Atypical rhizobia trigger nodulation and pathogenesis on the same legume hosts
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The emergence of commensalism and mutualism often derives from ancestral parasitism. However, in the case of rhizobium-legume interactions, bacterial strains displaying both
pathogenic and nodulation features on a single host have not been described yet. Here, we isolated such a bacterium from _Medicago_ nodules. On the same plant genotypes, the T4 strain can
induce ineffective nodules in a highly competitive way and behave as a harsh parasite triggering plant death. The T4 strain presents this dual ability on multiple legume species of the
Inverted Repeat-Lacking Clade, the output of the interaction relying on the developmental stage of the plant. Genomic and phenotypic clustering analysis show that T4 belongs to the
nonsymbiotic _Ensifer adhaerens_ group and clusters together with T173, another strain harboring this dual ability. In this work, we identify a bacterial clade that includes rhizobial
strains displaying both pathogenic and nodulating abilities on a single legume host. SIMILAR CONTENT BEING VIEWED BY OTHERS NOPP2 EFFECTOR OF _BRADYRHIZOBIUM ELKANII_ USDA61 IS A DETERMINANT
OF NODULATION IN _VIGNA RADIATA_ CULTIVARS Article Open access 19 October 2024 THE _BRADYRHIZOBIUM DIAZOEFFICIENS_ TYPE III EFFECTOR NOPE MODULATES THE REGULATION OF PLANT HORMONES TOWARDS
NODULATION IN _VIGNA RADIATA_ Article Open access 16 August 2021 RHIZOBIA USE A PATHOGENIC-LIKE EFFECTOR TO HIJACK LEGUMINOUS NODULATION SIGNALLING Article Open access 21 January 2021
INTRODUCTION Microbial symbionts are currently thought to emerge from transitions along the parasite-mutualist _continuum_ and these shifts are mainly driven by genetic, environmental and
ecological changes1. Shifts from mutualism to parasitism appear rare while the emergence of commensal and mutualistic organisms from parasitic ancestors are more frequent1,2. In the case of
the rhizobium-legume mutualism, rhizobia, which trigger nitrogen-fixing nodules on their host, are in general rather phylogenetically distant from pathogenic microbes3, and how mutualism
emerged in rhizobia remains unclear4,5. To our knowledge, a rhizobial strain harboring features of both pathogenic and nodulation abilities on a single host has not been identified so far.
Like other plants, legumes are challenged by the presence of many microbes including harmful and beneficial ones, and evolution resulted in perception mechanisms that allow plants to
recognize friends and foes6. Amongst the beneficial microorganisms interacting with legumes, rhizobia can confer a crucial advantage to their hosts when developing on nitrogen-poor
substrates. Rhizobia-legumes symbiotic interactions result in the formation of specialized root-derived organs, namely nodules, where rhizobia are hosted inside of plant cells. Within
nodules, rhizobia can fix atmospheric nitrogen and convert it into ammonium, a form of nitrogen assimilable by the host. As a benefit, rhizobia receives all nutrients from the plants7. In
agreement with the idea that mutualistic relationships can emerge from pathogenic associations, the interaction between rhizobia and legumes is accompanied by intricate molecular dialogues
and subsequent infection processes. These interactions involve various molecular actors, including exopolysaccharides, protein secretion systems and Nod factors, which not only contribute to
mutualism but also interfere with the signaling pathways of plant innate immunity2,8,9,10,11,12. For many years, nodule inhabitants have been considered as pure cultures of rhizobia because
of the tremendous densities of these bacteria within nodules and because of the molecular dialogue that takes place between the plant and the rhizobia in the rhizosphere that leads to
specific interactions. However, this view has been drastically challenged and it is now admitted that, in addition to nitrogen-fixing rhizobia, legume nodules host complex and diverse
microbial populations called the nodule accessory microbiome13,14,15. The roles of these microorganisms as well as the way they enter nodules remain largely unknown. In this study, our
primary objective was to characterize nodule endophytes, and thus we initially isolated bacterial strains from nodules of _Medicago littoralis_ R108 (formerly _Medicago truncatula_ R108)
inoculated with soil. Subsequently, we identified a distinctive strain of _Ensifer adhaerens_, referred to as T4, which exhibited divergent interactions with its host depending on the
developmental stage of the plant and on the presence or absence of bona fide rhizobia capable of inducing nodules. Notably, we discovered that T4 had the ability to co-occupy nodules induced
by other rhizobia, but it can also independently trigger nodule formation. Remarkably, we observed that the inoculation of young seedlings with T4 triggered the unexpected outcome of plant
death. We also showed that the versatile behavior of T4 was not restricted to the _Medicago_ genus and that it affected various legume species from the Inverted Repeat-Lacking Clade (IRLC),
a monophyletic subclade of the Papilionoideae. RESULTS _ENSIFER ADHAERENS_ T4 HAS VERSATILE INTERACTIONS WITH ITS HOST Bacterial endophytes were isolated from _Medicago littoralis_ R108
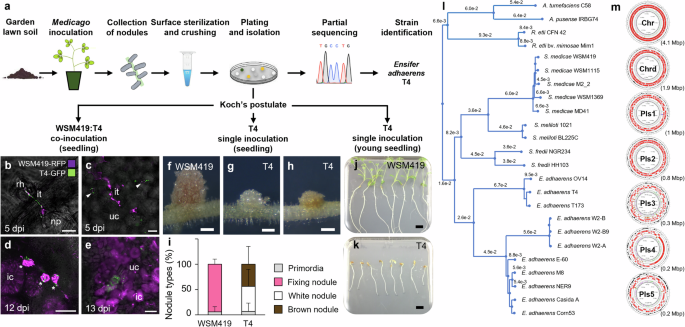
nodules (R108; formerly _Medicago truncatula_ ssp. _tricycla_ R10816,17) using garden lawn soil as an inoculant (Fig. 1a). Among the isolated bacteria, partial sequencing of _16S rRNA_,
_gyrB_, _rpoD_ and _recA_ suggested that isolates T1, T3 and T4 represented a single _Ensifer adhaerens_ (_E. adhaerens_) strain. The strain _E. adhaerens_ T4 (T4) was selected for further
investigations (Supplementary Data 1). While intending to ascertain, through co- and single inoculations on _M. truncatula_ A17 (A17) seedlings, that T4 was a bona fide nodule endophyte, we
observed that T4 was capable to both co-infect nodules together with the reference strain _Sinorhizobium medicae_ WSM419 (WSM419) and to trigger nodules by itself (Fig. 1b–i). Co-inoculation
experiments confirmed that T4 was indeed a nodule endophyte since both T4 and WSM419 co-occupied infection threads within root hair and nodule primordium, and that they were found inside
common nodule infected cells (Fig. 1b–e). These co-infection events were relatively rare and in cases of co-occurrence, WSM419 was the dominating population in the nodule (Fig. 1e). When T4
was inoculated alone, the induced nodules did not show the characteristic pinkish coloration of nitrogen-fixing nodules (such as those induced by WSM419 alone or co-occupied with T4).
Instead, T4 nodules were small, white or brown and without detectable nitrogenase activity, suggesting that T4 nodules were not functional (Fig. 1f–i and Fig. 2h). The ability of T4 to
trigger small white and brown nodules was not restricted to A17 since we also observed this trait in other species, including _M. truncatula_ F83005.5, _Medicago sativa_ cv. salina and
_Medicago sativa_ cv. Super GRI8 (Supplementary Fig. 1). Methylene blue staining of T4 brown nodules revealed the accumulation of phenolic compounds, which are typically found upon defense
reactions in plants (Supplementary Fig. 1). In addition to these interactions, we found that when T4 was inoculated alone on young germinating seedlings, T4 triggered the death of the host
(Fig. 1j, k). Such a particular dual interaction was also observed using _E. adhaerens_ strains T1 and T3 (Supplementary Data 1). To gain insight into the unique behavior of these _E.
adhaerens_ strains, we sequenced the genome of the T4 strain. Genome clustering analysis confirmed that T4 grouped with other _E. adhaerens_ strains and indicated that T4 was closely related
to _E. adhaerens_ T173, a bacterium isolated from _Melilotus albus_ nodules (18; Fig. 1l). The T4 genome was 8,451,442 bp and composed of seven circular replicons, one chromosome (Chr), one
chromid (Chrd) and five plasmids, Pls1 to Pls5, ranking the T4 genome among the largest _E. adhaerens_ genomes available today (Fig. 1m; Supplementary Fig. 2; Supplementary Data 2;
Supplementary Data 3). Interestingly, Pls3 presented typical features of rhizobial symbiotic mobile genetic elements as it carried a remarkably high density of transposase encoding genes as
well as genes potentially involved in the synthesis and secretion of Nod factors (NFs; Supplementary Fig. 2; Supplementary Fig. 3). Thus, our results highlight that T4 is an atypical _E.
