisms within the tumor microenvironment (TME)
that may lead to paucity of TILs have been found, including lack of tumor antigens, ineffective antigen presentation, defective T cell activation, and fibrotic stromal regions3,4,5. A number
of therapeutic approaches are under development to improve TIL levels within tumors and to trigger the CIC5,6. However, TIL levels of most tumors remain insufficient to support long-term
responses to ICI treatments due to an incomplete understanding of key operating mechanisms within the CIC. Tumor draining lymph nodes (TDLN) are the key sites of encounter between systemic
immune surveillance and tumor associated antigens (TAA)7 within the CIC. To initiate effective anti-tumor adaptive immunity, TAAs bound with major histocompatibility complex (MHC) molecules
on dendritic cells (DC) are presented to naïve T lymphocytes within paracortex of TDLNs, where stromal structures enable DCs to efficiently interact with TAA-specific naïve T lymphocytes8.
Co-stimulatory molecules on DCs are also required to interact with receptors on T cells for successful priming9. In situ cytokine profiles dictate the effector functions of primed T cells,
such as T helper 1 (Th1) cells vs. T helper 2 (Th2) cells10. With such important functions in driving anti-tumor immunity, TDLNs were shown to be integral to effective ICI response in recent
animal studies11. However, underlying mechanisms of how TDLNs impact lymphocyte infiltration into TME in humans remain poorly understood. To investigate the impact of TDLNs on lymphocyte
infiltration in human TMEs, we focus on paired human tumor-free TDLN samples from TNBC patients with hot vs. cold tumors. Within TDLNCold, preferred Th2-cell priming by mature DCs has been
found by correlation analysis of gene expression and spatial clustering analysis of multiplex immunofluorescent (mIF) staining on mature DCs and Th2 cells. Correspondingly, within paired
cold tumors, we observe increased Th2/Th1 ratios and more fibrotic microenvironments compared to hot tumors. Equally important, we found enrichment of IL4+ mast cells (MC) within sinus
regions of TDLNCold in both discovery and validation cohorts (_n_ = 43). Our findings not only unravel potential mechanisms of how TDLNs impact lymphocyte infiltration in TME, but also
provide a potential target (i.e., IL4+ MCs within TDLNs) to increase lymphocytes infiltration into tumors. In context of ICI treatment, combination treatment with MC inhibitors could be
helpful to facilitate ICI responses by enhancing lymphocyte infiltration. RESULTS SIMILAR STATIC IMMUNE PROFILES BETWEEN TDLNCOLD AND TDLNHOT This study utilized human triple-negative breast
cancer (TNBC) tumors with paired TDLNs. Since tumor invasion can substantially impact immune properties of TDLNs12, we focused on cases with available tumor-free TDLNs. Stromal TIL
percentage of each tumor was scored by a pathologist from H&E-stained slides13. Tumors were graded as cold (<10% stromal TIL), hot (>60% stromal TIL), or intermediate (10-60%
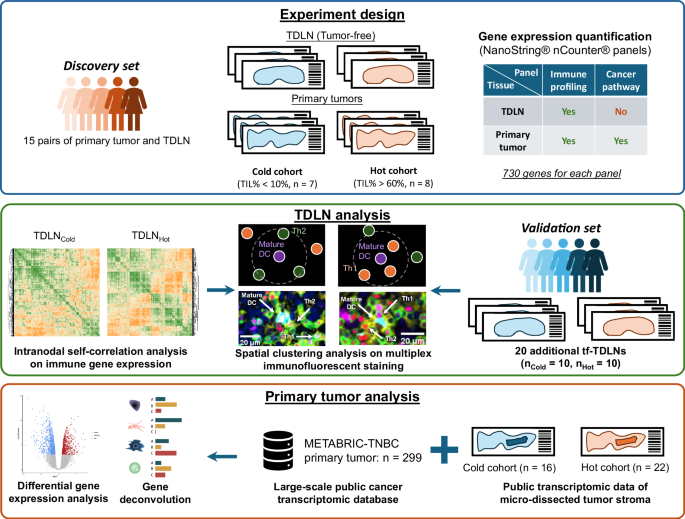
stromal TIL). We initially collected and analyzed 7 cold tumor-TDLNCold pairs and 8 hot tumor-TDLNHot pairs (Fig. 1, Table S1) as a Discovery set. FFPE samples were subjected to NanoString®
analyses (Data file S1, Fig. S1). By using the NanoString® Pan-Cancer immune panel, we quantified the expression of 730 immune genes of these 15 tumor-free TDLNs and paired tumors. Initial
analyses of the gene expression between TDLNCold vs. TDLNHot found no significantly differentially expressed genes (adjusted _p_-value < 0.10 and absolute log2 fold change > 1.0; Fig.
S2A), suggesting that TDLNCold and TDLNHot had similar overall immune transcriptomic profiles. As the major difference between cold vs. hot tumors is their T cell infiltration levels, we
examined genes related to T cell activation (Fig. S2C) and T cell proliferation (Fig. S2D) based on the panel annotation. Somewhat surprisingly, no significant changes in expression profiles
were found between TDLNCold and TDLNHot for T cell activation (Fig. S2E) and T cell proliferation (Fig. S2F). Further analysis of 14 immune cell scores (Data file S2) provided by NanoString
also showed no significantly different immune cell types between TDLNCold vs. TDLNHot (Fig. S2B, Data file S3). Since TDLN is the major site for antigen presentation by DCs, we also
estimated the cell abundances of major DC subtypes (i.e., cDC1, cDC2, and _CCR7_+ mature DC) based on a set of signatures from a single-cell RNA-sequencing dataset14 (Data file S2). Again,
no abundance differences were found in all three DC subtypes (Fig. S2G). DYSFUNCTIONAL IMMUNE COORDINATION REVEALED BY INTRANODAL SELF-ANALYSIS IN TDLNCOLD Given the lack of significant
differences in cell type abundance or static gene expression between TDLNCold vs. TDLNHot, we hypothesized that differences may lie in cell-cell interactions. To address this, we
investigated immune coordination within TDLNs draining cold vs. hot tumors. We developed an intranodal self-correlation analysis approach, a computational method combining correlation
analysis with unsupervised clustering algorithms (pseudocodes are available in Supplementary Methods). We first calculated Pearson’s correlation coefficient (PCC) of the expression values
for each pair of genes in TDLNCold vs. TDLNHot. Next, we applied two unsupervised clustering algorithms (i.e., hierarchical clustering and k-means clustering) with the same parameters to
group gene pairs based on the correlation similarities for TDLNCold and TDLNHot separately. Since gene-to-gene correlations can reflect gene regulatory relationships, and organized gene
regulatory relationships are necessary to perform normal cellular functions15,16, we proposed that the clustered pattern of gene-to-gene correlations can represent intranodal gene
regulation. We first implemented the hierarchical clustering algorithm on heatmaps with gene correlation matrices for TDLNCold vs. TDLNHot (Fig. 2A, left upper panel and right lower panel).
To directly address the difference in clustered patterns between TDLNCold and TDLNHot, we aligned the clustered orders of TDLNCold and TDLNHot into each other (Fig. 2A, right upper panel and
left lower panel). This combined analysis revealed that gene-to-gene correlations of TDLNHot were clustered in a more organized manner compared to TDLNCold. To quantify this observation, we
implemented k-means clustering for the PCCs of TDLNCold and TDLNHot separately, then calculated Silhouette scores for 2 to 12 clusters (Fig. 2B, Supplementary Methods). Significantly lower
Silhouette scores were observed in TDLNCold (_p_ = 6.0 × 10−4), indicating that the PCC similarities within a k-means cluster of TDLNCold was lower than TDLNHot, and the differences crossing
_k_-means clusters in TDLNCold is less distinguishable compared to TDLNHot. In summary, poorly organized gene-to-gene correlation in TDLNCold indicated that its immune coordination was
dysfunctional compared to TDLNHot, which might lead to the “cold” immunophenotype in its paired tumor. In addition, such dysfunctional intranodal immune coordination might impair the
robustness of the TDLN response to extra-nodal stimulation and tumor antigen. Beyond clustering patterns between TDLNCold and TDLNHot, we also compared the specific PCCs of each gene pair.
