Improving rice nitrogen-use efficiency by modulating a novel monouniquitination machinery for optimal root plasticity response to nitrogen
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Plant nitrogen (N)-use efficiency (NUE) is largely determined by the ability of root to take up external N sources, whose availability and distribution in turn trigger the
modification of root system architecture (RSA) for N foraging. Therefore, improving N-responsive reshaping of RSA for optimal N absorption is a major target for developing crops with high
NUE. In this study, we identified _RNR10_ (_REGULATOR OF N-RESPONSIVE RSA ON CHROMOSOME 10_) as the causal gene that underlies the significantly different root developmental plasticity in
response to changes in N level exhibited by the _indica_ (Xian) and _japonica_ (Geng) subspecies of rice. _RNR10_ encodes an F-box protein that interacts with a negative regulator of auxin
biosynthesis, DNR1 (DULL NITROGEN RESPONSE1). Interestingly, RNR10 monoubiquitinates DNR1 and inhibits its degradation, thus antagonizing auxin accumulation, which results in reduced root
responsivity to N and nitrate (NO3−) uptake. Therefore, modulating the RNR10-DNR1-auxin module provides a novel strategy for coordinating a desirable RSA and enhanced N acquisition for
future sustainable agriculture. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS PLASTID-LOCALIZED AMINO ACID METABOLISM COORDINATES RICE AMMONIUM TOLERANCE AND NITROGEN USE EFFICIENCY Article 21 August 2023 LOCAL AUXIN
BIOSYNTHESIS ACTS DOWNSTREAM OF BRASSINOSTEROIDS TO TRIGGER ROOT FORAGING FOR NITROGEN Article Open access 14 September 2021 TATCP6 IS REQUIRED FOR EFFICIENT AND BALANCED UTILIZATION OF
NITRATE AND PHOSPHORUS IN WHEAT Article Open access 16 February 2025 DATA AVAILABILITY Raw RNA-seq data are deposited at the National Center for Biotechnology Information’s Sequence Read
Archive (SRA) (accession number PRJNA986304). Proteomic identification and ubiquitination proteomics data are deposited at ProteomeXchange (accession number PXD043400). Source data are
provided with this paper. REFERENCES * Jia, Z., Giehl, R. & von Wirén, N. Nutrient-hormone relations: driving root plasticity in plants. _Mol. Plant_ 15, 86–103 (2022). Article CAS
PubMed Google Scholar * Liu, H., Liu, Q., Gao, X. & Fu, X. Role of nitrogen sensing and its integrative signaling pathways in shaping root system architecture. _Front. Agr. Sci. Eng._
9, 316–332 (2022). CAS Google Scholar * Masclaux-Daubresse, C. et al. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture.
_Ann. Bot._ 105, 1141–1157 (2010). Article PubMed PubMed Central Google Scholar * Kiba, T. & Krapp, A. Plant nitrogen acquisition under low availability: regulation of uptake and
root architecture. _Plant Cell Physiol._ 57, 707–714 (2016). Article CAS PubMed PubMed Central Google Scholar * Ma, W. et al. Auxin biosynthetic gene _TAR2_ is involved in low
nitrogen-mediated reprogramming of root architecture in _Arabidopsis_. _Plant J._ 78, 70–79 (2014). Article CAS PubMed Google Scholar * Liu, Y. & von Wirén, N. Integration of
nutrient and water availabilities via auxin into the root developmental program. _Curr. Opin. Plant Biol._ 65, 102117 (2022). Article CAS PubMed Google Scholar * Shao, A. et al. The
auxin biosynthetic _TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2.1-3A_ increases grain yield of wheat. _Plant Physiol._ 174, 2274–2288 (2017). Article CAS PubMed PubMed Central Google
Scholar * Tromas, A. et al. Auxin-binding protein 1 is a negative regulator of the SCF (TIR1/AFB) pathway. _Nat. Commun._ 4, 2496 (2013). Article PubMed Google Scholar * Zhang, L. et al.