adhaerens_ strain capable of versatile interactions with its host. T4 can behave as a nodule endophyte co-infecting nodules with bona fide rhizobia, it can induce the formation of nodules by
itself and it can behave as a pathogen triggering the death of the host. THE T4 INFECTION IS NFS-DEPENDENT AND RESULTS IN FIX- NODULES Sequence analysis confirmed that the T4 genome harbors
the determinants of nodulation on four loci of the T4 Pls3 (Fig. 2a; Supplementary Fig. 319). Blast searches against the NCBI nucleotide database indicated that genes involved in the
production, decoration, and secretion of T4 NFs were almost identical to those of T173 and WSM419 (Supplementary Fig. 3). Phylogenetic reconstruction based on _nodABCIJ_ genes indicated that
T4, T173 and WSM419 genes form a cluster distinct from that of _Sinorhizobium meliloti_ strains (Supplementary Fig. 3). The proximity between WSM419 and T4 _nod_ genes was further confirmed
by comparing the structure of WSM419 and T4 NFs (Supplementary Fig. 4). Confocal microscopy showed that T4 entered the plant roots through the root hair infection pathway (Fig. 2b). In
addition, the transcriptional fusion between the promoter of the early nodulin encoding gene _MtENOD11_ and the _GusA_ reporter gene was activated during T4 infection, suggesting that a
NFs-dependent molecular dialogue and infection process was taking place (Fig. 2c, d). This was further supported by the analysis of a T4Δ_nodABC_ deletion mutant unable to elicit nodules
(Fig. 2e) and by the fact that the presence of a nitrogen source inhibited the nodulation ability of T4 (Fig. 2f). In agreement with the fix_−_ phenotype of T4, we did not detect the
structural genes _nifHDK_ encoding the nitrogenase enzymatic complex in the T4 genome (Fig. 2a and h). T4 is thus a nod_+_ fix_−_ strain harboring core _nod_ genes and lacking core _nif_
genes. This makes T4 able to infect root hair in a NFs-dependent manner and to induce nodules that are ineffective for nitrogen fixation. THE T4 STRAIN IS A HIGHLY COMPETITIVE PARASITE We
next compared the ability of T4 to trigger nodules relative to WSM419. Upon single inoculation, we did not observe any difference in the kinetics of nodule induction when comparing T4 and
WSM419 (Supplementary Fig. 5). Furthermore, a histological time series analysis of T4 and WSM419 nodule development revealed that cortical divisions initiated synchronously (Supplementary
Fig. 7). However, during later stages of nodulation kinetics, we noticed that while WSM419 stopped to initiate nodules, T4 continued to elicit some. Thereby, more nodules accumulated in
T4-inoculated plants as compared to WSM419-inoculated ones (Supplementary Fig. 5; Fig. 2g). Upon T4 and WSM419 co-inoculation, T4 nodules (small white or brown) were easily distinguishable
from those of nitrogen-fixing symbionts (big and pink). Interestingly, when co-inoculated with equal bacterial densities, we systematically observed more nodules induced by T4 than by WSM419
(Fig. 2g). In addition, the nitrogenase activity was significantly reduced relative to plants inoculated with WSM419 alone, indicating that T4 affected the interaction between the host and
efficient symbionts (Fig. 2h). Under both single and co-inoculation conditions, the presence of T4 significantly reduced A17 leaf biomass by 53% and 42%, respectively, relative to WSM419
single inoculation (Supplementary Fig. 6). Similar results were observed using _M. sativa_ cv. salina as a host plant (Supplementary Fig. 6). Moreover, upon T4-WSM419 co-inoculation, we
observed a continuous formation of T4 nodules while the formation of WSM419 nodules was drastically impaired (Supplementary Fig. 6). This higher efficiency in triggering nodules was
confirmed using multiple lines of _Medicago_ (R108, _M. truncatula_ Ghor, _M. sativa_ cv. G969, cv. WL903, cv. Super GRI8 and cv. salina; Supplementary Fig. 6). In addition, co-inoculation
experiments using up to one thousand times more WSM419 cells than T4 ones only resulted in 34% of WSM419 nodules illustrating the high competitiveness of T4 for nodule formation
(Supplementary Fig. 6). Such a success for nodulation during competition was not only observed with WSM419 but was systematically encountered with nineteen other _S. medicae_ and _S.
meliloti_ strains, including strains that have originally been isolated from _M. truncatula_ (M102, M162, STM1643, T027, MD4, ML7 and 102F34; Fig. 2i). Similar experiments using either a
fix- mutant (WSM419∆_nifD_) or a wild type fix- strain (RM41) instead of T4 during competition with WSM419 did not show such a competitive phenotype, indicating that the T4 inability to fix
nitrogen was not the main determinant of its competitiveness (Fig. 2i). Taken together, these data indicate that T4 is particularly competitive for nodule induction and that the accumulation
of T4 fix- nodules instead of nitrogen-fixing ones limits plant development. T4 NODULES UNDERGO EARLY SENESCENCE AND DEFENSE REACTIONS A time series histological analysis of T4 and WSM419
nodule development indicated that rapidly after the initiation of nodule organogenesis, the development of T4 nodules stopped, suggesting an arrest of the meristematic activity (Fig. 3a-f;
Supplementary Fig. 7). During infection, as for WSM419, T4 reached the symbiotic cells and were internalized, however, within T4 nodules, symbiotic cells showed senescence features (Fig. 3g,
i; Supplementary Fig. 7). Indeed, as early as five dpi, 72% of T4-infected cells presented collapsed vacuoles and this phenotype reached 100% of cells by eight dpi while it was not observed
for WSM419-infected cells (Fig. 3g-j; Supplementary Fig. 7). In addition, from five dpi, the size of T4 infected and uninfected cells was significantly reduced relative to WSM419 (Fig.
3g-j; Supplementary Fig. 7). In agreement with these senescence features, we also reported the induction of senescence marker genes in T4 nodules, including _MtCYSTEINE PROTEASE2, 3, 5_
(_MtCP2, 3, 5_) and _MtNAC969_ (Fig. 3k20,21;). Taken together, these results indicated that T4 nodules prematurely underwent a senescence program. In agreement with the accumulation of
phenolic compounds, suggesting defense reactions in T4 nodules, the expression of ethylene biosynthesis and defense marker genes was induced (Supplementary Fig. 1; Supplementary Fig. 8). In
line with the defense and the senescence reactions described above, we also detected the reduced expression of key symbiotic genes required to prevent defense responses and nodule senescence
during _Medicago_-rhizobium symbiosis, including _MtSymbiotic CYSTEINE-RICH RECEPTOR-LIKE KINASE_ (_MtSymCRK_), _MtDEFECTIVE IN NITROGEN FIXATION2_ (_MtDNF2_) and _MtREGULATOR OF SYMBIOSOME
DIFFERENTIATION_ (_MtRSD;_ Fig. 3k22). In addition, we reported the non-induction of the _LEGHAEMOGLOBIN1_ gene in T4 nodules relative to WSM419 nodules (_MtLEGH1_; Fig. 3k). We thus
concluded that T4 nodules rapidly stopped their development and underwent induced senescence accompanied by defense reactions. T4 BACTEROIDS LOST VIABILITY BEFORE MORPHOLOGICAL
DIFFERENTIATION In agreement with the activation of senescence and defense reactions, live/dead staining of bacteroids revealed that from twelve dpi most intracellular T4 cells had lost
their viability (Fig. 3l-u). This result was further confirmed using T4-GFP and WSM419-RFP labeled-strains (Supplementary Fig. 9). Remarkably, even at 21 dpi, while most T4 bacteroids were
dead, some alive T4 bacteroids were detected, likely representing freshly released bacteria from infection threads (Fig. 3v, w). Confocal microscopy analyses also showed that T4 bacteroids
did not undergo the morphological differentiation typically observed for rhizobia during _Medicago_-rhizobium symbiosis (Fig. 3x–ab23). Such a differentiation process is mediated by plant
peptides that are maturated by the signal peptidase _MtDEFECTIVE IN NITROGEN FIXATION 1_ (_MtDNF1_) and targeted to the symbiosome24,25. In the _Mtdnf1-1_ mutant background, the loss of
viability of T4 was delayed, suggesting a contribution of plant-secreted peptides to the death of T4 bacteroids inside symbiotic cells (Fig. 3ac–aj). Together, these data showed the
premature death of T4 bacteroids prior to the morphological differentiation. T4 IS PATHOGENIC ON JUVENILE SEEDLINGS The strain T4 triggered plant death upon inoculation on young germinating
seedlings (Fig. 1j, k). While inoculation of WSM419 did not alter plant viability, T4 induced disease comparable to that of two well-known _Medicago_ pathogens, namely, _Xanthomonas
campestris_ pv. _campestris_ (_Xcc_) and _Xanthomonas euvesicatoria_ pv. _alfalfae_ (_Xea_; Supplementary Fig. 10). Interestingly, older A17 were no longer susceptible to T4. To investigate
this susceptible-to-resistant shift in A17, we inoculated germinating seedlings from different ages, ranging from zero to six days post-stratification (dps) and monitored their primary root
development as a quantitative read-out of survival (Fig. 4a, Supplementary Fig. 11). The strongest deleterious effect was observed when T4 was inoculated from zero to one dps. When
inoculated at zero dps, plant growth stopped early, after three days post-inoculation (dpi). When inoculated at one dps, root development was a bit less impacted and the growth arrest arose
later. For two dps inoculation, the negative impact of T4 was still significant but mild and from three to six dps no significant effect was observed (Fig. 4a). We thus observed a
susceptible-to-resistant shift of A17 which began after one dps. We also observed that reducing the bacterial concentration of the T4 inoculum attenuated symptoms (Supplementary Fig. 12). To
decipher if the T4 pathogenicity relied on the secretion of specific compounds, we inoculated A17 with the cell-free spent medium of A17 seedling-induced T4 liquid culture. This did not
impair plant viability suggesting that T4 bacterial cells are required to trigger pathogenicity (Supplementary Fig. 13). Aside from plant death and root growth arrest, T4-infected
symptomatic plants did not harbor morphological changes at the root level, however, plants displayed symptoms on cotyledons (no aperture; Fig. 4b–i). Indeed, seven days after the treatment
of susceptible plants (0-1 dps), all mock-inoculated plants displayed open cotyledons with the first leaf coming out while 96% of T4-inoculated plants harbored closed cotyledons indicating
that plant development was blocked (Fig. 4b–i). For resistant plants (3 dps and after), T4-inoculated plants did not present closed cotyledons anymore. We also observed the rapid
proliferation of T4 on susceptible A17 whole seedlings along the days following inoculation (Fig. 4j). In addition, we observed that T4 preferentially colonized A17 cotyledons and hypocotyls
rather than roots (Fig. 4k). At seven dpi, in susceptible plants, we observed the proliferation of T4-GFP inside the root tissues where they colonized the intercellular space of the root
cortex and reached the root vasculature (Fig. 4l, m). In resistant plants, the T4-GFP strain displayed a distinct colonization pattern with a reduced presence of bacteria on the root surface
and an entry in the plant tissues only through root hair infection threads without any colonization of intercellular spaces or vasculature (Fig. 4n). Interestingly, the inoculation of
mature WSM419-RPF nodules with T4-GFP did not have any impact on nodules and T4 was not detected within nodules. This suggested that nodules do not represent an entry point for T4
(Supplementary Fig. 14). Taken together, these data indicated that immediately after germination, from zero to one dps, A17 seedlings were susceptible to T4 triggering damping-off symptoms.
