Emerging many-to-one weighted mapping in hippocampus-amygdala network underlies memory formation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Memories are crucial for daily life, yet the network-level organizing principles governing neural representations of experiences remain unknown. Employing dual-site in vivo
recording in freely behaving male mice, here we show that hippocampal dorsal CA1 (dCA1) and basolateral amygdala (BLA) utilize distinct coding strategies for novel experiences. A small
assembly of BLA neurons emerged active during memory acquisition and persisted through consolidation, whereas most dCA1 neurons were engaged in both processes. Machine learning decoding
revealed that dCA1 population spikes predicted BLA assembly firing rate, suggesting that most dCA1 neurons concurrently index an episodic event by rapidly establishing weighted communication
with a specific BLA assembly – a process we term “many-to-one weighted mapping.” We also found that dCA1 reactivations preceded BLA assembly activity preferably during elongated and
enlarged dCA1 ripples. Using a closed-loop strategy, we demonstrated that suppressing BLA activity after large dCA1 ripples impaired memory. These findings highlight a many-to-one weighted
mapping mechanism underlying both the acquisition and consolidation of new memories. SIMILAR CONTENT BEING VIEWED BY OTHERS DISTINGUISHING EXAMPLES WHILE BUILDING CONCEPTS IN HIPPOCAMPAL AND
ARTIFICIAL NETWORKS Article Open access 20 January 2024 DE NOVO INTER-REGIONAL COACTIVATIONS OF PRECONFIGURED LOCAL ENSEMBLES SUPPORT MEMORY Article Open access 11 March 2022 INTEGRATING
NEW MEMORIES INTO THE HIPPOCAMPAL NETWORK ACTIVITY SPACE Article 18 February 2021 INTRODUCTION Converging evidence suggests that the formation of new memories involves a complex neural
network of brain regions, including the hippocampal dorsal CA1 (dCA1) and basolateral amygdala (BLA). How neuronal populations within these brain regions encode and exchange information is
yet to be fully understood1,2,3,4. The formation of episodic memories, or memories of events, can be divided into two processes: memory acquisition and consolidation. Memory acquisition is
rapid, indexing episodic events almost instantaneously; however, these memories are often fragile and only become stabilized after a slow transformation process known as memory
consolidation5,6. This process critically involves sleep, with dCA1 ripples, fast oscillations (100–300 Hz) that occur predominantly during slow-wave sleep and immobility, playing a crucial
role5,6,7. Despite this understanding, it is still unclear how the dCA1–BLA network enables such rapid indexing and gradual consolidation of new memories. Memories of events consist of three
primary components: where, when, and what8,9. Previous studies have demonstrated that the dCA1 encodes where and when by sequences, such that a substantial portion of dCA1 neurons fire
sequentially during spatial navigation or performing time-based tasks10,11. In contrast, no consensus has been reached regarding how dCA1 neurons encode specific events (what). Given the
continuous unfolding of episodic events in our daily life, the encoding of new events becomes intricately intertwined with the recall of past experiences. Consequently, dCA1 neurons become
highly entangled in the process of encoding both ongoing and past event information12, making it challenging to disentangle dCA1 spike patterns that specifically encode individual events.
Presently, there are two major hypotheses regarding how the hippocampus encodes episodic events (what). One prominent view holds that “(event) information is sparsely encoded in distributed
ensembles of hippocampal neurons”13. However, there is limited evidence supporting this sparse-coding hypothesis by dCA1 neurons13,14,15. In fact, recent findings suggest the contrary,
revealing that a substantial portion of dCA1 neurons (up to 50%), instead of a minority, are activated during fear memory procedures16,17,18,19. Moreover, ~30–42% of dCA1 neurons exhibit
co-activation when exposed to two different contexts16,20, indicating a substantial overlap of dCA1 neurons in encoding distinct contextual memories. These findings generally contradict the
sparse-coding hypothesis, at least within the dCA1 region. The other influential view suggests that the hippocampus functions to link or bind, rather than encode event information that is
otherwise represented in a distributed neural network21,22. This viewpoint, however, fails to address the precise mechanisms through which dCA1 activity effectively achieves such
linking/binding, and it has not been directly tested experimentally. To address this knowledge gap, our study employs a different approach to deduce dCA1 encoding principles by computing
activity correlations between dCA1 neurons and specific BLA assemblies. This approach leverages recent findings that BLA neurons form distinct assemblies to represent salient events and
memories23,24,25. RESULTS EMERGING DCA1–BLA COMMUNICATION UNDERLIES MEMORY FORMATION To investigate the encoding principles and communication dynamics of the dCA1 and BLA neuronal
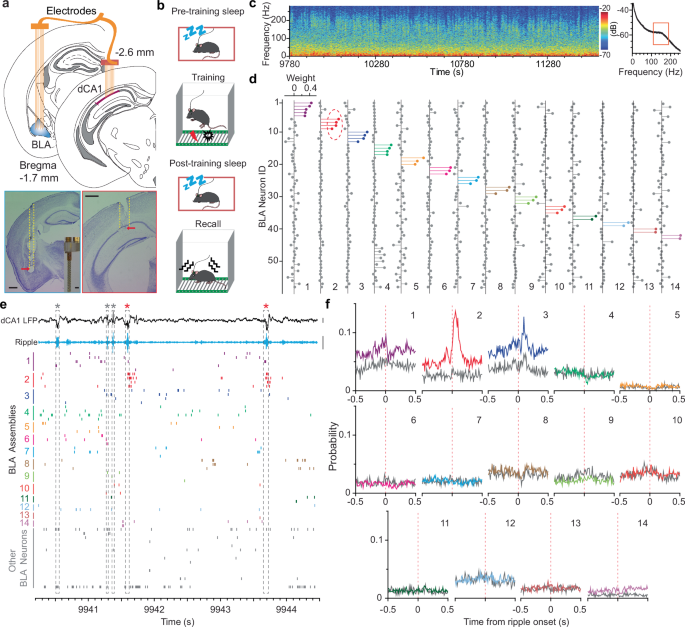
populations, we conducted dual-site in vivo recording of the dCA1 and BLA (up to 16 tetrodes per site; Fig. 1a). All mice received a contextual fear memory procedure that consisted of
pre-training sleep, training, post-training sleep, and fear recall test (Fig. 1b). Neuronal spikes and local field potentials (LFPs) were recorded throughout the entire process, which
enabled us to study dCA1–BLA neuronal ensemble dynamics at each memory stage, including acquisition, consolidation, and retrieval. We first identified slow-wave sleep (SWS) stages based on
prominent dCA1 delta26 and ripple oscillations (Fig. 1c). Next, we conducted an independent component analysis (ICA) of population spikes recorded during SWS to identify major BLA
assemblies, i.e., small groups of co-activated neurons27. As an example, the ICA identified 14 assemblies based on the activity of 58 BLA neurons recorded during post-training SWS (Fig. 1d;
Supplementary Fig. 1). We observed that one selective BLA assembly (#2) robustly increased its activity after a subset of dCA1 ripples (Fig. 1e). Subsequent cross-correlation analysis
confirmed this observation and revealed additional BLA assemblies that showed decreased activity (# 1&8), decreased activity followed by a rebound (#3), or little change of activity
(Fig. 1f). On average, the ICA algorithm identified 8.8 ± 1.0 BLA assemblies (mean ± s.e.m.; 2–6 neurons per assembly) across datasets recorded from 10 mice, with a minority of BLA neurons
not forming assemblies (Fig. 1d; Supplementary Figs. 2–4). Notably, often one BLA assembly (or neuron) from each dataset showed robust dCA1 ripple-modulated activation during post-, but not
pre-training sleep (Fig. 1f; Supplementary Figs. 2–4), suggesting the emergence of dCA1 ripple-to-BLA assembly communication after learning. Although there are other ripple-modulated BLA