Function of histone H2B monoubiquitination in transcriptional regulation of auxin biosynthesis in _Arabidopsis_. _Commun. Biol._ 4, 206 (2021). Article CAS PubMed PubMed Central Google
Scholar * Nakagawa, T. & Nakayama, K. Protein monoubiquitylation: targets and diverse functions. _Genes Cells_ 20, 543–562 (2015). Article CAS PubMed PubMed Central Google Scholar
* Zhang, S. et al. Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. _Plant Cell_ 33, 566–580 (2021). Article PubMed
Google Scholar * Xi, Z. Y. et al. Development of a wide population of chromosome single-segment substitution in the genetic background of an elite cultivar of rice (_Oryza sativa_ L.).
_Genome_ 49, 476–484 (2006). Article CAS PubMed Google Scholar * Jain, M. et al. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during
panicle and seed development, and regulation by light and abiotic stress. _Plant Physiol._ 143, 1467–1483 (2007). Article CAS PubMed PubMed Central Google Scholar * Kahloul, S. et al.
Structural, expression and interaction analysis of rice _SKP1-Like_ genes. _DNA Res._ 20, 67–78 (2012). Article PubMed PubMed Central Google Scholar * Wang, W. et al. Genomic variation
in 3,010 diverse accessions of Asian cultivated rice. _Nature_ 557, 43–49 (2018). Article CAS PubMed PubMed Central Google Scholar * Zheng, X. et al. Genomic signatures of domestication
and adaptation during geographical expansions of rice cultivation. _Plant Biotech. J._ 20, 16–18 (2022). Article Google Scholar * Chen, D. et al. Parkin mono-ubiquitinates Bcl-2 and
regulates autophagy. _J. Biol. Chem._ 285, 38214–38223 (2010). Article CAS PubMed PubMed Central Google Scholar * Pavlopoulos, E. et al. Neuralized1 activates CPEB3: a function for
nonproteolytic ubiquitin in synaptic plasticity and memory storage. _Cell_ 147, 1369–1383 (2011). Article CAS PubMed PubMed Central Google Scholar * Sun, H. et al. Heterotrimeric G
proteins regulate nitrogen-use efficiency in rice. _Nat. Genet._ 46, 652–656 (2014). Article CAS PubMed Google Scholar * Li, S. et al. Modulating plant growth-metabolism coordination for
sustainable agriculture. _Nature_ 560, 595–600 (2018). Article CAS PubMed PubMed Central Google Scholar * Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics
Analysis version 7.0 for bigger datasets. _Mol. Biol. Evol._ 33, 1870–1874 (2016). Article CAS PubMed PubMed Central Google Scholar * Felsenstein, J. Confidence limits on phylogenies:
an approach using the bootstrap. _Evolution_ 39, 783–791 (1985). Article PubMed Google Scholar * Wang, S. et al. Control of grain size, shape and quality by _OsSPL16_ in rice. _Nat.
Genet._ 44, 950–954 (2012). Article CAS PubMed Google Scholar * He, Y. et al. Programmed self-elimination of the _CRISPR/Cas9_ construct greatly accelerates the isolation of edited and
transgene-free rice plants. _Mol. Plant_ 11, 1210–1213 (2018). Article CAS PubMed Google Scholar * Huang, X. et al. Natural variation at the _DEP1_ locus enhances grain yield in rice.