To avoid spurious strong correlations driven by a single outlier and other data points with low variance, we implemented the leave-one-out comparison (LOOC) for each gene pair inspired by
the leave-one-out cross validation in machine learning (detail methods are described in Methods as well as Supplementary Methods). Among the most differentially correlated gene pairs based
on statistics from LOOC (Fig. S3), we identified that correlations of _TBX21_- and _GATA3_-CXCR1 pairs were both significantly different between TDLNCold and TDLNHot (_p__TBX21_-_CXCR1_ <
1.0 × 10−4 [Fig. 2C], p_GATA3_-_CXCR1_ = 1.7 × 10−3 [Fig. 2D])—but in opposite directions. _TBX21_-_CXCR1_ correlation was positive (_r_Cold = 0.846, _p_Cold = 0.02) in TDLNCold, but
negative (_r_Hot = −0.878, pHot = 4.1 × 10−3) in TDLNHot (Fig. 2C). _GATA3_-_CXCR1_ showed the opposite pattern (_r_Cold = −0.557, _p_Cold = 0.19; _r_Hot = 0.901, _p_Hot = 2.2 × 10−3; Fig.
2D). DCs have been described to downregulate _CXCR1_ during maturation within the T cell zone of TDLNs17. _TBX21_ (_T-bet_) and _GATA3_ are well-recognized markers of Th1 and Th2 cells,
respectively10. We hypothesized that intranodal correlations of _TBX21_- vs. _GATA3_-_CXCR1_ can reflect differential naïve CD4+ T cell polarization by mature DCs in TDLNCold vs. TDLNHot.
Within these contexts, our data suggested that in TDLNCold, _GATA3_ expression would be upregulated during the downregulation of _CXCR1_ in DC maturation and priming. Therefore, we further
hypothesized that mature DCs in TDLNCold would preferably prime naïve CD4+ T cells into Th2 cells. PREFERRED TH2 CELL POLARIZATION WITHIN TDLNCOLD To support the hypothesis of preferred Th2
polarization by mature DCs in TDLNCold, we implemented multiplex immunofluorescence (mIF) staining of TDLNCold and TDLNHot samples (Fig. S4, Fig. 3A and Fig. 3B, Data file S1). We used
DC-LAMP3 as a mature DC marker18 and T-bet/GATA3 as Th1/Th2 cell markers, combined with CD3 and CD4 T cell markers. Mature DC, Th1, and Th2 cells were defined by CD3-DC-LAMP3+ cell,
CD3+CD4+T-bet+ cell, and CD3+CD4+GATA3+ cell, respectively (Table S2, Fig. S5). First, we quantified the abundances of each cell type within TDLNs in two ways, cell densities (cells/mm2,
Fig. S6A) and relative cell abundances (cells/total cells, Fig. S6B). Consistent with our analysis of NanoString cell type scores, no difference in densities or relative abundances of any
cell type quantified by this mIF panel were observed. This indicated that abundances of mature DC, Th1, Th2, or CD3+CD4- cells within TDLNs were not associated with lymphocyte infiltration
into their paired tumors (Fig. S6A, B). Besides the overall abundances normalized to areas and total cell numbers, we further quantified the Th2 percentage relative to total CD4+ T cells
(CD3+CD4+ cells), and the ratio of Th2 to Th1 cell numbers. We found that both Th2% to total CD4+ T cells and Th2/Th1 ratios were significantly higher in TDLNCold compared to TDLNHot (p =
6.0 × 10−4 and _p_ = 6.0 × 10−4). This finding supports the preferred Th2 polarization in CD4+ T cells within TDLNCold (Fig. 3C, D). To investigate the potential mechanism for preferred Th2
polarization, we quantified the spatial relationships between mature DCs and Th1/Th2 cells. We implemented a spatial clustering algorithm, density-based spatial clustering of applications
(DBSCAN), a widely used spatial clustering algorithms in biological studies19,20,21, on mature DC, Th1, and Th2 cells (more details described in the Methods section). Based on their
intra-cluster cell compositions, we defined Th1-dominated clusters as clusters which contained both mature DC and Th1 cells, with Th1 higher than Th2 cell numbers. In contrast, Th2-dominated
clusters have mature DCs with higher Th2 than Th1 cells. Cluster compositions of each slide are provided in Data file S4. We calculated the percentages of Th1- and Th2-dominated clusters
relative to total cluster numbers for each TDLN. We then compared the cluster percentages between TDLNCold vs. TDLNHot for each Th-dominated cluster type. These showed that Th2-dominated
clusters were enriched in TDLNCold compared to TDLNHot (_p_ = 0.05, Fig. 3F), while percentage of Th1-dominated clusters were comparable (_p_ = 0.53, Fig. 3E). In sum, Th2 dominated clusters
were enriched within TDLNCold, indicating preferential Th2 cell polarization compared to Th1 cells (Fig. 3G). IL4 FROM MCS DRIVES PREFERRED TH2 POLARIZATION IN TDLNCOLD To gain further
insight into what may be associated with the preferential mature-DC-Th2 polarization within TDLNCold, we examined _IL4_ expression, an essential cytokine in Th2 polarization22,23,24.
Upregulation of _IL4_ was observed in TDLNCold compared to TDLNHot (Fig. 4A, _p_ = 0.02 with outlier exclusion; Fig. S2A, _p_ = 0.04, log2 fold change = −0.739 without outlier exclusion and
_p_-value adjustment). Since Th2 cell abundances were similar between TDLNCold and TDLNHot, we hypothesized that the upregulation of _IL4_ could be derived from other cell types. To
investigate the _IL4_ resources, we examined the correlation coefficients and slopes between _IL4_ expression and 14 cell type scores from NanoString® nCounter® Pan-Cancer immune profiling
panel (Fig. S7A) and found that the correlation between MC score and IL4 expression is the strongest with slope = 0.75 and _r_ = 0.56 (Fig. S7B, _p_ = 0.03) among all the 14 cell types.
