Expanded subsurface ocean anoxia in the atlantic during the paleocene-eocene thermal maximum
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The ocean has experienced substantial oxygen loss over recent decades, affecting marine ecosystems and fisheries. Investigating ocean deoxygenation during hyperthermal events, such
as the Paleocene-Eocene Thermal Maximum (PETM), offers insights into its future dynamics. Here, sediment cores from the South Atlantic reveal a pronounced decline in foraminifera-bound δ15N,
concurrent with an increase in marine barite δ34S and enhanced ocean productivity during the PETM. These findings suggest an expansion of oxygen-deficient zones (ODZs) from suboxia to
anoxia in the thermocline, with ammonium and sulfide accumulation. Model simulations indicate “ammonium-type” ODZs were driven by Southern Ocean warming and elevated productivity. Intense
fixed nitrogen loss at the upper boundary of these ODZs, along with increased oceanic phosphorus inventory, likely spurred a compensatory rise in N2 fixation. While the Pacific might
experience different oxygenation conditions during the PETM, parts of the Atlantic thermocline became anoxic, highlighting potential spatial variabilities of ocean deoxygenation under global
warming. SIMILAR CONTENT BEING VIEWED BY OTHERS ENHANCED OCEAN OXYGENATION DURING CENOZOIC WARM PERIODS Article Open access 31 August 2022 VERTICAL DECOUPLING IN LATE ORDOVICIAN ANOXIA DUE
TO REORGANIZATION OF OCEAN CIRCULATION Article 01 November 2021 A WELL-OXYGENATED EASTERN TROPICAL PACIFIC DURING THE WARM MIOCENE Article 28 June 2023 INTRODUCTION Oxygen (O2) is critical
to most forms of life. Due to its low solubility in water, the total amount of dissolved O2 in the ocean is less than 1% of that in the atmosphere. Consequently, O2 can become a limiting
resource in many marine environments. Over the past several decades, dissolved O2 levels in both open-ocean and coastal waters have declined, a trend mainly attributed to climate change and
anthropogenic nutrient input1,2,3. While the ongoing oceanic oxygen loss (i.e., ocean deoxygenation) is expected to persist due to rising global temperatures and increased ocean
stratification4, factors such as ocean ventilation and productivity may introduce complexity to these conditions. For example, climate models have yielded inconsistent predictions regarding
the future of ocean deoxygenation5,6. A pressing concern of ocean deoxygenation is the potential expansion of oxygen-deficient zones (ODZs) under global warming. These zones, with the lowest
O2 concentration, typically occur in the subsurface ocean at depths from 100 to 1000 meters. Changes in ODZs significantly influence the marine cycling of key elements, such as nitrogen,
sulfur, and carbon. Under suboxic conditions – where O2 concentrations drop below 5 μmol/kg4,7 – organic matter is respired via denitrification and anaerobic ammonia oxidation (anammox),
resulting in a net loss of biologically available nitrogen, or fixed nitrogen. In addition, ODZs are hotspots of nitrous oxide (N2O) production, a potent greenhouse gas ~ 300 times more
powerful than carbon dioxide over a 100-year period8. Although the modern ocean ODZs are mainly suboxic, they may have been more severely depleted of oxygen in the past. Under anoxic
conditions, where O2 concentrations fall to 0 μmol/kg and other electron acceptors like nitrate are limited, microbial sulfate reduction becomes the dominant pathway for organic matter
remineralization, leading to the accumulation of toxic hydrogen sulfide9. Such scenarios can lead to nitrate depletion in ODZs, with ammonium becoming the dominant form of fixed nitrogen,
thereby altering the marine nitrogen cycle10,11,12. The Paleocene-Eocene Thermal Maximum (PETM; ~ 56 million years ago), as a potential analog for high anthropogenic carbon emission
scenarios, provides important insights into the future evolution of ODZs. During the PETM, the estimated global mean surface temperature was 34.1 °C, exceeding present-day temperatures by 19
°C13,14. This temperature difference alone would have significantly reduced oxygen solubility, contributing to ocean deoxygenation. Indeed, evidence from marine barite sulfur isotopes
(δ34S, where δ34S = [(34S/32S)sample/(34S/32S)VCDT – 1] × 1000 [‰]) indicates large-scale ocean deoxygenation during the PETM, with ODZs evolving into anoxic and even sulfidic
conditions15,16. However, foraminifera-bound nitrogen isotopes (foraminifera-bound δ15N, where δ15N = [(15N/14N)sample/(15N/14N)air – 1] × 1000 [‰]) from the tropical North Pacific reveal a
decline during the PETM, which was explained as a rise in the upper ocean oxygen concentration caused by a reduction in biological productivity17. These findings suggest that the response of
ocean deoxygenation to global warming is more complex than previously thought, and the variations in ODZs during the PETM warrant further investigation. In this study, we aim to clarify the
extent of ODZs in the South Atlantic during the PETM by examining coupled records of foraminifera-bound δ15N, marine barite δ34S, and productivity on the same samples from Ocean Drilling
Program (ODP) Site 1263 and Site 1265, located on the southeastern margin of subtropical gyre at water depths above the paleo-carbonate compensation depth (Supplementary Fig. 1). This
multi-proxy reconstruction, in combination with modeling, allows for quantifying the impact of warming and productivity changes on the evolution of ODZs across the PETM. RESULTS AND
DISCUSSION NITROGEN-ISOTOPE AND PRODUCTIVITY RECONSTRUCTIONS The nitrogen isotope composition of foraminifera-bound organic matter (foraminifera-bound δ15N) is a reliable proxy for
reconstructing the past marine nitrogen cycling18. In today’s ODZs, water-column denitrification preferentially removes 14N, with isotope fractionation of 15 to 25‰19,20. This process
enriches the remaining nitrate in 15N, which then mixes with water outside the ODZs, raising the δ15N of nitrate in surrounding regions21,22. In areas where surface nitrate is fully
consumed, subsurface nitrate supplied to the euphotic zone is completely assimilated by phytoplankton and converted into organic matter. Foraminifera feeds on this organic matter, causing
foraminifera-bound δ15N to reflect the δ15N of subsurface nitrate in regions with complete surface nitrate consumption18. Consequently, foraminifera-bound δ15N records from ODZs and adjacent
regions have been used to reconstruct the historical expansion and contraction of ODZs23,24,25. Compared to bulk sedimentary δ15N, organic matter trapped within the mineral matrix of
planktic foraminifer shells is less vulnerable to isotopic alteration after burial, making foraminifera-bound δ15N an ideal proxy for preserving the original marine δ15N signature23. Our
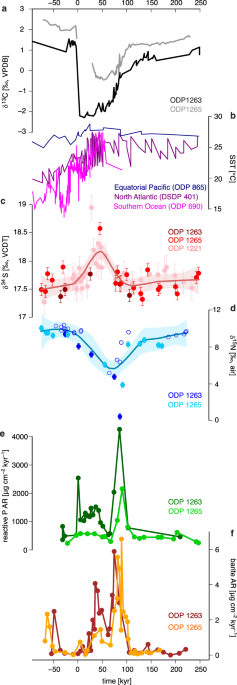
foraminifera-bound δ15N record based on mixed species shows values of approximately 10‰ before the PETM (Fig. 1), consistent with the previously reported foraminifera-bound δ15N values of
the early Cenozoic from the Pacific and Atlantic Ocean23. The foraminifera-bound δ15N drops from 10‰ to ~ 4‰ during the PETM, followed by a rapid recovery to the near pre-excursion value of
9‰. It should be noted that the single genus foraminifera δ15N record (based on _Morozovella_, _Acarinina_, and _Subbotina_) was recently published from Site 1263, although their record did
not span the whole PETM period due to changes in the foraminifera genus17. When plotted together, our mixed species foraminifera-bound δ15N are consistent with the published symbiont-bearing
single genus _Morozovella_ and _Acarinina_ δ15N, substantiating the decline of δ15N at the study sites. One datum point in our record showed an anomalously low δ15N of 0.4‰ at 85 kyr
relative to the onset of PETM. While the previous work did not observe a δ15N value close to 0‰, we keep this minimum datum as its nitrogen content falls within the reasonable range (2.5–4.5
μmol/g; Supplementary Table 1). The δ15N decline reconstructed from the South Atlantic also agrees with the near-shore δ15N record from the Tethys Ocean26 (Supplementary Fig. 2), implying