assemblies, they often exhibit decreased activity immediately after ripple events, or little modulation between pre- and post-training sleep (Fig. 1f; Supplementary Figs. 2–4). Based on a
permutation test, the proportions of ripple-modulated BLA assemblies are significantly higher than chance in each of the datasets during post-training sleep, as well as in most recording
sessions (7/10) during pre-training sleep (_P_ < 0.05; see Methods). To provide further evidence that these ripple-modulated BLA assemblies (or neurons) are involved in memory processes,
we analyzed their activity during memory acquisition and memory retrieval. Our results revealed that most of the post-training ripple-modulated BLA neurons (∼78%; 18/23) exhibited robust
prolonged activation during memory acquisition and retrieval stages (Fig. 2; Supplementary Figs. 2&3). These results suggest the involvement of a unique BLA assembly engaging in multiple
memory stages, including acquisition, consolidation, and retrieval. Subsequently, we termed a BLA assembly as a “BLA memory assembly” if it displayed robust activation during memory
acquisition and ripple-modulated activation during post-, but not pre-training sleep (Fig. 2c; Supplementary Fig. 4). Individual neurons within each BLA memory assembly or single BLA neurons
that met the above criteria were likewise termed “BLA memory neurons.” Notably, only one BLA assembly (or neuron) was identified as a memory assembly (or neuron) in each dataset based on
the above criteria. Overall, these BLA memory neurons comprise ∼5.3% (18/341) of the BLA neuronal population, indicating a sparse coding feature. Additionally, we found that communications
between dCA1 ripples and BLA memory assemblies (or neurons) were more prevalent during early-stage sleep compared to later-stage sleep after the contextual fear training (Supplementary Fig.
5). We next investigated whether BLA assemblies preexisted prior to learning or were newly formed during the learning process. Our ICA assembly analyses, based on separate pre-training or
post-training sleep datasets, largely identified the same groups of BLA neurons as assemblies (Supplementary Fig. 1). Importantly, further analyses revealed that most BLA assemblies
identified based on post-training sleep were already highly active during pre-training sleep (Supplementary Fig. 1), despite that their activity did not necessarily coincide with dCA1 ripple
events during pre-training sleep. These findings suggest that memory formation primarily recruits preconfigured or preexisting BLA assemblies, rather than forming new assemblies, a notion
consistent with recent findings3. MEMORY-ASSOCIATED RIPPLES ARE ENLARGED AND ELONGATED We next asked if the dCA1 ripple-to-BLA assembly communication conveyed unique information. To address
this, we classified dCA1 ripples into two categories: memory and non-memory associated ripples. Memory-associated ripples were defined if they occurred coinciding with BLA memory assembly
activation, while the remaining ripples were defined as non-memory ripples (Fig. 3a; see Methods). Our analyses revealed that memory-associated ripples had significantly larger amplitude and
longer duration (Fig. 3b; Supplementary Figs. 2&3). Moreover, these memory-associated ripples contained distinct contents, i.e., spikes of different groups of dCA1 neurons (Fig. 3c).
Comparing memory-associated ripples to non-memory ones, most dCA1 neurons showed further firing changes on top of their already increased activity: about one third showed upregulated
activity while another one third showed downregulated activity (Fig. 3d; Supplementary Figs. 2&3). These findings suggest that a unique population activity pattern of dCA1 neurons
communicates with a selective BLA assembly for relevant memory consolidation. We observed that the increased dCA1 activity during memory-associated ripples tend to be prolonged (Fig. 3d,
right). To verify this, we conducted an unbiased analysis on all dCA1 neurons that increased activity during memory _vs_. non-memory ripples. Our analyses confirmed that dCA1 neuronal
firings were elongated and more robust during memory-associated ripples across animals (Fig. 3e; Supplementary Figs. 2&3). These elongated dCA1 ripples and enhanced firings likely
signify the consolidation of newly formed memories28. Taken together, we propose a model of ripple-associated memory consolidation, as depicted in Fig. 3f. During non-memory ripples, most
dCA1 neurons exhibit a baseline probability of activation that represents preconfigured rigid motifs29,30. Upon memory consolidation, relevant dCA1 neurons exhibit upregulated and/or
prolonged activity during ripples, while the remaining dCA1 neurons show downregulated or no change in activity, which collectively represent specific information coding. DCA1 POPULATION
SPIKES PREDICT BLA MEMORY ASSEMBLY FIRING RATE We next asked if dCA1 population spikes can predict the firing rate of BLA memory assemblies or neurons. To address this, we implemented
generalized linear model (GLM) machine learning decoding31. Given that dCA1 neuronal activity preceded BLA memory assembly activity by ∼35 ms (Fig. 4 a&b), we used a shift of 35 ms
between dCA1 and BLA neurons for the GLM decoding. Overall, dCA1 and BLA neuronal spikes across multiple sliding windows (lasting 100 ms; Fig. 4c) before or after dCA1 ripples were
extracted: 50% of them were used for training of the GLM decoder, and the remaining 50% were used for decoding. We used correlation coefficients between the real and predicted firing rates
to indicate prediction power, resulting in a scale between –1 and 1 (Fig. 4d). Our findings demonstrated that the dCA1 population spikes predicted BLA memory assembly firing rate during the
post-, but not pre-training sleep (Fig. 4 d–f). This provides direct evidence of emerging weighted communications from dCA1 neurons to a selective BLA assembly in memory formation. In
contrast, dCA1 population spikes had limited prediction on BLA non-memory neurons, although the prediction power on a small subset of them appeared to be high during both pre- and
post-training sleep, which may reflect pre-existing dCA1–BLA communications in consolidating older memory traces (Fig. 4g). EMERGING MANY-TO-ONE WEIGHTED MAPPING UNDERLIES MEMORY FORMATION
Previous attempts to directly characterize dCA1 spike patterns that encode specific episodic events, such as experiencing shocks or air puffs2,32, have not yielded a framework for
understanding dCA1 information coding principles. Therefore, we employed a different approach to deduce the encoding principle of dCA1 neurons by analyzing their relationship with specific
BLA assemblies. This approach leverages our ability to isolate a distinct BLA memory assembly that is involved in memory acquisition, consolidation, and retrieval (Fig. 2). Our results
revealed that most dCA1 neurons exhibit BLA memory assembly-correlated activity during training and post-training sleep, but not pre-training sleep (Fig. 4a; Supplementary Figs. 2&3).
