Chiral plasmonic-dielectric coupling enables strong near-infrared chiroptical responses from helicoidal core-shell nanoparticles
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Helicoid plasmonic nanoparticles with intrinsic chirality are an emerging class of artificial chiral materials with tailorable properties. The ability to extend their chiroplasmonic
responses to the near-infrared (NIR) range is critically important for biomedical and nanophotonic applications, yet the rational design of such materials remains challenging. Herein, a
strategy employing chiral plasmon-dielectric coupling is proposed to manipulate the chiroptical responses into the NIR region with high optical anisotropy. Through this strategy, the
helicoid Au@Cu2O nanoparticles with structural chirality are designed and synthesized with tunable and enriched NIR chiroptical responses. Specially, a high optical anisotropy (_g_-factor)
with a value of 0.35 is achieved in the NIR region, and multi-band chiroptical behaviors are observed. Spectral and electromagnetic simulations elucidate that strong coupling between
chiroplasmonic core and chiral dielectric shell with high refractive index contributes to the rich and strong chiroptical responses, which are related to the interplay between various
emerged and enhanced electric and magnetic multipolar resonance modes proved by multipole expansion analysis. Moreover, the helicoid Au@Cu2O nanoparticles display greater polarization
rotation capability than the helicoid Au nanoparticles. This work offers mechanistic insights into chiral plasmon-dielectric coupling and suggests a general approach of creating NIR
chiroplasmonic materials. SIMILAR CONTENT BEING VIEWED BY OTHERS ENHANCED CHIROPTIC PROPERTIES OF NANOCOMPOSITES OF ACHIRAL PLASMONIC NANOPARTICLES DECORATED WITH CHIRAL DYE-LOADED MICELLES
Article Open access 05 January 2023 COLLECTIVE CHIROPTICAL ACTIVITY THROUGH THE INTERPLAY OF EXCITONIC AND CHARGE-TRANSFER EFFECTS IN LOCALIZED PLASMONIC FIELDS Article Open access 06 June
2024 ELECTROMAGNETIC CHIRALITY: FROM FUNDAMENTALS TO NONTRADITIONAL CHIROPTICAL PHENOMENA Article Open access 02 September 2020 INTRODUCTION Natural chiral materials, prevalent in molecular
and biological systems, manifest chiroptical properties derived from their intrinsic asymmetry. This inherent chirality plays a pivotal role in shaping the structure and functioning of these
materials within biological processes1,2,3,4. In recent years, the burgeoning field of nanoscience has witnessed rapid progresses in the design of artificial chiral nanomaterials. These
nanoengineered materials exhibit chiral properties that surpass those found in their natural counterparts, positioning them as promising candidates for a myriad of advanced
technologies4,5,6. For instance, artificial chiral nanomaterials have proven instrumental in enhancing the subtle chiral effects of molecules, contributing to advancements in
enantioselective sensing and catalysis7,8,9,10,11,12,13. Furthermore, their applications span a diverse of domains, including metamaterials, anti-counterfeiting and encryption,
optoelectronics, and spintronics, offering a versatile platform with the potential to revolutionize fields ranging from medicine to materials science2,7,14,15,16,17,18,19,20. Among different
artificial chiral nanomaterials, helicoid Au nanoparticles are emerging as a typical class of artificial chiral nanomaterials with intrinsic structural chirality21,22,23,24,25,26,27. The
strong chiroptical properties of these nanoparticles arise from their chiroplasmonic characteristics, stemming from the helicoid shape that creates a chiral three-dimensional (3D) continuous
route for plasmonic resonances. Consequently, helicoid Au nanoparticles demonstrate higher chiroptical properties, boasting dissymmetry factors (_g_-factors) over 0.1, surpassing those
observed in other discrete nanoparticles. Their chiral properties have also enabled their applications in chiral discrimination, biological regulation, and chiroplasmonic sensing7,23,24,28.
However, considering that most of the chiroptical responses in current helicoid Au nanoparticles are limited in the visible range, the ability to extend their chiroplasmonic responses to the
NIR range is critically important for their biomedical and nanophotonic applications, yet the rational design of such materials remains an ongoing challenge within the field. In this study,
we propose a strategy to extend the chiroptical responses into the NIR region by leveraging chiral plasmon-dielectric coupling. Employing this strategy, we synthesize the helicoid Au@Cu2O
nanoparticles with inherent structural chirality, resulting in enhanced chiroptical properties and achieving a strong NIR _g_-factor of up to 0.35. Through multipole expansion analysis,
spectral and 3D electromagnetic simulations, we elucidate the critical contribution of the coupling between the chiroplasmonic core and the chiral dielectric shell of the helicoid
nanoparticles. Notably, the helicoid Au@Cu2O nanoparticles exhibit a high polarization rotation capability, showcasing their potential for applications in chiral NIR metamaterials. RESULTS
SYNTHESIS OF THE HELICOID AU@CU2O NANOPARTICLES The growth of the helicoid Au@Cu2O nanoparticles with intrinsic chirality is directed by the helicoid Au nanoparticles via an epitaxial growth
protocol29. The similar crystal symmetry and relatively small lattice mismatch of 4.5% between Au and Cu2O allow epitaxial growth30,31. The helicoid Au nanoparticles employed herein were
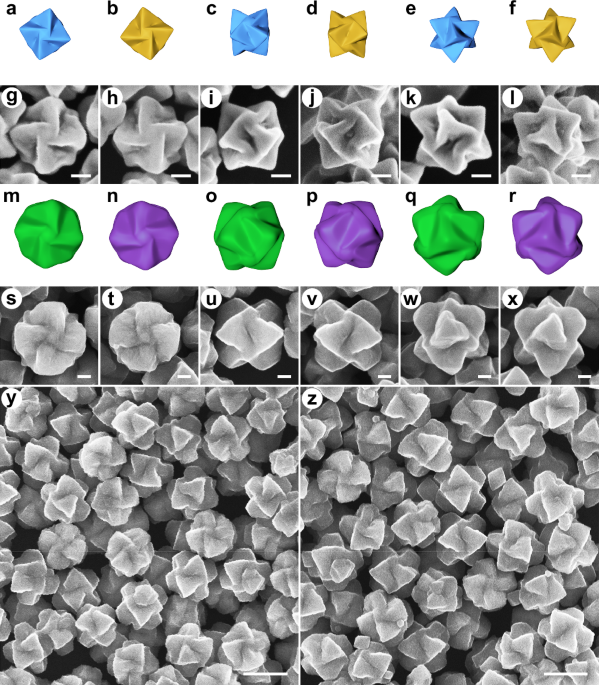
synthesized according to refs. 23,24. They are characterized with a chiral trisoctahedral morphology with windmill-like chiral features viewed from <100 > , <110 > , and
<111> directions (Fig. 1a–l and Supplementary Fig. 1). Utilizing the helicoid Au nanoparticles as templates, helicoid Cu2O shells are subsequently overgrown on Au nanoparticles using
sodium dodecyl sulfate as a stabilizing reagent. Scanning electron microscopy (SEM) images of the helicoid Au@Cu2O nanoparticles (Fig. 1s–x) illustrate that the helicoid Au@Cu2O
nanoparticles display distinct chiral morphologies viewed from different directions. These features are also displayed through visualization using their corresponding geometric models (Fig.