similar driving mechanisms between coastal and open-ocean settings. We also present the reactive phosphorus accumulation rate (reactive PAR) from the same sites as a measure of phosphorus
supply to the euphotic zone alongside the marine barite accumulation rate (barite AR), which serves as an indicator of export productivity27,28. Reactive PAR shows a four- to nine-fold
increase between 0 and 100 kyrs compared to the pre-PETM levels, peaking at 85 kyrs. This peak coincides with the foraminifera-bound δ15N minimum and the marine barite AR maximum. Notably,
the reactive phosphorus concentration is also higher between 0 and 100 kyrs, indicating that the increase in reactive PAR is not an artifact of mass accumulation rate (Supplementary Table
2). PRESENCE OF “AMMONIUM-TYPE” ODZS IN THE SOUTH ATLANTIC DURING THE PETM In the modern ocean, nitrate is the dominant form of fixed nitrogen in ODZs (“nitrate-type” ODZs; Fig. 2a). In
these ODZs, nitrate consumption through water-column denitrification is incomplete, resulting in large isotope fractionation of 15 to 25‰19,20. In contrast, denitrification in ocean
sediments is typically complete, removing fixed nitrogen with minimal isotope fractionation (Fig. 2c). Thus, lower rates of water-column denitrification relative to sedimentary
denitrification are expected to decrease mean ocean nitrate δ15N, leading to lower average foraminifera-bound δ15N values globally. The pronounced decline observed in most foraminifera-bound
δ15N records during the PETM, if interpreted at face value, could generally suggest a contraction of nitrate-type ODZs or a reduction in global water-column denitrification relative to
sedimentary denitrification. In the tropical North Pacific, this decline has been previously attributed to reduced ocean suboxia, driven by decreased productivity17. However, this
interpretation is unlikely to apply to the South Atlantic. First, reactive PAR and marine barite AR in the South Atlantic indicate an increase in productivity, not a decline (Fig. 2 and
Supplementary Table 3). Second, independent evidence from foraminiferal Cr-isotope and Ce anomalies, reconstructed from sites at intermediate paleo-water depths in the Atlantic, points to
the presence of oxygen-free water during this period29,30. Third, the concurrent increase in mean ocean sulfate δ34S and δ18O preserved in marine barite indicates the accumulation of
hydrogen sulfide within some ODZs15,16. Although the exact locations of these sulfide-rich ODZs remain unclear due to sulfate’s long residence time in the ocean, microbial sulfate reduction
only occurs after nitrate’s depletion, owing to thermodynamic constraints9. Consequently, the dominant form of fixed nitrogen in sulfide-rich ODZs must be ammonium, not nitrate
(“ammonium-type” ODZs; Fig. 2b). Unlike nitrate-type ODZs, ammonium-type ODZs follow different fixed nitrogen loss pathways and may express weaker isotope effects, which could lead to lower
δ15N values, similar to those observed during the Cretaceous oceanic anoxic events (OAEs)11,12,31,32. We propose that the decline in foraminifera-bound δ15N in the South Atlantic during the
PETM is a consequence of further expansion of suboxia to anoxia in the thermocline water, developing ammonium-type ODZs, which also hosts the previously identified hydrogen sulfide. In these
ODZs, when ammonium is introduced into the euphotic zone through mixing, nitrification-coupled anammox/denitrification occurs at the upper boundary of the chemocline. This process
represents a different pathway of fixed nitrogen loss, which is not present in today’s nitrate-type ODZs33 (Fig. 2d). In the modern Black Sea, ammonium-type ODZs exist below the euphotic
zone (Fig. 2b), where ammonium concentrations are > 20 μM with δ15N of 4‰34,35. Due to salinity stratification in the upper Black Sea, the upward transport of ammonium is slow, resulting
in the majority of ammonium being removed through nitrification-coupled anammox/denitrification in the anoxic-suboxic transition zone. Consequently, the residual nitrate concentration at the
top of the suboxic zone is up to 4 μM, with δ15N of 10 to 14‰34,35. Simple mass-balance calculation suggests a net isotope fractionation effect as low as 2‰ associated with fixed nitrogen
loss in the water column, much weaker than nitrate-type ODZs19,20,21,22. The weakly expressed isotope effect is similar to that of sedimentary denitrification; although the organism-level
isotope effect is identical to that seen in water-column denitrification, the quantitative consumption of nitrate in sediments leaves behind a minimal isotope fingerprint on the seawater
above. Due to the high sea-surface temperature (SST) during the PETM13, the South Atlantic subtropical gyre likely experienced stronger stratification, limiting upward ammonium transport
into the euphotic zone, similar to the conditions observed in the modern Black Sea. If ammonium-type ODZs were prominent during the PETM, this would have increased the proportion of fixed
nitrogen loss through pathways with weak isotope fractionation (i.e., nitrification-coupled anammox/denitrification at the upper boundary of ammonium-type ODZs and/or sedimentary
denitrification) in the global ocean, resulting in a reduction in the mean ocean fixed nitrogen δ15N compared to the pre-PETM condition. In addition, it has been suggested that nitrate-type
ODZs were contracted in the tropical North Pacific due to reduced upwelling during the PETM17. The combined effect of smaller nitrate-type ODZs and expanded ammonium-type ODZs would lower
the mean ocean fixed nitrogen δ15N without necessitating changes in sedimentary denitrification rates. We use a simple one-box model to quantify the effect of ammonium-type ODZs on the mean
ocean fixed nitrogen δ15N during the PETM, and compare it with all existing foraminifera-bound δ15N data17,32,36 (Fig. 3a). In this model, N2 fixation input is assumed to be balanced by
fixed nitrogen loss through sedimentary denitrification, water-column denitrification in nitrate-type ODZs, and nitrification-coupled anammox/denitrification in ammonium-type ODZs
(“Methods”). Compared to the modern ocean, the mean ocean fixed nitrogen δ15N is higher in the pre-PETM (11.3‰ vs. 5‰), indicating more extensive suboxia and an absence of ammonium-type ODZs
(Fig. 3b). The mean ocean fixed nitrogen δ15N shifts towards a lower value of 7.8‰ during the PETM. Assuming no change in sedimentary denitrification, this decline could be explained as a
result of increased nitrogen loss at the upper boundary of ammonium-type ODZs, which constitutes, on average, ~ 30% of water-column fixed nitrogen loss. The same framework might also explain
the particularly low δ15N during the Cretaceous OAE232, where most water-column fixed nitrogen loss occurs in ammonium-type ODZs. One consequence of the intense fixed nitrogen loss at the
upper boundary of ammonium-type ODZs in the South Atlantic is nitrogen limitation in the euphotic zone. Despite this limitation, we observe a rise in productivity during the PETM (Fig. 1),
suggesting that additional fixed nitrogen was supplied to the euphotic zone, most likely through increased N2 fixation. Modern ocean observations indicate that N2-fixing microbes thrive in
water with low N:P ratios37. During the PTEM, it has been suggested that intense weathering led to an increase in the oceanic phosphorus inventory38,39. The phosphorus increase, coupled with
the significant nitrogen loss at the upper boundary of the ammonium-type ODZs, is expected to drive a decrease in the N:P ratio of surface waters, leading to a substantial rise in N2
fixation and thus boosting productivity in the South Atlantic during the PETM. To better constrain the timing and magnitude changes in marine nitrogen cycling in the South Atlantic across
the PETM, we develop a multi-box isotope-enabled model that specifies fixed nitrogen species in different water layers (see “Methods”, Supplementary Fig. 3 and Supplementary Table 4).