Expression of multiple MC markers and the MC score were significantly upregulated in TDLNCold (Fig. 4B, _p__TPSAB1_ = 2.3 × 10−3 without the outlier; Fig. S8A–D, _p__KIT_ = 0.23, _p__CMA1_ =
0.09, _p__TPSAB1_ = 0.02, _p__IL3RA_ = 0.40 with outliers and without _p_-value adjustment) based on our NanoString data and corresponding MC signatures. Due to the low abundance of MCs
within lymph nodes, this limits the accuracy of gene deconvolution methods25,26,27. Bulk transcriptomic data with strict statistical adjustments could underestimate any modest enrichment of
MCs within TDLNCold based on differential gene expression analysis and comparisons of estimated immune cell abundances. As such, to examine MCs more accurately within TDLNCold compared to
TDLNHot, we quantified MC densities by performing immunohistochemical (IHC) staining for CD117+ cells (Fig. 4C and Fig. 4E, Fig. S9A, Data file S1). We found that MC densities in TDLNCold
were significantly higher than TDLNHot (Fig. 4G, _p_ = 2.3 × 10−3, Fig. S10A). To further validate this finding, we collected another cohort with 10 TDLNsCold- and 10 TDLNsHot-tumor pairs
(Table S3) as an independent validation cohort. The same definition of cold/hot tumors was applied to this independent validation cohort with the same pathologist. We similarly performed
CD117 IHC staining and quantified MC densities (Fig. 4D, F, Fig. S9B). These results confirmed that MC densities within TDLNs can accurately reflect T cell infiltration of paired tumors
(Fig. 4H, AUC = 0.80, _p_-value = 0.01, Fig. S10B, C, Data file S1). To confirm the IL4 expression on MCs, we co-stained IL4 and CD117 on 30 ROIs (_n_Cold = 15, _n_Hot = 15, 1 mm × 0.62 mm)
from 22 TDLN slides (_n_Cold = 11 and _n_Hot = 11), including samples from both discovery and validation cohorts (Data file S1, S5). We identified IL4+ MCs (IL4+CD117+ cells) within both
TDLNCold and TDLNHot (Fig. 4I, J, Data file S5, Fig. S11, S12). The cell densities of IL4+ MCs were significantly higher in TDLNCold compared to TDLNHot (Fig. 4K, _p_ = 0.01). Another
important question was whether IL4+ MCs need to be close to mature DC and T cells to skew the Th2 polarization. Considering the potential of IL4 to diffuse across different regions within
TDLNs, we hypothesized that IL4 producing MCs may not need to be directly adjacent to T cells or DCs to influence their biology. Based on the qualitative observation of sinus enrichment of
MCs from our H-DAB staining (Fig. S9A, B), we hypothesized that IL4+ MCs would also accumulate within the sinus region and IL4 could diffuse into surrounding regions to skew Th2 cell
polarization. Guided by a clinical pathologist expert in lymphoma and lymph node structure research, we stratified the ROIs into B cell zones, T cell zones, and sinus regions (Fig. 4I, J,
Fig. S12) based on CD3 staining. We found that densities of MCs were higher in the sinus (_p_ < 1.0 × 10−4) and T cell zones (_p_ < 1.0 × 10−4) than B cell zones (Fig. S10D). Comparing
sinus and T cell zones, there was a trend (_p_ = 0.07, 2-sided Wilcoxon matched-pairs signed rank test) towards more MCs within sinuses (Fig. S10D). Densities of IL4+ MCs were also higher
within sinuses (_p_ < 1.0 × 10−4) and T cell zones (_p_ = 8.0 × 10−4) than B cell zones (Fig. 4M), while sinuses were also slightly enriched for IL4+ MCs (_p_ = 0.08, 2-sided Wilcoxon
matched-pairs signed rank test) compared to T cell zones. These spatial patterns indicate that IL4+ MCs within sinuses and T cell zones would associate with Th2 polarization in direct and
indirect manners. Lastly, we compared the densities of IL4+ MCs between TDLNCold and TDLNHot within each TDLN region (Fig. 4N). TDLNCold has more IL4+ MCs than TDLNHot within both T cell
zones (_p_ = 4.2 × 10−3) and sinuses (_p_ = 0.03). In addition to densities of IL4+ MCs, we also calculated the percentages of IL4+ MCs in relation to total MCs. Higher percentages of IL4+
MCs in TDLNCold were observed as compared to TDLNHot when considering entire TDLNs (Fig. 4L, _p_ = 6.4 × 10−3). Within sinuses, but not T cell zones, percentages of IL4+ MCs were
significantly higher in TDLNCold compared to TDLNHot (_p_Sinus = 9.3 × 10−3, _p_T cell zone = 0.41, Fig. 4O). TDLN-DRIVEN TYPE 2 IMMUNITY IN TUMOR MICROENVIRONMENT To complete the
cancer-immunity cycle, we sought to connect our findings within TDLNs with the immune profiles of their paired tumors. Two NanoString® panels (i.e., nCounter® Pan-Cancer Immune Profiling
panel and Cancer Pathway panel) representing more than 1000 immuno-oncological genes were used to investigate the TME of these primary tumors. Starting from differential gene expression
analysis, we observed upregulation of type 2 immunity-related genes (Fig. S13A, C) in cold primary tumors compared to hot. Specifically, PDGFC and VEGFA, two growth factors secreted by
tissue-repairing macrophages (TsRpM), were upregulated in cold tumors based on the Immune Profiling panel (Fig. S13B). Pro-fibrosis genes, including _INHBB_, _LAMA5_, _PPARGC1A_, and
_COL11A1_ were upregulated in cold tumors based on the Cancer Pathway panel (Fig. S13D). Similarly, we implemented self-correlation analysis for gene expressions from the Immune Profiling
panel of cold vs. hot primary tumors (same as what was done for TDLNs). We found that the clustering pattern of self-correlation matrix was less organized in cold tumors compared to hot
ones, indicating that immune coordination was dysfunctional in cold tumors as well (Fig. S13E). In addition to the gene expression profiles, we estimated immune cell abundances from
NanoString® annotation and corresponding nCounter® analysis (Data file S2). All immune cell types, except for MCs, were lower in cold tumors as compared to hot tumors (Fig. S13F). We looked
further into the relative abundances and gene expression differences between cold vs. hot tumors. First, we calculated the Th2/Th1 ratios by dividing the Th2 cell type score to the Th1 cell
type score from NanoString® nSolver analyzer v4.0 for each sample. We found that Th2/Th1 ratios were significantly negatively correlated with _PTPRC_ (CD45) and _CD3D_ expression in TNBC
tumors (Fig. 5A, B), which supported the Th2/Th1 axis being a key feature of immune infiltration. As predicted from our TDLN results, we found significantly higher Th2/Th1 ratios in cold
paired primary tumors as compared to hot (Fig. 5E, _p_ = 6.0 × 10−4). To further validate in a larger cohort, we utilized the METABRIC database with all available TNBC primary tumor samples
and corresponding transcriptomic data. METABRIC-TNBC tumor samples were stratified into cold vs. hot samples based on PTPRC expression (bottom/top 35 percentile), respectively28. The
abundances of Th2 and Th1 cells were estimated by MCP-Counter method using the same signatures from NanoString®. Th2/Th1 ratios were calculated by dividing the estimated abundances. Similar
negative correlations between Th2/Th1 ratios and _PTPRC_/_CD3D_ (Fig. 5H/I) were found in METABRIC-TNBC samples as from our in-house samples. METABRIC-TNBC samples also showed Th2/Th1 ratios
in cold tumors were significantly higher than in hot tumors (Fig. 5L, _p_ < 1.0 × 10−4). Increased Th2/Th1 ratios within cold tumors mirrored the preferential Th2 cell polarization in
paired TDLNCold. We further hypothesized that relative Th2 cell enrichment is associated with type 2 immune response limiting immune infiltration into cold tumors. Specifically, relative Th2
cell enrichment could increase IL4 production which would activate TsRpMs29,30,31. TsRpM could then secrete multiple growth factors (e.g., platelet-derived growth factors, PDGFs) to
stimulate cancer associated fibroblasts32,33,34,35 (CAFs), which could limit lymphocyte infiltration by creating a fibrotic TME36,37,38. In support of this hypothesis, we examined the
relative expression of molecules mentioned above using both in-house and METABRIC-TNBC samples. We found that the relative expression of _IL4_ to T cell marker _CD3D_ was significantly
higher in cold tumors (Fig. 5F, _p_NanoString = 1.2 × 10−3; Fig. 5M, _p_METABRIC-TNBC < 1.0×10−4). Relative expression of _PDGFC_ to macrophage marker CD68 was significantly higher in
cold tumors (Fig. 5G, _p_NanoString = 3.0 × 10−4; 5 N, _p_METABRIC-TNBC < 1.0 × 10−4). We also investigated the correlations between Th2, TsRpM, and CAF cell type scores (Data file S2)
using the MCP-counter method39. These showed that Th2, TsRpM, and CAF were significantly positively correlated with each other in both in-house and METABRIC datasets (Fig. 5C, D, J, K).