This suggests the involvement of the majority of dCA1 neurons compared to a small assembly of BLA neurons (5.3%) in memory acquisition and consolidation. To further uncover the extent of
dCA1 involvement in communication with the BLA, we divided the dCA1 neurons into subgroups based on their high or low activity in correlation with the BLA memory assembly (Fig. 5a). Our GLM
decoding results showed that not only the higher-, but also lower-weight dCA1 neurons predicted the BLA assembly firing rate (Fig. 5 b&c; Supplementary Figs. 2&3). This suggests that
decoding the BLA assembly activity involves contributions from many dCA1 neurons, rather than just a few. Taken together, we propose a model for memory formation: an emerging many-to-one
weighted mapping from dCA1 neurons to a selective BLA assembly underlies the formation of a new memory (Fig. 5d). In a follow-up analysis, we compared the spatial information coding
properties between the higher- and lower-weight dCA1 neurons as classified above. Our results revealed that subsets of both groups exhibit place cell characteristics (Supplementary Fig. 6).
We found no difference in spatial information coding capacity between the higher- and lower-weight dCA1 neurons (Supplementary Fig. 6). These results align with previous findings and support
the notion that memory coding (non-spatial) and spatial coding are represented by partially overlapping groups of dCA1 neurons33,34. CLOSED-LOOP OPTOINHIBITION DURING POST-TRAINING SLEEP
IMPAIRS MEMORY To determine if dCA1 ripple-coincided BLA activity is necessary for memory consolidation, we employed a closed-loop optoinhibition approach. We microinjected
AAV-CaMKII-stGtACR235 into the BLA bilaterally and then implanted two optic fibers slightly above the injection sites, which enabled later optoinhibition of BLA pyramidal neurons. Meanwhile,
we implanted four tetrodes into the dCA1 to record ripple activity (Fig. 6a). After 2–3 weeks to allow viral expression, mice underwent a contextual fear conditioning procedure, followed by
closed-loop, delayed, or no-stimulation of the BLA (Fig. 6b). This manipulation lasted two hours during post-training rest/sleep, similar to that reported previously36,37. Our analysis
showed that large-amplitude dCA1 ripples had a greater correlation with BLA memory assembly activity (Fig. 2c; Supplementary Figs. 2&3). Therefore, we used a high threshold (8 s.d.) to
trigger closed-loop optoinhibitions of the BLA, aiming to disrupt information flow into the BLA immediately after large ripple events (Fig. 6c). Our offline analysis confirmed that the
closed-loop group mice received the optoinhibition within a short latency of ∼10 ms after ripple detection, whereas the delayed group had an extended latency of ∼160 ms (Fig. 6d).
Behaviorally, the closed-loop group mice exhibited significantly reduced freezing compared to the delayed or no-stimulation groups, indicating impaired contextual fear memory (Fig. 6e).
These findings suggest a crucial role of dCA1–BLA communication in memory formation. DCA1 COMMUNICATES WITH BLA THROUGH A RELAY REGION Anatomically, dCA1 and BLA are not directly connected
by synapses38,39. Therefore, dCA1 potentially communicates with BLA through a relay region. This notion aligns with our finding that the dCA1-to-BLA information flow takes about 35 ms,
indicating a di-synaptic communication. It appears that only the entorhinal, perirhinal, and ectorhinal cortices receive direct inputs from the dCA1 and project directly to the BLA, based on
comprehensive anterograde and retrograde tracing studies shown in open-source databases, including the Mouse Connectome Project (Fig. 7a) and Allen Brain Atlas – Mouse Connectivity (Fig.
7b). Theoretically, information relayed from dCA1 to BLA could utilize a many→fewer→one or many→many→one principle (Fig. 7c). The former notion gains support in findings from immediate early
gene studies, demonstrating that a large portion of dCA1 neurons (up to 50%) are activated during memory processing, whereas a moderate portion of entorhinal neurons (∼25%), and only a
small portion of BLA neurons (∼10%) are activated in the same process16,17,18. These differences are further amplified with decreasing neuron numbers, from ∼400 k in dCA1, ∼330 k in
deep-layer entorhinal cortex, to only ∼83 k in BLA of rats40,41,42,43. DISCUSSION Our findings suggest that most dCA1 neurons concurrently index an episodic event by rapidly establishing
weighted communications with a specific BLA assembly. We refer to this process as “many-to-one weighted mapping.” The involvement of a significant portion of dCA1 neurons in encoding a
specific event presents several theoretical advantages. Firstly, this mechanism offers immense encoding capacity due to the numerous potential combinations of dCA1 weighted mappings.