Assuming that 1) the oceanic nitrogen inventory is always in a steady state, and 2) the sedimentary denitrification rate is stable at 4 Tmol N/yr, approximately 92% of ammonification occurs
in the subsurface while the rest in the deep ocean, similar to the modern ocean37. We use constraints from the reconstructed foraminifera-bound δ15N, marine sulfate δ34S, and
nutrient/productivity records to calculate the rates of water-column nitrogen loss in ODZs and N2 fixation across the PETM. Our simulation captures the transition in the predominant form of
fixed nitrogen, shifting from nitrate to ammonium in the subsurface ocean in response to ODZs’ expansion in the South Atlantic during the PETM. Prior to the onset of the PETM, suboxia had
developed in the subsurface ocean, where water-column denitrification in nitrate-type ODZs is responsible for 60% of total fixed nitrogen loss (Fig. 4a, b). Assuming sustained anoxia within
ODZs for 100 kyrs, as constrained by marine sulfate isotope data15,16, water-column denitrification must have increased, with a gradual loss of nitrate from the subsurface ocean from 20 μM
to 4.7 μM at 81 kyrs from the onset of the PETM (Fig. 4c). Due to significant consumption of subsurface nitrate, nitrate-type ODZ nitrogen loss briefly decreases until the nitrate
concentration begins to recover. Simultaneously, ammonium accumulates and reaches a maximum concentration of 16.3 μM. As the ammonium is transported to the surface ocean,
nitrification-coupled anammox/denitrification becomes the dominant pathway (90%) of water-column nitrogen loss in the model, with a net isotope fractionation effect of 2‰ (sensitivity tests
in Supplementary Fig. 4). To compensate for the intense fixed nitrogen loss, N2 fixation has to exceed the pre-PETM level by a factor of 4.7 (Fig. 4b). Elevated N2 fixation rates would
stimulate biological productivity, consistent with the reactive PAR and marine barite AR records during the PETM27,28. When dissolved oxygen resumes in ODZs, the marine nitrogen cycle
returns to the pre-PETM structure. This model exercise demonstrates that the δ15N decline in the South Atlantic during the PETM is a combined effect of 1) the development of ammonium-type
ODZs, which results in a decrease in mean ocean fixed nitrogen δ15N, and 2) increased N2 fixation rates above ammonium-type ODZs, (Fig. 4d and sensitivity tests in Supplementary Fig. 4).
Another potential mechanism for the δ15N decline is greater isotope effects (up to 7‰) associated with alternative (V- and Fe-based) nitrogenases40. However, the deep ocean probably remains
largely oxygenated during the PETM, which would not cause further Mo removal from seawater into sediments41. Thus, it is unlikely that the global oceanic Mo inventory during the PETM is low
enough to favor alternative nitrogenases in triggering the δ15N decline. MECHANISMS FOR EXPANDED SUBSURFACE ANOXIA IN THE ATLANTIC The expansion of ODZs to the extent of anoxia in the South
Atlantic during the PETM is most likely driven by a combination of global warming and enhanced biological productivity. The oxygenation of the subsurface ocean in the South Atlantic is
strongly influenced by O2 supply at high latitudes, particularly from the Southern Ocean surface water. Therefore, SST in the Southern Ocean plays a crucial role in controlling the extent of
ODZs by affecting O2 solubility. Before the PETM, the SST in the Southern Ocean was already ~ 13 °C higher than today (Supplementary Fig. 5). The temperature effect alone would have reduced
O2 solubility by 90 μmol/kg42, preconditioning the ODZs for further expansion. This is consistent with extensive suboxic conditions in the pre-PETM ocean characterized by the high δ15N.
When we further consider an increase in global mean surface temperature by 5.6 °C in the PETM, attributed to nearly doubling atmospheric CO2 concentration, the entire ocean would have been,
on average, 19 °C warmer than today, with the SST in the Southern Ocean higher than 25 °C13. The cumulative temperature effect on O2 solubility is equivalent to a 30% reduction in the
dissolved O2 inventory. Furthermore, intensified warming at high latitudes, through reducing the meridional SST gradient, weakens the ventilation of intermediate and deep waters primarily
originating from the Southern Ocean30. Benthic foraminiferal δ13C and Nd-isotope evidence support a less vigorous ocean overturning during the PETM43,44. Using the Earth system model cGENIE,
we simulate the impact of temperature change on the oceanic O2 distribution (see “Methods”). Compared to today’s ODZs, the ODZs during the PETM have expanded to cover approximately
one-third of the global ocean area in the subsurface ocean, mainly in the Atlantic, eastern tropical Pacific, and Tethys Ocean (Fig. 5). In these areas, the minimum O2 concentration could
have reached zero. We also notice the simulated O2 concentration in some regions of the Pacific remains > 5 μmol/kg, indicating that the response of ODZs to global warming is more complex
in the Pacific compared to the Atlantic, which may be associated with paleogeography restriction and/or local productivity. In addition to global warming, enhanced biological productivity
in the South Atlantic during the PETM could have further intensified the expansion of ODZs by increasing O2 consumption in the thermocline water. While a more stratified ocean would limit
the vertical supply of nutrients to fuel productivity45, it has been suggested that terrestrial weathering has increased globally by 50–60% over 100 kyrs, bringing more phosphorus into the
ocean and promoting productivity during the PETM39,46. The increase in carbon export from the euphotic zone enhances the remineralization of sinking particulate organic matter in the
twilight zone27. As higher seawater temperatures accelerate microbial metabolic activities47, oxygen demand in the interior of the ocean could have become dramatically higher, contributing
to widespread ODZs during the PETM. Despite different residence times of nitrogen and sulfur in the ocean (103 vs. 106 years), we find coupled perturbations to both cycles during the
transient ocean deoxygenation event in the early Cenozoic hypothermal. With widespread suboxia present in the pre-PETM ocean, ODZs are preconditioned to evolve toward anoxia in the South
Atlantic, where ammonium becomes the dominant form of fixed nitrogen. The short-lived accumulation of ammonium in the thermocline water aligns with the coeval sulfide accumulation15,16,
underscoring that significant portions of ODZs have reached anoxic conditions. Ammonium-type ODZs and a compensatory increase in N2 fixation above them offer a plausible explanation of δ15N
decline during the PETM, which might apply to the Cretaceous OAEs and yet to be observed in other Cenozoic warm periods. Our findings suggest that the Paleocene-Eocene warming, alongside
productivity changes, could have led to complex and regionally variable impacts on ocean deoxygenation. While the tropical North Pacific may have undergone less severe deoxygenation or even
an increase in upper ocean oxygen17, the Atlantic Ocean likely experienced intense deoxygenation, marked by the expansion of ODZs enriched with ammonium and hydrogen sulfide. These results
highlight the spatial variability in ocean deoxygenation under extreme climate warming scenarios. In addition, to accurately predict future ocean change in response to anthropogenic warming,
it is crucial to improve our understanding of the future trajectory of the biological carbon pump, a topic currently lacking consensus in Earth System models48. METHODS SITE LOCATION AND
AGE MODEL Sediment samples were collected from ODP Leg 208 Site 1263 Hole C and Hole D (28°31.98′S, 2°46.78′E) drilled at 2717 m water depth and Site 1265 Hole A (28°50.10′S, 2°38.36′E)
drilled at 3060 m water depth on Walvis Ridge. These sites were located on the southeastern margin of the subtropical gyre in the South Atlantic during the PETM (35 °S, 10 °E; Supplementary
Fig. 1). The paleo-water depths of Site 1263 and Site 1265 were approximately 1500 m and 2000 m, respectively49. Composite depth (mcd) allows for stratigraphic correlations among holes43,50.