These strong correlations across key components in type 2 immunity further support the hypothesis that type 2 immune response modulates immune infiltration into tumors. To further support a
more fibrotic TME in cold tumors, we re-analyzed another public transcriptomic dataset (GSE88715). In this previous study, the authors collected 38 TNBC primary tumors and stratified these
tumors into four groups (i.e., immune desert [ID], marginal restricted [MR], stromal restricted [SR] and fully inflamed [FI]) based on the immune infiltration levels and locations from
clinical pathologists36. They micro-dissected the samples into epithelium and stroma regions and quantified their gene expression via microarrays for each region. To align their groups with
our cold/hot definitions based on stromal TIL percentages (i.e., TIL% > 60% as hot, while TIL% <10% as cold), we considered ID and MR as the cold tumors (_n_ = 16), and SR and FI as
the hot tumors (_n_ = 22). First, we compared the gene expression profiles between cold and hot tumors within stroma (Fig. S14A) and epithelium (Fig. S14B) regions. Within the stroma regions
(Fig. S14C), cold tumors upregulated tissue repairing and fibrosis related genes (i.e., _FGF1_, _COL10A1_, _MMP11_, _COL5A2_), while cytotoxicity and CD8+ T cell markers were upregulated in
hot tumors (i.e., _CD8A_, _CD8B_, _GZMB_, _CXCL13_). In contrast, the expression profiles of epithelium regions were similar between cold and hot tumors (Fig. S14B). From cell abundances
estimated by MCP-Counter, T cells and CD8+ T cells were significantly enriched in stroma regions of hot tumors (Fig. 5O, P, _p_adj = 1.7 × 10−3, Fig. S14D), while CAF was the only
significantly upregulated cell type in stroma of cold tumors (_p_adj = 2.2 × 10−3, Fig. 5Q, R, Fig. S14D). These results suggest higher stromal CAFs in cold tumors compared to hot tumors,
leading to a more fibrotic TME in cold tumors. We further compared T cell scores between epithelium and stroma regions within cold primary tumors and found a significantly lower T cell score
in epithelium vs. stroma regions (Fig. S14E). Associated with this T cell stasis within stroma, more CAFs were found in stroma (Fig. S14F) compared to epithelium regions. DISCUSSION To gain
insights into tumor extrinsic factors that regulate immune infiltration into tumors, we investigated the impact of TDLNs by implementing a machine-learning-inspired intranodal
self-correlation analysis of gene expression data, with further spatial analysis of mIF staining. Our results provide valuable insights into how anti-tumor immunity may unfold over time
systemically (Fig. S15). Our initial results showed that immune cell abundances and static gene expressions were not different between TDLNCold and TDLNHot. Rather, correlations between
immune components and their clustering patterns were significantly different between TDLNCold and TDLNHot. Following initial findings from our intranodal self-correlation analysis, we
further implemented mIF staining to investigate CD4+ T cell polarization in TDLNs. In line with results from self-correlation analysis, spatial analysis shows enrichment of mature-DC-Th2
clusters in TDLNCold, suggesting that mature DCs within TDLNCold may preferentially polarize naive CD4+ T cells to Th2 cells. We also found that Th2 cell polarization preference was
associated with increased IL4 production by MCs in TDLNCold. Downstream, preferred Th2 polarization within TDLNCold was associated with higher Th2 compared to Th1 cells in paired cold
tumors. This Th2 enrichment was found to be linked to a type 2 immune response within cold tumors, along with more activating tissue repair macrophages and cancer-associated fibroblasts. Low
abundance of MCs within lymph nodes limited the ability of gene deconvolution approaches from bulk transcriptomic data with strict statistical adjustments to uncover potential MC enrichment
and _IL4_ upregulation from comparative analysis25,26,27. Going beyond bulk transcriptomic analysis, we implemented mIF and IHC staining to accurately quantify MCs along with IL4 expression
in our initial cohort and an additional independent validation cohort. We found consistent enrichment of MCs and IL4 expression on MCs within the sinus regions of TDLNCold. Correlation
analysis has been applied to transcriptomic data since early 2000s40,41,42. In cancer immunology, it has been successfully used to unravel various biological insights, e.g., identification
of potential therapeutic targets43, biomarkers44, dysregulated pathways, and key regulatory changes45. In our study, we combined two distinct features of correlation analysis into our
intranodal self-correlation analysis. Focusing initially on clustering patterns, we discovered dysfunctional immune coordination in TDLNCold compared to TDLNHot. Inspired by leave-one-out
cross validation, an evaluation method used in machine learning, we further identified specific gene pairs (i.e., _TBX21_- vs. _GATA3_-_CXCR1_) representing coordinated cellular regulations.
Importantly, both major findings derived from self-correlation analysis of transcriptomic data were validated by spatial analysis of mIF staining, highlighting the capacity of correlation
analysis to discover cellular dysregulations. Our findings consistently revealed that even though there are no overall cell abundance differences (except for MCs) between TDLNCold vs
TDLNHot, there are gene correlation, relative Th2/Th1 ratio or percentage of Th2 cells to total CD4+ T cells and spatial relationship differences that are biologically consistent. Several
papers have shown that spatial organization, rather than numerical differences, of immune cells within TDLNs46,47 or tumors48 correlate with clinical outcomes. In this study, preferential
Th2 polarization in TDLNCold vs. TDLNHot is suggested by increased relative Th2 cell abundances (to Th1 or to total CD4+ T cells) and different spatial distributions of mature-DC-Th cells,
rather than overall Th cell abundances. These findings suggest that preferential Th2 polarization could only impact the relative abundances of Th2 cells to Th1 and total CD4+ T cells, but
not the overall abundances between TDLNCold vs. TDLNHot. Potential mechanism of the similar overall Th cell abundances is the rapid and similar egress rates of Th cells such that these cells
do not accumulate within TDLNs49, supported by our similar expression levels of Th2 egress related genes. MCs play important roles in immune activation50, wound healing51, allergy52, and
tumor immunology53. Tumor infiltrating mast cells (TIMC) have found to associate with prognosis in multiple cancer types, including breast cancer54, pancreatic cancer, ovarian cancer, and
liver cancer55. In prostate adenocarcinoma models, treatment with MC degranulation inhibitor (cromolyn) inhibited tumor growth56. However, the immuno-oncological functions of MCs in either
TME or systemic immunity are still poorly understood57,58. Both in vivo experiments with a MC-deficient mouse breast cancer model and in vitro co-culture experiments demonstrated that MCs
promoted tumor growth by promoting estrogen receptor activities in breast cancer cells59. In our study, we found that IL4+ MCs are associated with preferred Th2 polarization in TDLNs by
enriching within the sinus and T cell zones of TDLNs associated with cold TNBC tumors. Similarly, preferred Th2 cell polarization in TDLNs was also identified by De Monte et al.60 in
pancreatic ductal adenocarcinomas, in which the TME has limited lymphocyte infiltration and enrichment of Th2 cells44. In their mouse model, basophils within TDLNs were found as the primary
source of IL4 production, and their recruitment from blood partially relied upon the chemokine CCL7/MCP3 released from “alternatively activated” monocytes60. Other examples of Th1 vs. Th2
priming preference driving divergent downstream immune responses were shown in two recent elegant animal studies. In an animal model of contact hypersensitivity induced by the hapten
fluorescein isothiocyanate, injection of diphtheria toxin increased the population of CD11b+ type 2 conventional DCs (cDC2) in skin-draining lymph nodes, which significantly increased the
Th2-dependent skin inflammation61. In addition, a recent animal study of lung cancer showed that IFNγ within mediastinal lymph nodes (mLNs) skewed regulatory T (Treg) cells to become
Th1-like effector Treg cells which suppressed cDC1s and led to mLN-specific failure of cytotoxic T cell activation62. Our findings suggest a potential therapeutic opportunity in modulating
MCs within TDLNs to promote T cell infiltration into tumors and enhance immunotherapies. Consistent with this view, a recent study using humanized melanoma mouse model demonstrated that
anti-PD1 treatment combined with sunitinib could deplete MCs and achieve complete regression of tumors, while anti-PD1 treatment only could not63. Downstream to TDLNs, we also found an
increased Th2/Th1 ratio within cold tumors, which is consistent with previous studies44,64. Major cytokines secreted by Th2 cells, IL-4, and IL-13, could stimulate tumor-associated
macrophages (TAM) to promote tissue-repairing functions and create an anti-inflammatory immune contexture65,66,67,68. Specifically, tissue-repairing TAMs could secrete IL-10, TGF-β, VEGFs,
PDGFs, MMPs, and osteopontins69 to induce tissue fibrosis through fibroblasts. In our findings, upregulation of _VEGFA_ and _PDGFC_ were associated with limited immune infiltration.