Secondly, it provides robustness and redundancy, thereby enhancing single-trial learning and minimizing potential disruptions from background or external noises9,44. It is noteworthy that
single-trial learning and memory formation are recognized as features of biological intelligence in comparison to long-training sessions of artificial intelligence45. On the other hand, the
small proportion of participating BLA neurons (5.3%) may contribute to simplicity and efficiency in readout. In essence, we propose that many-to-one weighted mappings are responsible for
creating distinct memories within network representations. Specifically, when intermittent dCA1 firings occur that represent any significant mental or cognitive process spanning a few
hundred milliseconds, they constitute an exclusive population activity pattern. This patterned firing enables the establishment of a unique weighted mapping from many dCA1 neurons to a
specific BLA assembly that represents, in a primitive form, the same significant event, likely through rapid long-term potentiation46. In other words, distinct dCA1 population activity
patterns communicate with corresponding BLA assemblies to rapidly index various episodic memories. It is possible that this dCA1 population activity pattern simultaneously communicates with
multiple neuronal assemblies that are distributed across the brain through collateral projections47,48,49. This would allow dCA1 reactivations during ripples to bind distributed assemblies
across brain regions to form a network representation of long-term memories. In support, we and others have shown that ripple-triggered optoinhibition of the BLA (Fig. 6) or the medial
prefrontal cortex50 impairs learning and memory formation. Few studies have investigated the interactions between hippocampus and amygdala at the neuronal ensemble level during memory
formation2,3. One study reported increased correlation between dCA1 neurons and selective BLA neurons during fear memory formation2. Our study extended this finding by showing that
population spikes in dCA1 collectively predicted the firing rate of a selective BLA assembly during ripples. Another study found that the BLA assemblies are preconfigured before memory
acquisition3. Our results confirmed this finding and further demonstrated that the activity of a unique BLA assembly was preceded by dCA1 ripples during post- but not pre-training sleep. We
speculate that the recruitment of preexisting BLA assemblies to establish communication with the dCA1 enables rapid and efficient circuit mapping29,51,52,53. Together, an emerging
hippocampus-to-amygdala communication appears to underline the acquisition and consolidation of new salient memories. One outstanding question concerns how similar contextual fear
experiences are encoded by the dCA1–BLA network. Prior research has shown that lesions of the dorsal hippocampus abolish the discrimination of similar contexts, indicating its crucial role
in context discrimination54. It is plausible that different subpopulations of dCA1 neurons each encode a distinct context, albeit with a considerable amount of overlap between the dCA1
subpopulations (∼30–42%), based on previous work16,20. This distinction appears to amplify upstream of CA1, where less- or even non-overlapping subpopulations of neurons within the CA3 and
dentate gyrus are involved in distinguishing different contexts16,20. In contrast, we speculate that the same assembly of BLA neurons will be activated in multiple fear contexts. This notion
is supported by prior studies implicating the central role of the BLA in fear generalization55,56. Additionally, our preliminary findings revealed that many BLA memory neurons also
responded to novel contexts after the contextual fear training (Supplementary Fig. 7), indicating that these BLA neurons exhibit generalized fear responses to multiple contexts. In our
experiments, we used relatively strong footshocks to induce salient memory, resulting in a many-to-one weighted mapping from dCA1 to BLA. Notably, the weights of correlation vary greatly
across dCA1 neuron–BLA assembly pairs, forming a continuum spectrum from low to high values. We speculate that for less salient memories, the overall range of dCA1-BLA communication weights
will be narrower (i.e., indicating a weaker association). Accordingly, the proportion of dCA1 neurons that significantly contribute to memory formation will also reduce. One caveat of the
current study is the lack of an experiment that directly tests our proposed model of many-to-one weighted mapping. Here, we propose two possible experiments for future investigation. The
first experiment involves conducting retrograde tracing using transsynaptic viruses, such as the modified rabies virus57 or pseudorabies virus58. Based on our many-to-one model, the
infection of one or a few neurons within the BLA will result in exponential labeling of many neurons in upstream projection regions. The second experiment involves conducting high-resolution
focal stimulation of multiple sites within a projection region while recording from the BLA59. If our model is correct, these focal stimulations will activate the same assembly of BLA
neurons, albeit at varying levels of activation (i.e., weighted communication). Our research also demonstrated that memory-associated dCA1 ripples are elongated, consistent with a recent
study showing that prolonging dCA1 ripples after learning improves memory28. We found that the elongation of ripples is accompanied by an overall prolongation and enhancement of neuronal
firings in the dCA1, with some neurons upregulating and others downregulating their activity. Additionally, our results revealed that memory-associated ripples were enlarged in amplitude,
contrary to a previous study28, which may reflect species differences between mice and rats60. Together, our findings suggest that elongated and enlarged dCA1 ripples signify the
consolidation of newly acquired memories, while shorter and smaller ripples may indicate baseline activity or preconfigured dCA1 rigid motifs29,52. METHODS MICE Male C57BL/6 mice were
purchased from the Jackson Laboratory (stock #000664). Mice were 3–4 months old at the time of surgery; after surgery, they were singly housed in cages (40 × 20 × 25 cm) containing corn cob
and cotton material and kept on a 12 h light/dark cycle with _ad libitum_ access to food and water. Experimental procedures were approved by the Institutional Animal Care and Use Committees
of Drexel University (protocol # LA-23-740) and were in accordance with the National Research Council _Guide for the Care and Use of Laboratory Animals_. STEREOTAXIC SURGERY Surgery
procedures were similar to that used in our lab24. In brief, mice were anesthetized with ketamine/xylazine mixture (∼100/10 mg/kg, i.p.) and kept on a heating pad at 37 °C. For in vivo
electrophysiology recording, mice received implantation of two electrode arrays (8–16 tetrodes each) into the BLA and dCA1, respectively61. For closed-loop optoinhibition, mice received