The ages of these samples were reported as time relative to the onset of the negative carbon isotope excursion (CIE) using the astronomically tuned timescale based on the carbon isotope
record from ODP Site 69050. Mass accumulation rate (MAR) was calculated as the product of the sedimentation rate and the dry bulk density obtained from ODP reports49. ANALYSIS OF
FORAMINIFERA-BOUND NITROGEN ISOTOPES Examinations under a light microscope and scanning electron microscope showed that foraminifera were overall small throughout the PETM interval. The
dominant genus of planktic foraminifera across the PETM were _Morozovella_ and _Acarinina_. Around 5 mg of size fractions (125–250 μm) of mixed-species foraminifera were carefully weighed
and subsequently crushed into smaller pieces using a glass stick and combusted foil. The foraminifera were then meticulously transferred into 15 ml centrifuge tubes. Next, all samples
underwent a 3-min ultrasonication process in 2% sodium polyphosphate with a pH of 8, followed by three thorough rinses with Milli- Q water to remove any potential external sedimentary
contaminants51. To minimize nitrogen contamination associated with metal oxides, a reductive cleaning method was performed using dithionite citrate reagent52, where the samples were soaked
in the reagent and placed in an 80 °C water bath for 1 h with intermittent shaking. Afterward, the samples were rinsed three times with Milli-Q water. Then, the samples were soaked in a
10–15% sodium hypochlorite solution, thoroughly shaken, and left on an orbital shaker overnight (> 12 h) to remove any remaining organic nitrogen contamination. Following these steps, the
samples were rinsed three times with Milli-Q water and dried overnight in an oven at ~ 60 °C. After cleaning, the foraminifera were dissolved using HCl, and the released nitrogen was
oxidized to nitrate using persulfate oxidation25. At least 2 mg of the cleaned foraminifera samples were carefully weighed using clean weighing paper and then transferred into 4 ml combusted
glass vials. 60 µl of 4 N hydrochloric acid was added to the foraminifera samples. The released organic matter was oxidized to nitrate by a low-N potassium persulfate solution. The samples
were then transferred to an autoclave and subjected to 1 h of treatment at 120 °C to ensure complete oxidation. Two amino acid isotope references, USGS 64 (δ15N = + 1.76‰) and USGS 65 (δ15N
= + 20.68‰), as well as persulfate solution blanks, were used during the oxidation as standards. Following autoclaving, the samples were centrifuged at 6000 rpm for 10 min, and the resulting
solution containing nitrate was transferred to a new, clean, pre-combusted 4 ml vial. pH adjustments were then carried out to ensure a final neutral pH. The ‘denitrifier method’ was used to
measure the isotopic composition of the converted nitrate, which was quantitatively converted into N2O using denitrifying bacteria _P. chlororaphis_ that lacks N2O reductase53. The
resulting N2O was analyzed for δ15N on a customized Gas Bench coupled to a Delta V Plus isotope ratio mass spectrometer at Boston College. Isotope values were calibrated relative to air54
using two nitrate isotope references: the international nitrate standards IAEA-N3 (δ15N = + 4.7‰) and USGS-34 (δ15N = − 1.8‰)55. An in-house coral standard (CBS-II) was used as a quality
control for the overall protocol25. The precision of the entire protocol, as determined by the coral standard, was better than 0.3‰ (1-σ). The foraminifera-bound nitrogen content remains
stable between 2.5 and 4.5 μmol/g and has no apparent correlation with the foraminifera-bound δ15N (see Supplementary Table 1), indicating negligible diagenetic impact. There is one lowest
data point of 0.4‰ at 85 kyrs relative to the onset of the PETM. Although this data point is not replicated due to limited materials, its nitrogen content falls into the same range as other
samples. ANALYSIS OF SEDIMENTARY PHOSPHORUS FRACTIONS We crushed freeze-dried sediments and passed them through a 150 μm sieve to homogenize particle size. Five components of sedimentary P
(i.e., water-soluble P, oxide-associated P, authigenic P, detrital P, organic P) were extracted from about 0.1 g samples into known volumes of extractants, using the modified sequential P
extraction (SEDEX) procedure56,57. We analyzed P concentrations via the molybdenum blue colorimetric method at low pH conditions58 with an EnSpire® multimode microplate spectrophotometer at
Southern University of Science and Technology. The absorbance was at a wavelength of 850 nm. Results were reported as the means with 1-σ errors of triplicate runs on individual components.
The long-term analytical error of reactive P concentration was ± 1.32 μmol P/g sediment using the same deep-sea sample as the in-house standard, similar to those from other sedimentary P
studies59. The reactive P concentration was calculated as the sum of water-soluble P, oxide-associated P, authigenic P, and organic P concentrations. The reactive P accumulation rate
(reactive PAR) was calculated as the product of the concentration of reactive P and MAR (Table 2). ONE-BOX MODEL FOR MEAN OCEAN FIXED NITROGEN Δ15N We build a steady-state one-box model to
explore how the development of ammonium-type ODZs affects mean ocean fixed nitrogen δ15N values. In a steady-state, nitrogen input through N2 fixation (_J__fix_) is balanced by the total
fixed nitrogen loss through sedimentary denitrification (_J__sed_), water-column denitrification (_J__wc_) in nitrate-type ODZs, and water-column nitrification-coupled
anammox/denitrification (_J__N/D_) in ammonium-type ODZs (Eq. 1). $${J}_{{fix}}={J}_{{sed}}+{J}_{{wc}}+{J}_{N/D}$$ (1) We define _f__sed_ as the fraction of sedimentary denitrification to
the total nitrogen loss, and _f__N/D_ as the fraction of nitrification-coupled anammox/denitrification to the water-column nitrogen loss. Assuming the newly fixed nitrogen δ15N value
(_δ__fix_) is constant at − 1‰ (60), and the globally mean N-isotope fractionations associated with _J__sed_ and _J__wc_ are 0 and 20‰, respectively19,20, we achieve isotope balance as in
Eq. 2. $$-1={\delta }_{{DIN}}\,{f}_{{sed}}+(1-{f}_{{sed}})[{({\delta }_{{DIN}}-20)1-f}_{(N/D)}+({\delta }_{{DIN}}-{\varepsilon }_{N/D})\,{f}_{N/D}]$$ (2) where _δ__DIN_ denotes the mean
ocean fixed nitrogen δ15N; _ε__D/N_ denotes the N-isotope fractionation associated with nitrification-coupled anammox/denitrification. In the modern ocean, where _J__N/D_ = 0 and δ15NDIN =
5‰, _f__N/D_ is 0% and _f__sed_ is 70%. Solving for _δ__DIN_: $${\delta }_{{DIN}}=19-(20-{\varepsilon }_{N/D})\,{f}_{N/D}-20{f}_{{sed}}+(20-{\varepsilon }_{N/D})\,{f}_{s{ed}}\,{f}_{N/D}$$
(3) Based on Eq. 3, we generate the contour plots of mean ocean-fixed nitrogen δ15N in Fig. 3b for _ε__N/D_ of 2‰ and 10‰. We also calculate the mean sedimentary δ15N or foraminifera-bound
δ15N values from the compiled modern, pre-PETM, PETM, and OAE2 data, and add them to the contour plot. This simple exercise indicates that ammonium-type ODZs have been more widespread in the
OAE2 than the PETM, but have not occurred in the pre-PETM and modern. THE PETM NITROGEN CYCLE MODEL To further reconstruct the marine nitrogen cycle in the South Atlantic across the PETM,
we establish a multi-box isotope-enabled model by dividing the ocean into four nitrogen reservoirs (i.e., surface fixed nitrogen, subsurface nitrate, subsurface ammonium, and deep nitrate
reservoirs) and simultaneously monitor changes in the nitrogen concentrations and δ15N compositions (see model topology in Supplementary Fig. 3 and Supplementary Table 4). Before exploring
perturbations in nitrogen cycling, we assume an initial steady state for the pre-PETM ocean, where all the reservoirs are mass and isotope balanced (Supplementary Fig. 3a). Similar to the
mean ocean δ15N model above, _J__fix_ in the surface ocean is equal to the sum of _J__sed_ in the deep ocean and _J__wc_ and _J__N/D_ in the subsurface ocean (Eq. 4). In ammonium-type ODZs,
a part of ammonium supplied toward the surface ocean gets nitrified and subsequently undergoes anammox/denitrification in the water column. _J__N/D_ can be calculated as a function of the
fraction of nitrification-coupled anammox/denitrification to total ammonium supply from the subsurface (_k_), the subsurface ammonium concentration (_C__subNH4_), and the mixing rate at the
upper boundary of the subsurface ocean (_v__upper_). In nitrate-type ODZs, _J__N/D_ is negligible as _C__subNH4_ is close to 0.