Additionally, we found strong correlations between Th2 cell, TsRpM, and CAF, which are consistent with the Th2-TsRpM-CAF axis limiting immune infiltration36. In a recent study with three
different breast cancer mouse models, inhibition of Th2 function with suplatast tosilate significantly improved infiltration of CD8+ T cells and reduced M2-like macrophages with reduction of
IL4 production70. By combining the ICB treatments, inhibition of Th2 function improved ICB efficacy without increasing potential adverse effects70. Based on this Th2-TsRpM-CAF axis, various
potential therapeutic targets are being developed and some drugs have been shown to be effective in combination with ICI treatments68. Due to inherent limitations of research from human
patient samples, our findings are observational and correlative by nature. However, our conclusions are derived from multiple different experimental techniques (including intra-nodal
self-correlation analysis of transcriptomic data, cell abundance analysis, and spatial clustering analysis of histological staining), and further validated with an independent patient cohort
with total 43 TDLN-tumor pairs. It is impossible to directly manipulate TDLNs or tumors in live patients. While there are inbred mouse models with cold vs. hot tumors, these are of
different tumor types with different mutational burden. Our findings are on a single tumor type, triple negative breast cancer, with different levels of immune infiltration. These valuable
human findings provide a direct and more precise representation of human biology than animal studies. In sum, our results highlight the important roles of TDLNs in regulating T cell
infiltration into tumors, and the value of paired TDLN-tumor studies to identify key mechanisms within the entire CIC. These findings provide translationally valuable insights (i.e.,
association between TDLN immune profile and type 2 immunity within tumors) and potential therapeutic strategies (i.e., modulate IL4+ MCs within TDLNs) to enhance lymphocyte infiltration into
tumors. METHODS STUDY DESIGN Specimens for both discovery and validation cohorts were collected and biobanked under IRB 07047 at City of Hope (Note S1). Secondary research use of specimens
in this study was approved under IRB 12195 and IRB 11273 at City of Hope. Patient characteristics of discovery and validation cohorts are summarized in Table S1 and Table S3. TDLNs in both
discovery and validation cohorts were sentinel lymph nodes without any metastasis. TDLNs were neoadjuvant treatment-free and collected at diagnosis with paired primary tumors. The
classification of tumor samples as TNBC was performed by clinical pathologists. The stratification of “cold” (_n_Discovery = 7, _n_Validation = 14) and “hot” (_n_Discovery = 8, _n_Validation
= 14) tumors was performed by clinical pathologists examining the stromal TIL percentages in H&E-stained slides (<10% and >60%), respectively13. QUANTIFICATION OF GENE EXPRESSION
AND CELL TYPE SCORE THROUGH NANOSTRING FOR TDLNS AND PAIRED TUMORS RNA was extracted from 10 µm thick slices of unbaked formalin fixed paraffin embedded (FFPE) tissue using Qiagen miRNeasy
FFPE kits. RNA transcripts related to immuno-oncology were detected using NanoString® PanCancer Immune Panel with nCounter® technology (NanoString Technologies) for both TDLN and primary
tumors. RNA transcripts related to oncology were detected using NanoString® PanCancer Cancer Pathway Panel. The regions for RNA extraction were selected by a clinical pathologist with valid
TDLN regions (Fig S1, Data file S1). RNA concentration was assessed with the Nanodrop spectrophotometer ND-1000 and Qubit 3.0 Fluorometer (Thermo Fischer Scientific). RNA fragmentation and
quality control were further determined by 2100 Bioanalyzer (Agilent). Total RNA was hybridized overnight at 65 °C for 14 to 18 h as per manufacturers’ recommendations. Post-hybridization,
probe-target mixture was purified by nCounter Prep Station and then quantified with nCounter Digital Analyzer. Quality control and normalization of data were performed with nSolver Analysis
Software version 4.0 and nCounter Advanced Analysis Software version 2.0.115, in which the measured gene expression values were normalized to the geometric mean of 40 housekeeping genes. The
cell type scores were calculated as the mean of the log2 expression levels for all the probes included in the final calculation for that specific cell type (Data file S2). INTRANODAL
SELF-CORRELATION ANALYSIS FOR GENE EXPRESSION The log2 transformed normalized gene expression was used in self-correlation analysis. The Pearson correlation coefficient (PCC) for each gene
pair was calculated within “cold” and “hot” cohorts, separately. The hierarchical clustering for gene-to-gene correlation matrices of “cold” and “hot” cohorts was implemented separately by
the hierarchical clustering algorithm in ComplexHeatmap (v2.10.0) R package with its default settings. The clustering orders of the “cold” or “hot” cohort were mapped to the other cohort
(i.e., “hot” and “cold” cohort) respectively to directly compare the different clustering results. To further quantify the differences of cluster patterns, the K-means clustering algorithm
(Python sklearn.cluster.KMeans function, v1.1.1) was implemented to both correlation matrices and average silhouette scores were calculated from 2 to 12 clusters by Python function
sklearn.metrics.silhouette_score (v1.1.1), respectively. The statistical comparisons for specific gene pairs were conducted by cocor R package v1.1.3 (i.e., Fisher’s z test) with two
independent groups settings71. To avoid the potential high correlation driven by single outlier, a customized comparison approach (i.e., leave-one-out comparison, LOOC) inspired by
leave-one-out cross validation was implemented with R scripts. Generally, for each gene pair in each cohort, a set of PCCs was calculated by iteratively leaving a sample out of the PCC
calculation until each sample was left for one time. Then the means of LOO PCC sets between TDLNCold and TDLNHot was compared by multiple _t_ test with FDR _p_-value adjustment implemented
in R language (Supplementary Methods). MULTIPLEX IMMUNOFLUORESCENCE STAINING Formalin-fixed paraffin-embedded (FFPE) tumor-negative TDLNs were used to perform immunofluorescent staining and
spatial analysis. FFPE tissue samples were sectioned at a thickness of 3μm, baked, and placed on positively charged glass slides. They were deparaffinized with xylene and rehydrated with
decreasing gradient concentrations (100%, 95%, 70%, and 0%) of ethanol, respectively. Antigen retrieval was performed with AR9 buffer (pH9) (Akoya Biosciences) or AR6 buffer (pH6) (Akoya
Biosciences) using a microwave oven. Slides were washed with MilliQ water and then with TBS (Biocare Medical) with 0.1% Tween-20 (TBST) followed by blocking with Antibody Diluent Background
Reducing (S3022, Agilent Dako). For mature-DC-Th cell panel, the following primary antibodies were incubated for 1 h at room temperature: anti-human CD3 (polyclonal; DAKO; 1:1000);
anti-human CD4 (clone EP204; Cell Marque; 1:200); anti-human GATA3 (clone L50-823; Biocare Medical; 1:1000); anti-human T-bet (clone 4B10; Abcam; 1:500); anti-human DC-LAMP (clone 1010E1.01;
Novus; 1:1000). For IL4-MC panel, the following primary antibodies were incubated for 1 h at room temperature: anti-human CD3 (polyclonal Rb A0452; DAKO; 1:1000); anti-human IL-4 (clone C1
Mu ab239508; Abcam; 1:500); anti-human CD117 (clone YR145 Rb ab32363; Abcam; 1:2000). The slides were then incubated with Mach 2 Rabbit or Mouse HRP-Polymer (Biocare Medical), and then
immunofloursecently labeled using Opal Fluorophores (Akoya Biosciences). To continue with the next primary antibody staining, the next epitope was exposed using microwave treatment as
described above. DAPI (Akoya Biosciences; 1:100) was stained after the final marker and the slides were mounted using ProLong Gold Antifade Reagent (P36930, Thermo Fisher). Slides were
imaged using the Vectra 3 automated quantitative pathology imaging system (Akoya Biosciences). For mature-DC-Th cell panel, multispectral images were unmixed and multi-component tifs were
exported using inForm Software (Akoya Biosciences) and tif images were stitched for each patient using a customized QuPath (v0.2.0-m9) script72. The cell segmentation was implemented by
“Cell detection” function in QuPath considering DAPI expression. The cell phenotyping was implemented by thresholding approach for each marker and further defined by the marker status (Table
S2). The marker expression was visualized by heatmaps and density plots to examine the phenotyping. For IL4-MC panel, the same unmix and export procedure as mature-DC-Th cell panel was
implemented. ROIs with 1 mm wide and 0.62 mm height were selected randomly from the QuPath (v0.4.4) stitched whole TDLN slides. The representative areas of B cell zones, T cell zones, and
sinuses in each ROI were annotated by clinic pathologists based on CD3 staining. A QuPath pixel classifier was trained for each ROI to split the ROI into B cell zone, T cell zone, and sinus
subregions. The cell segmentation was implemented by “Cell detection” function in QuPath considering DAPI expression. The cell phenotyping was implemented by thresholding approach for each
marker and further defined by the marker status. The marker expression was visualized by heatmaps and density plots to examine the phenotyping. SPATIAL CLUSTERING FOR MATURE DC, TH1 AND TH2
CELLS The measurement table exported from QuPath included the _x_–_y_ coordinates of each cell on a 2D space and the cell phenotypes (i.e., mature DC, Th1, and Th2 cells). For the spatial
clustering of mature DC, Th1, and Th2 cells, DBSCAN was implemented with Python function (sklearn.cluster.DBSCAN)73. The default minimum number of core cells (minPts = 5) was used for all
the slides. The maximum distance between two cells for one to be considered as in the neighborhood of the other (epsilon) was determined by selecting the elbow points on the k-distance
graphs generated by Python function (sklearn.neighbors.NearestNeighbors) with default settings for each slide. The elbow point selection was implemented by Python function
(kneed.KneeLocator) (Data S4). For all the other parameters in DBSCAN, the default settings were used. The detailed cluster assignment of each cell in each TDLN was also provided in Data S4.