_Nat. Genet._ 41, 494–497 (2009). Article CAS PubMed Google Scholar * Wang, S. et al. The _OsSPL16-GW7_ regulatory module determines grain shape and simultaneously improves rice yield
and grain quality. _Nat. Genet._ 47, 949–954 (2015). Article CAS PubMed Google Scholar * Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions
in plants. _Plant Physiol._ 146, 368–376 (2008). Article CAS PubMed PubMed Central Google Scholar * Wang, F. et al. Biochemical insights on degradation of _Arabidopsis_ DELLA proteins
gained from a cell-free assay system. _Plant Cell_ 21, 2378–2390 (2009). Article CAS PubMed PubMed Central Google Scholar * Wu, K. et al. Enhanced sustainable green revolution yield via
nitrogen-responsive chromatin modulation in rice. _Science_ 367, eaaz204 (2020). Article Google Scholar * Zhao, Q. et al. A plant-specific in vitro ubiquitination analysis system. _Plant
J._ 74, 524–533 (2013). Article CAS PubMed Google Scholar * Perkins, D. N., Pappin, D. J., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching
sequence databases using mass spectrometry data. _Electrophoresis_ 20, 3551–3567 (1999). Article CAS PubMed Google Scholar * Ho, C. H., Lin, S. H., Hu, H. C. & Tsay, Y. F. CHL1
functions as a nitrate sensor in plants. _Cell_ 138, 1184–1194 (2009). Article CAS PubMed Google Scholar * Loqué, D. et al. Additive contribution of AMT1;1 and AMT1;3 to high-affinity
ammonium uptake across the plasma membrane of nitrogen-deficient _Arabidopsis_ roots. _Plant J._ 48, 522–534 (2006). Article PubMed Google Scholar * Stamatakis, A. RAxML version 8: a tool
for phylogenetic analysis and post-analysis of large phylogenies. _Bioinformatics_ 30, 1312–1313 (2014). Article CAS PubMed PubMed Central Google Scholar * Bandelt, H. J., Forster, P.
& Rohl, A. Median-joining networks for inferring intraspecific phylogenies. _Mol. Biol. Evol._ 16, 37–48 (1999). Article CAS PubMed Google Scholar * Li, H. & Durbin, R. Fast and
accurate long-read alignment with Burrows-Wheeler transform. _Bioinformatics_ 26, 589–595 (2010). Article PubMed PubMed Central Google Scholar * Layer, R. M. et al. LUMPY: a
probabilistic framework for structural variant discovery. _Genome Biol._ 15, R84 (2014). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank G. Xu
(Nanjing Agricultural University) for critical suggestions. This research was supported by the National Key Research and Development Program of China (No. 2021YFF1000400 to Shan Li), Jiangsu
Province Key Research and Development Program (No. BE2022335-2 to Shan Li), the National Natural Science Foundation of China (No. 32122065 to Shan Li), the Jiangsu Natural Science
Foundation (No. BK20200540 to Shan Li), Fundamental Research Funds for the Central Universities (No. KJYQ2022001 to Shan Li) and Jiangsu Collaborative Innovation Center for Modern Crop
Production. Work in N.P.H.’s laboratory was supported by the BBSRC-Newton ‘Rice’ Initiative (grant no. BB/N013611/1 to N.P.H.) and also by BBSRC Response Modes grant no. BB/S013741/1 to
N.P.H. AUTHOR INFORMATION Author notes * These authors contributed equally: Yunzhi Huang, Zhe Ji. AUTHORS AND AFFILIATIONS * State Key Laboratory of Crop Genetics & Germplasm Enhancement
and Utilization, Nanjing Agricultural University, Nanjing, China Yunzhi Huang, Yujun Tao, Shuxian Wei, Wu Jiao, Yongzhi Fang, Peng Jian, Chengbo Shen, Yaojun Qin, Siyu Zhang, Shunqi Li,
Xuan Liu, Shuming Kang, Yanan Tian, Qingxin Song & Shan Li * Department of Biology, University of Oxford, Oxford, UK Zhe Ji & Nicholas P. Harberd * State Key Laboratory for
Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University, Guangzhou, China Shaokui Wang * Jiangsu Collaborative Innovation Center for Modern Crop
Production, Nanjing Agricultural University, Nanjing, China Shan Li Authors * Yunzhi Huang View author publications You can also search for this author inPubMed Google Scholar * Zhe Ji View
author publications You can also search for this author inPubMed Google Scholar * Yujun Tao View author publications You can also search for this author inPubMed Google Scholar * Shuxian Wei
View author publications You can also search for this author inPubMed Google Scholar * Wu Jiao View author publications You can also search for this author inPubMed Google Scholar * Yongzhi
Fang View author publications You can also search for this author inPubMed Google Scholar * Peng Jian View author publications You can also search for this author inPubMed Google Scholar *
Chengbo Shen View author publications You can also search for this author inPubMed Google Scholar * Yaojun Qin View author publications You can also search for this author inPubMed Google
Scholar * Siyu Zhang View author publications You can also search for this author inPubMed Google Scholar * Shunqi Li View author publications You can also search for this author inPubMed
Google Scholar * Xuan Liu View author publications You can also search for this author inPubMed Google Scholar * Shuming Kang View author publications You can also search for this author
inPubMed Google Scholar * Yanan Tian View author publications You can also search for this author inPubMed Google Scholar * Qingxin Song View author publications You can also search for this
author inPubMed Google Scholar * Nicholas P. Harberd View author publications You can also search for this author inPubMed Google Scholar * Shaokui Wang View author publications You can
also search for this author inPubMed Google Scholar * Shan Li View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Shan Li, Y.H. and Z.J.