$${J}_{{fix}}={J}_{{sed}}+{J}_{{wc}}+k{{C}_{{subNH}4}v}_{{upper}}$$ (4) where _k_ remains unchanged at 0.8, indicating that 80% of the ammonium mixed up from the subsurface is lost through
nitrification-coupled anammox/denitrification; _v__upper_ is set to be 3 × 1015 m3/yr61. In an oligotrophic ocean like the South Atlantic, biological assimilation ( _J__assi_) will take up
all the fixed nitrogen in the surface ocean supplied from (i) N2 fixation and (ii) the subsurface nitrate and ammonium (if it exists) reservoirs through vertical mixing (Eq. 5). Thus, we
assume no N-isotope fractionation during biological assimilation of nitrate in an oligotrophic ocean (Eq. 6). $${J}_{{fix}}+[{C}_{{subNO}3}+(1-k){{C}_{{subNH}4}}]v_{{upper}}={J}_{{assi}}$$
(5) $${\delta }_{{fix}}\,{J}_{{fix}} + \left[\right.{\delta }_{{subNO}3}{C}_{{subNO}3}+{\delta }_{{subNH}4}{C}_{{subNH}4} \\ -({\delta }_{{subNH}4}-{\varepsilon
}_{N/D})(1-k){{C}_{{subNH}4}\left]\right.v}_{{upper}}={\delta }_{{bio}}\,{J}_{{assi}}$$ (6) where _C__subNO3_ denotes the subsurface nitrate concentration; _δ__subNO3_, _δ__subNH4_, and
_δ__bio_ denote the δ15N of subsurface nitrate, subsurface ammonium, and biomass, respectively. We set _δ__fix_ = − 1‰ (60) and _ε__N/D_ = 2‰ (taken from the Black Sea) throughout the model
run. In the subsurface ocean, ammonium sourced from biomass degradation (i.e., ammonification) will be subsequently (i) oxidized to nitrogen oxides through nitrification (_J__nitr_) and (ii)
supplied to the surface ocean (Eq. 7). The latter does not exist until ammonium starts to accumulate in ODZs. These processes have no isotope fractionation (Eq. 8).
$${f}_{{subammo}}\,{J}_{{assi}}={J}_{{nitr}}+{C}_{{subNH}4}{v}_{{upper}}$$ (7) $${\delta }_{{bio}}\,{f}_{{subammo}}\,{J}_{{assi}}={\delta }_{{subNH}4}\,{J}_{{nitr}}+{\delta
}_{{subNH}4}{C}_{{subNH}4}{v}_{{upper}}$$ (8) where _f__subammo_ denotes the constant fraction of ammonification in the subsurface ocean to biological assimilation. Nitrate in the subsurface
ocean is controlled by nitrification, water-column denitrification, and nitrate exchange with the surface and the deep ocean (Eq. 9). Nitrate exchange fluxes are a function of the
subsurface nitrate concentration, the deep nitrate concentrations (_C__deep_), and the mixing rate at the upper and lower boundaries of the subsurface ocean. Water-column denitrification
expresses strong N-isotope fractionation (_ε__wc_) as observations in today’s ocean, which will drive the overall marine δ15N toward higher values (Eq. 10).
$${J}_{{nitr}}+{C}_{{deep}}{v}_{{lower}}={J}_{{wc}}+{C}_{{subNO}3}({v}_{{upper}}+{v}_{{lower}})$$ (9) $${{\delta }_{{subNH}4}\,J}_{{nitr}} +{\delta
}_{{deep}}{C}_{{deep}}{v}_{{lower}}=\left({\delta }_{{subNO}3}-{\varepsilon }_{{wc}}\right){J}_{{wc}} \\ +{\delta }_{{subNO}3}{C}_{{subNO}3}({v}_{{upper}}+{v}_{{lower}})$$ (10) where
_δ__deep_ denotes deep nitrate δ15N; _v__lower_ denotes the mixing rate at the lower boundary of the subsurface ocean, which is set to be 1 × 1014 m3/yr61. In the deep ocean, nitrate remains
the dominant form of fixed nitrogen due to deep-sea ventilation. Ammonification in the deep ocean is balanced by sedimentary denitrification and nitrate exchange between the subsurface and
the deep ocean (Eq. 11). Due to complete nitrate consumption in sediment porewaters, N-isotope fractionation associated with sedimentary denitrification is negligible (Eq. 12).
$${f}_{{deepammo}}\,{J}_{{assi}}+{C}_{{subNO}3}{v}_{{lower}}={C}_{{deep}}{v}_{{lower}}+{J}_{{sed}}$$ (11) $${\delta }_{{bio}}\,{f}_{{deepammo}}\,{J}_{{assi}}+{\delta
}_{{subNO}3}{C}_{{subNO}3}{v}_{{lower}}={\delta }_{{deep}}({C}_{{deep}}{v}_{{lower}}+{J}_{{sed}})$$ (12) where _f__deepammo_ denotes the constant fraction of ammonification in the deep ocean
to biological assimilation, which is equivalent to 1 – _f__subammo_. At the initial steady state, our model achieves equilibrium when approximately 92% of organic matter is recycled through
ammonification in the subsurface ocean (i.e., _f__subammo_ = 0.92) while the rest is recycled in the deep ocean (_f__deepammo_ = 0.08), similar to the present day37. This yields the
N-isotope fractionation of 19.2‰ associated with water-column denitrification in nitrate-type ODZs, which corresponds to the deep nitrate δ15N of 9.7‰ and the biomass δ15N of 9.3‰,
consistent with the foraminifera-bound δ15N values in the pre-PETM. To further model the response of nitrogen cycling to ocean deoxygenation, we use constraints from the foraminifera-bound
δ15N, marine sulfate δ34S, and productivity records. To reproduce the observed N-isotope decline, we make the following assumptions: * i. The oceanic nitrogen inventory remains constant
throughout the model run. Nitrogen input through N2 fixation is set to always compensate for nitrogen output. The ocean δ15N changes with these processes. * ii. The timing of anoxia within
ODZs is between 0 and 100 kyrs relative to the onset of the PETM, as constrained by the positive sulfur and oxygen isotope excursions of marine sulfate15,16. Once ODZs become oxygen-free,
ammonium sourced from biomass degradation can no longer be substantially oxidized to nitrogen oxides (Supplementary Fig. 3b). Thus, we force the ratio of nitrification to ammonification to
decrease at the onset, cease within the time interval (as an extreme case), and recover at 100 kyrs when the oxygen concentration returns to the pre-PETM level. * iii. Water-column nitrogen
loss fluxes are a function of subsurface fixed nitrogen concentrations. With intensified ocean deoxygenation, water-column denitrification in nitrate-type ODZs increases until subsurface
nitrate is progressively depleted. The increasing fixed nitrogen loss through water-column denitrification in nitrate-type ODZs and nitrification-coupled anammox/denitrification in
ammonium-type ODZs will be compensated by increased N2 fixation, which further influences biological assimilation to reflect changes in productivity. With these assumptions, our model
reconstructs the evolution of marine nitrogen cycling in response to a transient expansion and recovery of ODZs during the PETM. When dissolved oxygen resumes in ODZs, elevating
nitrification reduces the ammonium concentration and, subsequently, ammonium-type ODZ nitrogen loss and assimilation. In addition, we test the sensitivity of the model to N2 fixation. In the
sensitivity test, we force a constant N2 fixation flux, while keeping the rest parameters and forcing same as above (Supplementary Fig. 4b). In this scenario, N2 fixation cannot compensate
nitrogen loss during the expansion of ODZs. The oceanic nitrogen inventory is reduced by 12% during the PETM. The deficiency of nitrogen in the ocean will cause a reduction in biological
assimilation by ~ 70%, which is opposed to productivity records27,28. With a constant N2 fixation flux, the drawdown of δ15N could only be achieved with a close-to-zero isotope fractionation
effect associated with nitrification-coupled anammox/denitrification in ammonium-type ODZs. However, this scenario overall fits foraminifera-bound δ15N poorer compared to that of a constant