The cluster annotation was determined by the cell composition of each cluster. Specifically, the mature-DC-Th1 dominated clusters must include mature DC and Th1 cells and if Th2 cells were
included as well, more Th1 cells should be in these clusters compared to Th2 cells and vice versa to mature-DC-Th2 dominated clusters. The Python scripts used here are available in the
GitHub repository with full details. H-DAB STAINING FFPE tissue samples were sectioned at a thickness of 5μm, baked, and placed on positively charged glass slides. Slides were loaded on a
Ventana Discovery Ultra (Ventana Medical Systems, Roche Diagnostics) automated IHC staining machine for deparaffinization, rehydration, endogenous peroxidase activity inhibition, and heat
inactivation antigen retrieval (pH 8.5). Anti-human CD117, c-kit (Clone: YR145; Cell Marque; 1:50) rabbit antibody was incubated followed by DISCOVERY anti-Rabbit HQ and DISCOVERY
anti-HQ-HRP. The stains were then visualized with DISCOVERY ChromoMap DAB and counterstained with hematoxylin (Ventana) and sealed with coverslips. The whole slide images were acquired with
NanoZoomer S360 Digital Slide Scanner (Hamamatsu) and viewed by NDP.view image viewer software. The whole slide of each sample was analyzed by QuPath v0.3.272. The cell segmentation waw
implemented by “Cell detection” function in QuPath considering hematoxylin OD intensity. CD117+ cells (i.e., MCs) were identified by “Training object classifier” function with manually
selected CD117+ cells and random-forest algorithm. STATISTICS AND REPRODUCIBILITY Graphs and statistics were performed using Graphpad Prism 9 (v9.4), R (v4.1.3), and Python (v3.9.13) as
described. Statistics described were generated using multiple 2-sided Mann–Whitney tests with Holm–Sidak _p_-value adjustment, Kruskal–Wallis test with Dunn’s multiple comparison, paired
2-sided Student’s _t_ test, 2-sided Fisher’s z test, or 2-sided Mann–Whitney tests. No statistical method was used to predetermine the sample size. All outliers were identified and removed
by Grubbs’ method (alpha = 0.05) or ROUT method with _Q_ = 1% in Graphpad Prism 9. The investigators were blinded to allocation during experiments and outcome assessment. Calculated _p_
values are displayed as “ns”, _p_ > 0.05; *, _p_ ≤ 0.05; **, _p_ ≤ 0.01; ***, _p_ ≤ 0.001; ****, _p_ ≤ 0.0001. Experimental specific detailed statistic methods are described in
corresponding figure legends. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The
NanoString data (pan-cancer pathway panel) generated in this study have been deposited in the GEO database under accession code GSE272453. The NanoString data (pan-cancer immune profiling
panel) generated in this study have been deposited in the GEO database under accession code GSE272455 (Pan-cancer immune profiling panel). The raw data of mIF staining in this study are
provided in the Supplementary Datasets on FigShare [https://doi.org/10.6084/m9.figshare.25157960]. The detailed spatial clustering data from mIF staining in this study are provided in the
Supplementary Datasets on FigShare [https://doi.org/10.6084/m9.figshare.25157924]. The processed data used in the figures/Supplementary Figs. is provided in Source Data on FigShare
[https://doi.org/10.6084/m9.figshare.26384302]. Source data are provided with this paper. CODE AVAILABILITY All scripts used in this study are available in GitHub
[https://github.com/weihuaguo/tdln_cold_hot_tnbc] and Zenodo [https://doi.org/10.5281/zenodo.13126674]. REFERENCES * Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic
mechanisms of sensitization to checkpoint inhibition. _Cell_ 184, 596–614.e14 (2021). Article CAS PubMed PubMed Central Google Scholar * Chen, D. S. & Mellman, I. Oncology meets
immunology: the cancer-immunity cycle. _Immunity_ 39, 1–10 (2013). Article PubMed Google Scholar * Duan, Q., Zhang, H., Zheng, J. & Zhang, L. Turning cold into hot: firing up the
tumor microenvironment. _Trends Cancer_ 6, 605–618 (2020). Article CAS PubMed Google Scholar * van der Woude, L. L., Gorris, M. A. J., Halilovic, A., Figdor, C. G. & de Vries, I. J.
M. Migrating into the tumor: a roadmap for T cells. _Trends Cancer_ 3, 797–808 (2017). Article PubMed Google Scholar * Bonaventura, P. et al. Cold tumors: a therapeutic challenge for
immunotherapy. _Front. Immunol._ 10, 168 (2019). Article CAS PubMed PubMed Central Google Scholar * Liu, Y.-T. & Sun, Z.-J. Turning cold tumors into hot tumors by improving T-cell
infiltration. _Theranostics_ 11, 5365–5386 (2021). Article CAS PubMed Google Scholar * Marzo, A. L. et al. Tumor antigens are constitutively presented in the draining lymph nodes. _J.
Immunol._ 162, 5838 LP–5835845 (1999). Article Google Scholar * Grant, S. M., Lou, M., Yao, L., Germain, R. N. & Radtke, A. J. The lymph node at a glance—how spatial organization
optimizes the immune response. _J. Cell Sci._ 133, jcs241828 (2020). Article CAS PubMed PubMed Central Google Scholar * Murphy, K. & Weaver, C. _Janeway’s Immunobiology_ (Garland
Science, 2016). * Bailey, S. R. et al. Th17 cells in cancer: the ultimate identity crisis. _Front. Immunol._ 5, 276 (2014). Article PubMed PubMed Central Google Scholar * Goode, E. F.,
Roussos Torres, E. T. & Irshad, S. Lymph node immune profiles as predictive biomarkers for immune checkpoint inhibitor response. _Front. Mol. Biosci._ 8 (2021). * Kohrt, H. E. et al.
Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. _PLoS Med._ 2, e284 (2005). Article PubMed PubMed Central Google Scholar * Salgado, R. et
al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. _Ann. Oncol._ 26, 259–271 (2015). Article CAS
PubMed Google Scholar * Hoffmann, C. et al. PD-L1 and ICOSL discriminate human secretory and helper dendritic cells in cancer, allergy and autoimmunity. _Nat. Commun._ 13, 1983 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar * Zaborowski, A. B. & Walther, D. Determinants of correlated expression of transcription factors and their target genes.
_Nucleic Acids Res._ 48, 11347–11369 (2020). Article CAS PubMed PubMed Central Google Scholar * Liu, Z.-P. Quantifying gene regulatory relationships with association measures: a
comparative study. _Front. Genet._ 8, 96 (2017). Article ADS PubMed PubMed Central Google Scholar * Lipscomb, M. F. & Masten, B. J. Dendritic cells: immune regulators in health and
disease. _Physiol. Rev._ 82, 97–130 (2002). Article CAS PubMed Google Scholar * de Saint-Vis, B. et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC
maturation, is transiently expressed in MHC class II compartment. _Immunity_ 9, 325–336 (1998). Article PubMed Google Scholar * Tibi, M. et al. A telencephalon cell type atlas for
goldfish reveals diversity in the evolution of spatial structure and cell types. _Sci. Adv._ 9, eadh7693 (2024). Article Google Scholar * Dhainaut, M. et al. Spatial CRISPR genomics
identifies regulators of the tumor microenvironment. _Cell_ 185, 1223–1239.e20 (2022). Article CAS PubMed PubMed Central Google Scholar * Russell, A. J. C. et al. Slide-tags enables
single-nucleus barcoding for multimodal spatial genomics. _Nature_ 625, 101–109 (2024). Article ADS CAS PubMed Google Scholar * Hsieh, C. S., Heimberger, A. B., Gold, J. S., O’Garra, A.