conceived and designed this study. Y.H. performed most of the experiments; Y.H., Y. Tao, S. Wei, S.K. and C.S. conducted map-based cloning. Y.H., S.Z., Y.F., Y.Q., P.J. Shunqi Li, X.L., and
Y. Tian constructed transgenic rice plants and performed field experiments and biochemical experiments. Y.H. and Z.J. conducted phylogenetic analysis. W.J. performed bioinformatic analysis.
S. Wang provided SSSL lines. Z.J. and Shan Li wrote the manuscript. Z.J., Q.S., N.P.H., S. Wang and Shan Li revised the manuscript. All authors discussed the results and contributed to the
manuscript. CORRESPONDING AUTHOR Correspondence to Shan Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature
Plants_ thanks Nicolaus von Wirén and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
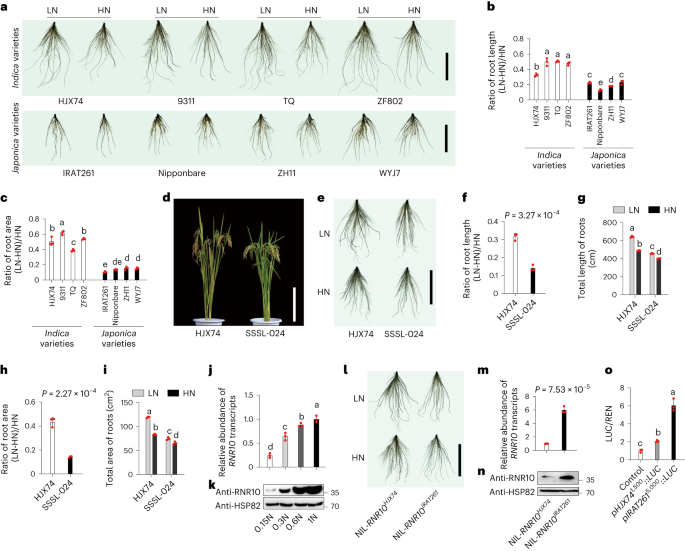
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 N-DEPENDENT DEVELOPMENTAL PLASTICITY RESPONSES OF
_INDICA_ AND _JAPONICA_ RICE PLANTS. A, 21-day-old rice plants grown at LN (0.375 mM NH4NO3) versus HN (1.25 mM NH4NO3) supply. Scale bar, 10 cm. B, C, The absolute value of total length (B)
and total area (C) of visible roots of 10-day old _indica_ and _japonica_ varieties. Source data EXTENDED DATA FIG. 2 _RNR10_ WAS IDENTIFIED BY FINE-SCALE MAPPING, AND ITS SEQUENCE DIVERGES
BETWEEN _INDICA_ AND _JAPONICA_. A, Successive maps of the candidate gene, _RNR10_, using indicated recombination break points and linked DNA markers to an ~ 3.3 kb segment flanked by the
markers P241 and P242 on chromosome 10. The gene structure of _RNR10_ is shown underneath, where the thick black bars represent the protein-coding sequence, and the start and stop codons are
labelled as ATG and TGA, respectively. The 3496-bp SV and 604-bp deletion in the _RNR10__IRAT261_ promoter relative to the _RNR10_ start ATG (nucleotide 1), are shown. B, Agarose gel
electrophoresis of PCR amplified products from _RNR10_ promoter, open reading frame and protein coding region, respectively. C, D, Agarose gel electrophoresis of PCR amplified products using
primer pairs flanking the 3496-bp segment in the promoter region (C) and the 604-bp segment in the open reading frame (D) from our collection of 12 _indica_ and 12 _japonica_ varieties. E,
Agarose gel electrophoresis shows that there is no detectable difference in the size of the protein coding region. F, The differences in _RNR10_ transcript level between _indica_ and
_japonica_ varieties. Different letters denote significant differences (_P_ < 0.05) from a Duncan’s multiple range test. Exact _P_ values are listed in Supplemental Information Table 11.