Later on, between two and three dps, A17 became resistant to T4. T4 TRIGGERS DISEASE ON VARIOUS IRLC LEGUME SPECIES Besides A17, T4 also triggered disease symptoms leading to plant death on
young seedlings of various _Medicago_ species and lines including _M. truncatula_ Parragio, F83005.5 and Ghor, _M. littoralis_ R108, _M. sativa_ cv. Super GRI8, WL903, G969, Salina, Oleron.
T4 also triggered death of other species from the Inverted Repeat-Lacking Clade (IRLC) including _Melilotus albus, Melilotus officinalis_ and _Trigonella calliceras_. However, T4 showed
faint to no effect on the viability of _Trifolium repens_, _Trifolium pratens_ and _Trifolium subterraneum_, _Pisum sativum_, _Vicia hirsuta, Lens culinaris_ or on the phylogenetically more
distant legumes _Glycine max_, _Lotus japonicus_ Gifu, _Sesbania rostrata, Astragalus_, _Galega orientalis_ and _Galega officinalis_ (Fig. 5a). T4 had no pathogenic effect on _Medicago
polymorpha ciliaris_, a species on which T4 induced nodules indicating that pathogenicity and ability to nodulate can be uncoupled (Fig. 5a). In agreement and despite that NFs were shown to
suppress immunity2, we did not detect any virulence defect when using a T4Δ_nodABC_ mutant unable to produce NFs, demonstrating that NFs were not required for T4 pathogenicity (Fig. 5b). Our
results indicate that T4 can infect a range of legume species belonging to the IRLC, triggering deleterious effects in most of them. T4 BELONGS TO A CLADE CONTAINING NODULATING-PARASITIC
STRAINS Although similar in their organization, with large synthenic regions, the genomes of T4 and T173 displayed significant specificities. For instance, 1344 and 1801 genes were specific
to T173 and T4, respectively, and the Pls5 was specific to T4 (Supplementary Fig. 15). As demonstrated above, T4 and T173 strains belonged to the same phylogenetic cluster and were clearly
separated from most other _E. adhaerens_ sequenced strains (Fig. 1l). In agreement, biolog phenotypic microarrays confirmed that the two strains displayed strong similarities at the
phenotypic level (Fig. 5d; Supplementary Fig. 16; Supplementary Fig. 17; Supplementary Fig. 18; Supplementary Data 6). We evaluated whether T173 and four other _E. adhaerens_ strains,
namely, Casida A, R-7457, BR819 and OV1426,27,28,29,30,31 shared with T4, the dual ability to induce nodules and to trigger plant death, by inoculating young A17 seedlings. Our results
showed that T173 was the only other _E. adhaerens_ strain capable to both induce nodules and to trigger plant death on A17 seedlings (Fig. 5c). Genomic comparison of the sequenced strains
T4, T173, OV14, Casida A and WSM419 indicated that a reduced number of coding sequence families were exclusively shared between T4 and T173 (885 CDS families; Supplementary Fig. 19,
Supplementary Data 7). These T4-T173 specific CDS families were specifically enriched on Pls2 and Pls4 suggesting that virulence determinants of strain T4 and T173 might be carried on these
plasmids (Supplementary Fig. 19). Therefore, T4 and T173 represent closely related strains able to induce nodules as well as to behave as parasites on a single host plant. DISCUSSION It has
recently been proposed that the symbiotic response was mostly in place in the most recent ancestor of the root nodule symbiosis-forming species more than 90 million years ago32.
Nevertheless, very little is known about how mutualistic symbionts can emerge from parasitic or commensal associations. The evolutionary trajectory of nodulation has been investigated in the
past, notably through the artificial transfer of the nodulation ability towards non-nodulating bacterial species. This has been carried out with success using the phytopathogenic bacteria
_Ralstonia solanacearum_ on _Mimosa pudica_ or using _Agrobacterium tumefaciens_ on _Phaseolus vulgaris_ and _Leucaena esculenta_33,34. In addition, phylogenetic proximity was observed
between pathogenic and symbiotic bacteria with sometimes blurry boundaries. For instance, the non-pathogenic strain IRBG74 is genomically very close to pathogenic _Agrobacteria_ but it
nodulates sesbania35. We isolated and characterized an atypical bacterial strain, namely _Ensifer adhaerens_ T4 (T4), that naturally displays the ability to trigger drastic disease and to
induce nodules on a single legume host species depending on the plant developmental stage. Remarkably, we found that another _E. adhaerens_ strain, namely T17318, also shared such an
atypical behavior. Our findings are in agreement with previous research works reporting that T173 elicited large numbers of small white fix- nodules on several legume species including
_Medicago sativa_, _Medicago lupulina, Melilotus albus_ and _Macropitilum atropurpureum_, however, its pathogenic behavior was not reported18. In addition, we reported the very high
competitiveness of the T4 for nodule formation under competition with known efficient and inefficient symbiotic strains. We demonstrated that such a trait was conserved in T173
(Supplementary Fig. 20). Recent phylogenetic studies demonstrated that the _Ensifer_ (syn. _Sinorhizobium_) genus is actually subdivided into two main clades, a symbiotic clade and a
nonsymbiotic clade, containing _Sinorhizobium_ strains and _Ensifer_ strains, respectively36,37. Here, we showed that T4 groups together with _E. adhaerens_ species and therefore belongs to
the nonsymbiotic clade. To date, the T4 strain represents the third known _Ensifer_ species belonging to the nonsymbiotic clade while harboring the core _nodABC_ genes, together with T173
and _Ensifer sesbaniae_ that harbor _nodABC_ and _nifHDK_ loci, the latter being capable to fix nitrogen36,38. Within the _E. adhaerens_ species, although harboring substantial genomic
differences, T4 and T173 group together and form a phylogenetic cluster relatively distinct from other sequenced _E. adhaerens_ strains, suggesting the existence of a taxonomic group
exhibiting such a particular pathogenic/nodulating phenotype. The T4 and T173 closest genome available corresponds to that of _E. adhaerens_ OV14, a non-pathogenic bacterium isolated from
the rhizosphere of _Brassica napus_, which is used for plant genetic transformation as an alternative to _Agrobacterium_30,31. The reliability of such a T4-T173 taxonomic group is supported
by the fact that strains T4 and T173 are far from being clonal and that they have been isolated from two distinct continents, in France and in Canada, respectively18. The organization of T4
and T173 genomes, especially for replicons carrying _nod_ genes, is substantially different suggesting either old and divergent or independent and more recent horizontal acquisitions. Based
on sequence analysis (Supplementary Fig. 3), it seems reasonable to speculate that _E. adhaerens_ T4 and T173 nodulation genes were acquired from either one, or two distinct _S. medicae_
strains. The T4-T173 cluster is also supported by the similarity of metabolic capacities that are shared between T4 and T173. The symbiotic plasmids of T4 and T173 were remarkably compact
(T4, 295 kbp; T173, 204 kbp) compared to the size of symbiotic plasmids from other _S. medicae_ or _S. meliloti_ species (WSM419, 1245 kbp; SU277, 1024 kbp; WSM1115, 1128 kbp; 1021, 1354
kbp). Furthermore, these two plasmids display a high proportion of repeated regions (11% and 27%, for T173 and T4, respectively) which is a typical feature of instability and suggests an
ongoing genetic erosion of those replicons. Nevertheless, until now T4 and T173 symbiotic plasmids have been maintained. The capacity of these atypical _E. adhaerens_ strains to form and
colonize nodules at high frequency without fixing nitrogen might represent an evolutionary asset allowing the diversification of _E. adhaerens_ ecological niches. Indeed, we were able to
demonstrate that, as regular nitrogen-fixing symbionts, the T4 strain is also able to resume growth from senescent nodules (Supplementary Fig. 21). However, it should be noted that the