1m–r). Along the <100> orientation, the windmill-like structure of the L-handed helicoid Au@Cu2O nanoparticles rotates counterclockwise, whereas that of the D-handed helicoid Au@Cu2O
nanoparticles rotates clockwise. When observing along the <111> axis, the trigonal pyramids of the helicoid Au@Cu2O nanoparticles also exhibit clockwise or counterclockwise rotation,
directed by the chirality of the Au templates. Viewing from the <110> axis, the intersection of two neighboring pyramids of the L- or D-handed helicoid Au@Cu2O nanoparticles is tilted
in opposite directions, due to clockwise or counterclockwise rotation of the pyramidal components. Low-magnification SEM images (Fig. 1y, z) demonstrate low dispersity in size and shape of
both enantiomers of the helicoid Au@Cu2O nanoparticles. The above SEM results suggest that the helicoid Au nanoparticles not only serve as a substrate for the deposition of Cu2O, but also
determine the rotation and chirality of the Cu2O shells. Compared with the original helicoid Au nanoparticles, the helicoid Au@Cu2O nanoparticles possess blunter trigonal pyramids with
relatively smaller rotation degree, specifically for angle 1 from 73.2° to 133.0° and for angle 2 from 26.0° to 16.2°, respectively, shown in Supplementary Fig. 2. The valley between the two
adjacent pyramids also becomes shallower after the overgrowth of Cu2O. Furthermore, the crystal structure and composition of the as-synthesized helicoid Au@Cu2O nanoparticles are
investigated. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) studies (Fig. 2a–c) reveal a continuous lattice fringe of 0.30 nm on the shell area, corresponding to the
(110) plane of Cu2O. Selected area electron diffraction (SAED) patterns of the shell area show a typical pattern of the <100> direction, suggesting the single-crystalline nature of
the Cu2O shell. Furthermore, scanning transmission electron microscopy (STEM) images, energy dispersive X-ray (EDX) mapping, and EDX line scan (Fig. 2d-g) offer additional validation of the
well-defined core-shell architecture, with uniform Cu2O shells grown on the surface of the Au cores. X-ray diffraction (XRD) patterns (Fig. 2h) of the helicoid Au@Cu2O nanoparticles
demonstrate the cubic structures and high crystallinity of Cu2O shells and Au cores. The predominance of Cu+ as the primary constituent in the Cu2O shells is also unveiled by the X-ray
photoelectron spectroscopy (XPS) technique (Fig. 2i). The main peaks at 932.45 and 952.35 eV correspond to Cu+ 2_p_3/2 and Cu+ 2_p_1/2 peaks of Cu+, respectively. The existence of peaks at
934.58 (Cu2+ 2_p_3/2) and 954.85 (Cu2+ 2_p_1/2) eV originating from Cu2+ is due to oxidation of a small amount of Cu2O32. CHIROPTICAL PROPERTY OF THE HELICOID AU@CU2O NANOPARTICLES Cu2O, a
p-type semiconductor with high refractive index (above 2.7 in the visible region), has been incorporated with metal nanoparticles to tune their plasmonic
characteristics30,31,33,34,35,36,37,38,39,40. Nonetheless, the role of Cu2O in chiroplasmonics remains elusive. Herein, the robust epitaxial growth of Cu2O on the helicoid Au nanoparticles
provides a basis to investigate the chiroplasmonic coupling between Cu2O and Au. To tune their chiroplasmonic properties, the helicoid Au@Cu2O nanoparticles with different Cu2O thicknesses
are rationally synthesized through the precise modulation of the concentrations of CuCl2 solution. As determined by inductively coupled plasma (ICP) techniques, the helicoid Au@Cu2O variants
with different Cu2O thicknesses, denoted as Au@Cu2O-1 to Au@Cu2O-8, have molar ratios of Cu2O and Au of 0.27, 0.46, 0.62, 0.72, 0.94, 1.09, 1.37, and 1.52, respectively. The calculated Cu2O
thicknesses of the helicoid Au@Cu2O nanoparticles are documented in Supplementary Table 1, ranging from the thinnest shell at 14.6 nm to the thickest at 73.2 nm. SEM images of typical L-
and D-handed helicoid Au@Cu2O nanoparticles, depicted in Fig. 3, show their similar chiral shapes to those in Fig. 1. Furthermore, these helicoid Au@Cu2O nanoparticles exhibit strong
chiroplasmonic properties that can be fine-tuned by controlling the thickness of the Cu2O shells (Fig. 3 and Supplementary Figs. 4, 5). Reverse chiroptical signals with nearly identical
values are observed with the enantiomers of the helicoid Au@Cu2O nanoparticles (Fig. 3), which further indicates the chirality transfer from the helicoid Au nanoparticles to the helicoid
Cu2O shells. Notably, the helicoid Au@Cu2O nanoparticles exhibit strong chiroptical responses with enriched spectral features in the NIR region. After the coating of Cu2O shells, both the
extinction and _g_-factor spectra of the helicoid nanoparticles experience red-shift to the NIR region. More importantly, a high _g_-factor in the NIR region is observed. As depicted in Fig.
4a and Supplementary Figs. 4, 5, the helicoid Au@Cu2O nanoparticles exhibit chiroptical responses with tunable intensity and wavelength dependent on Cu2O shell thicknesses. Before Cu2O
coating, the D-handed helicoid Au nanoparticles show a main positive band with a _g_-factor of 0.16 at 610 nm. By changing the thickness of Cu2O shells, the wavelength of the maximum
positive band in the _g_-factor spectra of the D-handed helicoid Au@Cu2O nanoparticles can be fine-tuned from 786 to 970 nm. Compared with the D-handed helicoid Au nanoparticle, the
introduction of Cu2O shell induces adjustable red-shifts ranging from 176 to 360 nm. Meanwhile, their corresponding _g_-factor values are enhanced with increased shell thicknesses. The
D-handed helicoid Au@Cu2O-6 nanoparticles manifest the strongest chiroptical signals with a _g_-factor value of up to 0.35 observed at 942 nm. In comparison to the helicoid Au nanoparticles,
there is a 111% improvement in optical anisotropy and a spectral red-shift of 332 nm. The _g_-factor of 0.35 is among the highest _g_-factor in the NIR region from discrete chiral
nanoparticles (Supplementary Table 2)10,14,21,22,23,41,42,43,44,45,46. Moreover, the helicoid Au@Cu2O nanoparticles are stable even after storage for ten months (Supplementary Fig. 6),
compared with the chiral Au nanorods from chiral-micelle-directed syntheses41,42,47. Apart from the strong chiroptical responses, Fig. 4a also shows that the helicoid Au@Cu2O nanoparticles
display enriched chiroptical responses, echoing their complex extinction spectra in Supplementary Fig. 5a, b. Fig 4b, demonstrating the _g_-factor spectra of the enantiomers of the helicoid
Au@Cu2O-6 nanoparticles, depicts rich chiroptical signals as many as six clear well-defined peaks from visible to NIR regions. Specifically, the D-handed helicoid Au@Cu2O-6 nanoparticles
exhibit three positive peaks at 616, 726, and 942 nm, respectively, and three negative bands at 570, 668, and 842 nm, respectively. The L-handed counterparts have exactly mirror-symmetric
_g_-factor spectra with reverse signals appearing at the same wavelengths with those of the D-handed helicoid Au@Cu2O-6 nanoparticles. Their extinction spectra are almost identical
(Supplementary Fig. 4). Richer chiroptical properties of the helicoid Au@Cu2O nanoparticles over the helicoid Au nanoparticles are also clearly observed in Fig. 4c. In addition, multiple
Cotton peaks observed in the helicoid Au@Cu2O nanoparticles generate seven crossing points, more than twice that of the helicoid Au nanoparticles. The enriched optical features endow the
helicoid Au@Cu2O nanoparticles potential for chiral sensing and advanced optical technologies. To elucidate the underlying mechanism responsible for the strong chiroptical property of the
helicoid Au@Cu2O nanoparticles, we conducted a comprehensive theoretical analysis employing the finite-difference time-domain (FDTD) method. The theoretically predicted chiroptical spectra
of the helicoid Au@Cu2O nanoparticles exhibit large spectral red-shifts, intensity enhancement, as well as rich Cotton peaks compared to the helicoid Au nanoparticles. These results match
well with the experimental findings and confirm that coating the helicoid Au cores with Cu2O shells is an efficient strategy for tuning their chiroptical properties (Supplementary Figs. 8,
9). The enhanced and enriched chiroptical responses of the helicoid Au@Cu2O nanoparticles can be directly correlated with the high refractive index of Cu2O (above 2.7). Spectral simulation
of core-shell helicoid nanoparticles with chiral Au cores and chiral dielectric shells with different refractive indices is performed. As Fig. 4d illustrates, as refractive index of
dielectric shell progressively increases, spectral red-shifts, enhanced chiroptical signals and emergence of multiple peaks are observed. Furthermore, the richer chiroptical responses
corroborate with the appearance of multi-peaks illustrated in simulated extinction spectra (Supplementary Fig. 10). These results also indicate that the chiroptical responses of the helicoid
core-shell nanoparticles can be altered by introducing other shell materials, such as CuO (Supplementary Figs. 7, 11). Multipole expansion analysis further reveals that the helicoid Au@Cu2O
nanoparticles exhibit many newly emerged and enhanced resonance modes, including the electric dipole, the magnetic dipole, the electric quadrupole, and the magnetic quadrupole, compared
with the helicoid Au nanoparticles (Supplementary Fig. 12). The detailed analysis in Supplementary Information indicates that the extensive interplay between these electric and magnetic