microinjection of AAV viruses (AAV1-CKIIa-stGtACR2-FusionRed; 0.25 μl; ∼1013 GC/ml; _Addgene_ 105669) and implantation of two optic fibers (diameter 200 μm) into the BLA bilaterally;
meanwhile, they received implantation of 4 tetrodes into the dCA1 unilaterally. AAV viruses were microinjected through a syringe pump (_World Precision Instruments_) over 5 min (50 nL/min),
with an addition of 5 min before removal of the injection needle (34 gauge, beveled). The BLA coordinates were AP –1.7 mm, ML 3.4 mm, DV 3.9 mm; the dCA1 coordinates were AP –2.6 mm, ML 1.8
mm, and DV 1.1 mm. IN VIVO ELECTROPHYSIOLOGY Each tetrode consisted of four wires (90% platinum 10% iridium; 18 μm diameter; _California Fine Wire_). A microdrive was used to couple with the
electrode bundle, similar to that used in our lab24,26. Neural signal was preamplified, digitized, and recorded using a _Blackrock Neurotech_ CerePlex except one dataset that was recorded
using a _Plexon_ acquisition system; meanwhile, animals’ behaviors were recorded. With the _Blackrock_ system, the local field potentials (LFPs) were digitized at 2 kHz and filtered at 500
Hz low cut; spikes were digitized at 30 kHz and filtered between 600–6000 Hz. For the _Plexon_ system, the LFPs were digitized at 1 kHz and filtered between 0.7–300 Hz; spikes were digitized
at 40 kHz and filtered between 400–7000 Hz. The tetrode arrays were gradually lowered daily until we recorded clear ripples and a substantial number of neurons; otherwise, mice were
excluded from the study. The recorded spikes were sorted using the MClust 3.524; key datasets were manually verified using _Plexon_ Offline Sorter. In total, spikes from 10 mice were used
for analyses in this study; the neuron numbers in BLA and dCA1 were 58/55, 41/63, 36/52, 35/40, 35/18, 33/64, 31/41, 29/50, 29/38, and 14/15, respectively. ASSEMBLY DETECTION BY INDEPENDENT
COMPONENT ANALYSIS (ICA) ICA was performed as described previously27. In brief, spike counts of each neuron were binned at 25 ms and z-scored to generate a neuronal population activity
matrix (neurons × bins). Coactivity patterns were then extracted from this data matrix in two major steps. Firstly, the number of significant coactivation patterns was estimated by
calculating the independent components (ICs) of the data matrix with variances above a threshold derived from an analytical probability function for uncorrelated data (Marchenko-Pastur
distribution; Supplementary Fig. 1). Secondly, fastICA was used to extract the coactivity patterns from the projection of the data matrix into the subspace spanned by the significant ICs. In
simple terms, this method first finds the significant ICs and then rotates them to match the ideal assembly patterns. These detected assembly patterns often were comprised of a small number
of neurons with high weights, along with a larger group of neurons with low or zero weights. Neurons whose weights exceeded 2 s.d. from the mean weight of each assembly pattern were
classified as members of an assembly, as described in previously62,63. The activation strength of each assembly was calculated by projecting the columns of the z-scored spike matrix onto the
axis defined by the corresponding assembly pattern (Supplementary Fig. 1). The activation events were identified when the activation strength exceeded 5 s.d. from the mean activation
strength of each assembly, as described previously3. Notably, using other thresholds, including 4 s.d. and 6 s.d., reached the same conclusions. To investigate the significance of activation
event rates, we computed the activation strength of surrogate assemblies (500 surrogates for each assembly) generated by randomly permuting the data matrix. For all activation strength
calculations, the assembly patterns extracted from post-training SWS sessions were utilized as templates. RIPPLES Ripples were band-pass filtered between 100–250 Hz and ripple envelope was
smoothed with a Gaussian kernel (s.d. = 4 ms)64. Ripple amplitudes were defined as the peak values of ripple envelopes. For analyses, we used amplitudes exceeding 5 s.d. above the mean,
except for Fig. 3, where amplitudes exceeding 3 s.d. above the mean were analyzed. Ripple onsets and offsets were defined as the points where ripple amplitudes exceeded 1 s.d. above the mean
before and after the corresponding ripple peaks (Fig. 3a). Ripple length was defined as the duration between the onset and offset; only ripples longer than 20 ms were used for further
analysis. MEMORY AND NONMEMORY ASSOCIATED RIPPLES We defined BLA memory assemblies or neurons if they displayed robust activation during memory acquisition and ripple-modulated activation
during post-, but not pre-training sleep (Fig. 2c). dCA1 ripples (recorded during the post-training sleep) were defined as memory-associated ripples if the corresponding BLA memory assembly
(or neuron) exhibited high activation (2 s.d. above the median) within 150 ms of the ripple onset. The remaining ripples were defined as non-memory ripples (Fig. 3a). RIPPLE CONTENTS
(RELATED TO FIG. 3C) Firing difference score was defined as M | N = abs(M–N) / (M + N). M and N are the mean firing rates of each dCA1 neuron calculated ±100 ms within the onset of memory
and non-memory associated ripples, respectively. M1 | M2 was defined as abs(M1–M2) / (M1 + M2), in which M1 and M2 are the mean firing rates of each dCA1 neuron calculated ±100 ms within the
onset of two randomly divided groups of memory-associated ripples. N1 | N2 was similarly defined as M1 | M2 except for non-memory ripples. RIPPLE-MODULATED BLA ASSEMBLIES AND PERMUTATION
TEST Ripple modulation was determined by correlating the spikes of individual BLA assembly with respect to dCA1 ripple events using a peri-event analysis with a bin size of 5 ms. A Gaussian
kernel (s.d. = 15 ms) was then applied to smooth the peri-ripple histogram. Assemblies that showed a difference from the baseline by a z-score of 3.3 or greater for three or more consecutive
bins (within ± 150 ms from ripple onset) were defined as ripple-modulated assemblies, as described previously26. To determine if the proportion of dCA1 ripple-modulated BLA assemblies was
greater than chance, we conducted a permutation test. Specifically, we randomly shuffled the timing of dCA1 ripple events 100 times followed by cross-correlation analyses to calculate the
proportion of BLA assemblies modulated by the shuffled ripple events. Next, we performed a Chi-squared test to compare the proportions of BLA assemblies modulated by real and shuffled ripple
events. Our results revealed a significant difference (_P_ < 0.05) in each of the animals during post-training sleep and in most (7/10) of the animals during pre-training sleep. GLM
DECODING We constructed generalized linear models (GLMs) with a log link function to predict spike counts of individual BLA assemblies or neurons during ripples based on population spike