ecological success of this clade of nodule-triggering soil bacteria does not only rely on their ability to achieve nodulation and to resume growth post nodule senescence but likely results
from multiple factors such as their fitness in the soil in the presence of competitors and/or to their ability to benefit from their host through various mechanisms. Notably, the pathogenic
behavior of the T4-T173 clade likely contributes to its ecological success. Moreover, it would be relevant to better determine the geographical distribution of such strains. The nodulation
ability has been independently and frequently lost during evolution indicating an apparent selection against symbiosis39. Such mutualism breakdowns have been proposed to be due to the spread
of non-fixing cheater symbionts exploiting the benefit of the host association without returning any benefit to the host1,39. The discovery of the T4-T173 clade and its unexpected
nodulating pathogenic strains suggests that the trade-off between cost and benefit in nodulation can be even more unbalanced than when considering only non-fixing rhizobia. Such a clade
might contribute to the selective pressure acting against symbiosis in legumes. It has been proposed that cheaters and mutualists often coexist because of tradeoffs between presence of
cheaters, plant benefits from mutualists and costly induction of defenses40. When it behaves as an endophyte, the T4 strain follows the typical features of a cheating organism. Except that
the T4 uses its own NFs to enter the nodule in contrast to other nodule endophytes hijacking symbiont’s NFs signaling41. In agreement with our observations, it has also been shown that
cheaters are rather present at low levels within _Medicago sativa_ nodules mixed-infected with mutualists42. The presence of cheaters is likely masked by the dominant mutualists and they
thus escape sanction. In addition, it has been reported that non-rhizobial nod− and/or fix− nodule endophytes better survive post-nodulation than mutualistic rhizobia43. This reflects that,
below a certain threshold, cheaters thrive within nodules and that nodules represent a niche for the proliferation of non-beneficial bacteria. Our study reports the isolation of a unique
clade of parasitic microbes harboring and using NFs. Hence, the atypical behavior of T4 renders this strain very valuable as a tool to better understand the molecular frontiers existing
between parasites and mutualists, the evolutionary trajectory of legume-rhizobium mutualistic interactions as well as transition mechanisms occurring along the parasitism-mutualism
_continuum_. In addition, T4 can also be used to better understand the genetic control of nodule immunity and chronic infection during legume-rhizobium symbiosis. METHODS TRAPPING,
ISOLATION, DNA EXTRACTION AND IDENTIFICATION OF NODULE ENDOPHYTES Wild type _M. littoralis_ R108 seedlings (Formerly _M. truncatula ssp. tricycla_16,17 were grown in 1.5 L pots containing a
mixture of sterile sand-perlite (1:3, v:v) and inoculated with 50 mL of non-sterile garden lawn soil collected in Limours-en-Hurepoix, France, N 48.649137°, E 2.069144°. 30-day-old nodulated
roots were harvested, washed under tap water to remove adhering soil particles, sterilized with NaOCl (6%) for 5 min and washed three times with sterile water. Pink (nitrogen-fixing) as
well as white and brown (non-fixing) nodules were collected, pooled and crushed in 1.5 mL tubes containing 200 µL of YEM medium44. Nodule extracts were plated on YEM agar medium and
incubated for 48 h at 30 °C. Nodule endophyte colonies were isolated according to their color, shape, shiningness, exopolysaccharide secretion and antibiotic resistance. Bacterial DNA was
extracted using NucleoSpin Microbial DNA Mini kit according to the manufacturer’s recommendation (https://www.mn-net.com). Partial _16S rRNA_, _gyrB, rpoD_ and _recA_ encoding sequences were
amplified by PCR using Q5 High-Fidelity DNA Polymerase according to the manufacturer’s recommendation (https://www.neb-online.fr/). PCR products were sequenced (www.eurofinsgenomics.eu) and
blast on NCBI for sequence homology (https://blast.ncbi.nlm.nih.gov/). Primers used for PCR and sequencing are provided in (Supplementary Data 4). PLANT MATERIAL AND GROWTH CONDITIONS
Experiments were essentially performed using _Medicago truncatula ssp. truncatula_ ecotype Jemalong A1745. The A17 transgenic derivatives, A17 _proENOD11::gusA_46 and A17 _Mtdnf1-1_24 were
also used. Other legume species were used, including _Glycine max, Lotus japonicus_ Gifu_, Sesbania rostrata, Astragalus sp., Galega orientalis, Galega officinalis, Medicago truncatula_
(Parragio, F83005.5, Ghor)_, Medicago littoralis_ R108, _Medicago sativa_ (WL903, G969, Salina, Oleron and Super GRI8), _Medicago polymorpha ciliaris, Trigonella calliceras, Melilotus albus,
Melilotus officinalis, Trifolium pratense, Trifolium repens_, _Trifolium subterraneum_, _Pisum sativum_ var. caméor, _Vicia hirsuta_ and _Lens culinaris_. Seeds were scarified with
sandpaper and surface-sterilized with NaOCl (1.5 g of active chlorine per 1 L of water) supplemented with one droplet of liquid soap for 30 min. Three washes were performed with sterile
water. Seeds were stratified for two days at 4 °C under darkness on water agar plates and then transferred for 22 h (for pathogenic assay) or for 70 h (for symbiotic assay) at 24 °C under
darkness for germination. Seedlings were grown in vitro on Buffered Nodulation Media (BNM47) or in 1.5 L pots containing a mixture of sand-perlite (1:3, v:v). Plants were grown in controlled
environmental chambers under 16 h: 8 h, light: dark, 24 °C: 24 °C, day: night, 60% relative humidity and 200 μE light intensity. Plants grown in sand-perlite mixture were watered with 1 g/L
of nitrogen-free nutritive solution (NPK 0-15-40; Plantprod). MICROBIAL MATERIALS AND GROWTH CONDITIONS _Ensifer adhaerens_, _Sinorhizobium medicae_, _Sinorhizobium meliloti_, _Xanthomonas_
sp. and _Escherichia coli_ strains physically used in this study are provided in Supplementary Data 8. SOLID CULTURE OF RHIZOBIA Strains were grown on YEB plates for 48 h at 30 °C48. LIQUID
CULTURE OF RHIZOBIA Bacteria were grown in 20 mL of YEB for 48 h at 30 °C. PREPARATION OF RHIZOBIA AND _XANTHOMONAS_ SUSPENSION Liquid cultures were centrifuged for 20 min at 3200 × _g_,
washed twice and adjusted at OD600 nm: 0.1. For in vitro assays, 1 mL of bacterial suspensions was used per plate containing eight seedlings. For assay in pots containing sand:perlite, 50 mL
of bacterial suspension was used per pot containing five seedlings. SYMBIOTIC ASSAYS, CO-INOCULATION ASSAYS AND PATHOGENIC ASSAYS SYMBIOTIC ASSAYS 22 h post-stratification, seedlings were
transferred on BNM plates and 48 h after transfer, seedlings were root-inoculated with 1 mL of a bacterial suspension at OD600 nm: 0.1 (For symbiotic assays, seedlings were inoculated 70 h
post-stratification). CO-INOCULATION ASSAYS Co-inoculation assays were performed as described for symbiotic assays with a mixture of two bacterial suspensions (1:1, v:v) adjusted to final
OD600 nm: 0.1 each. PATHOGENIC ASSAYS A total of 22 h post-stratification, whole seedlings were infected by dipping in bacterial suspension for 1 h and next transferred to BNM plate. (For
pathogenic assays, seedlings were inoculated 22 h post-stratification). For both WSM419 and T4, an OD600 nm of 0.1 corresponded to a concentration of 108 cfu/mL as determined with a spiral
plater (easySpiral; www.interscience.com). WSM419 AND T4 MUTANT CONSTRUCTIONS T4-GFP Spontaneous rifampicin-resistant _E. adhaerens_ T4 mutant was first generated49_. E. adhaerens_ T4 was
then tagged on the chromosome using pBK-miniTn7-gfp3 construct50. Transformation was done by conjugation using _E_. _coli_ SM10λpir51 harboring the helper plasmid pUX-BF1352 and the
mobilizer plasmid pRK60053. _E. adhaerens_ T4-GFP derivatives were screened for GFP fluorescence and genotyped by _16S rRNA_ gene sequencing. In this strain derivative, the insertion was