resonances plays a critical role in generating the strong and rich chiroptical characteristics of the helicoid Au@Cu2O nanoparticles across different spectral ranges. The high dielectric
constant of Cu2O, along with the large size and anisotropic geometry of the helicoid Au@Cu2O nanoparticles, induces phase retardation effects and redistribution of charges, generating
multipolar resonances30,37,48. The generation of multiple Cotton peaks is also intuitively shown in Supplementary Fig. 13. In terms of the spectral red-shits, increased charge separation
with higher dielectric constants and shell thicknesses reduces the restoring force for the surface charges, resulting in the shift of the resonance modes toward lower energy induced by the
coated Cu2O shells (Supplementary Fig. 12). Equation (1) directly illustrates the relationship between the spectral red-shifts and the dielectric constants and the thicknesses of the shell,
where _λ_, _λ_p, _ε_∞, _n_, and _Φ_ denote the resonance wavelength of Au, the bulk plasmon wavelength of Au without medium effect, the high frequency of the Au core, the refractive index of
surrounding medium, and the volume fraction of shell layer, respectively49,50. Additionally, dielectric shells with increased dielectric constants and shell thicknesses lead to greater
charge imbalance at the Au-Cu2O interface, enhancing the extinction cross-sections and resulting in the stronger chiroptical signals for the helicoid Au@Cu2O nanoparticles30. $${\lambda
}^{2}={\lambda }_{{{{\rm{p}}}}}^{2}\left[{\varepsilon }^{\infty }+2{n}_{{{{\rm{ethanol}}}}}^{2}+2\varPhi
\frac{\left({n}_{{{{{\rm{Cu}}}}}_{2}{{{\rm{O}}}}}^{2}-{n}_{{{{\rm{ethanol}}}}}^{2}\right)}{3}\right]$$ (1) In addition to refractive index, the strong coupling between chiroplasmonic and
chiral dielectric nanomaterials contributes to the extraordinary chiroptical property of the helicoid Au@Cu2O nanoparticles. To elucidate the coupling effect, five models are built for
spectral and electromagnetic field simulations, including the helicoid Cu2O nanoparticles, the Au cube@helicoid Cu2O nanoparticles, the helicoid Au@helicoid Cu2O nanoparticles, the helicoid
Au@Cu2O sphere nanoparticles, and the helicoid Au nanoparticles. The simulated spectra results demonstrate that the coupling of chiral Au cores and Cu2O shells realizes stronger chiroptical
signals, compared with the helicoid Au nanoparticles and the helicoid Cu2O nanoparticles (Supplementary Fig. 14). Note that the chiral Cu2O shells also contribute to the chiroptical
properties of the helicoid Au@Cu2O nanoparticles, although their contribution is considerably weaker (Supplementary Fig. 14). More importantly, electromagnetic field simulations indicate
that the strong coupling effect of the helicoid Au cores with high electric fields and the helicoid Cu2O shells with strong magnetic fields results in enhancements of both electric and
magnetic fields (Fig. 5b, c, Supplementary Figs. 16b, c, 17, and 18a,c,e) and greater asymmetric responses to left and right circularly polarized light (LCP and RCP) in both the electric
field and magnetic field (Supplementary Figs. 16d, e, 19, and 20a, c, e), expressing as (|_E_LCP | / | _E_0 | - | _E_RCP | / | _E_0 | ) and (|_B_LCP | / | _B_0 | - | _B_RCP | / | _B_0 | ),
respectively. The enhanced asymmetric electromagnetic fields of the helicoid Au@helicoid Cu2O nanoparticles provide strong evidence for their observed stronger chiroptical signals. The
mapping of electromagnetic fields distributions of the Au cube@helicoid Cu2O nanoparticles and the helicoid Au@Cu2O sphere nanoparticles further reveals that the unique helicoid morphologies
of both the Au cores and Cu2O shells contribute to the enhanced chiroptical properties of the helicoid Au@Cu2O nanoparticles (Fig. 5b, c, Supplementary Figs. 16–20b–d). Furthermore, the
plasmonic and dielectric coupling from the helicoid Au@Cu2O nanoparticles gives rise to larger _Q_-factor (Supplementary Fig. 21) and mode volumes, specifically 6.38 × 104 nm3 for the
helicoid Au nanoparticles and 9.02 × 104 nm3 for the helicoid Au@Cu2O nanoparticles. This coupling not only reduces optical loss but also enlarges resonance regions for interaction with
molecules. To comprehensively uncover the chiral fields of the helicoid Au@Cu2O nanoparticles, optical chirality (_C_) is introduced to visualize the local asymmetric fields51,52. _C_ is a
metric quantifying local density of the chirality of electromagnetic fields generated by chiral systems, which can evaluate light-matter interactions for small chiral molecules and has
recently been proved to be meaningful for predicting dissymmetric factor in chiral sensing applications2,51,52,53,54,55,56. _C_ is obtained by calculation according to Eq. (2), where _E_
(_E_) and _B_ (_B_) are the real (complex) electric and magnetic fields, respectively, and _ε_0 and _μ_0 are the permittivity and permeability of a vacuum, respectively2,51,54,57,58. _C_,
affected by both electric and magnetic fields, can provide a chiral field distribution. As demonstrated in Fig. 5d, e and Supplementary Figs. 22–24, benefiting from the strong chiral
plasmon-dielectric coupling and the helicoid morphologies of Au cores and Cu2O shells, the helicoid Au@helicoid Cu2O nanoparticles display enhanced optical chirality compared to the helicoid
Au and the helicoid Cu2O nanostructures, especially from the cross-sections at position of 50, 100, and 150 nm. The optical chirality of the helicoid nanoparticles, combining the simulated
chiroptical spectra of helicoid Au@dielectric shell nanoparticles with different refractive indices and the asymmetric electromagnetic fields of the helicoid nanoparticles, provides
compelling evidence that the enhanced _g_-factor originates from the Cu2O coating. Furthermore, among the five chiral nanostructures, the helicoid Au@helicoid Cu2O nanoparticles exhibit the
highest surface optical chirality where interaction with chiral molecules occurs, which is benefited from the coupling between the asymmetric plasmonics from the helicoid Au core and the
asymmetric dielectric environment from the helicoid Cu2O shell. The observed enhancement in optical chirality and asymmetric electromagnetic fields of the helicoid Au@Cu2O nanoparticles is
anticipated to boost their chiral sensing capabilities. This improvement is attributed to not only their near-field radiative coupling and antenna effects but also to the better scale
matching between the highly confined electromagnetic fields around the nanoparticles and the chiral molecules, compared to circularly polarized light (CPL)59,60,61. $$C=\frac{{\varepsilon
}_{0}}{2}E\cdot \nabla \times E+\frac{1}{2{\mu }_{0}}B\cdot \nabla \times B=-\frac{\omega \,{\varepsilon }_{0}}{2}{{{\rm{Im}}}}\left({{{{\boldsymbol{E}}}}}{*}\cdot
{{{\boldsymbol{B}}}}\right)$$ (2) POLARIZATION ROTATION BY THE HELICOID AU@CU2O NANOPARTICLES The strong and enriched chiroptical responses of the helicoid Au@Cu2O nanoparticles make them a
promising candidate for anti-counterfeiting and encryption applications based on polarization rotation. In this respect, the polarization-rotating capability of helicoid Au@Cu2O
nanoparticles is investigated. As depicted in Fig. 6a, the helicoid Au@Cu2O nanoparticles, positioned between mutually orthogonal polarizer and analyzer configurations, induce a certain
degree of light rotation due to their chirality, resulting in transmitted light that can be recorded. By rotating the analyzer clockwise and counterclockwise, macroscopic colors of
transmitted light display asymmetric change, from light red to yellow for the L-handed helicoid Au@Cu2O-4 nanoparticles, while reverse color transition for the D-handed helicoid Au@Cu2O-4
nanoparticles (Fig. 6b). Transmission spectra shown in Fig. 6c, d and Supplementary Fig. 25 provide understandable explanations. Fig. 6c presents the transmission spectra consisting of peaks
at 929, 742, and 639 nm when the L-handed helicoid Au@Cu2O-4 nanoparticles are under rotation angle of −6°, while peaks at 853, 682, and 587 nm for rotation angle of 6°. The exactly reverse
transmission spectra are observed for the D-handed helicoid Au@Cu2O-4 nanoparticles (Fig. 6d). Furthermore, macroscopic colors of transmitted light display richness with dependence on Cu2O
shell thicknesses (Fig. 6e and Supplementary Fig. 26). Greater polarization-rotation ability of the helicoid Au@Cu2O nanoparticles is indicated by more obvious asymmetrical macroscopic
colors when rotate the analyzer 1° and −1°, compared with the helicoid Au nanoparticles (Supplementary Fig. 27). The polarization-rotating ability and abundant macroscopic colors displayed
by the helicoid Au@Cu2O nanoparticles show their availability for encryption and anti-counterfeiting. DISCUSSION In conclusion, we synthesized the helicoid Au@Cu2O nanoparticles that exhibit
chiral geometry inherited from the helicoid Au nanoparticles. The helicoid Au@Cu2O nanoparticles demonstrate strong chiroptical activity with a _g_-factor value of 0.35, along with rich
chiroptical signals spanning from visible to NIR area. The multipole expansion analysis, along with systematic spectral and 3D electromagnetic simulations, confirms that the enhanced and
enriched chiroptical responses of the helicoid Au@Cu2O nanoparticles stem from the interplay between various newly emerged and enhanced electric and magnetic multipolar resonance modes.