oceanic nitrogen inventory. INTERMEDIATE-COMPLEXITY EARTH SYSTEM MODEL CGENIE We utilize the late-Paleogene configuration of the carbon-centric Grid Enabled Integrated Earth system model
(cGENIE) after Ref. 62 and modify two parameters in the initial set-up for the pre-PETM to reconcile proxy information. First, we assume the pre-PETM atmospheric CO2 concentration is 1120
ppm, exceeding the preindustrial CO2 concentration (280 ppm) by a factor of four. The × 4 CO2 concentration is chosen because (i) it falls within the range of × 2 to × 8 CO2 projected by
previous studies13,29,30,45,63 and (ii) the resulting pre-PETM surface water temperatures and dissolved O2 concentrations match proxy observations (Supplementary Fig. 5 and Table 3). Second,
we change the initial ocean temperature to 13 °C for the pre-PETM. While the default configuration is 10 °C, the latest proxy and data assimilation results suggest warmer bottom water
temperatures at ~ 13 °C in the pre-PETM13,64,65,66,67. Before carbon emission, the model is spun up to achieve long-term equilibrium. The simulated minimum O2 concentration is consistent
with redox proxy records that the development of suboxic and anoxic waters is mainly restricted in the Atlantic and Tethys in the pre-PETM. The atmospheric _p_O2 level could be another
factor affecting oxygen in the ocean. The previous study suggested that after the PETM, the atmospheric _p_O2 level experienced a gradual rise from ~ 17% in the early Eocene to as high as
23% in the Oligocene, followed by a decline toward the present value of 21%68. If true, the initial atmospheric _p_O2 level and, consequently, the dissolved O2 concentration in the ocean
during the PETM would be about 20% lower than the present-day level. However, considering debates on the atmospheric _p_O2 history, our cGENIE simulations stick with the modern-like
atmospheric _p_O2 level of 21% in this study, as most of the previous modeling work did30,62. We test different carbon emission scenarios following the rates and magnitudes of carbon
emissions suggested by previous studies62,63,69,70, ranging from a release of 3000 Pg C (low-emission) to 10,900 Pg C (high-emission) within 5000 years, and investigate the responses of the
oceanic O2 concentration. All the scenarios agree with enhanced ocean deoxygenation, where the minimum O2 concentration in the subsurface Atlantic, Tethys, and Eastern Pacific may have
reached anoxia during the PETM (Supplementary Fig. 6). These results strongly support the “ammonium ocean” mechanism in the subsurface South Atlantic. We also note that compared to the South
Atlantic, the minimum O2 concentration is overall higher in the tropical North Pacific – consistent with proxy data (Supplementary Table 3) – and more variable to different scenarios. While
discussing the Pacific is beyond the scope of this study, we focus on the South Atlantic, where all the simulations agree on subsurface ocean anoxia during the PETM. Our cGENIE results are
also consistent with the previous General Circulation Model (GCM) results for future climate warming (SSP5-8.5)17, where the subsurface O2 concentration mainly increases at low latitudes but
decreases at ~ 30°N and ~ 30°S. In other words, the mechanism of ocean oxygen rise/decline is unlikely to apply to the whole ocean – while some parts of the tropical Pacific undergo
enhanced oxygenation, the subtropical Atlantic is experiencing significant oxygen loss. DATA AVAILABILITY All data generated in this study are provided in the Supplementary Information. CODE
AVAILABILITY Codes are described in the text and Supplementary Information. REFERENCES * Schmidtko, S., Stramma, L. & Visbeck, M. Decline in global oceanic oxygen content during the
past five decades. _Nature_ 542, 335–339 (2017). Article ADS PubMed CAS Google Scholar * Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. _Science_ 359,
eaam7240 (2018). Article PubMed Google Scholar * Kwiatkowski, L. et al. Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production
decline from CMIP6 model projections. _Biogeosciences_ 17, 3439–3470 (2020). Article ADS CAS Google Scholar * Oschlies, A., Brandt, P., Stramma, L. & Schmidtko, S. Drivers and
mechanisms of ocean deoxygenation. _Nat. Geosci._ 11, 467–473 (2018). Article ADS CAS Google Scholar * Fu, W., Primeau, F., Keith Moore, J., Lindsay, K. & Randerson, J. T. Reversal
of increasing tropical ocean hypoxia trends with sustained climate warming. _Glob. Biogeochem. Cycles_ 32, 551–564 (2018). Article ADS CAS Google Scholar * Busecke, J. J. M., Resplandy,
L., Ditkovsky, S. J. & John, J. G. Diverging fates of the Pacific Ocean oxygen minimum zone and its core in a warming world. _AGU Adv._ 3, e2021AV000470 (2022). Article ADS Google
Scholar * Kwiecinski, J. V. & Babbin, A. R. A high-resolution atlas of the eastern tropical pacific oxygen deficient zones. _Glob. Biogeochem. Cycles_ 35, e2021GB007001 (2021). Article
ADS CAS Google Scholar * IPCC in _Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on_
Climate Change, (eds Masson-Delmotte, V. et al.) (Cambridge University Press, 2021). * Jørgensen, B. B. in _The Major Biogeochemical Cycles and their Interaction_ (eds Bolin, B. & Cook,
R. B.) 477–5095 (John Wiley 1983). * Meyer, K. M. & Kump, L. R. Oceanic euxinia in Earth history: causes and consequences. _Annu. Rev. Earth. Planet. Sci._ 36, 251–288 (2008). Article
ADS CAS Google Scholar * Higgins, M. B., Robinson, R. S., Husson, J. M., Carter, S. J. & Pearson, A. Dominant eukaryotic export production during ocean anoxic events reflects the
importance of recycled NH4+. _Proc. Natl. Acad. Sci. USA_ 109, 2269–2274 (2012). Article ADS PubMed PubMed Central CAS Google Scholar * Naafs, B. D. A. et al. Fundamentally different
global marine nitrogen cycling in response to severe ocean deoxygenation. _Proc. Natl. Acad. Sci. USA_ 116, 24979–24984 (2019). Article ADS PubMed PubMed Central CAS Google Scholar *
Tierney, J. E. et al. Spatial patterns of climate change across the Paleocene–Eocene Thermal Maximum. _Proc. Natl. Acad. Sci. USA_ 119, e2205326119 (2022). Article PubMed PubMed Central
CAS Google Scholar * Judd, E. J. et al. A 485-million-year history of Earth’s surface temperature. _Science_ 385, eadk3705 (2024). Article PubMed CAS Google Scholar * Yao, W., Paytan,
A. & Wortmann, U. G. Large-scale ocean deoxygenation during the Paleocene-Eocene Thermal Maximum. _Science_ 361, 804–806 (2018). Article ADS PubMed CAS Google Scholar * Yao, W.,
Paytan, A. & Wortmann, U. G. Effects of a transient marine sulfur reservoir on seawater δ18OSO4 during the Paleocene-Eocene Thermal Maximum. _Geochim. Cosmochim. Acta_ 269, 257–269
(2020). Article ADS CAS Google Scholar * Moretti, S. et al. Oxygen rise in the tropical upper ocean during the Paleocene-Eocene Thermal Maximum. _Science_ 383, 727–731 (2024). Article
ADS PubMed CAS Google Scholar * Ren, H., Sigman, D. M., Thunell, R. C. & Prokopenko, M. G. Nitrogen isotopic composition of planktonic foraminifera from the modern ocean and recent
sediments. _Limnol. Oceanogr._ 57, 1011–1024 (2012). Article ADS CAS Google Scholar * Casciotti, K. L. Nitrogen and oxygen isotopic studies of the marine nitrogen cycle. _Annu. Rev. Mar.