checked by PCR and sequencing. The insertion is located downstream the _nodM_ locus. T4Δ_NODABC_ Flanking regions of _nodABC_ were PCR amplified using Phusion Taq polymerase, OCB2262/OCB2263
and OCB2264/2265 as primers and T4 genomic DNA as template. PCR products were cloned into pJET1-2 (Thermo Scientific™) and then subsequently juxtaposed as _Sac_I-_Nco_I and _Nco_I-_Sal_I
fragments into _Sal_I-_Sac_I-digested pJQ200mp1954 giving pLS368-1. The absence of mutation in the construct was checked by DNA sequencing. Plasmid was introduced in T4 by triparental mating
using pRK600 as a helper53. Single-crossover genomic integration of pLS368-1 was generated by selecting for gentamycin (Gm) resistance. The resulting strain was then propagated in the
absence of antibiotic and cells having lost the plasmid by a second recombination event were selected by plating on TYC supplemented with 5% sucrose (Suc). SucR GmS colonies were screened by
PCR analysis using OCB2266/OCB2267. WSM419Δ_NIFD_ Flanking regions of _nifD_ were PCR amplified using the primers OCB1684/OCB1685 and OCB1682/OCB1683 and WSM419 genomic DNA as template. PCR
products were cloned into pGEM-T (Promega™) and then subsequently juxtaposed as _Sal_I-_BamH_I and _Bam_HI-_Sac_I fragments into _Sal_I-_Sac_I-digested pJQ200mp1954 giving pLS296-1. The
absence of mutation in the construct was checked by DNA sequencing. Plasmid was introduced in WSM419 by triparental mating using the helper plasmid pRK201355. Single-crossover genomic
integration of pLS296-1 was generated by selecting for gentamycin (Gm) resistance. The resulting strain was propagated in the absence of antibiotic and cells having lost the plasmid by a
second recombination event were selected by plating on TYC supplemented with 5% sucrose (Suc). SucR GmS colonies were screened by PCR analysis using OCB1866/OCB1867. Primers used to
construct bacterial mutants are provided in Supplementary Data 4. ACETYLENE REDUCTION ASSAY Acetylene reduction assays were carried out using a protocol modified from ref. 56. Briefly, a
21-dpi-nodulated-root system from a single plant was placed in a 21-mL glass vial sealed with a rubber septum in the presence of 200 µL of water. 500 µL of acetylene gas was injected into
each vial and a 2-h incubation was performed. For each sample, 1 mL of gas was injected for analysis. Ethylene production was measured by gas chromatography using a gas chromatograph 7820 A
(Agilent Technologies) equipped with a GS-Alumina column (50 m × 0.53 mm). H2 and N2 were used as carrier and makeup gases, respectively. Column temperature and gas flow were set at 120 °C
and 7.5 mL/min, respectively. MICROSCOPY AND SAMPLE PREPARATION DESCRIPTION OF TECHNOVIT SAMPLES Zero dpi samples, 1 cm of primary root segment was collected 1 cm above the root apex. two
dpi samples, 1 cm of primary root segment was taken above the root apex marked at zero dpi (this position has been chosen to maximize the recovery of nodule organogenesis events, according
to Shen et al., 2019 showing the maximum response to LCO in the first cm of root above the root apex). 5 dpi samples, nodule primordia. 8, 12, 16, and 21 dpi samples, nodules. TECHNOVIT
RESIN INCLUSION Samples were fixed for 30 min in 0.05 M sodium cacodylate buffer, pH 7, 1% (v/v) glutaraldehyde, and 4% (v/v) formaldehyde under vacuum (∼500 mm Hg), incubated overnight at 4
°C and washed two times for 30 min with sodium cacodylate buffer. Once dehydrated by successive ethanol bath series (10%, 30%, 50%, 70%, 90%, 100%, 100%, 100%, 1 h each), ethanol was
progressively replaced by Technovit 7100 (www.kulzer-technik.com) using ethanol:Technovit solutions [(v/v), 3:1, 1:1, 1:3, 0:3, 0:3, 0:3, 1 h each] at 4 °C and under agitation. Samples were
included in Technovit resin using Teflon Histoform S embedding molds (Heraeus Kulzer). 5-µm-thick sections were carried out using an RM2155 microtome (www.leica-microsystems.com) and a TC-65
tungsten carbide blade (www.leica-microsystems.com). Samples were stained for 10 min in Toluidine Blue 0.02% (w/v). VIBRATOME SEMI-THIN SECTIONS Nodule samples were embedded in agarose (6%,
w/v) and sectioned using vibratome (VT1200S; www.leica-microsystems.com). The vibratome was set up as follows: speed, 0.60 mm/s; amplitude, 2.55 mm; thickness, 60 µm and continuous mode.
Sections were kept in Tris-HCl, 50 mM pH: 7.2 for subsequent analysis. LIVE/DEAD STAINING Nodule sections were stained for 20 min under darkness using the LIVE/DEAD BacLight Bacterial
Viability Kit (SYTO9, 3.34 μM and PI, 20 μM in Tris-HCl, 50 mM pH 7.2 (www.thermofisher.com). Sections were washed in Tris-HCl, 50 mM pH 7.2 prior observation. DAPI STAINING Free-living
bacteria and bacteroids from crushed nodules were stained for 10 min with DAPI (4, 6-diamidino-2-phenylindole) 50 µg/mL. CONFOCAL LASER SCANNING MICROSCOPY Fluorescent signals were detected
by confocal laser scanning microscopy (LSM880; www.zeiss.fr). Images were acquired and processed using ZEN2.3 lite software (www.zeiss.fr). FLUORESCENT BACTERIA Fluorescent signals from _S.
medicae_ WSM419-RFP (excitation wavelength, 561 nm; detection wavelength, 606–633 nm) and _E. adhaerens_ T4-GFP (excitation wavelength, 488 nm; detection wavelength, 498-567 nm). LIVE/DEAD
Fluorescent signals from alive SYTO9-stained rhizobia (excitation wavelength, 488 nm; detection wavelength, 501–559 nm) and from dead IP-stained rhizobia (excitation wavelength, 561 nm;
detection wavelength, 606–633 nm). DAPI Fluorescent signals from DAPI-stained rhizobia (excitation wavelength, 405 nm; detection wavelength, 425–514 nm). ANALYSIS OF LIGHT MICROSCOPY
PICTURES Pictures were treated with ImageJ software or observed and captured thanks to a microscope BX53 (OLYMPUS) and the cellSens Standard software (OLYMPUS). PROMOTER:GUS GENE EXPRESSION
PATTERN Histochemical GUS staining was performed as described in ref. 57. 2-dpi _M. truncatula_ A17 transgenic plants expressing the _proENOD11::gusA_ reporter construct were inoculated with
WSM419 or T446. Root samples were vacuum infiltrated for 30 min (∼500 mm Hg) in X‐gluc staining buffer (50 mM phosphate buffer (pH 7.2), 1 mM potassium ferricyanide, 1 mM potassium
ferrocyanide, 0.1% (w/v) SDS, 1 mM EDTA and 1.25 mM 5‐bromo‐4‐chloro‐3‐indolyl‐beta‐d‐GlcA containing cyclohexylammonium salts) and incubated overnight at 37 °C under darkness. Samples were
fixed in 50 mM phosphate buffer (pH 7.2), 1% (v/v) glutaraldehyde and 4% (v/v) formaldehyde for 15 min under vacuum (∼500 mm Hg). Pictures were acquired using a stereomicroscope Stemi 305
(ZEISS). RT-QPCR GENE EXPRESSION PROFILING ALONG THE NODULATION KINETICS DESCRIPTION OF SAMPLES 0- and 2-dpi samples, primary root lacking 0.5 cm of root below hypocotyl and 0.5 cm of root
above root apex. 5-dpi samples, primary root holding nodule primordia, lacking 0.5 cm of root below hypocotyl and 0.5 cm of root above root apex. 8-dpi samples, nodules with 0.5 cm of
subtenting root. 12-, 16- and 21-dpi samples, nodule with minimum subtending root. RNA EXTRACTION Total RNA extractions were performed from frozen tissues using TRIzol reagent (Ambion). RNA
samples were treated with the TURBO DNA-free Kit (Ambion) according to the manufacturer’s recommendations. REVERSE TRANSCRIPTION Full-length cDNA were synthesized from 800 ng of total ARN
using the SuperScript II Reverse Transcriptase kit (Invitrogen) in presence of Ribolock RNase Inhibitor (Thermo Scientific). RT-QPCR ANALYSIS RT-qPCR was performed on five times diluted cDNA
using LightCycler FastStart DNA Master SYBR Green I kit and a LightCycler 480 II according to the manufacturer’s instructions (Roche). Cycle threshold and primer specificities were
determined with the LightCycler 480 software release 1.5.0 SP4. Primer efficiencies were calculated with LinReg PCR: Analysis of Real-Time PCR Data, version 2016.1. _MtACT11_ and _MtRNA
RECOGNITION MOTIF_ reference genes were used for gene expression normalization. Information concerning primers used for RT-qPCR gene expression analyses are provided in Supplementary Data 4.