These responses are attributed to the synergistic effects of the helicoid Au cores with high electric fields and the helicoid Cu2O shells with strong magnetic fields. The critical role of
the helicoid morphologies of both the Au cores and the Cu2O shells is also highlighted. This study not only broadens the application of plasmonic and dielectric coupling to helicoid
nanoparticles but also demonstrates its potential as a powerful approach to create NIR-active chiroplasmonic materials with high _g_-factors, which distinguishes this work from refs. 62,63.
In addition, the great polarization rotation capability exhibited by the helicoid Au@Cu2O nanoparticles suggests their potential utility in encryption and anti-counterfeiting. Furthermore,
the strong chiroptical properties and diverse compositions of the helicoid Au@Cu2O nanoparticles enable advanced applications, such as chirality sensing, enantioselective catalysis, etc. Our
work enriches the library of helicoid inorganic nanoparticles and suggests an efficient strategy for boosting the chiroplasmonic properties of chiral nanoparticles through the core-shell
configuration. METHODS CHEMICALS Tetrachloroauric trihydrate (HAuCl4·3H2O, ≥ 49.0% Au basis) and sodium borohydride (NaBH4, 99.99%) were obtained from Sigma-Aldrich. Hexadecylpyridinium
bromide hydrate (CPB, > 96%), hexadecylpyridinium chloride monohydrate (CPC, > 98%), l-/d-cysteine (l-/d-Cys, > 99.0%), and L-ascorbic acid (AA, > 99.0%) were obtained from TCI.
Cetyltrimethylammonium bromide (CTAB, > 99%) was obtained from Acros Organics. Silver nitrate (AgNO3, 99.8%) was obtained from Sinopharm Chemical reagent. Copper chloride dihydrate
(CuCl2·2H2O, AR, ≥ 99.0%), hydroxylammonium chloride (NH2OH·HCl, AR, ≥ 98.5%), and ethanol were obtained from XILONG SCIENTIFIC. Trisodium citrate dihydrate (99%) was obtained from Acros
Organics. Sodium hydroxide (NaOH, AR, ≥ 96.0%) was obtained from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd. Sodium dodecyl sulfate (SDS, AR, 99%) was obtained from Beijing
Chemical Works. Ultrapure de-ionized (DI) water (18.25 MΩ cm) was used throughout the experiments. SYNTHESIS OF AU NANOSPHERES Au nanospheres were synthesized according to ref. 64. In a
typical synthesis, the Au nanospheres were obtained after six synthetic procedures as follows: (1) Synthesis of small Au nanoparticles: 10 mL of 100 mM CTAB solution was kept in a 30 °C
water bath for at least 10 min. Then, 0.25 mL of 10 mM HAuCl4 solution and 0.6 mL of 10 mM NaBH4 solution were added in sequence under vigorous stirring. After stirring for 2 min, the
mixture was kept undisturbed in a 30 °C water bath for 30 min. (2) Synthesis of Au nanorods: 40 mL of 100 mM CTAB solution was kept in a 30 °C water bath for at least 10 min. Then, 2 mL of
10 mM HAuCl4 solution, 0.24 mL of 10 mM AgNO3 solutions, 0.32 mL of 100 mM AA solution, and 48 μL of the small Au nanoparticles were added in sequence. The mixture was kept undisturbed in a
30 °C water bath for 2 h. The product was collected by centrifugation (15778× _g_, 10 min), and washed with H2O and centrifuged once again. Finally, the product was redispersed in 40 mL of
10 mM CTAB solution. (3) Secondary growth of large Au nanorods: 2 mL of 10 mM HAuCl4 solution and 0.4 mL of 100 mM AA solution were added in sequence to the Au nanorod solutions obtained by
(2). The mixture was kept undisturbed in a 40 °C water bath for 1 h. The product was collected by centrifugation (15778× _g_, 10 min) and redispersed in 40 mL of 10 mM CTAB solution. (4)
Etching of the large Au nanorods: The large Au nanorod solution was kept in a 40 °C water bath for at least 10 min. 0.8 mL of 10 mM HAuCl4 solution was added. The mixture was kept
undisturbed in a 40 °C water bath for 12–16 h. The product was collected by centrifugation (15778× _g_, 10 min), and was washed with 20 mM of CPC solution and centrifuged twice again.
Finally, the product was redispersed in 40 mL of 20 mM CPC solution. (5) Secondary growth of Au nanoparticles: 200 mL of 100 mM CPC solution was kept in a 30 °C water bath for at least 10
min. Then, 4 mL of 10 mM HAuCl4 solution, 0.6 mL of 100 mM AA solution, and 40 mL of the Au nanoparticles obtained by (4) were added in sequence. The mixture was kept undisturbed in a 30 °C
water bath for 1 h. The product was collected by centrifugation (15778× _g_, 3 min) and redispersed in 40 mL of 10 mM CTAB solution. (6) Synthesis of Au nanospheres: The Au nanoparticle
solution obtained by (5) was kept in a 40 °C water bath for at least 10 min. 0.8 mL of 10 mM HAuCl4 solution was added. The mixture was kept undisturbed in a 40 °C water bath for 12–16 h.
