Engineered lactobacilli display anti-biofilm and growth suppressing activities against Pseudomonas aeruginosa
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Biofilms are an emerging target for new therapeutics in the effort to address the continued increase in resistance and tolerance to traditional antimicrobials. In particular, the distinct
nature of the biofilm growth state often means that traditional antimcirobials, developed to combat planktonic cells, are ineffective. Biofilm treatments are designed to both reduce pathogen
load at an infection site and decrease the development of resistance by rendering the embedded organisms more susceptible to treatment at lower antimicrobial concentrations. In this work,
we developed a new antimicrobial treatment modality using engineered lactic acid bacteria (LAB). We first characterized the natural capacity of two lactobacilli, L. plantarum and L.
rhamnosus, to inhibit P. aeruginosa growth, biofilm formation, and biofilm viability, which we found to be dependent upon the low pH generated during culture of the LAB. We further
engineered these LAB to secrete enzymes known to degrade P. aeruginosa biofilms and show that our best performing engineered LAB, secreting a pathogen-derived enzyme (PelAh), degrades up to
85% of P. aeruginosa biofilm.
As an important virulence factor for pathogenic microbes, biofilms are associated with an expanding array of pathologies, including various airway, gastrointestinal, and ocular infections,
endocarditis, periodontitis, osteomyelitis, cystitis, and chronic wounds1,2,3,4,5,6,7. Biofilms represent a distinct growth state, morphologically distinguished by bacteria residing within a
self-produced matrix of extracellular polymeric substances (EPS), that may include proteins, extracellular DNA (eDNA), polysaccharides, and lipids8. Within the biofilm, isogenic cells
exhibit phenotypic diversity that is driven by the discrete microenvironments created by metabolite, ion, gas, and antimicrobial gradients within the biofilm. Biomedically, this phenotypic
diversity manifests as distinct tolerances or resistances to traditional antimicrobials as well as the host immune system9,10. Additionally, biofilms stabilize surface colonization and are
frequently less susceptible to traditional methods of surface decontamination, exacerbating the recalcitrance to treatment. Thus, clearance of mature biofilms is an essential component for
the successful resolution of numerous infections, especially those that are chronic or recurrent in nature.
Invasive burn wounds and chronic wounds, or wounds that fail to progress through the later stages of the normal healing process, are commonly contaminated or colonized by a multitude of
biofilm-forming organisms. Standard treatments for these wound types include nanocrystalline silver, silver sulphadiazine, iodine, or topical antibiotics. However, these treatments are often
ineffective at reducing wound infection, add unnecessary expense, and/or inhibit the healing process11,12,13,14. Further, extensive use of these treatments has bred a large population of
multi-drug-resistant microbes for which new treatments that target both planktonic and biofilm cells are necessary.
A widespread biofilm targeting strategy is the enzymatic degradation of biofilm polymer(s) to decrease surface adhesion and return the entrained bacteria to a more treatable
phenotype15,16,17,18,19,20,21. Rapid advancement in synthetic biology and probiotic therapies have led to interest in developing engineered bacteriotherapies or live biotherapeutic products.
These “smart”, bacteria-based therapeutic delivery vectors provide sustained delivery of the therapeutic and dynamically respond to environmental signals, while retaining their innate
probiotic qualities22,23,24,25. Recent examples of bacteriotherapies include the delivery of enzymes, antimicrobials, metabolites, or anti-inflammatory proteins to combat metabolic
deficiencies, tumors, inflammation, biofilms, and infections22,26,27,28,29,30,31,32. In this study, we construct and assess the utility of genetically engineered probiotic bacteria as
anti-biofilm and antimicrobial agents against the common wound pathogen Pseudomonas aeruginosa.
We selected lactic acid bacteria (LAB) as the chassis strains for the bacteriotherapy due to their broad-spectrum antimicrobial and wound healing capacities, genetic tractability, and
well-characterized expression systems for the production and secretion of heterologous proteins. Furthermore, Several LAB have been shown to impair the growth of drug-resistant P. aeruginosa
clinical isolates33. More specifically, Lactobacillus plantarum and Lactobacillus rhamnosus, the species used in this work, enhance the outcome of mouse P. aeruginosa infection models,
increase epithelial migration, and are equally as effective as current treatments when applied to human burn wounds34,35,36,37. We add to this body of evidence, showing that L. plantarum
WCFS1 and L. rhamnosus GG (LGG) are effective inhibitors of planktonic growth, biofilm formation, and the viability of biofilm-embedded cells (biofilm viability) of the burn wound isolate P.
aeruginosa PA14 (PA14). We further increase the usefulness of L. plantarum and LGG by engineering them to secrete enzymes known to degrade PA14 biofilms and demonstrate the efficacy of this
design for degradation of mature PA14 biofilms.
