A conserved fungal morphogenetic kinase regulates pathogenic growth in response to carbon source diversity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Fungal pathogens must exhibit strong nutritional plasticity, effectively sensing and utilizing diverse nutrients to support virulence. How the signals generated by nutritional sensing are
efficiently translated to the morphogenetic machinery for optimal growth and support of virulence remains incompletely understood. Here, we show that the conserved morphogenesis-related
kinase, CotA, imparts isoform-specific control over Aspergillus fumigatus invasive growth in host-mimicking environments and during infection. CotA-mediated invasive growth is responsive to
exogenous carbon source quality, with only preferred carbon sources supporting hyphal morphogenesis in a mutant lacking one of two identified protein isoforms. Strikingly, we find that the
CotA protein does not regulate, nor is cotA gene expression regulated by, the carbon catabolite repression system. Instead, we show that CotA partially mediates invasive growth in specific
carbon sources and virulence through the conserved downstream effector and translational repressor, SsdA. Therefore, A. fumigatus CotA accomplishes its conserved morphogenetic functions to
drive pathogenic growth by translating host-relevant carbon source quality signals into morphogenetic outputs for efficient tissue invasive growth.
Invasive aspergillosis, caused mainly by Aspergillus fumigatus, is the most threatening clinical manifestation of Aspergillus spp. and is initiated by the inhalation of conidia that deposit
in the airways. In immunosuppressed hosts, these inhaled conidia are able to undergo germination, hyphal extension and tissue invasion which leads to fungal dissemination and critical
outcomes1. The human lung represents a hostile environment where germinating Aspergillus conidia must rapidly adapt and respond to a series of challenges including, limited availability of
nutrients, fluctuations of oxygen saturation and temperature, and generation of reactive oxygen and nitrogen species by the host defense mechanisms2,3,4,5. Successful adaptation allows the
invading pathogen to restore cellular homeostasis and acquire nutrients that are not easily available6. The Aspergilli display impressive nutritional and metabolic plasticity that allow
efficient growth and development, supporting virulence in a variety of host settings2,7.
How the signals generated by nutrient sensing and utilization are transmitted to specific morphogenetic machinery for support of fungal pathogenesis has been a focus of study for many
years8. Unfortunately, this question remains poorly understood in pathogens like Aspergillus. Here, through a screen to identify protein kinases required for invasive growth in a
host-mimicking environment, we uncovered a previously unrecognized role for a conserved morphogenetic protein kinase in the pathobiology of invasive aspergillosis. Through a series of in
vitro and in vivo studies, we show that expression of two CotA kinase protein isoforms, long and short, is required for tissue invasive hyphal morphogenesis. We show that loss of the long
isoform imparts strict, carbon-source mediated growth requirements on A. fumigatus that is independent of the carbon catabolite repression (CCR) system. Finally, we show that the putative
translational repressor, SsdA, partially regulates the carbon source-dependent phenotypes. Taken together, our findings uncover new biological mechanisms underpinning pathogenic fungal
growth in host-relevant carbon source environments.
To identify protein kinases that are necessary for growth in the nutritional landscape of the host lung, we first generated a lung environment-mimicking culture medium by combining a
homogenized mouse lung tissue suspension, buffered to pH 7, with Noble agar (termed, Lung Agar). We then screened an A. fumigatus protein kinase disruption mutant library, generated
previously by our laboratory, for colony formation on this Lung Agar9 (Supplementary Fig. 1). As a growth control, we used glucose minimal media (GMM) buffered to pH 710. Of the 118 mutants
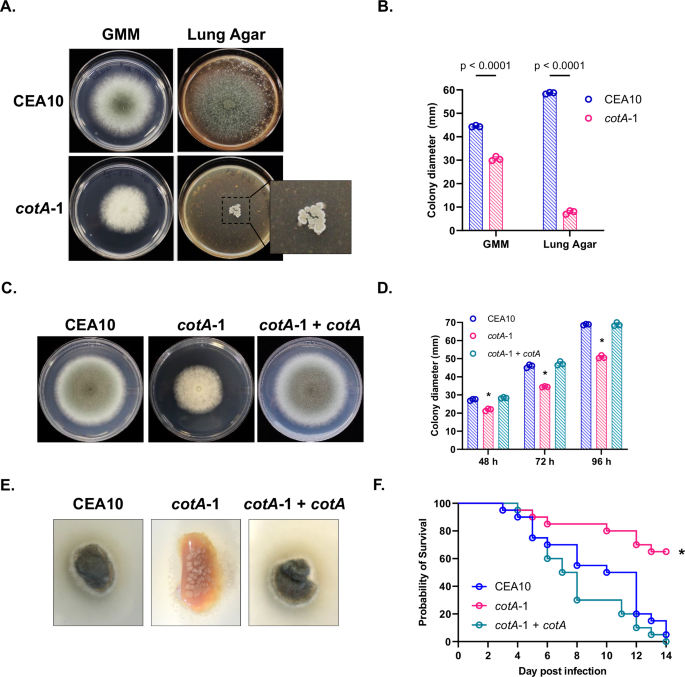
screened, only one mutant strain displayed a pronounced reduction in growth accompanied by an inability to support normal hyphal extension on Lung Agar, in comparison to growth on GMM (Fig.
1A). This mutant (cotA-1) bears a disruption at the 5’ end of the gene encoding the conserved morphogenetic Nuclear Dbf2-Related (NDR) protein kinase, CotA (AFUB_068890)9. As shown in Fig.
1A–D, the cotA-1 mutant develops a moderate growth defect in comparison to the parental strain, CEA10 (p