The product was collected by centrifugation (15778× _g_, 10 min), and was washed with 20 mM CPC solution and centrifuged twice again. Finally, the Au nanospheres was redispersed in 40 mL of
20 mM CPC solution. SYNTHESIS OF THE HELICOID AU NANOPARTICLES The helicoid Au nanoparticles were synthesized according to refs. 23,24. In a typical synthesis, 2.025 mL of H2O, 1.35 mL of
100 mM CPB solution, and 0.45 mL of 100 mM CPC solution were mixed well and then kept in a 30 °C water bath for at least 10 min. Then 0.2 mL of 10 mM HAuCl4 solution, 1.4 mL of 100 mM AA
solution, 12.5 μL of 100 μM l-/d-Cys solution, and 15 μL of the Au nanosphere seed solutions were added in sequence. The mixture was kept undisturbed in a 30 °C water bath for 2 h. The
product was collected by centrifugation (2616 × _g_, 3 min), and then washed with 1 mM CTAB solution and centrifuged once again. Finally, the product was redispersed in 0.5 mL of 1 mM CTAB
solution. SYNTHESIS OF THE HELICOID AU@CU2O NANOPARTICLES The helicoid Au@Cu2O nanoparticles with different shell thicknesses were acquired using an epitaxial growth method by changing the
concentration of CuCl2 solution29. In a typical synthesis, 3.2 mL of H2O, 0.1 mL of CuCl2 solution (3, 5, 7, 9, 11, 13, 15, 17 mM), and 0.5 mL of 300 mM SDS solution were subsequently added
to a 20 mL vial under stirring in a 30 °C water bath. After incubating for 10 min, 0.5 mL of 100 mM trisodium citrate solution, 0.25 mL of the helicoid Au nanoparticle solution, 0.125 mL of
1.0 M NaOH solution, and 0.325 mL of 200 mM NH2OH·HCl solution were then subsequently added to the vial under stirring (200 rpm). Upon adding NH2OH·HCl solution, the color of the mixed
solutions changes to green, indicating the coating of Cu2O shells. After reacting for 10 min, the products were collected by centrifugation (4651× _g_, 5 min) and redispersed in 5 mL of
H2O/ethanol (1:1) solutions. Then the solution was centrifuged again (4651× _g_, 5 min) and washed with ethanol twice. The final products were redispersed in 0.25 mL of ethanol. The L-handed
helicoid Au nanoparticles were used for the synthesis of the L-handed helicoid Au@Cu2O nanoparticles, while the D-handed helicoid Au nanoparticles for the D-handed helicoid Au@Cu2O
nanoparticles. Their molar ratios of Cu2O and Au obtained by ICP techniques are 0.27, 0.46, 0.62, 0.72, 0.94, 1.09, 1.37, and 1.52, respectively. The helicoid Au@Cu2O nanoparticles with
increased shell thicknesses are named after helicoid Au@Cu2O-1, helicoid Au@Cu2O-2, helicoid Au@Cu2O-3, helicoid Au@Cu2O-4, helicoid Au@Cu2O-5, helicoid Au@Cu2O-6, helicoid Au@Cu2O-7, and
helicoid Au@Cu2O-8, respectively. POLARIZATION ROTATION OF THE HELICOID AU@CU2O NANOPARTICLES The light from a fiber-coupled halogen lamp (> 400 nm) as white-light illumination source
passes the horizontally linear polarizer, a cuvette containing the helicoid Au@Cu2O nanoparticles solutions, and the vertically linear polarizer as analyzer. A cross-polarized state was
attained, termed 0°, wherein light could not penetrate. Rotating the analyzer from −6° (clockwise) to 6° (counterclockwise), transmitted light was observed by naked eyes and recorded by a
mobile phone. To record transmission spectra, a UV-Vis spectrophotometer was used. The sample chamber housed the horizontally linear polarizer, the cuvette containing the helicoid Au@Cu2O
nanoparticles and the vertically linear polarizer arranged in sequence from light source to detector. Rotating the analyzer from −6° (clockwise) to 6° (counterclockwise), transmission
spectra were recorded. Before taking each spectral measurement at each rotating angle, baseline was determined using ethanol. DIFFERENTIAL RESPONSE OF THE HELICOID AU@CU2O NANOPARTICLES The
polarization-dependent UV-Vis studies were performed to verify the contribution of different resonance modes of the helicoid Au@Cu2O nanoparticles under LCP and RCP light. Specifically, a
linear polarizer and a quarter wave plate were assembled to generate LCP and RCP light and placed between light source and cuvette in the sample room of the UV-Vis spectrometer. The
extinction spectra of the helicoid Au@Cu2O nanoparticles and those under LCP and RCP light were obtained (Supplementary Fig. 13). The calculated _g_-factor spectra of the L-handed and
D-handed helicoid Au@Cu2O nanoparticles were obtained according to Eq. (3), where ExtLCP and ExtRCP denote the extinction of the helicoid Au@Cu2O nanoparticles under LCP and RCP light,
respectively. $$g=\frac{2\left({{{{\rm{Ext}}}}}_{{{{\rm{LCP}}}}}-{{{{\rm{Ext}}}}}_{{{{\rm{RCP}}}}}\right)}{\left({{{{\rm{Ext}}}}}_{{{{\rm{LCP}}}}}+{{{{\rm{Ext}}}}}_{{{{\rm{RCP}}}}}\right)}$$
(3) INSTRUMENTATION SEM images were obtained with a ZEISS Gemini Sigma 300 operating at 10 kV. TEM images, HRTEM images, STEM, EDX mapping images, and SAED patterns were acquired using a
FEI Tecnai G2 F30 S-TWIN operating at 300 kV. XRD patterns were acquired using a Bruker D8 ADVANCE X-ray diffractometer with Cu _K_α radiation. XPS spectra were from an ESCALAB-MKII
spectrometer equipped with an Al _K_α X-ray source using C 1 _s_ (284.8 eV) as reference. The specific composition of the helicoid Au@Cu2O nanoparticles was analyzed by inductively coupled
plasma-optical emission spectroscopy (ICP-OES) on a Thermo Scientific iCAP 7000 series spectrometer (Thermo Scientific, USA). Circular dichroism (CD) spectra and extinction spectra were
recorded using a Chirascan plus spectrometer and a Shimadzu UV-1800 spectrometer with a quartz cell of 10 mm path length at room temperature, respectively. The _g_-factor spectra were
calculated from the measured CD spectra and extinction spectra. The fiber-coupled halogen lamp OSL2, and the horizontally and vertically linear polarizers WP50L-VIS (420−700 nm) are from
Thorlabs. The transmission spectra were acquired using a Shimadzu UV-1800 spectrometer. NUMERICAL SIMULATIONS All theoretically predicted _g_-factor spectra, extinction spectra, and
electromagnetic fields of nanoparticles were attained by the FDTD method based on the commercial software (FDTD, Ansys Lumerical Solutions, Inc.). The model sizes of the D-handed helicoid Au
nanoparticles and D-handed helicoid Au@Cu2O nanoparticles were consistent with their morphological parameters from SEM images. The permittivity of Au came from Johnson and Christy data in
the software’s database, and the optical properties of Cu2O and CuO were provided by Carl G. Ribbing and Arne Roos65,66. The dielectric medium surrounding the helicoid Au nanoparticle is
water with refractive index of 1.33, while ethanol with refractive index of 1.36 for the helicoid Au@Cu2O nanoparticle. The mesh was set as 1 nm for all directions to ensure structural
detail. The boundary conditions of FDTD were PML (perfectly matched layer) for all directions. To set the incident light as circularly polarized light, two TFSF (total-field scattered-field)
light sources with vertical polarization direction were combined, meanwhile, the phase difference was set as 90° and −90° for LCP and RCP light, respectively. The _g_-factor spectra were
calculated as Eq. (4). _C_ext,LCP and _C_ext,RCP represent extinction cross-section of chiral nanoparticles at LCP and RCP incidences, respectively. They are average results of three
directions along the <100 > , <110 > , and <111> axes. The simulation time was set as 1000 s to ensure the convergence of the results. The spectra were recorded by Analysis
Group, which was provided by the FDTD solution software and consisted of frequency domain power monitors for measuring extinction cross sections. The theoretical average spectra of the
helicoid Au@Cu2O nanoparticles from 42 orientations were also obtained. The incident illumination of an electromagnetic wave was set along the _z_ direction. Under the fixed-illumination
condition, the nanoparticles were rotated. As shown in Supplementary Fig. 9, the polar angle (_Φ_) was changed from 0° to 180° in 30° increments and the azimuthal angle (_φ_) was
simultaneously changed from 0° to 150° in 30° increments. Simulated electric and magnetic fields of the helicoid Cu2O nanoparticles, the Au cube@helicoid Cu2O nanoparticles, the helicoid
Au@helicoid Cu2O nanoparticles, the helicoid Au@Cu2O sphere nanoparticles, and the helicoid Au nanoparticles were obtained under the incidence of LCP and RCP light along the <111> axis
of the nanostructures at 720, 860, 900, 920, and 620 nm, respectively. These wavelengths were determined according to CD spectra of the five nanostructures along the <111> axis
(Supplementary Fig. 15). The sizes of Au cube and Cu2O sphere in the Au cube@helicoid Cu2O nanoparticle and the helicoid Au@Cu2O sphere nanoparticle are 220 and 480 nm, respectively. CD
spectra and extinction spectra along the <111> axis of the nanostructures were calculated as Eqs. (5) and (6). To map the chiral electromagnetic field distributions of the five
nanostructures at different cross-sections, electromagnetic fields distributions of different _xy_ cross-sections at different fixed _z_ values were monitored under the illumination of the
LCP/RCP light along the <111> orientation of the chiral nanostructures, i.e. along the _z_ axis, as displayed by Supplementary Fig. 17. The multipolar contribution to the scattering
spectra of the helicoid Au and the helicoid Au@Cu2O nanoparticles was analyzed through multipole expansion from the calculated electromagnetic-field vectors67. The _Q_-factor values were
calculated according to Eq. (7), where _λ_ is the wavelength of the decomposed extinction peak and Δ_λ_ is the corresponding full width at half maxima68,69,70,71,72,73. The mode volume (_V_)
was obtained by calculation according to Eqs. (8) and (9), where _W_(\(\vec{{{{\bf{r}}}}}\)) denotes the electromagnetic energy density, _E_(\(\vec{{{{\bf{r}}}}}\)) and
_B_(\(\vec{{{{\bf{r}}}}}\)) are the electric and magnetic fields, respectively, _μ_0 is the permeability and _ε_(\(\vec{{{{\bf{r}}}}}\)) is the dielectric permittivity68,70,71,74,75.