& Murphy, K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. _Proc. Natl Acad. Sci._ 89,
6065–6069 (1992). Article ADS CAS PubMed PubMed Central Google Scholar * Swain, S. L., Weinberg, A. D., English, M. & Huston, G. IL-4 directs the development of Th2-like helper
effectors. _J. Immunol._ 145, 3796 LP–3793806 (1990). Article Google Scholar * Seder, R. A., Paul, W. E., Davis, M. M. & Fazekas de St Groth, B. The presence of interleukin 4 during in
vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. _J. Exp. Med._ 176, 1091–1098 (1992). Article CAS PubMed Google Scholar
* Jin, H. & Liu, Z. A benchmark for RNA-seq deconvolution analysis under dynamic testing environments. _Genome Biol._ 22, 102 (2021). Article CAS PubMed PubMed Central Google
Scholar * Momeni, K., Ghorbian, S., Ahmadpour, E. & Sharifi, R. Unraveling the complexity: understanding the deconvolutions of RNA-seq data. _Transl. Med. Commun._ 8, 21 (2023). Article
Google Scholar * Sutton, G. J. et al. Comprehensive evaluation of deconvolution methods for human brain gene expression. _Nat. Commun._ 13, 1358 (2022). Article ADS CAS PubMed PubMed
Central Google Scholar * Curtis, C. et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. _Nature_ 486, 346–352 (2012). Article CAS PubMed
PubMed Central Google Scholar * Bao, K. & Reinhardt, R. L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. _Cytokine_ 75, 25–37 (2015). Article CAS
PubMed PubMed Central Google Scholar * Gieseck, R. L., Wilson, M. S. & Wynn, T. A. Type 2 immunity in tissue repair and fibrosis. _Nat. Rev. Immunol._ 18, 62–76 (2018). Article CAS
PubMed Google Scholar * Voehringer, D., Reese, T. A., Huang, X., Shinkai, K. & Locksley, R. M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil
cells of the innate immune system. _J. Exp. Med._ 203, 1435–1446 (2006). Article CAS PubMed PubMed Central Google Scholar * Knipper, J. A. et al. Interleukin-4 receptor α signaling in
myeloid cells controls collagen fibril assembly in skin repair. _Immunity_ 43, 803–816 (2015). Article CAS PubMed PubMed Central Google Scholar * Pellicoro, A., Ramachandran, P.,
Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. _Nat. Rev. Immunol._ 14, 181–194 (2014). Article CAS PubMed Google
Scholar * Eitner, F. et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. _J. Am. Soc. Nephrol._ 19, 281 LP–281289 (2008). Article Google Scholar * Son,
D., Na, Y. R., Hwang, E.-S. & Seok, S. H. Platelet-derived Growth Factor-C (PDGF-C) Induces Anti-apoptotic Effects on Macrophages through Akt and Bad Phosphorylation*. _J. Biol. Chem._
289, 6225–6235 (2014). Article CAS PubMed PubMed Central Google Scholar * Gruosso, T. et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers.
_J. Clin. Invest._ 129, 1785–1800 (2019). Article PubMed PubMed Central Google Scholar * Koppensteiner, L., Mathieson, L., O’Connor, R. A. & Akram, A. R. Cancer associated
fibroblasts—an impediment to effective anti-cancer T cell immunity. _Front. Immunol._ 13, 887380 (2022). Article CAS PubMed PubMed Central Google Scholar * Sahai, E. et al. A framework
for advancing our understanding of cancer-associated fibroblasts. _Nat. Rev. Cancer_ 20, 174–186 (2020). Article CAS PubMed PubMed Central Google Scholar * Becht, E. et al. Estimating
the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. _Genome Biol._ 17, 218 (2016). Article PubMed PubMed Central Google Scholar *
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. _BMC Bioinform._ 9, 559 (2008). Article Google Scholar * Klebanov, L. & Yakovlev, A.
Diverse correlation structures in gene expression data and their utility in improving statistical inference. _Ann. Appl Stat._ 1, 538–559 (2007). Article MathSciNet Google Scholar * Lai,
Y., Wu, B., Chen, L. & Zhao, H. A statistical method for identifying differential gene–gene co-expression patterns. _Bioinformatics_ 20, 3146–3155 (2004). Article CAS PubMed Google
Scholar * Horvath, S. et al. Analysis of oncogenic signaling networks in glioblastoma identifies <em>ASPM</em> as a molecular target. _Proc. Natl Acad. Sci._ 103, 17402
LP–17417407 (2006). Article ADS Google Scholar * Thorsson, V. et al. The immune landscape of cancer. _Immunity_ 48, 812–830.e14 (2018). Article CAS PubMed PubMed Central Google
Scholar * McKenzie, A. T., Katsyv, I., Song, W.-M., Wang, M. & Zhang, B. DGCA: a comprehensive R package for differential gene correlation analysis. _BMC Syst. Biol._ 10, 106 (2016).
Article PubMed PubMed Central Google Scholar * Blenman, K. R. M. et al. Sentinel lymph node B cells can predict disease-free survival in breast cancer patients. _NPJ Breast Cancer_ 4, 28
(2018). Article PubMed PubMed Central Google Scholar * Chang, A. Y. et al. Spatial organization of dendritic cells within tumor draining lymph nodes impacts clinical outcome in breast
cancer patients. _J. Transl. Med._ 11, 242 (2013). Article PubMed PubMed Central Google Scholar * Wortman, J. C. et al. Spatial distribution of B cells and lymphocyte clusters as a
predictor of triple-negative breast cancer outcome. _NPJ Breast Cancer_ 7, 84 (2021). Article CAS PubMed PubMed Central Google Scholar * Mandl, J. N. et al. Quantification of lymph node
transit times reveals differences in antigen surveillance strategies of naïve CD4+ and CD8+ T cells. _Proc. Natl Acad. Sci._ 109, 18036–18041 (2012). Article ADS CAS PubMed PubMed
Central Google Scholar * Sumpter, T. L., Balmert, S. C. & Kaplan, D. H. Cutaneous immune responses mediated by dendritic cells and mast cells. _JCI Insight_ 4, e123947 (2019). Article
PubMed PubMed Central Google Scholar * Komi, D. E. A., Khomtchouk, K. & Santa Maria, P. L. A review of the contribution of mast cells in wound healing: involved molecular and
cellular mechanisms. _Clin. Rev. Allergy Immunol._ 58, 298–312 (2020). Article CAS PubMed Google Scholar * Galli, S. J. & Tsai, M. Mast cells in allergy and infection: versatile
effector and regulatory cells in innate and adaptive immunity. _Eur. J. Immunol._ 40, 1843–1851 (2010). Article CAS PubMed PubMed Central Google Scholar * Varricchi, G. et al. Are mast
cells MASTers in cancer? _Front. Immunol._ 8, 424 (2017). Article PubMed PubMed Central Google Scholar * Dabiri, S. et al. The presence of stromal mast cells identifies a subset of
invasive breast cancers with a favorable prognosis. _Mod. Pathol._ 17, 690–695 (2004). Article PubMed Google Scholar * Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of
tumor infiltrating myeloid cells. _Cell_ 184, 792–809.e23 (2021). Article CAS PubMed Google Scholar * Pittoni, P. et al. Mast cell targeting hampers prostate adenocarcinoma development
but promotes the occurrence of highly malignant neuroendocrine cancers. _Cancer Res._ 71, 5987–5997 (2011). Article CAS PubMed Google Scholar * Aponte-López, A. & Muñoz-Cruz, S. in
_Tumor Microenvironment: Hematopoietic Cells—Part B_ (ed. Birbrair, A.) 159–173 (Springer International Publishing, 2020). https://doi.org/10.1007/978-3-030-49270-0_9. * Aponte-López, A.,
Fuentes-Pananá, E. M., Cortes-Muñoz, D. & Muñoz-Cruz, S. Mast cell, the neglected member of the tumor microenvironment: role in breast cancer. _J. Immunol. Res._ 2018, 2584243 (2018).