Data are mean ± s.e.m. (_n_ = 3 biologically independent samples). Source data EXTENDED DATA FIG. 3 THE ROOT AND SHOOT PHENOTYPES OF THE NIL PLANTS. A, The ratio [(LN-HN)/HN] of total length
of visible roots. B, The absolute value of total length of visible roots. C, The ratio [(LN-HN)/HN] of total area of visible roots. D, The absolute value of total area of visible roots.
E-I, Mature plant height (E), the number of tillers per plant (F), the number of secondary branches per panicle (G), the number of grains per panicle (H), and grain yield per plant (I) of
the NIL plants. J, The ratio [(LN-HN)/HN] of root to shoot biomass ratio. K, L, The changes in the ratios [(LN-HN)/HN] of root biomass (K) and shoot biomass (L) over time. M, N, The absolute
values of root biomass (L) and shoot biomass (M). A, C, E-I, _P_ values were generated from two-sided Student’s _t_ tests. B, D, J-L, Different letters denote significant differences (_P_
< 0.05) from a Duncan’s multiple range test. Exact _P_ values are listed in Supplemental Information Table 11. A-D, J-N, Data are mean ± s.e.m. (_n_ = 3 biologically independent samples).
E, Data are mean ± s.e.m. (_n_ = 16 biologically independent samples). F-I, Data are mean ± s.e.m. (_n_ = 12 biologically independent samples). Source data EXTENDED DATA FIG. 4 THE HIGHER
EXPRESSION OF _RNR10_ DRIVEN BY THE _RNR10__IRAT261_ PROMOTER IN THE HJX74 BACKGROUND RESULTED IN REDUCED ROOT GROWTH AND NO3− UPTAKE. A, Morphology of mature HJX74 and
HJX74/_pRNR10__IRAT261__::RNR10-GFP_ plants. Scale bar, 10 cm. B, Root _RNR10_ transcript abundance. Transcript abundance was measured relative to HJX74 (set to 1). C, Root RNR10-GFP protein
abundance. HSP82 severs as loading control. The pictures of western blots represent one of the three experiments performed independently with similar results. D, Root systems of HJX74 and
HJX74/_pRNR10__IRAT261__::RNR10-GFP_ plants. Scale bar, 10 cm. E, The ratio [(LN-HN)/HN] of total length of visible roots. F, The absolute value of total length of visible roots. G, The
ratio [(LN-HN)/HN] of total area of visible roots. H, The absolute value of total area of visible roots. I, The ratio [(LN-HN)/HN] of root to shoot biomass ratio. J, The absolute value of
the ratio of root to shoot biomass. K-N, Mature plant height (K), the number of tillers per plant (L), the number of grains per panicle (M), and grain yield per plant (N). B, E, G, I, K-N,
_P_ values were generated from two-sided Student’s _t_ tests. F, H, J, Different letters denote significant differences (_P_ < 0.05) from a Duncan’s multiple range test. Exact _P_ values
are listed in Supplemental Information Table 11. B, E-J, Data are mean ± s.e.m. (_n_ = 3 biologically independent samples). K, Data are mean ± s.e.m. (_n_ = 16 biologically independent
samples). L-N, Data are mean ± s.e.m. (_n_ = 12 biologically independent samples). Source data EXTENDED DATA FIG. 5 _RNR10_ TRANSCRIPT ABUNDANCE, RNR10 PROTEIN ACCUMULATION AND RSA OF ZH11,