counts in dCA1 across specific time windows31. Ripples detected during pre- and post-training sleeps, and all dCA1 and BLA neurons were included for the analysis. Spike counts of each neuron
or assembly were binned in 100-ms bins relative to ripple onset: −300 to −200 ms, −200 to −100 ms, −100 to 0 ms, 0 to 100 ms for dCA1, and −65 to 35 ms, 35 to 135 ms, 135 to 235 ms, 235 to
335 ms for BLA. We used dCA1 population spike counts in different time bins to predict the spike count of a single BLA assembly or neuron. We randomly partitioned the ripples into two
equally sized datasets: one of them was used to train the GLM decoder, and the other was used for the test. For the test phase, the model derived from the training phase was applied to the
dCA1 population spike data in the test set, yielding predictions for the predicted BLA spike counts across ripples. Lastly, we conducted correlation coefficient analysis between the
predicted and real BLA spike counts to measure GLM decoding power on a scale from −1 to 1. SPATIAL INFORMATION ANALYSIS To quantify spatial information coding, firing rate maps of individual
dCA1 neurons were generated in 1 × 1 cm spatial bins in NeuroExplorer and smoothed by a Gaussian filter (filter width, 5 bins) before being sent to MATLAB for further analyses. Spatial
information content for each dCA1 unit was calculated using the formula: $${Information\; content}({bits}/{spike})={\sum}_{{{{\rm{i}}}}}{{{{\rm{p}}}}}_{{{{\rm{i}}}}}\frac{{{{{\rm{\lambda
}}}}}_{i}}{{{{\rm{\lambda }}}}}{\log }_{2}\frac{{{{{\rm{\lambda }}}}}_{{{{\rm{i}}}}}}{{{{\rm{\lambda }}}}},$$ (1) where λi is the mean firing rate in the i-th bin, λ is the overall mean
firing rate, and pi is the probability of the animal’s being in the i-th bin, as described previously65. Only units with peak firing rates higher than 0.4 Hz in any spatial bin were used for
further analysis66. FEAR CONDITIONING (IN VIVO RECORDING) The fear-conditioning chamber used in the experiment was a square chamber measuring 25 × 25 × 32 cm, with a 36-bar shock grid floor
(_Med Associates_). The behaviors of the mice were recorded using either the _Blackrock Neurotech_ NeuroMotive or _Plexon_ CinePlex video system. During training, the mice were first
allowed to explore the footshock chamber for ∼3 minutes. They then received up to 10 mild footshocks (0.75 mA, 0.5 s), with a 2–3 min interval between shocks. Note that we used
direct-current footshocks to minimize electromagnetic noise, so occasionally mice missed a shock if they stood on two positively or negatively charged grids. One minute after the last shock,
the mice were returned to their home cages. Approximately 2 hours later, the mice were placed back in the footshock chamber for a 5-min contextual fear test. Subsequently, as a control
test, mice were placed into a neutral chamber (40 cm in diameter, 35 cm in height) for 5 min. Neural activity was recorded continuously, including the pre-training sleep (1–2 hours),
training (∼0.5 hour), post-training sleep (1–2 hours), contextual-fear and neutral-chamber tests (5 min each). FEAR CONDITIONING (CLOSED-LOOP OPTOINHIBITION) Three groups of mice were used:
1) closed-loop; 2) delayed; and 3) no-stimulation groups. All mice were singly housed and received daily habituations of in vivo recording for 1–2 weeks. All mice underwent a contextual fear
procedure that consisted of three footshocks (0.75 mA, 2 s; 1–1.5 min apart), similar to that described previously67. Freezing behaviors were automatically scored using _Med Associates_
VideoFreeze67. To conduct closed-loop optoinhibition, we used the _Open Ephys_ recording system and _Opto Engine_ lasers. Only large dCA1 ripples (raw 100–250 Hz filtered traces) with peak
amplitude exceeding 8 s.d.68 were used to trigger bilateral optoinhibition of the BLA (0.5 mW; 150 ms), either immediately (closed-loop group) or after a delay of 150 ms (delayed-stimulation
group). HISTOLOGY To mark the final recording sites, we made electrical lesions by passing 20-second, 10-μA currents through two or more tetrodes. Mice were deeply anesthetized and
intracardially perfused with ice-cold PBS or saline, followed by 10% formalin. The brains were removed and postfixed in formalin for at least 24 hours. The brains were sliced into coronal
sections of 50-μm thickness using _Leica_ vibratome. Sections from the dual-site recording mice were stained with cresyl violet for microscopic examination of electrode placements, whereas
other sections were mounted with Mowiol mounting medium mixed with DAPI for microscopic fluorescent examination of viral vector expression and optical fiber placements. STATISTICS Sample
sizes were based on previous similar studies in our labs24,26. To determine firing-rate change during dCA1 ripples, the value that deviates from the mean by a _z_-score of >3.3 (_P_ <
0.001) for at least three consecutive bins (bin = 5 ms) was considered significant. Other statistical analyses include Analysis of Variance (ANOVA) followed by post-hoc Bonferroni, Wilcoxon
rank-sum test, and Student’s _t_ test. All statistical tested are two-sided when applicable; _P_ values of 0.05 or lower were considered significant. REPORTING SUMMARY Further information on
research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Key dataset used in this study has been deposited to a public repository:
https://figshare.com/articles/dataset/_b_Emerging_many-to-one_weighted_mapping_in_hippocampus-amygdala_network_underlies_memory_formation_b_/27104269 Source data are provided with this
paper. CODE AVAILABILITY Key MATLAB scripts used in this study have been deposited to a public repository: https://github.com/DVWangLab. REFERENCES * Kitamura, T. et al. Engrams and circuits
crucial for systems consolidation of a memory. _Science_ 356, 73–78 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Girardeau, G., Inema, I. & Buzsaki, G.
Reactivations of emotional memory in the hippocampus-amygdala system during sleep. _Nat. Neurosci._ 20, 1634–1642 (2017). Article CAS PubMed Google Scholar * Miyawaki, H. & Mizuseki,
K. De novo inter-regional coactivations of preconfigured local ensembles support memory. _Nat. Commun._ 13, 1272 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Kim, W.
B. & Cho, J. H. Encoding of contextual fear memory in hippocampal-amygdala circuit. _Nat. Commun._ 11, 1382 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Walker,
M. P. & Stickgold, R. Sleep-dependent learning and memory consolidation. _Neuron_ 44, 121–133 (2004). Article CAS PubMed Google Scholar * Diekelmann, S. & Born, J. The memory
function of sleep. _Nat. Rev. Neurosci._ 11, 114–126 (2010). Article CAS PubMed Google Scholar * Buzsaki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and
planning. _Hippocampus_ 25, 1073–1188 (2015). Article PubMed PubMed Central Google Scholar * Ergorul, C. & Eichenbaum, H. The hippocampus and memory for “what,” “where,” and “when.