Sci._ 8, 379–407 (2016). Article ADS Google Scholar * Sigman, D. M. & Fripiat, F. in _Encyclopedia of Ocean Sciences_ 3rd edn (eds Kirk Cochran, J., Bokuniewicz, H. J. & Yager,
P. L.) 263–278 (Academic, 2019). * Sigman, D. M. et al. Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin. _Glob. Biogeochem. Cycles_ 19,
GB4022 (2005). Article ADS Google Scholar * Peters, B. D., Lam, P. J. & Casciotti, K. L. Nitrogen and oxygen isotope measurements of nitrate along the US GEOTRACES Eastern Pacific
Zonal Transect (GP16) yield insights into nitrate supply, remineralization, and water mass transport. _Mar. Chem._ 201, 137–150 (2018). Article CAS Google Scholar * Kast, E. et al.
Nitrogen isotope evidence for expanded ocean suboxia in the early Cenozoic. _Science_ 364, 386–389 (2019). Article ADS PubMed CAS Google Scholar * Auderset, A. et al. Enhanced ocean
oxygenation during Cenozoic warm periods. _Nature_ 609, 77–82 (2022). Article ADS PubMed PubMed Central CAS Google Scholar * Wang, X. T. et al. Oceanic nutrient rise and the late
Miocene inception of Pacific oxygen-deficient zones. _Proc. Natl. Acad. Sci. USA_ 119, e2204986119 (2022). Article PubMed PubMed Central CAS Google Scholar * Junium, C. K., Dickson, A.
J. & Uveges, B. T. Perturbation to the nitrogen cycle during rapid Early Eocene global warming. _Nat. Commun._ 9, 3186 (2018). Article ADS PubMed PubMed Central Google Scholar * Ma,
Z. et al. Carbon sequestration during the Palaeocene-Eocene Thermal Maximum by an efficient biological pump. _Nat. Geosci._ 7, 382–388 (2014). Article ADS CAS Google Scholar * Griffith,
E. M. et al. Benthopelagic decoupling: The marine biological carbon pump during Eocene hyperthermals. _Paleoceanogr. Paleoclimatol._ 36, e2020PA004053 (2021). Article Google Scholar *
Zhou, X. et al. Expanded oxygen minimum zones during the late Paleocene-early Eocene: Hints from multiproxy comparison and ocean modeling. _Paleoceanography_ 31, 1532–1546 (2016). Article
ADS Google Scholar * Remmelzwaal, S. R. C. et al. Investigating ocean deoxygenation during the PETM through the Cr isotopic signature of foraminifera. _Paleoceanogr. Paleoclimatol._ 34,
917–929 (2019). Article ADS Google Scholar * Ruvalcaba Baroni, I., Van Helmond, N. A. G. M., Tsandev, I., Middelburg, J. J. & Slomp, C. P. The nitrogen isotope composition of
sediments from the proto‐North Atlantic during Oceanic Anoxic Event 2. _Paleoceanography_ 30, 923–937 (2015). Article ADS Google Scholar * Zhai, R., Zeng, Z., Zhang, R. & Yao, W. The
response of nitrogen and sulfur cycles to ocean deoxygenation across the Cenomanian-Turonian boundary. _Glob. Planet. Change_ 227, 104182 (2023). Article Google Scholar * Peng, X. et al.
Revisiting nitrification in the Eastern Tropical South Pacific: A focus on controls. _J. Geophys. Res. Oceans_ 121, 1667–1684 (2016). Article ADS CAS Google Scholar * Velinsky, D. J.,
Fogel, M. L., Todd, J. F. & Tebo, B. M. Isotopic fractionation of dissolved ammonium at the oxygen-hydrogen sulfide interface in anoxic waters. _Geophys. Res. Lett._ 18, 649–652 (1991).
Article ADS CAS Google Scholar * Murray, J. W., Fuchsman, C., Kirkpatric, J., Paul, B. & Konovalov, S. K. Species and δ15N signatures of nitrogen transformations in the suboxic zone
of the Black Sea. _Oceanography_ 18, 36–47 (2005). Article Google Scholar * Tesdal, J.-E., Galbraith, E. D. & Kienast, M. Nitrogen isotopes in bulk marine sediment: linking seafloor
observations with subseafloor records. _Biogeosciences_ 10, 101–118 (2013). Article ADS Google Scholar * Sarmiento, J. L. & Gruber, N. _Ocean Biogeochemical Dynamics_ (Princeton
University Press, 2013). * Komar, N. & Zeebe, R. E. Redox-controlled carbon and phosphorus burial: A mechanism for enhanced organic carbon sequestration during the PETM. _Earth Planet.
Sci. Lett._ 479, 71–82 (2017). Article ADS CAS Google Scholar * Papadomanolaki, N. M., Sluijs, A. & Slomp, C. P. Eutrophication and deoxygenation forcing of marginal marine organic
carbon burial during the PETM. _Paleoceanogr. Paleoclimatol._ 37, e2021PA004232 (2022). Article ADS PubMed PubMed Central Google Scholar * Zhang, X., Sigman, D. M., Morel, F. M. &
Kraepiel, A. M. Nitrogen isotope fractionation by alternative nitrogenases and past ocean anoxia. _Proc. Natl. Acad. Sci. USA_ 111, 4782–4787 (2014). Article ADS PubMed PubMed Central
CAS Google Scholar * Dickson, A. J. A molybdenum-isotope perspective on Phanerozoic deoxygenation events. _Nat. Geosci._ 10, 721–726 (2017). Article ADS CAS Google Scholar * Weiss, R.
F. The solubility of nitrogen, oxygen and argon in water and seawater. _Deep Sea Res. Oceanogr. Abstr._ 17, 721–735 (1970). Article ADS CAS Google Scholar * Nunes, F. & Norris, R. D.
Abrupt reversal in ocean overturning during the Palaeocene/Eocene warm period. _Nature_ 439, 60–63 (2006). Article ADS PubMed CAS Google Scholar * Abbott, A. N., Haley, B. A., Tripati,
A. K. & Frank, M. Constraints on ocean circulation at the Paleocene–Eocene Thermal Maximum from neodymium isotopes. _Clim. Past_ 12, 837–847 (2016). Article Google Scholar * Winguth,
A. M. E., Thomas, E. & Winguth, C. Global decline in ocean ventilation, oxygenation, and productivity during the Paleocene-Eocene Thermal Maximum: Implications for the benthic
extinction. _Geology_ 40, 263–266 (2012). Article ADS CAS Google Scholar * Pogge von Strandmann, P. A. E. et al. Lithium isotope evidence for enhanced weathering and erosion during the
Paleocene-Eocene Thermal Maximum. _Sci. Adv._ 7, eabh4224 (2021). Article ADS PubMed PubMed Central CAS Google Scholar * Laws, E. A., Falkowki, P. G. Jr., Smith, W. O., Ducklow, H.
& McCarthy, J. J. Temperature effects on export production in the open ocean. _Glob. Biogeochem. Cycles_ 14, 1231–1246 (2000). Article ADS CAS Google Scholar * Henson, S. A. et al.