ZH11/_PACT::RNR10-FLAG_ AND ZH11/_RNR10_ PLANTS. A, _RNR10_ mRNA abundance in ZH11/_pAct::RNR10-Flag-_1, ZH11/_pAct::RNR10-Flag-_2 and ZH11/_rnr10_ plants, relative to ZH11 (set to 1). B,
Comparison of RNR10 protein abundance detected by an anti-DDDDK-tag antibody in ZH11 versus ZH11/_pAct::DNR1-Flag_. HSP82 severs as loading control. C, Gene structure of the _RNR10_ gene
showing the location of the CRISPR/Cas9-generated 1-bp deletion in the ZH11/_rnr10_ mutant. D, Comparison of RNR10 protein abundance detected by an anti-RNR10 antibody in ZH11 versus
ZH11/_rnr10_. E-G, The absolute values of total length of visible roots (E), total area of visible roots (F) and root to shoot biomass ratio(G). A, E-G, Different letters denote significant
differences (_P_ < 0.05) from a Duncan’s multiple range test. Exact _P_ values are listed in Supplemental Information Table 11. Data are mean ± s.e.m. (_n_ = 3 biologically independent
samples). The experiments in B and D were repeated independently at least three times with similar results. Source data EXTENDED DATA FIG. 6 PHYLOGENETIC RELATIONSHIP AMONG THE RICE F-BOX
PROTEINS AND THEIR HOMOLOGOUS GENES, AND AMINO ACID SEQUENCE ALIGNMENT OF THE FBA SUBFAMILY. A, Phylogenetic tree of _RNR10_ and its homologous genes, constructed in MEGA11 using the
Neighbour-Joining method. _RNR10_ is indicated by the star. B, Alignment of the protein sequences of members in the FBA subfamily. Black represents identical amino acids. The numbers
indicate the positions of the amino acids. EXTENDED DATA FIG. 7 RNR10 INTERACTS WITH DNR1 AND OSKS. A-D, Four unique peptide sequences were identified by immunoprecipitation followed by mass
spectrometry assays. E-G, Three amino acids were identified by mass spectrometric analysis. H, I, SFLC assays. cLUC-tagged RNR10 was co-transformed into tobacco leaves along with
nLUC-tagged OSK1 (H) or OSK20 (I). J, K, Co-IP experiments. Flag-tagged RNR10 was co-transformed into rice protoplasts with HA-tagged OSK1 (J) or OSK20 (K). The experiments in J and K were
repeated independently at least three times with similar results. Source data EXTENDED DATA FIG. 8 ROOT PHENOTYPES AND THE RATIO OF ROOT TO SHOOT BIOMASS OF NIL-_RNR10__HJX74_-_DNR1__HJX74_,
NIL-_RNR10__IRAT261_, NIL-_DNR1__IRAP9_, AND NIL-_RNR10__IRAT261_-_DNR1__IRAP9_. A, Root systems of NIL-_RNR10__HJX74_-_DNR1__HJX74_, NIL-_RNR10__IRAT261_, NIL-_DNR1__IRAP9_, and
NIL-_RNR10__IRAT261_-_DNR1__IRAP9_ plants. Scale bar, 10 cm. B, The ratio [(LN-HN)/HN] of total length of visible roots. C, The absolute value of total length of visible roots. D, The ratio
[(LN-HN)/HN] of total area of visible roots. E, The absolute value of total area of visible roots. F, 15NO3− uptake rates. G, The ratio [(LN-HN)/HN] of root to shoot biomass ratio. H, The
absolute value of ratio of root to shoot biomass. B-H, Different letters denote significant differences (_P_ < 0.05) from a Duncan’s multiple range test. Exact _P_ values are listed in