$$g=\frac{2 * \left({C}_{{{{\rm{ext}}}},{{{\rm{LCP}}}}}-{C}_{{{{\rm{ext}}}},{{{\rm{RCP}}}}}\right)}{\left({C}_{{{{\rm{ext}}}},{{{\rm{LCP}}}}}+{C}_{{{{\rm{ext}}}},{{{\rm{RCP}}}}}\right)}$$
(4) $${{{\rm{CD}}}}={C}_{{{{\rm{ext}}}},{{{\rm{LCP}}}}}-{C}_{{{{\rm{ext}}}},{{{\rm{RCP}}}}}$$ (5)
$${{{\rm{ext}}}}=\frac{{C}_{{{{\rm{ext}}}},{{{\rm{LCP}}}}}+{C}_{{{{\rm{ext}}}},{{{\rm{RCP}}}}}}{2}$$ (6) $$Q \,=\frac{\lambda }{\Delta \lambda }$$ (7) $$V=\left[\int
W\left(\vec{{{{\bf{r}}}}}\right){{{{\rm{d}}}}}^{3}\,\vec{{{{\bf{r}}}}}\right]/\max \left[W\left(\vec{{{{\bf{r}}}}}\right)\right]$$ (8)
$$W\left(\vec{{{{\bf{r}}}}}\right)=\frac{1}{2}{{\mathrm{Re}}}\left\{\frac{{{{\rm{d}}}}\left[\omega \,\varepsilon \left(\vec{{{{\bf{r}}}}}\right)\right]}{{{{\rm{d}}}}\omega
}\right\}{\left|E\left(\vec{{{{\bf{r}}}}}\right)\right|}^{2}+\frac{1}{2}{\mu }_{0}{\left|B\left(\vec{{{{\bf{r}}}}}\right)\right|}^{2}$$ (9) CU2O THICKNESSES OF THE HELICOID AU@CU2O
NANOPARTICLES The Cu2O shell thicknesses are calculated following a method from a previous report of our group, as Eq. (10)76. _n_Au/_n_Cu2O represents the molar ratio of Au and Cu2O of the
helicoid Au@Cu2O nanoparticles obtained by ICP techniques. _b_ denotes the length of the regular octahedron from the helicoid Au@Cu2O nanoparticles viewed from the <111> axis, as shown
red lines in Supplementary Fig. 3. _a_ is the thickness of the Cu2O shell.
$$\frac{{n}_{{{{\rm{Au}}}}}}{{n}_{{{{\rm{C}}}}{{{{\rm{u}}}}}_{2}{{{\rm{O}}}}}}=\frac{{\left(b-a\right)}^{3}}{{b}^{3}-{\left(b-a\right)}^{3}}$$ (10) REPORTING SUMMARY Further information on
research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY The data that support the findings of this study are available from the
corresponding author upon request. Source data are provided with this paper. REFERENCES * Nam, K. T. & Kim, H. Gold meets peptides in a hybrid coil. _Science_ 371, 1311–1311 (2021).
Article ADS CAS PubMed Google Scholar * Hendry, E. et al. Ultrasensitive detection and characterization of biomolecules using superchiral fields. _Nat. Nanotechnol._ 5, 783–787 (2010).
Article ADS CAS PubMed Google Scholar * Im, S. W. et al. Chiral surface and geometry of metal nanocrystals. _Adv. Mater._ 32, 1905758 (2020). Article CAS Google Scholar * Tan, L.,
Fu, W., Gao, Q. & Wang, P.-P. Chiral plasmonic hybrid nanostructures: a gateway to advanced chiroptical materials. _Adv. Mater._ 36, 2309033 (2024). Article CAS Google Scholar * Duan,
Y. & Che, S. Chiral mesostructured inorganic materials with optical chiral response. _Adv. Mater._ 35, 2205088 (2023). Article CAS Google Scholar * Ma, W. et al. Chiral inorganic
nanostructures. _Chem. Rev._ 117, 8041–8093 (2017). Article ADS CAS PubMed Google Scholar * Kim, R. M. et al. Enantioselective sensing by collective circular dichroism. _Nature_ 612,
470–476 (2022). Article ADS CAS PubMed Google Scholar * Negrín-Montecelo, Y. et al. Chiral generation of hot carriers for polarization-sensitive plasmonic photocatalysis. _J. Am. Chem.
Soc._ 144, 1663–1671 (2022). Article PubMed Google Scholar * Shukla, N. & Gellman, A. J. Chiral metal surfaces for enantioselective processes. _Nat. Mater._ 19, 939–945 (2020).
Article ADS CAS PubMed Google Scholar * Wang, G. et al. Chiral plasmonic triangular nanorings with SERS activity for ultrasensitive detection of amyloid proteins in Alzheimer’s disease.
_Adv. Mater._ 33, 2102337 (2021). Article CAS Google Scholar * Liu, Z. et al. Enantiomeric discrimination by surface‐enhanced raman scattering-chiral anisotropy of chiral nanostructured
gold films. _Angew. Chem. Int. Ed._ 59, 15226–15231 (2020). Article CAS Google Scholar * Okur, S. et al. An enantioselective e‐nose: an array of nanoporous homochiral MOF films for
stereospecific sensing of shiral odors. _Angew. Chem. Int. Ed._ 60, 3566–3571 (2020). Article Google Scholar * Bainova, P. et al. Plasmon-assisted chemistry using chiral gold helicoids:
toward asymmetric organic catalysis. _ACS Catal._ 13, 12859–12867 (2023). Article CAS Google Scholar * Xu, L. et al. Enantiomer-dependent immunological response to chiral nanoparticles.
_Nature_ 601, 366–373 (2022). Article ADS CAS PubMed Google Scholar * Namgung, S. D. et al. Circularly polarized light-sensitive, hot electron transistor with chiral plasmonic
nanoparticles. _Nat. Commun._ 13, 5081 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Wang, W. et al. The development of chiral Nanoparticles to target NK cells and
CD8+ T cells for cancer immunotherapy. _Adv. Mater._ 34, 2109354 (2022). Article CAS Google Scholar * Liu, Z. et al. Photomagnetic-chiral anisotropy of chiral nanostructured gold films.
_Chem_ 8, 186–196 (2022). Article CAS Google Scholar * Peng, Y. et al. Realization of VIS–NIR dual-modal circularly polarized light detection in chiral perovskite bulk crystals. _J. Am.