_Learn Mem._ 11, 397–405 (2004). Article PubMed PubMed Central Google Scholar * Sugar, J. & Moser, M. B. Episodic memory: Neuronal codes for what, where, and when. _Hippocampus_ 29,
1190–1205 (2019). Article PubMed Google Scholar * Buzsaki, G. & Tingley, D. Space and Time: The Hippocampus as a Sequence Generator. _Trends Cogn. Sci._ 22, 853–869 (2018). Article
PubMed PubMed Central Google Scholar * Eichenbaum, H. Time cells in the hippocampus: a new dimension for mapping memories. _Nat. Rev. Neurosci._ 15, 732–744 (2014). Article CAS PubMed
PubMed Central Google Scholar * Moser, M. B. & Moser, E. I. Distributed encoding and retrieval of spatial memory in the hippocampus. _J. Neurosci._ 18, 7535–7542 (1998). Article CAS
PubMed PubMed Central Google Scholar * Kandel, E. R., Dudai, Y. & Mayford, M. R. The molecular and systems biology of memory. _Cell_ 157, 163–186 (2014). Article CAS PubMed Google
Scholar * Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. _Curr. Opin. Neurobiol._ 7, 217–227 (1997). Article CAS PubMed Google
Scholar * Josselyn, S. A. and S. Tonegawa, Memory engrams: Recalling the past and imagining the future. Science. 367, eaaw4325 (2020) * Ramirez, S. et al. Creating a false memory in the
hippocampus. _Science_ 341, 387–391 (2013). Article ADS CAS PubMed Google Scholar * Tayler, K. K. et al. Reactivation of neural ensembles during the retrieval of recent and remote
memory. _Curr. Biol._ 23, 99–106 (2013). Article CAS PubMed Google Scholar * Zelikowsky, M. et al. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential
components of contextual fear. _J. Neurosci._ 34, 8462–8466 (2014). Article PubMed PubMed Central Google Scholar * Liu, X. et al. Optogenetic stimulation of a hippocampal engram
activates fear memory recall. _Nature_ 484, 381–385 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Leutgeb, S. et al. Distinct ensemble codes in hippocampal areas CA3
and CA1. _Science_ 305, 1295–1298 (2004). Article ADS CAS PubMed Google Scholar * Fortin, N. J., Agster, K. L. & Eichenbaum, H. B. Critical role of the hippocampus in memory for
sequences of events. _Nat. Neurosci._ 5, 458–462 (2002). Article CAS PubMed PubMed Central Google Scholar * Eichenbaum, H. Hippocampus: cognitive processes and neural representations
that underlie declarative memory. _Neuron_ 44, 109–120 (2004). Article CAS PubMed Google Scholar * Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal
contextual memory engram. _Nature_ 513, 426–430 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Liu, J., Lin, L. & Wang, D. V. Representation of Fear of Heights by
Basolateral Amygdala Neurons. _J. Neurosci._ 41, 1080–1091 (2021). Article CAS PubMed PubMed Central Google Scholar * Liu, J. et al. Neural Coding of Appetitive Food Experiences in the
Amygdala. _Neurobiol. Learn Mem._ 155, 261–275 (2018). Article PubMed Google Scholar * Wang, D. V. et al. Mesopontine median raphe regulates hippocampal ripple oscillation and memory
consolidation. _Nat. Neurosci._ 18, 728–735 (2015). Article CAS PubMed PubMed Central Google Scholar * Lopes-dos-Santos, V., Ribeiro, S. & Tort, A. B. Detecting cell assemblies in
large neuronal populations. _J. Neurosci. Methods_ 220, 149–166 (2013). Article PubMed Google Scholar * Fernandez-Ruiz, A. et al. Long-duration hippocampal sharp wave ripples improve
memory. _Science_ 364, 1082–1086 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Hall, A. F. & Wang, D. V. The two tales of hippocampal sharp-wave ripple content:
The rigid and the plastic. _Prog. Neurobiol._ 221, 102396 (2023). Article PubMed Google Scholar * Grosmark, A. D. & Buzsaki, G. Diversity in neural firing dynamics supports both rigid
and learned hippocampal sequences. _Science_ 351, 1440–1443 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Rothschild, G., Eban, E. & Frank, L. M. A
cortical-hippocampal-cortical loop of information processing during memory consolidation. _Nat. Neurosci._ 20, 251–259 (2017). Article CAS PubMed Google Scholar * McEchron, M. D., Tseng,
W. & Disterhoft, J. F. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. _J. Neurosci._ 23, 1535–1547 (2003).
Article CAS PubMed PubMed Central Google Scholar * Tanaka, K. Z. et al. The hippocampal engram maps experience but not place. _Science_ 361, 392–397 (2018). Article ADS CAS PubMed
Google Scholar * Aronov, D., Nevers, R. & Tank, D. W. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. _Nature_ 543, 719–722 (2017). Article ADS CAS PubMed
PubMed Central Google Scholar * Mahn, M. et al. High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. _Nat. Commun._ 9, 4125 (2018). Article ADS
PubMed PubMed Central Google Scholar * Girardeau, G. et al. Selective suppression of hippocampal ripples impairs spatial memory. _Nat. Neurosci._ 12, 1222–1223 (2009). Article CAS
PubMed Google Scholar * Maingret, N. et al. Hippocampo-cortical coupling mediates memory consolidation during sleep. _Nat. Neurosci._ 19, 959–964 (2016). Article CAS PubMed Google
Scholar * van Groen, T. & Wyss, J. M. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. _J.
Comp. Neurol._ 302, 515–528 (1990). Article PubMed Google Scholar * Oh, S. W. et al. A mesoscale connectome of the mouse brain. _Nature_ 508, 207–214 (2014). Article ADS CAS PubMed
PubMed Central Google Scholar * Berdel, B., Morys, J. & Maciejewska, B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. _Int J. Dev.
Neurosci._ 15, 755–765 (1997). Article CAS PubMed Google Scholar * Mulders, W. H., West, M. J. & Slomianka, L. Neuron numbers in the presubiculum, parasubiculum, and entorhinal area
of the rat. _J. Comp. Neurol._ 385, 83–94 (1997). Article CAS PubMed Google Scholar * Merrill, D. A., Chiba, A. A. & Tuszynski, M. H. Conservation of neuronal number and size in the
entorhinal cortex of behaviorally characterized aged rats. _J. Comp. Neurol._ 438, 445–456 (2001). Article CAS PubMed Google Scholar * Schmidt, B., Marrone, D. F. & Markus, E. J.