Supplemental Information Table 11. Data are mean ± s.e.m. (_n_ = 3 biologically independent samples). Source data EXTENDED DATA FIG. 9 GENE STRUCTURE, ROOT SIZE AND ABOVE-GROUND PHENOTYPES
OF NIL-_DNR1__HJX74_, NIL-_DNR1__IRAP9_, AND NIL-_DNR1__IRAP9__/RNR10_. A, Gene structure of the _RNR10_ gene showing the location of the CRISPR/Cas9-generated 5-bp deletion in the
NIL-_DNR1__IRAP9__/rnr10_ mutant. B, C, The absolute value of total length of visible roots (B) and total area of visible roots (C). D-F, The number of tillers per plant (D), the number of
secondary branches per panicle (E) and the number of grains per panicle (F) of NIL-_DNR1__HJX74_, NIL-_DNR1__IRAP9_ and NIL-_DNR1__IRAP9__/rnr10_. B-F, Different letters denote significant
differences (_P_ < 0.05) from a Duncan’s multiple range test. Exact _P_ values are listed in Supplemental Information Table 11. B, C, Data are mean ± s.e.m. (_n_ = 3 biologically
independent samples). D-F, Data are mean ± s.e.m. (_n_ = 12 biologically independent samples). Source data EXTENDED DATA FIG. 10 EFFECTS ON DNR1 AND RNR10 ABUNDANCE BY EXTERNAL N, AND
_RNR10_ EXPRESSION PATTERN. A, B, Shoot mRNA and protein abundances of DNR1 (A) and RNR10 (B) in HJX74 grown in nutrient solutions with different N supply (0.15 N, 0.1875 mM NH4NO3; 0.3 N,
0.375 mM NH4NO3; 0.6 N, 0.75 mM NH4NO3; 1 N, 1.25 mM NH4NO3). Transcript abundance was measured relative to 0.15 N (set to 1). HSP82 severs as loading control. The experiments in A and B
were repeated independently at least three times with similar results. C, Expression profile of _RNR10_ in root, stem, leaf, node, and panicle tissues. _RNR10_ transcript abundance in root
was set to 1. A-C, Different letters denote significant differences (_P_ < 0.05) from a Duncan’s multiple range test. Exact _P_ values are listed in Supplemental Information Table 11.
Data are mean ± s.e.m. (_n_ = 3 biologically independent samples). Source data SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLE Supplementary Tables 1–11. SOURCE DATA SOURCE
DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Statistical source data. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5
Statistical source data. SOURCE DATA FIG. 6 Statistical source data. SOURCE DATA FIG. 7 Statistical source data. SOURCE DATA FIGS. 1, 3, 4, 5 AND 6 Unprocessed blots and gels. SOURCE DATA
EXTENDED DATA FIGS. 1, 3, 4, 5, 8, 9 AND 10 Statistical source data. SOURCE DATA EXTENDED DATA FIGS. 2, 4, 5, 7 AND 10 Unprocessed blots and gels. RIGHTS AND PERMISSIONS Springer Nature or
its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Huang, Y., Ji, Z., Tao, Y. _et al._ Improving rice nitrogen-use efficiency by modulating a novel monouniquitination machinery for optimal root plasticity response to nitrogen. _Nat. Plants_
9, 1902–1914 (2023). https://doi.org/10.1038/s41477-023-01533-7 Download citation * Received: 22 November 2022 * Accepted: 01 September 2023 * Published: 05 October 2023 * Issue Date:
November 2023 * DOI: https://doi.org/10.1038/s41477-023-01533-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