Article PubMed PubMed Central Google Scholar * Majorini, M. T. et al. Infiltrating mast cell–mediated stimulation of estrogen receptor activity in breast cancer cells promotes the
luminal phenotype. _Cancer Res._ 80, 2311–2324 (2020). Article CAS PubMed Google Scholar * De Monte, L. et al. Basophil recruitment into tumor-draining lymph nodes correlates with Th2
inflammation and reduced survival in pancreatic cancer patients. _Cancer Res._ 76, 1792–1803 (2016). Article PubMed Google Scholar * Miller, H. L. et al. Altered ratio of dendritic cell
subsets in skin-draining lymph nodes promotes Th2-driven contact hypersensitivity. _Proc. Natl Acad. Sci._ 118, e2021364118 (2021). Article CAS PubMed PubMed Central Google Scholar *
Zagorulya, M. et al. Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. _Immunity_ 56,
386–405.e10 (2023). Article CAS PubMed PubMed Central Google Scholar * Somasundaram, R. et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. _Nat.
Commun._ 12, 346 (2021). Article CAS PubMed PubMed Central Google Scholar * Xiao, Y. et al. Multi-omics profiling reveals distinct microenvironment characterization and suggests immune
escape mechanisms of triple-negative breast cancer. _Clin. Cancer Res._ 25, 5002 LP–5005014 (2019). Article Google Scholar * Liu, Y. & Cao, X. The origin and function of
tumor-associated macrophages. _Cell Mol. Immunol._ 12, 1–4 (2015). Article PubMed Google Scholar * Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and
fibrosis. _Immunity_ 44, 450–462 (2016). Article CAS PubMed PubMed Central Google Scholar * Bouchery, T. & Harris, N. L. Specific repair by discerning macrophages. _Science_ 356,
1014 (2017). Article ADS CAS PubMed Google Scholar * Mehta, A. K., Kadel, S., Townsend, M. G., Oliwa, M. & Guerriero, J. L. Macrophage biology and mechanisms of immune suppression
in breast cancer. _Front. Immunol._ 12, 626 (2021). Article Google Scholar * Watanabe, S., Alexander, M., Misharin, A. V. & Budinger, G. R. S. The role of macrophages in the resolution
of inflammation. _J. Clin. Invest._ 129, 2619–2628 (2019). Article PubMed PubMed Central Google Scholar * Chen, Y. et al. Pharmaceutical targeting Th2-mediated immunity enhances
immunotherapy response in breast cancer. _J. Transl. Med._ 20, 615 (2022). Article CAS PubMed PubMed Central Google Scholar * Diedenhofen, B. & Musch, J. cocor: a comprehensive
solution for the statistical comparison of correlations. _PLoS ONE_ 10, e0121945 (2015). Article PubMed PubMed Central Google Scholar * Bankhead, P. et al. QuPath: Open source software
for digital pathology image analysis. _Sci. Rep._ 7, 16878 (2017). Article ADS PubMed PubMed Central Google Scholar * Ester, M., Kriegel, H.-P., Sander, J. & Xu, X. A density-based
algorithm for discovering clusters in large spatial databases with noise. In: _Proc. 2nd International Conference on Knowledge Discovery and Data Mining_ (eds Simoudis, E., Han, J. &
Fayyad, U.) 226–231 (AAAI Press, 1996). Download references ACKNOWLEDGEMENTS The authors would like to thank Michele Kirschenbaum for obtaining patient consent, procuring tissue samples, and
obtaining clinical information. We thank Dr. Russell Rockne and Dr. Andrei Rodin for suggestions on algorithm improvement. We thank Dr. Kim Margolin for valuable translational suggestions.
We thank Dr. Haiqing Li for critical review of this manuscript. We appreciate the funding resource of Stand Up To Cancer and Breast Cancer Research Foundation grant SU2C-BCRF 2015-001 (PPL).
AUTHOR INFORMATION Author notes * Lei Wang Present address: International Cancer Center, Shenzhen University Medical School, 518060, Shenzhen, Guangdong, China * Min Hui Lim Present
address: Genomics Core, Cleveland Clinic, Cleveland, OH, 44106, USA * Christina H. Wei Present address: Pathology Laboratory Administration, Los Angeles General Medical Center, Los Angeles,
CA, 90033, USA * Caroline Hoffmann Present address: Owkin, Inc., New York, NY, 10003, USA AUTHORS AND AFFILIATIONS * Department of Immuno-Oncology, Beckman Research Institute, City of Hope,
Duarte, CA, USA Weihua Guo, Jiayi Tan, Lei Wang, Colt A. Egelston, Diana L. Simons, Min Hui Lim, Shawn Solomon, Caroline Hoffmann & Peter P. Lee * Irell & Manella Graduate School of
Biological Sciences, City of Hope Comprehensive Cancer Center, Duarte, CA, 91010, USA Jiayi Tan * Department of Surgery, City of Hope Comprehensive Cancer Center, Duarte, CA, 91010, USA
Aaron Ochoa * Mork Family Department of Chemical Engineering & Material Science, University of Southern California, Los Angeles, CA, 90089, USA Lu Wang * Department of Medical Oncology,
City of Hope, Duarte, CA, 91010, USA James Waisman * Department of Pathology, City of Hope, Duarte, CA, 91010, USA Christina H. Wei, Joo Song & Daniel Schmolze Authors * Weihua Guo View
author publications You can also search for this author inPubMed Google Scholar * Jiayi Tan View author publications You can also search for this author inPubMed Google Scholar * Lei Wang
View author publications You can also search for this author inPubMed Google Scholar * Colt A. Egelston View author publications You can also search for this author inPubMed Google Scholar *
Diana L. Simons View author publications You can also search for this author inPubMed Google Scholar * Aaron Ochoa View author publications You can also search for this author inPubMed
Google Scholar * Min Hui Lim View author publications You can also search for this author inPubMed Google Scholar * Lu Wang View author publications You can also search for this author
inPubMed Google Scholar * Shawn Solomon View author publications You can also search for this author inPubMed Google Scholar * James Waisman View author publications You can also search for
this author inPubMed Google Scholar * Christina H. Wei View author publications You can also search for this author inPubMed Google Scholar * Caroline Hoffmann View author publications You
can also search for this author inPubMed Google Scholar * Joo Song View author publications You can also search for this author inPubMed Google Scholar * Daniel Schmolze View author
publications You can also search for this author inPubMed Google Scholar * Peter P. Lee View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
W.G. and P.P.L. conceptualized experiments; W.G., J.T., Lei W., C.A.E., D.L.S., A.O., M.L., S.S. and J.S. conducted experiments; W.G., Lei W. and Lu W. analyzed experimental data; W.G. and
P.P.L. wrote the original manuscript; W.G., J.T., Lei W., Lu W., C.A.E., D.L.S., M.L., S.S., J.W., C.W., C.H., D.S. and P.P.L. revised and finalized the manuscript. CORRESPONDING AUTHOR
Correspondence to Peter P. Lee. ETHICS DECLARATIONS COMPETING INTERESTS C.H. serves as advisor and has received honoraria from Nanobiotix and Owkin. All other authors declare that they have
no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Jo Van Ginderachter and the other, anonymous, reviewer(s) for their contribution to the peer review
of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES DATA FILE S1 DATA FILE S2 DATA FILE S3
REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International
License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived
from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line
to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Guo, W., Tan, J., Wang, L. _et al._ Tumor draining lymph nodes connected to cold triple-negative breast cancers are characterized by Th2-associated
microenvironment. _Nat Commun_ 15, 8592 (2024). https://doi.org/10.1038/s41467-024-52577-y Download citation * Received: 30 March 2023 * Accepted: 10 September 2024 * Published: 04 October
2024 * DOI: https://doi.org/10.1038/s41467-024-52577-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