T4 COLONIZATION ANALYSIS At 10 dpi, T4-inoculated A17 plantlets were collected and rinsed twice with sterile water for 15 seconds under gentle agitation. Cotyledon, hypocotyl and root
organs were separated using a sterile razor blade. The fresh weight of individual organs was determined before grinding the material with two mm diameter glass beads in 600 µL of sterile
water using a Fastprep-96 (MP biomedicals) for 2.5 min at 1800 rpm. Suspensions were diluted in sterile water and 10 µL of each dilution were spotted on solid TY medium supplemented with
kanamycin (50 µg/mL). Plates were incubated for two days at 28 °C before colony counting. BACTERIAL GENOMIC DNA EXTRACTION, PACBIO LIBRARY PREPARATION AND GENOME SEQUENCING T4 genome
sequencing was performed at King Abdullah University of Science and Technology (KAUST, Saudi Arabia). Fresh and pure bacterial culture was used for total genomic DNA extraction using Sigma’s
GenElute bacterial genomic DNA kit (Sigma Aldrich, Germany) following the manufacturer’s protocol. DNA quality and quantity was assessed by using NanoDrop 2000 (Thermo Fisher Scientific,
USA) and Qubit dsDNA BR assay kit (Thermo Fisher Scientific, USA). DNA was size selected to 10 kb using the BluePippin™ Size-Selection System (Sage Science, USA), following the High-PassTM
DNA Size Selection of ~20 kb SMRTbellTM Templates manual. The SMRTbell™ template library was prepared according to the instructions from Pacific Biosciences’s “Procedure & Checklist - 20
kb Template Preparation using BluePippin™ Size-Selection System” guide. The SMRT cells were run at the KAUST Bioscience Core Labs on the PacBio _RSII_ (Pacific Biosciences, USA) sequencing
platform using P6-C4 chemistry. GENOME ASSEMBLY AND ANNOTATION PacBio reads were assembled into seven circular contigs by using Flye v.2.9.1 (https://github.com/fenderglass/Flye58) with
default parameters. Circularization of contigs was automatically performed by Flye, circularity has been checked by aligning contigs against themselves using Gepard59. OriC sites were
identified using Ori-Finder 2 and replicons were restarted according to OriC sites when detected60. Genome annotation was conducted using the Microscope platform interface
(https://mage.genoscope.cns.fr61). PHYLOGENETIC RECONSTRUCTION OF BACTERIA BASED ON WHOLE GENOMES The phylogenetic tree was generated using the Microscope platform interface
(https://mage.genoscope.cns.fr61). The genomic similarity was estimated using Mash software computing a distance between two genomes (https://github.com/marbl/Mash). This distance is
correlated to the ANI like: D ≈ 1-ANI. From all the pairwise distances of the genomes set, a tree is constructed dynamically using the neighbor-joining javascript package
(https://www.npmjs.com/package/neighbor-joining). The tree displays clustering annotations. The clustering has been computed from all-pairs distances ≤ 0.06 ( ≈ 94% ANI) that correspond to
the ANI standard to define a species group using the Louvain Community Detection (https://github.com/taynaud/python-louvain). PHYLOGENETIC RECONSTRUCTION OF LEGUME SPECIES BASED ON _MATK_
SEQUENCES The evolutionary history of legume species was inferred by using the Maximum Likelihood method and Tamura-Nei model62. Chloroplastic _matK_ sequences, retrieved from NCBI, have
been used (Supplementary Data 5). Log likelihood: -10895,59. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Neighbor-Join and BioNJ
algorithms were applied to a matrix of pairwise distances estimated using the Tamura-Nei model. The scale corresponds to the number of substitutions per site. Codon positions included were
1st+2nd+3rd+Noncoding. 2597 positions were considered in the final dataset. The phylogenetic reconstruction has been done using MEGA X63. PHENOTYPE MICROARRAY ANALYSIS The Phenotype
Microarrays data were analyzed using the R package OPM version 1.0.664. For the original data generated in this study, means of the area under the curve for the replicates of each strain
were calculated using OPM. For the data reused from36 containing only one replicate, the area under the curve was calculated using OPM. Data were normalized to 100 relative to the maximal
value found in each microplate (Supplementary Data 6). The heatmap was drawn using the heatmap function of the OPM package with default clustering method for the combined PM01 and PM02
plates. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data related to this
study are included in the manuscript, in Supplementary information and in Supplementary Data. The genomic data of the _E. adhaerens_ T4 strain are accessible on MicroScope - Microbial Genome
Annotation & Analysis Platform (https://mage.genoscope.cns.fr) as well as on NCBI under the BioProject ID: PRJNA1066792. All genetic materials used in this study are available on
request to Pascal Ratet or Benjamin Gourion ([email protected]; [email protected]). The _E. adhaerens_ T4 strain is also available at the CIRM-CFBP French Collection for Plant
Associated Bacteria (https://cirm-cfbp.fr/) under the accession number CFBP 9181. Source data are provided with this paper. REFERENCES * Drew, G. C., Stevens, E. J. & King, K. C.
Microbial evolution and transitions along the parasite-mutualist continuum. _Nat. Rev. Microbiol_ 19, 623–638 (2021). Article PubMed PubMed Central CAS Google Scholar * Liang, Y. et al.
Nonlegumes respond to rhizobial Nod factors by suppressing the innate immune response. _Science_ 341, 1384–1387 (2013). Article ADS PubMed CAS Google Scholar * Nishiguchi, M. K. et al.
Deciphering evolutionary mechanisms between mutualistic and pathogenic symbioses. _Vie et. milieu_ 58, 87–106 (2008). PubMed CAS Google Scholar * Sachs, J. L., Skophammer, R. G., Bansal,
N. & Stajich, J. E. Evolutionary origins and diversification of proteobacterial mutualists. _Proc. Biol. Sci._ 281, 20132146 (2014). PubMed PubMed Central Google Scholar * Sachs, J.
L., Skophammer, R. G. & Regus, J. U. Evolutionary transitions in bacterial symbiosis. _Proc. Natl Acad. Sci. USA_ 108, 10800–10807 (2011). Article ADS PubMed PubMed Central CAS
Google Scholar * Zipfel, C. & Oldroyd, G. E. Plant signalling in symbiosis and immunity. _Nature_ 543, 328–336 (2017). Article ADS PubMed CAS Google Scholar * Poole, P.,
Ramachandran, V. & Terpolilli, J. Rhizobia: from saprophytes to endosymbionts. _Nat. Rev. Microbiol_ 16, 291–303 (2018). Article PubMed CAS Google Scholar * Aslam, S. N. et al.
Bacterial polysaccharides suppress induced innate immunity by calcium chelation. _Curr. Biol._ 18, 1078–1083 (2008). Article PubMed CAS Google Scholar * Jones, K. M. et al. Differential
response of the plant _Medicago truncatula_ to its symbiont _Sinorhizobium meliloti_ or an exopolysaccharide-deficient mutant. _Proc. Natl Acad. Sci. USA_ 105, 704–709 (2008). Article ADS
PubMed PubMed Central CAS Google Scholar * Okazaki, S., Kaneko, T., Sato, S. & Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system.
_Proc. Natl Acad. Sci. USA_ 110, 17131–17136 (2013). Article ADS PubMed PubMed Central CAS Google Scholar * Okazaki, S. et al. Rhizobium-legume symbiosis in the absence of Nod factors:
two possible scenarios with or without the T3SS. _ISME J._ 10, 64–74 (2016). Article PubMed CAS Google Scholar * Teulet, A. et al. The rhizobial type III effector ErnA confers the
ability to form nodules in legumes. _Proc. Natl Acad. Sci. USA_ 116, 21758–21768 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Brown, S. P., Grillo, M. A., Podowski,
J. C. & Heath, K. D. Soil origin and plant genotype structure distinct microbiome compartments in the model legume _Medicago truncatula_. _Microbiome_ 8, 139 (2020). Article PubMed
PubMed Central CAS Google Scholar * Hansen, B. L. et al. Cooperation, Competition, and Specialized Metabolism in a Simplified Root Nodule Microbiome. _mBio_ 11,
https://doi.org/10.1128/mBio.01917-20 (2020). * Martínez-Hidalgo, P. & Hirsch, A. M. The nodule microbiome: N2-fixing rhizobia do not live alone. _Phytobiomes_ 1, 70–82 (2017). Article
Google Scholar * Choi, I. S. et al. Plastid phylogenomics uncovers multiple species in _Medicago truncatula_ (Fabaceae) germplasm accessions. _Sci. Rep._ 12, 21172 (2022). Article ADS
PubMed PubMed Central CAS Google Scholar * Hoffmann, B., Trinh, T. H., Leung, J., Kondorosi, A. & Kondorosi, E. A new _Medicago truncatula_ line with superior in vitro regeneration,
transformation, and symbiotic properties isolated through cell culture selection. _Mol. Plant Microbe Interact._ 10, 307–315 (1997). Article CAS Google Scholar * Bromfield, E. S. P. et
al. Ensifer, Phyllobacterium and Rhizobium species occupy nodules of _Medicago sativa_ (alfalfa) and _Melilotus alba_ (sweet clover) grown at a Canadian site without a history of
cultivation. _Microbiol. (Read.)_ 156, 505–520 (2010). Article CAS Google Scholar * Geddes, B. A. et al. Minimal gene set from _Sinorhizobium_ (_Ensifer_) _meliloti_ pSymA required for
efficient symbiosis with Medicago. _Proc. Natl Acad. Sci. USA_ 118, https://doi.org/10.1073/pnas.2018015118 (2021). * Perez Guerra, J. C. et al. Comparison of developmental and
stress-induced nodule senescence in _Medicago truncatula_. _Plant Physiol._ 152, 1574–1584 (2010). Article PubMed PubMed Central Google Scholar * de Zelicourt, A. et al. Dual involvement
of a _Medicago truncatula_ NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. _Plant J._ 70, 220–230 (2012). Article PubMed Google Scholar *
Gourion, B., Berrabah, F., Ratet, P. & Stacey, G. Rhizobium-legume symbioses: the crucial role of plant immunity. _Trends Plant Sci._ 20, 186–194 (2015). Article PubMed CAS Google
Scholar * Mergaert, P. et al. Eukaryotic control on bacterial cell cycle and differentiation in the _Rhizobium_-legume symbiosis. _Proc. Natl Acad. Sci. USA_ 103, 5230–5235 (2006). Article
ADS PubMed PubMed Central CAS Google Scholar * Van de Velde, W. et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. _Science_ 327, 1122–1126 (2010).