Chem. Soc._ 143, 14077–14082 (2021). Article CAS PubMed Google Scholar * Lv, J. et al. Biomimetic chiral photonic crystals. _Angew. Chem. Int. Ed._ 58, 7783–7787 (2019). Article CAS
Google Scholar * Lu, J. et al. Enhanced optical asymmetry in supramolecular chiroplasmonic assemblies with long-range order. _Science_ 371, 1368–1374 (2021). Article ADS CAS PubMed
Google Scholar * Lee, H.-E. et al. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. _Nature_ 556, 360–365 (2018). Article ADS CAS PubMed Google Scholar
* Cho, N. H. et al. Uniform chiral gap synthesis for high dissymmetry factor in single plasmonic gold nanoparticle. _ACS Nano_ 14, 3595–3602 (2020). Article CAS PubMed Google Scholar *
Lv, X. et al. Engineering the intrinsic chirality of plasmonic Au@Pd metamaterials for highly sensitive chiroplasmonic hydrogen sensing. _Adv. Mater._ 35, 2305429 (2023). Article CAS
Google Scholar * Wu, F. et al. Synthesis of chiral Au nanocrystals with precise homochiral facets for enantioselective surface chemistry. _Nano Lett._ 22, 2915–2922 (2022). Article ADS
CAS PubMed Google Scholar * Zheng, J. et al. Halide-assisted differential growth of chiral nanoparticles with threefold rotational symmetry. _Nat. Commun._ 14, 3783 (2023). Article ADS
PubMed PubMed Central Google Scholar * Kim, H. et al. Capacitive enhancements of the chiroptical response in plasmonic helicoids. _Adv. Opt. Mater._ 11, 2300205 (2023). Article ADS CAS
Google Scholar * Sun, X. et al. Tunable reversal of circular dichroism in the seed-mediated growth of bichiral plasmonic nanoparticles. _ACS Nano_ 16, 19174–19186 (2022). Article CAS
PubMed Google Scholar * Wu, F. et al. Surface topographical engineering of chiral Au nanocrystals with chiral hot spots for plasmon-enhanced chiral discrimination. _Nano Lett._ 23,
8233–8240 (2023). Article ADS CAS PubMed Google Scholar * Hong, J. W., Wi, D. H., Lee, S. U. & Han, S. W. Metal-semiconductor heteronanocrystals with desired configurations for
plasmonic photocatalysis. _J. Am. Chem. Soc._ 138, 15766–15773 (2016). Article CAS PubMed Google Scholar * Zhang, L., Jing, H., Boisvert, G., He, J. Z. & Wang, H. Geometry control
and optical tunability of metal-cuprous oxide core-shell nanoparticles. _ACS Nano_ 6, 3514–3527 (2012). Article CAS PubMed Google Scholar * Liu, D.-Y. et al. Distinctive enhanced and
tunable plasmon resonant absorption from controllable Au@Cu2O nanoparticles: experimental and theoretical modeling. _J. Phys. Chem. C._ 116, 4477–4483 (2012). Article CAS Google Scholar *
Xu, W. et al. Continuous tuning of Au-Cu2O janus nanostructures for efficient charge separation. _Angew. Chem. Int. Ed._ 59, 22246–22251 (2020). Article CAS Google Scholar * Jing, H.,
Large, N., Zhang, Q. & Wang, H. Epitaxial growth of Cu2O on Ag allows for fine control over particle geometries and optical properties of Ag-Cu2O core-shell nanoparticles. _J. Phys.
Chem. C._ 118, 19948–19963 (2014). Article CAS Google Scholar * Huang, M. H., Rej, S. & Chiu, C. Y. Facet-dependent optical properties revealed through investigation of polyhedral
Au-Cu2O and bimetallic core-shell nanocrystals. _Small_ 11, 2716–2726 (2015). Article CAS PubMed Google Scholar * Kuo, M.-Y. et al. Au@Cu2O core@shell nanocrystals as dual-functional
catalysts for sustainable environmental applications. _Appl. Catal., B_ 242, 499–506 (2019). Article CAS Google Scholar * Lu, B. et al. Hollow Au-Cu2O core-shell nanoparticles with
geometry-dependent optical properties as efficient plasmonic photocatalysts under visible light. _Langmuir_ 32, 3085–3094 (2016). Article CAS PubMed Google Scholar * Shi, X. et al.
Plasmon enhancement effect in Au gold nanorods@Cu2O core-shell nanostructures and their use in probing defect states. _Langmuir_ 31, 1537–1546 (2015). Article CAS PubMed Google Scholar *
Wang, H. J., Yang, K. H., Hsu, S. C. & Huang, M. H. Photothermal effects from Au-Cu2O core-shell nanocubes, octahedra, and nanobars with broad near-infrared absorption tunability.
_Nanoscale_ 8, 965–972 (2016). Article ADS CAS PubMed Google Scholar * Zhang, S. et al. Plasmon modes induced by anisotropic gap opening in Au@Cu2O nanorods. _Small_ 12, 4264–4276
(2016). Article ADS CAS PubMed Google Scholar * Zhang, L., Blom, D. A. & Wang, H. Au–Cu2O core–shell nanoparticles: a hybrid metal-semiconductor heteronanostructure with
geometrically tunable optical properties. _Chem. Mater._ 23, 4587–4598 (2011). Article CAS Google Scholar * González-Rubio, G. et al. Micelle-directed chiral seeded growth on anisotropic
gold nanocrystals. _Science_ 368, 1472–1477 (2020). Article ADS PubMed Google Scholar * Zhuo, X., Vila-Liarte, D., Wang, S., Jimenez de Aberasturi, D. & Liz-Marzán, L. M. Coated
chiral plasmonic nanorods with enhanced structural stability. _Chem. Mater._ 35, 5689–5698 (2023). Article CAS Google Scholar * Im, S. W., Jo, E., Kim, R. M., Han, J. H. & Nam, K. T.
32‐Symmetric chiral gold nanoplates with near‐infrared circular dichroism. _Adv. Opt. Mater._ 11, 2300037 (2023). Article CAS Google Scholar * Ni, B. et al. Chiral seeded growth of gold
nanorods into fourfold twisted nanoparticles with plasmonic optical activity. _Adv. Mater._ 35, 2208299 (2023). Article CAS Google Scholar * Karst, J. et al. Chiral scatterometry on
chemically synthesized single plasmonic nanoparticles. _ACS Nano_ 13, 8659–8668 (2019). Article CAS PubMed Google Scholar * Kim, Y. & Nam, J.-M. Mechanically interlocked gold
nanocatenanes. _Nat. Synth._ 1, 649–657 (2022). Article ADS Google Scholar * Chen, Y. et al. Stabilizing chiral gold nanorods from chiral-micelle-directed synthesis by sulfide treatment
for chiroplasmonic sensing. _ACS Appl. Nano Mater._ 7, 1503–1508 (2024). Article CAS Google Scholar * Zhang, L. & Wang, H. Cuprous oxide nanoshells with geometrically tunable optical
properties. _ACS Nano_ 5, 3257–3267 (2011). Article CAS PubMed Google Scholar * Hirakawa, T. & Kamat, P. V. Charge separation and catalytic activity of Ag@TiO2 core-shell composite
clusters under UV-irradiation. _J. Am. Chem. Soc._ 127, 3928–3934 (2005). Article CAS PubMed Google Scholar * Chen, W.-T., Yang, T.-T. & Hsu, Y.-J. Au-CdS core-shell nanocrystals
with controllable shell thickness and photoinduced charge separation property. _Chem. Mater._ 20, 7204–7206 (2008). Article CAS Google Scholar * Chen, Y. et al. Multidimensional
nanoscopic chiroptics. _Nat. Rev. Phys._ 4, 113–124 (2021). Article Google Scholar * Lu, X., Wang, X., Liu, Y. & Ding, T. Optical dipole-induced anisotropic growth of semiconductors: A
facile strategy toward chiral and complex nanostructures. _Proc. Natl Acad. Sci. USA_ 120, e2216627120 (2023). Article CAS PubMed PubMed Central Google Scholar * Wang, Y.-F. et al.