Uncertain response of ocean biological carbon export in a changing world. _Nat. Geosci._ 15, 248–254 (2022). Article ADS CAS Google Scholar * Zachos, J. C. et al. Proceedings of the
Ocean Drilling Program, Initial Reports, 208, 1–112 (College Station, 2004). * Zachos, J. C. et al. Rapid acidification of the ocean during the Paleocene-Eocene thermal maximum. _Science_
308, 1611–1615 (2005). Article ADS PubMed CAS Google Scholar * Ren, H. et al. Foraminiferal isotope evidence of reduced nitrogen fixation in the ice age Atlantic Ocean. _Science_ 323,
244–248 (2009). Article PubMed CAS Google Scholar * Mehra, O. P. & Jackson, M. L. Iron oxide removal from soils and clays by a dithionite– citrate system buffered with sodium
bicarbonate. _Clays Clay Miner._ 7, 317–327 (1960). Google Scholar * Sigman, D. M. et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. _Anal.
Chem._ 73, 4145–4153 (2001). Article PubMed CAS Google Scholar * Weigand, M. A., Foriel, J., Barnett, B., Oleynik, S. & Sigman, D. M. Updates to instrumentation and protocols for
isotopic analysis of nitrate by the denitrifier method. _Rapid Commun. Mass Spectrom._ 30, 1365–1383 (2016). Article ADS PubMed CAS Google Scholar * Böhlke, J. K. & Coplen, T. B.
Interlaboratory comparison of reference materials for nitrogen-isotope-ratio measurements. In _Reference and Intercomparison Materials for Stable Isotopes of Light Elements_ 51–66
(International Atomic Energy Agency, 1995). * Ruttenberg, K. C. Development of a sequential extraction method for different forms of phosphorus in marine sediments. _Limnol. Oceanogr._ 37,
1460–1482 (1992). Article ADS CAS Google Scholar * Anderson, L. D. & Delaney, M. L. Sequential extraction and analysis of phosphorus in marine sediments: streamlining of the SEDEX
procedure. _Limnol. Oceanogr._ 45, 509–515 (2000). Article ADS CAS Google Scholar * Murphy, J. & Riley, J. P. A modified single solution method for the determination of phosphate in
natural waters. _Anal. Chim. Acta_ 27, 31–36 (1962). Article CAS Google Scholar * Faul, K. L. & Paytan, A. Phosphorus and barite concentrations and geochemistry in Site 1221
Paleocene/Eocene Boundary sediments. _Proceedings of the Ocean Drilling Program, Scientific Results_, 199 (eds Wilson, P. A., Lyle, M. & Firth, J. V.) 477–5095 (John Wiley, 2005). *
Gruber, N. in _Nitrogen in the Marine Environment_ (eds Capone, D. G., Bronk, D. A., Mulholland, M. R. & Carpenter, E. J.) 1–50 (Elsevier, 2008). * Deutsch, C., Sigman, D. M., Thunell,
R. C., Meckler, A. N. & Haug, G. H. Isotopic constraints on glacial/interglacial changes in the oceanic nitrogen budget. _Glob. Biogeochem. Cycles_ 18, GB4012 (2004). Article ADS
Google Scholar * Ridgwell, A. & Schmidt, D. N. Past constraints on the vulnerability of marine calcifiers to massive carbon dioxide release. _Nat. Geosci._ 3, 196–200 (2010). Article
ADS CAS Google Scholar * McInerney, F. A. & Wing, S. L. The Paleocene–Eocene Thermal Maximum: a perturbation of carbon cycle, climate, and biosphere with implications for the future.
_Annu. Rev. Earth Planet. Sci._ 39, 489–516 (2011). Article ADS CAS Google Scholar * McCarren, H., Thomas, E., Hasegawa, T., Röhl, U. & Zachos, J. C. Depth dependency of the
Paleocene-Eocene carbon isotope excursion: Paired benthic and terrestrial biomarker records (Ocean Drilling Program Leg 208, Walvis Ridge). _Geochem. Geophys. Geosyst._ 9, Q10008 (2008).
Article ADS Google Scholar * Thomas, D. J., Zachos, J. C., Bralower, T. J., Thomas, E. & Bohaty, S. Warming the fuel for the fire: Evidence for the thermal dissociation of methane
hydrate during the Paleocene-Eocene thermal maximum. _Geology_ 30, 1067–1070 (2002). Article ADS CAS Google Scholar * Bornemann, A. et al. Persistent environmental change after the
Paleocene–Eocene Thermal Maximum in the eastern North Atlantic. _Earth Planet. Sci. Lett._ 394, 70–81 (2014). Article ADS CAS Google Scholar * Tripati, A. K. & Elderfield, H. Abrupt
hydrographic changes in the equatorial Pacific and subtropical Atlantic from foraminiferal Mg/Ca indicate greenhouse origin for the thermal maximum at the Paleocene-Eocene Boundary.
_Geochem. Geophys. Geosyst._ 5, Q02006 (2004). Article ADS Google Scholar * Falkowski, P. G. The rise of oxygen over the past 205 million years and the evolution of large placental
mammals. _Science_ 309, 2202–2204 (2005). Article ADS PubMed CAS Google Scholar * Gutjahr, M., Dickson, A. J. & Uveges, B. T. Very large release of mostly volcanic carbon during the
Palaeocene–Eocene Thermal Maximum. _Nature_ 548, 573–577 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Cui, Y. et al. Slow release of fossil carbon during the
Palaeocene–Eocene Thermal Maximum. _Nat. Geosci._ 4, 481––4485 (2011). Article ADS Google Scholar Download references ACKNOWLEDGEMENTS We thank A. Paytan for discussion and Y. Hu and J.
Liao for their assistance with sample preparation. This research was supported by the National Natural Science Foundation of China (42376049), National Key R&D Program of China
(2022YFF0802900), Guangdong Natural Science Foundation (2024A1515012537), Science and Technology Innovation Committee of Shenzhen Municipality (20231114150312001) grants to W.Y., and Boston
College Start-up funds to X.T.W. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ocean Science and Engineering, Southern University of Science and Technology, Shenzhen,
Guangdong, China Weiqi Yao, Ruixiang Zhai, Ruiling Zhang & Yilin Liu * Department of Earth and Environmental Sciences, Boston College, Chestnut Hill, MA, USA Tianshu Kong, Xingchen Tony
Wang & Ruixiang Zhai Authors * Weiqi Yao View author publications You can also search for this author inPubMed Google Scholar * Tianshu Kong View author publications You can also search
for this author inPubMed Google Scholar * Xingchen Tony Wang View author publications You can also search for this author inPubMed Google Scholar * Ruixiang Zhai View author publications You
can also search for this author inPubMed Google Scholar * Ruiling Zhang View author publications You can also search for this author inPubMed Google Scholar * Yilin Liu View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.Y. and X.T.W. conceived the project. T.K. performed nitrogen isotope analyses. Y.L. performed
phosphorus analyses. W.Y., R.Zhai and R.Zhang contributed to the computations. W.Y. provided samples and composed the manuscript with contributions from T.K. and X.T.W. W.Y., T.K., X.T.W.,
R.Zhai, R.Zhang and Y.L. contributed to data interpretation and the final manuscript. CORRESPONDING AUTHORS Correspondence to Weiqi Yao or Xingchen Tony Wang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Bernhard Naafs and the other anonymous reviewers for their
contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION TRANSPARENT PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material.
You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yao, W., Kong, T., Wang, X.T. _et al._ Expanded subsurface ocean anoxia in
the Atlantic during the Paleocene-Eocene Thermal Maximum. _Nat Commun_ 15, 9053 (2024). https://doi.org/10.1038/s41467-024-53423-x Download citation * Received: 26 March 2024 * Accepted: 11
October 2024 * Published: 20 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53423-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