Article ADS PubMed Google Scholar * Wang, D. et al. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. _Science_ 327, 1126–1129 (2010). Article ADS
PubMed PubMed Central CAS Google Scholar * Casida, L. E. _Ensifer adhaerens_ gen. nov., sp. nov.: a bacterial predator of bacteria in soil†. _Int. J. Syst. Evolut. Microbiol._ 32,
339–345 (1982). Google Scholar * Moreira, F. M. S., Gillis, M., Pot, B., Kersters, K. & Franco, A. A. Characterization of rhizobia isolated from different divergence groups of tropical
leguminosae by comparative polyacrylamide gel electrophoresis of their total proteins. _Syst. Appl. Microbiol._ 16, 135–146 (1993). Article Google Scholar * Munoz, E., Villadas, P. J.
& Toro, N. Ectopic transposition of a group II intron in natural bacterial populations. _Mol. Microbiol_ 41, 645–652 (2001). Article PubMed CAS Google Scholar * Willems, A. et al.
Description of new Ensifer strains from nodules and proposal to transfer _Ensifer adhaerens_ Casida 1982 to Sinorhizobium as _Sinorhizobium adhaerens_ comb. nov. Request for an Opinion.
_Int. J. Syst. Evolut. Microbiol._ 53, 1207–1217 (2003). Article CAS Google Scholar * Wendt, T., Doohan, F. & Mullins, E. Production of _Phytophthora infestans_-resistant potato
(_Solanum tuberosum_) utilising _Ensifer adhaerens_ OV14. _Transgenic Res_ 21, 567–578 (2012). Article PubMed CAS Google Scholar * Rudder, S., Doohan, F., Creevey, C. J., Wendt, T. &
Mullins, E. Genome sequence of _Ensifer adhaerens_ OV14 provides insights into its ability as a novel vector for the genetic transformation of plant genomes. _BMC Genomics_ 15, 268 (2014).
Article PubMed PubMed Central Google Scholar * Libourel, C. et al. Comparative phylotranscriptomics reveals ancestral and derived root nodule symbiosis programmes. _Nat. Plants_ 9,
1067–1080 (2023). Article PubMed PubMed Central CAS Google Scholar * Martinez, E., Palacios, R. & Sanchez, F. Nitrogen-fixing nodules induced by _Agrobacterium tumefaciens_
harboring _Rhizobium phaseoli_ plasmids. _J. Bacteriol._ 169, 2828–2834 (1987). Article PubMed PubMed Central CAS Google Scholar * Marchetti, M. et al. Experimental evolution of a plant
pathogen into a legume symbiont. _PLoS Biol._ 8, e1000280 (2010). Article PubMed PubMed Central Google Scholar * Cummings, S. P. et al. Nodulation of Sesbania species by Rhizobium
(Agrobacterium) strain IRBG74 and other rhizobia. _Environ. Microbiol_ 11, 2510–2525 (2009). Article PubMed PubMed Central CAS Google Scholar * Fagorzi, C. et al. Symbiotic and
nonsymbiotic members of the genus ensifer (syn. sinorhizobium) are separated into two clades based on comparative genomics and high-throughput phenotyping. _Genome Biol. Evol._ 12, 2521–2534
(2020). Article PubMed PubMed Central CAS Google Scholar * Kuzmanovic, N., Fagorzi, C., Mengoni, A., Lassalle, F. & diCenzo, G. C. Taxonomy of Rhizobiaceae revisited: proposal of a
new framework for genus delimitation. _Int. J. Syst. Evol. Microbiol._ 72, https://doi.org/10.1099/ijsem.0.005243 (2022). * Bromfield, E. S. P., Cloutier, S. & Hynes, M. F. _Ensifer
canadensis_ sp. nov. strain T173(T) isolated from _Melilotus albus_ (sweet clover) in Canada possesses recombinant plasmid pT173b harbouring symbiosis and type IV secretion system genes
apparently acquired from _Ensifer medicae_. _Front Microbiol_ 14, 1195755 (2023). Article PubMed PubMed Central Google Scholar * Griesmann, M. et al. Phylogenomics reveals multiple
losses of nitrogen-fixing root nodule symbiosis. _Science_ 361, https://doi.org/10.1126/science.aat1743 (2018). * Gano-Cohen, K. A. et al. Interspecific conflict and the evolution of
ineffective rhizobia. _Ecol. Lett._ 22, 914–924 (2019). Article PubMed Google Scholar * Zgadzaj, R. et al. A legume genetic framework controls infection of nodules by symbiotic and
endophytic bacteria. _PLoS Genet_ 11, e1005280 (2015). Article PubMed PubMed Central Google Scholar * Checcucci, A. et al. Mixed nodule infection in _Sinorhizobium meliloti_-_Medicago
sativa_ symbiosis suggest the presence of cheating behavior. _Front Plant Sci._ 7, 835 (2016). Article PubMed PubMed Central Google Scholar * Muresu, R. et al. Coexistence of
predominantly nonculturable rhizobia with diverse, endophytic bacterial taxa within nodules of wild legumes. _FEMS Microbiol Ecol._ 63, 383–400 (2008). Article PubMed CAS Google Scholar
* Hofer, A. W. Methods for distinguishing between legume bacteria and their most common contaminant1. _Agron. J._ 27, 228–230 (1935). Article Google Scholar * Young, N. D. et al. The
_Medicago_ genome provides insight into the evolution of rhizobial symbioses. _Nature_ 480, 520–524 (2011). Article ADS PubMed PubMed Central CAS Google Scholar * Journet, E.-P. et al.
_Medicago truncatula_ ENOD11: A novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. _Mol. Plant-Microbe Interact._ 14, 737–748 (2001).
Article PubMed CAS Google Scholar * Ehrhardt, D. W., Atkinson, E. M. & Long, S. R. Depolarization of alfalfa root hair membrane potential by _Rhizobium meliloti_ Nod factors.
_Science_ 256, 998–1000 (1992). Article ADS PubMed CAS Google Scholar * Krall, L. et al. Detergent extraction identifies different VirB protein subassemblies of the type IV secretion
machinery in the membranes of _Agrobacterium tumefaciens_. _Proc. Natl Acad. Sci. USA_ 99, 11405–11410 (2002). Article ADS PubMed PubMed Central CAS Google Scholar * Crotti, E. et al.
Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. _Environ. Microbiol_ 11, 3252–3264 (2009). Article
PubMed CAS Google Scholar * Koch, B., Jensen, L. E. & Nybroe, O. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative
bacteria at a neutral chromosomal site. _J. Microbiol Methods_ 45, 187–195 (2001). Article PubMed CAS Google Scholar * Simon, R., Priefer, U. & Pühler, A. A broad host range
mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. _Bio/Technol._ 1, 784–791 (1983). Article CAS Google Scholar * Bao, Y., Lies, D. P.,
Fu, H. & Roberts, G. P. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. _Gene_ 109, 167–168 (1991). Article
PubMed CAS Google Scholar * Finan, T. M., Kunkel, B., De Vos, G. F. & Signer, E. R. Second symbiotic megaplasmid in _Rhizobium meliloti_ carrying exopolysaccharide and thiamine
synthesis genes. _J. Bacteriol._ 167, 66–72 (1986). Article PubMed PubMed Central CAS Google Scholar * Quandt, J. & Hynes, M. F. Versatile suicide vectors which allow direct
selection for gene replacement in Gram-negative bacteria. _Gene_ 127, 15–21 (1993). Article PubMed CAS Google Scholar * Figurski, D. H. & Helinski, D. R. Replication of an
origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. _Proc. Natl Acad. Sci. USA_ 76, 1648–1652 (1979). Article ADS PubMed PubMed Central CAS
Google Scholar * Koch, B. & Evans, H. J. Reduction of acetylene to ethylene by soybean root nodules. _Plant Physiol._ 41, 1748–1750 (1966). Article PubMed PubMed Central CAS Google
Scholar * Pichon, M. et al. _Rhizobium meliloti_ elicits transient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa. _Plant Cell_ 4,
1199–1211 (1992). PubMed PubMed Central CAS Google Scholar * Kolmogorov, M., Yuan, J., Lin, Y. & Pevzner, P. A. Assembly of long, error-prone reads using repeat graphs. _Nat.