Disambiguating the similar: the dentate gyrus and pattern separation. _Behav. Brain Res_ 226, 56–65 (2012). Article PubMed Google Scholar * Wiltgen, B. J. et al. Context fear learning in
the absence of the hippocampus. _J. Neurosci._ 26, 5484–5491 (2006). Article CAS PubMed PubMed Central Google Scholar * Coppolino, S. & Migliore, M. An explainable artificial
intelligence approach to spatial navigation based on hippocampal circuitry. _Neural Netw._ 163, 97–107 (2023). Article PubMed Google Scholar * Lynch, M. A. Long-term potentiation and
memory. _Physiol. Rev._ 84, 87–136 (2004). Article CAS PubMed Google Scholar * Ciocchi, S. et al. Brain computation. Selective information routing by ventral hippocampal CA1 projection
neurons. _Science_ 348, 560–563 (2015). Article ADS CAS PubMed Google Scholar * Swanson, L. W., Sawchenko, P. E. & Cowan, W. M. Evidence for collateral projections by neurons in
Ammon’s horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. _J. Neurosci._ 1, 548–559 (1981). Article CAS PubMed PubMed Central Google Scholar *
Peyrache, A. et al. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. _Nat. Neurosci._ 12, 919–926 (2009). Article CAS PubMed Google Scholar * den
Bakker, H. et al. Sharp-wave-ripple-associated activity in the medial prefrontal cortex supports spatial rule switching. _Cell Rep._ 42, 112959 (2023). Article Google Scholar * Dragoi, G.
& Tonegawa, S. Preplay of future place cell sequences by hippocampal cellular assemblies. _Nature_ 469, 397–401 (2011). Article ADS CAS PubMed Google Scholar * Dragoi, G. The
generative grammar of the brain: a critique of internally generated representations. _Nat. Rev. Neurosci._ 25, 60–75 (2024). Article CAS PubMed Google Scholar * Farooq, U. et al.
Strengthened Temporal Coordination within Pre-existing Sequential Cell Assemblies Supports Trajectory Replay. _Neuron_ 103, 719–733.e7 (2019). Article CAS PubMed PubMed Central Google
Scholar * Frankland, P. W. et al. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. _Behav. Neurosci._ 112, 863–874 (1998). Article CAS
PubMed Google Scholar * Dunsmoor, J. E. & Paz, R. Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. _Biol. Psychiatry_ 78, 336–343 (2015). Article PubMed Google
Scholar * Dymond, S. et al. Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. _Behav. Ther._ 46, 561–582 (2015). Article PubMed Google
Scholar * Wickersham, I. R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. _Neuron_ 53, 639–647 (2007). Article CAS PubMed PubMed
Central Google Scholar * Card, J. P. & Enquist, L. W. Transneuronal circuit analysis with pseudorabies viruses. _Curr. Protoc. Neurosci._ 68, 1 5 1–1 5 39 (2014). Article PubMed
Google Scholar * Wu, F. et al. Monolithically Integrated muLEDs on Silicon Neural Probes for High-Resolution Optogenetic Studies in Behaving Animals. _Neuron_ 88, 1136–1148 (2015). Article
CAS PubMed PubMed Central Google Scholar * Mou, X. et al. Comparing Mouse and Rat Hippocampal Place Cell Activities and Firing Sequences in the Same Environments. _Front Cell
Neurosci._ 12, 332 (2018). Article CAS PubMed PubMed Central Google Scholar * Lin, L. et al. Large-scale neural ensemble recording in the brains of freely behaving mice. _J. Neurosci.
Methods_ 155, 28–38 (2006). Article PubMed Google Scholar * El-Gaby, M. et al. An emergent neural coactivity code for dynamic memory. _Nat. Neurosci._ 24, 694–704 (2021). Article CAS
PubMed PubMed Central Google Scholar * van de Ven, G. M. et al. Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples. _Neuron_
92, 968–974 (2016). Article PubMed PubMed Central Google Scholar * Karlsson, M. P. & Frank, L. M. Awake replay of remote experiences in the hippocampus. _Nat. Neurosci._ 12, 913–918
(2009). Article CAS PubMed PubMed Central Google Scholar * Skaggs, W. E. et al. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences.
_Hippocampus_ 6, 149–172 (1996). Article CAS PubMed Google Scholar * Roux, L. et al. Sharp wave ripples during learning stabilize the hippocampal spatial map. _Nat. Neurosci._ 20,
845–853 (2017). Article CAS PubMed PubMed Central Google Scholar * Opalka, A. N. & Wang, D. V. Hippocampal efferents to retrosplenial cortex and lateral septum are required for
memory acquisition. _Learn Mem._ 27, 310–318 (2020). Article CAS PubMed PubMed Central Google Scholar * Wang, D. V. & Ikemoto, S. Coordinated Interaction between Hippocampal
Sharp-Wave Ripples and Anterior Cingulate Unit Activity. _J. Neurosci._ 36, 10663–10672 (2016). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We
thank Dr. Vitor Lopes-dos-Santos for discussions on the ICA analysis and Drs. Gideon Rothchild and Hualou Liang for discussions on the GLM decoding. We thank Dr. Wen-Jun Gao for comments on
an earlier version. This work was supported by the National Institutes of Health grants R01MH119102 (D.V.W.) and F31MH134582 (A.F.H.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Neurobiology & Anatomy, Drexel University College of Medicine, Philadelphia, PA, 19129, USA Jun Liu, Arron F. Hall & Dong V. Wang Authors * Jun Liu View author
publications You can also search for this author inPubMed Google Scholar * Arron F. Hall View author publications You can also search for this author inPubMed Google Scholar * Dong V. Wang
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization & Methodology: J.L., D.V.W.; Investigation: J.L., A.F.H., D.V.W.;
Writing—original draft: J.L., D.V.W.; Writing—review & editing: J.L., A.F.H., D.V.W. CORRESPONDING AUTHOR Correspondence to Dong V. Wang. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Sadegh Nabavi, and the other, anonymous, reviewers for their contribution to the
peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, J., Hall, A.F. & Wang, D.V. Emerging
many-to-one weighted mapping in hippocampus-amygdala network underlies memory formation. _Nat Commun_ 15, 9248 (2024). https://doi.org/10.1038/s41467-024-53665-9 Download citation *
Received: 14 September 2023 * Accepted: 18 October 2024 * Published: 26 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53665-9 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