Engineering high-performance dielectric chiral shells with enhanced chiral fields for sensitive chiral biosensor. _Rare Met._ 43, 1197–1206 (2024). Article CAS Google Scholar * Yao, K.
& Liu, Y. Enhancing circular dichroism by chiral hotspots in silicon nanocube dimers. _Nanoscale_ 10, 8779–8786 (2018). Article CAS PubMed Google Scholar * Schäferling, M., Dregely,
D., Hentschel, M. & Giessen, H. Tailoring enhanced optical chirality: design principles for chiral plasmonic nanostructures. _Phys. Rev. X_ 2, 031010 (2012). Google Scholar * Zhang, L.
et al. Chiral gold nanorods with five-fold rotational symmetry and orientation-dependent chiroptical properties of their monomers and dimers. _Angew. Chem. Int. Ed._ 62, e202312615 (2023).
Article CAS Google Scholar * Lu, X., Wang, X., Wang, S. & Ding, T. Polarization-directed growth of spiral nanostructures by laser direct writing with vector beams. _Nat. Commun._ 14,
1422 (2023). Article ADS CAS PubMed PubMed Central Google Scholar * Tang, Y. & Cohen, A. E. Optical chirality and its interaction with matter. _Phys. Rev. Lett._ 104, 163901
(2010). Article ADS PubMed Google Scholar * Kang, L., Ren, Q. & Werner, D. H. Leveraging superchiral light for manipulation of optical chirality in the near-field of plasmonic
metamaterials. _ACS Photonics_ 4, 1298–1305 (2017). Article CAS Google Scholar * Mun, J. et al. Electromagnetic chirality: from fundamentals to nontraditional chiroptical phenomena.
_Light.: Sci. Appl._ 9, 139 (2020). Article ADS CAS PubMed Google Scholar * Both, S. et al. Nanophotonic chiral sensing: how does it actually work? _ACS Nano_ 16, 2822–2832 (2022).
Article CAS PubMed Google Scholar * Yang, Y., Miller, O. D., Christensen, T., Joannopoulos, J. D. & Soljacic, M. Low-loss plasmonic dielectric nanoresonators. _Nano Lett._ 17,
3238–3245 (2017). Article ADS CAS PubMed Google Scholar * Govorov, A. O., Fan, Z., Hernandez, P., Slocik, J. M. & Naik, R. R. Theory of circular dichroism of nanomaterials
comprising chiral molecules and nanocrystals: plasmon enhancement, dipole interactions, and dielectric effects. _Nano Lett._ 10, 1374–1382 (2010). Article ADS CAS PubMed Google Scholar
* Niu, W. et al. Selective synthesis of single-crystalline rhombic dodecahedral, octahedral, and cubic gold nanocrystals. _J. Am. Chem. Soc._ 131, 697–703 (2009). Article CAS PubMed
Google Scholar * ROOS, C. G. R. A. “Copper Oxides (Cu2O, CuO)” in HANDBOOK OF OPTICAL CONSTANTS OF SOLIDS II. 875-882 (1991). * Johnson, P. & Christy, R. Optical constants of transition
metals: Ti, V, Cr, Mn, Fe, Co, Ni, and Pd. _Phys. Rev. B_ 9, 5056–5070 (1974). Article ADS CAS Google Scholar * Alaee, R., Rockstuhl, C. & Fernandez-Corbaton, I. An electromagnetic
multipole expansion beyond the long-wavelength approximation. _Opt. Commun._ 407, 17–21 (2018). Article ADS CAS Google Scholar * Francs, G. C. d., Derom, S., Vincent, R., Bouhelier, A.
& Dereux, A. Mie plasmons: modes volumes, quality factors and coupling strengths (Purcell factor) to a dipolar emitter. _Int. J. Opt_. 175162, (2012). * Pan, J. et al. Low-threshold
plasmonic lasing based on high-Q dipole void mode in a metallic nanoshell. _Opt. Lett._ 37, 1181–1183 (2012). Article ADS CAS PubMed Google Scholar * Wan, M., Gu, P., Liu, W., Chen, Z.
& Wang, Z. Low threshold spaser based on deep-subwavelength spherical hyperbolic metamaterial cavities. _Appl. Phys. Lett._ 110, 031103 (2017). Article ADS Google Scholar * Gu, P. et
al. High-Q and intense lattice plasmon resonance in hexagonal nonclose packed thin silver nanoshells array. _J. Phys. Chem. C._ 128, 6431–6437 (2024). Article CAS Google Scholar * Gu, P.
et al. Experimental observation of sharp cavity plasmon resonances in dielectric-metal core-shell resonators. _Appl. Phys. Lett._ 107, 141908 (2015). Article ADS Google Scholar * Gu, P.,
Chen, J., Wan, M., Chen, Z. & Wang, Z. Comparative studies on the quality factors of whispering gallery modes and hybrid plasmon photon modes. _Opt. Express_ 25, 9295–9304 (2017).
Article ADS CAS PubMed Google Scholar * Xiang, C., Chan, C. K. & Wang, J. Proposal and numerical study of ultra-compact active hybrid plasmonic resonator for sub-wavelength lasing
applications. _Sci. Rep._ 4, 3720 (2014). Article PubMed PubMed Central Google Scholar * Klusmann, C., Oppermann, J., Forster, P., Rockstuhl, C. & Kalt, H. Identification of
dielectric, plasmonic, and hybrid modes in metal-coated whispering-gallery-mode resonators. _ACS Photonics_ 5, 2365–2373 (2018). Article CAS Google Scholar * Wu, F. et al. Heteroepitaxial
growth of Au@Pd core–shell nanocrystals with intrinsic chiral surfaces for enantiomeric recognition. _Rare Met._ 43, 225–235 (2024). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by National Key R&D Program of China (2022YFE0113000), National Natural Science Foundation of China (Nos. 22374144, 22072144, 22102171, and
22204160), and Natural Science Foundation of Jilin Province (No. YDZJ202201ZYTS341). AUTHOR INFORMATION Author notes * These authors contributed equally: Xiali Lv, Yu Tian. AUTHORS AND
AFFILIATIONS * State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, China Xiali Lv, Yu Tian, Fengxia Wu,
Xiaoxi Luan, Fenghua Li, Guobao Xu & Wenxin Niu * School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, China Xiali Lv, Xiaoxi Luan, Guobao
Xu & Wenxin Niu * State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, China Zhili Shen & Kun Liu Authors * Xiali Lv
View author publications You can also search for this author inPubMed Google Scholar * Yu Tian View author publications You can also search for this author inPubMed Google Scholar * Fengxia
Wu View author publications You can also search for this author inPubMed Google Scholar * Xiaoxi Luan View author publications You can also search for this author inPubMed Google Scholar *
Fenghua Li View author publications You can also search for this author inPubMed Google Scholar * Zhili Shen View author publications You can also search for this author inPubMed Google
Scholar * Guobao Xu View author publications You can also search for this author inPubMed Google Scholar * Kun Liu View author publications You can also search for this author inPubMed
Google Scholar * Wenxin Niu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.L.L. performed the research and wrote the paper; Y.T.
conducted the numerical simulations; F.X.W. and X.X.L assisted in the data analysis of the research; Z.L.S assisted in the experimental characterizations of the research; F.H.L., G.B.X.,
K.L., and W.X.N. revised the research; W.X.N. supervised the project. CORRESPONDING AUTHOR Correspondence to Wenxin Niu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interest. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Junsuk Rho and the other, anonymous, reviewers for their contribution to the peer review of this work.
A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION REPORTING SUMMARY PEER REVIEW FILE SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do
not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lv, X., Tian, Y., Wu, F. _et al._ Chiral plasmonic-dielectric coupling
enables strong near-infrared chiroptical responses from helicoidal core-shell nanoparticles. _Nat Commun_ 15, 9234 (2024). https://doi.org/10.1038/s41467-024-53705-4 Download citation *
Received: 25 March 2024 * Accepted: 18 October 2024 * Published: 25 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53705-4 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