Biotechnol._ 37, 540–546 (2019). Article PubMed CAS Google Scholar * Krumsiek, J., Arnold, R. & Rattei, T. Gepard: a rapid and sensitive tool for creating dotplots on genome scale.
_Bioinformatics_ 23, 1026–1028 (2007). Article PubMed CAS Google Scholar * Luo, H., Zhang, C. T. & Gao, F. Ori-Finder 2, an integrated tool to predict replication origins in the
archaeal genomes. _Front Microbiol_ 5, 482 (2014). Article PubMed PubMed Central Google Scholar * Vallenet, D. et al. MicroScope: an integrated platform for the annotation and
exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. _Nucleic Acids Res._ 48, D579–D589 (2019). PubMed Central Google Scholar * Tamura,
K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. _Mol. Biol. Evol._ 10, 512–526 (1993). PubMed CAS
Google Scholar * Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. _Mol. Biol. Evol._ 35, 1547–1549
(2018). Article PubMed PubMed Central CAS Google Scholar * Vaas, L. A. et al. opm: an R package for analysing OmniLog(R) phenotype microarray data. _Bioinformatics_ 29, 1823–1824
(2013). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS This work has been supported by the grant ANR-21-CE20-0016-01-PATHOSYM attributed by the French National
Research Agency to PR. PR is supported by the French Laboratory of Excellence “SPS” ANR-10-LABX-0040-SPS, ANR-17-EUR-0007 and EUR SPS-GSR which is managed by the French National Research
Agency under the program “Investissements d’avenir” ANR-11-IDEX-0003-02. BG and FV are supported by the French Laboratory of Excellence “TULIP” ANR-10-LABX-41 and ANR-11-IDEX-0002-02. VG is
supported by the IdEX Université de Paris ANR-18-IDEX-0001. FPP and FM are from MetaToul platform (Toulouse metabolomics & fluxomics facilities, www.metatoul.fr) which is part of the
French National Infrastructure for Metabolomics and Fluxomics MetaboHUB-ANR-11-INBS-0010. ER was supported by the grant FP7-609398 attributed in the frame of the Agreenskills+ postdoctoral
fellowship programme which depends on the EU’s Seventh Framework Programme. The Laboratory of Bioinformatics Analyses for Genomics and Metabolism - LABGeM (CEA/Genoscope & CNRS UMR8030),
France Génomique and the French Bioinformatics Institute (funded as part of the “Investissement d’Avenir” program managed by Agence Nationale pour la Recherche, ANR-10-INBS-09 and
ANR-11-INBS-0013) are acknowledged for their support within the MicroScope annotation platform. We thank Sylvie Fournier and Guillaume Marti from the Metatoul platform for their help in the
NFs characterization. We thank Dr. Julie Cullimore (LIPME, France), Dr. Benoit Alunni (I2BC, France), Dr. Peter Mergaert (I2BC, France) and Dr. Ouartsi Akilafor (Université Badji
Mokhtar-Annaba, Algeria) for providing most rhizobial strains used for T4-competitiveness assays. We thank Dr. Gabriella Endré (Szeged Research Centre for Biology, Hungary) for providing
WSM419-RFP and WSM419-GFP derivatives. We thank Dr. Eden S. P. Bromfield (Ottawa research and development centre, Canada) for providing the _E. adhaerens_ T173 strain. We thank Dr. Anne
Willems (Ghent University, Belgium) for providing _E. adhaerens_ Casida A, R-7457 and BR819 strains from the BCCM/LMG collection (https://bccm.belspo.be/). We thank Dr. Ewen Mullins (Teagasc
Crops Research Centre, Ireland) for providing the _E. adhaerens_ OV14 strain. We thank again Dr. Benoit Alunni and Dr. Peter Mergaert for providing seeds of legumes from the IRLC. We thank
Dr. George C. diCenzo (Queen’s University, Canada) for providing published biolog datasets. AUTHOR INFORMATION Author notes * Kévin Magne Present address: Université Paris-Saclay, INRAE,
AgroParisTech, Institute Jean-Pierre Bourgin for Plant Sciences (IJPB), 78000, Versailles, France * Eleonora Rolli Present address: Department of Food, Environmental and Nutritional Sciences
(DeFENS), University of Milan, 20133, Milan, Italy AUTHORS AND AFFILIATIONS * Université Paris-Saclay, CNRS, INRAE, Université Evry, Institute of Plant Sciences Paris-Saclay, 91190, Gif sur
Yvette, France Kévin Magne, Sophie Massot, Elhosseyn Ait-Salem, Ilona Pires, Adrien Jugan, Eleonora Rolli, Gautier Bernal, Véronique Gruber & Pascal Ratet * Université Paris Cité, CNRS,
INRAE, Institute of Plant Sciences Paris-Saclay, 91190, Gif sur Yvette, France Kévin Magne, Sophie Massot, Elhosseyn Ait-Salem, Ilona Pires, Adrien Jugan, Eleonora Rolli, Gautier Bernal,
Véronique Gruber & Pascal Ratet * Laboratoire des Interactions Plantes Microbes Environnement, Université de Toulouse, INRAE, CNRS, 31326, Castanet-Tolosan, France Tifaine Folletti,
Laurent Sauviac, Fabienne Maillet, Chrystel Gibelin, Fabienne Vailleau & Benjamin Gourion * DARWIN21, Biological and Environmental Sciences and Engineering Division, King Abdullah
University of Science and Technology, Thuwal, 23955, Saudi Arabia Maged M. Saad, Abdul Aziz Eida & Heribert Hirt * Computational Bioscience Research Center, King Abdullah University of
Science and Technology, Thuwal, Saudi Arabia Salim Bougouffa * iMEAN, 31077, Toulouse, France Raphaël Forquet & Rémi Peyraud * Laboratoire de Recherche en Sciences Végétales, CNRS, UPS,
Toulouse INP, Université de Toulouse, Toulouse, France Virginie Puech-Pages * Metatoul-AgromiX Platform, MetaboHUB, National Infrastructure for Metabolomics and Fluxomics, LRSV, Toulouse,
France Virginie Puech-Pages Authors * Kévin Magne View author publications You can also search for this author inPubMed Google Scholar * Sophie Massot View author publications You can also
search for this author inPubMed Google Scholar * Tifaine Folletti View author publications You can also search for this author inPubMed Google Scholar * Laurent Sauviac View author
publications You can also search for this author inPubMed Google Scholar * Elhosseyn Ait-Salem View author publications You can also search for this author inPubMed Google Scholar * Ilona
Pires View author publications You can also search for this author inPubMed Google Scholar * Maged M. Saad View author publications You can also search for this author inPubMed Google
Scholar * Abdul Aziz Eida View author publications You can also search for this author inPubMed Google Scholar * Salim Bougouffa View author publications You can also search for this author
inPubMed Google Scholar * Adrien Jugan View author publications You can also search for this author inPubMed Google Scholar * Eleonora Rolli View author publications You can also search for
this author inPubMed Google Scholar * Raphaël Forquet View author publications You can also search for this author inPubMed Google Scholar * Virginie Puech-Pages View author publications You
can also search for this author inPubMed Google Scholar * Fabienne Maillet View author publications You can also search for this author inPubMed Google Scholar * Gautier Bernal View author
publications You can also search for this author inPubMed Google Scholar * Chrystel Gibelin View author publications You can also search for this author inPubMed Google Scholar * Heribert
Hirt View author publications You can also search for this author inPubMed Google Scholar * Véronique Gruber View author publications You can also search for this author inPubMed Google
Scholar * Rémi Peyraud View author publications You can also search for this author inPubMed Google Scholar * Fabienne Vailleau View author publications You can also search for this author
inPubMed Google Scholar * Benjamin Gourion View author publications You can also search for this author inPubMed Google Scholar * Pascal Ratet View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS P.R., B.G., F.V., and K.M. designed research; K.M., S.M., T.F., L.S., E.A.S., I.P., M.M.S., A.A.E., S.B., A.J., E.R., V.P.P., F.M.,
G.B., C.G., and B.G. performed research; K.M., T.F., L.S., E.A.S., I.P., M.M.S., A.A.E., S.B., A.J., E.R., R.F., V.P.P., H.H., V.G., R.P., F.V., B.G., and P.R. analyzed data; K.M. and B.G.
wrote the manuscript. CORRESPONDING AUTHORS Correspondence to Benjamin Gourion or Pascal Ratet. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests PEER REVIEW
PEER REVIEW INFORMATION _Nature Communications_ thanks Euan James, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is
available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4
SUPPLEMENTARY DATA 5 SUPPLEMENTARY DATA 6 SUPPLEMENTARY DATA 7 SUPPLEMENTARY DATA 8 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Magne, K., Massot, S., Folletti, T. _et al._ Atypical rhizobia
trigger nodulation and pathogenesis on the same legume hosts. _Nat Commun_ 15, 9246 (2024). https://doi.org/10.1038/s41467-024-53388-x Download citation * Received: 12 October 2023 *
Accepted: 09 October 2024 * Published: 26 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53388-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