The feasibility of L. plantarum and LGG as therapeutic vectors was first analyzed by characterizing their innate capacity to inhibit PA14 growth using an agar-well diffusion assay and
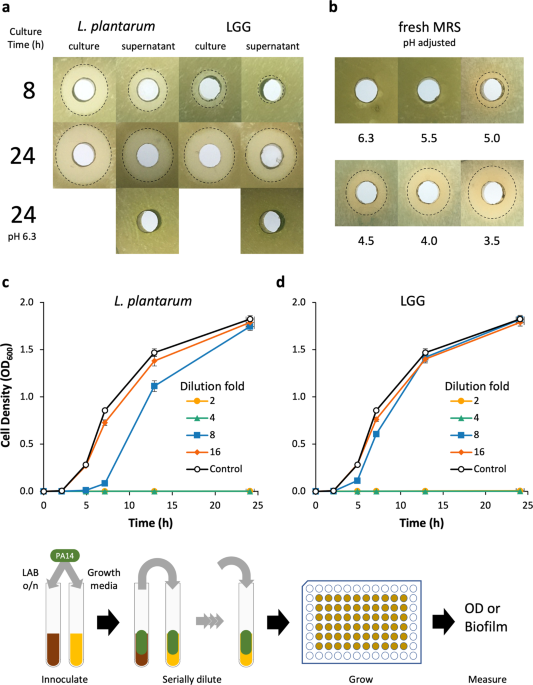
dilutions of LAB cultures in a modified MIC assay. The agar-well diffusion assay was used to determine the aeration and duration of LAB culture that maximally inhibited PA14 growth. When L.
plantarum cultures were grown aerobically in a test tube or flask, growth inhibition of PA14 moderately increased (Supplementary Fig. 1). However, culture aeration had no impact on LGG
growth inhibition of PA14. Early phase L. plantarum and LGG cultures (grown ≤4 h) and supernatants failed to inhibit PA14 growth, while late-stage (≥8 h) cultures and supernatants of both
organisms inhibited PA14 growth (Fig. 1a, Supplementary Fig. 1). The 24 h cultures of L. plantarum were marginally more inhibitory than those of LGG; yet the supernatants of both LAB
exhibited similar growth inhibition against PA14. Generally, we found that PA14 growth inhibition increased with LAB culture duration and cultures were more inhibitory than cell-free
supernatants. The pH of 24 h supernatants was 3.8–3.9 and when we adjusted their pH back to the starting pH of 6.3, we observed no growth inhibition. To determine if the inhibitory activity
was due to pH alone or a factor that was active at low pH, we adjusted the pH of fresh MRS down to that of spent media and evaluated its inhibitory activity against PA14. Decreasing the
medium pH increased growth inhibition and when adjusted to pH 3.5, the medium had similar inhibitory activity as that of a 24 h LAB culture of pH 3.8–3.9.
a Agar well diffusion assay of L. plantarum and LGG cultures and supernatants grown in MRS medium. Culture time was either 8 or 24 h. pH adjustment abrogates inhibitor activity of
supernatants. b Agar well diffusion assay of pH-adjusted fresh MRS medium. The pH of the acid-adjusted medium is located below the plate image to which it refers. Cultures of planktonic PA14
with c L. plantarum and d LGG supernatants show inhibition at low dilution factors. n = 9 from three separate experiments for all conditions. Error bars represent ±1 SEM. Workflow for c and
d is shown: The LAB supernatants were inoculated with PA14, which were serially diluted into fresh PA14 cultures. Each line represents a different dilution factor of the LAB supernatant.
We also used a modified minimum inhibitory concentration (MIC) assay to more quantitatively evaluate the inhibition of PA14 growth by L. plantarum and LGG supernatants over time and
determine the relative quantity of spent supernatant necessary for bioactivity. Supernatants from 24 h cultures of L. plantarum and LGG diluted up to 25% (i.e., 4× dilution) of the culture
volume completely inhibited PA14 growth (Fig. 1c, d). L. plantarum supernatant diluted 8× still retained some inhibitory activity, while 8× dilution of LGG supernatants had no inhibitory
activity relative to growth medium alone. A dilution of 16×, or greater, of either LAB culture supernatant failed to inhibit PA14 growth.
Having characterized L. plantarum and LGG inhibition of planktonic PA14 cells, we also analyzed the impact L. plantarum and LGG supernatants had on PA14 biofilm formation and biofilm
viability (i.e., viability of cells in the biofilm matrix). We used the modified MIC assay workflow to evaluate the inhibition of PA14 biofilm formation. The supernatants from L. plantarum
and LGG cultures inhibited PA14 biofilm formation in a concentration-dependent manner (Fig. 2a). Only at dilutions greater than 16× did biofilm form at detectable levels. The MRS media
control also inhibited PA14 biofilm formation, but only when undiluted or diluted 2-fold.
a Inhibition of PA14 biofilm formation by L. plantarum and LGG. Workflow for a was similar to that shown in Fig. 1. b Inhibition of viability of mature PA14 biofilms by L. plantarum and LGG
determined by measuring XTT reduction (absorbance at 475 nm). LAB cultures were diluted in PBS. c Inhibition of PA14 biofilm viability by L. plantarum and LGG compared to pH of culture
diluted in PBS or MRS adjusted to a specific pH. Workflow for b and c is shown. n = 9 from three separate experiments for all conditions. Error bars represent ±1 SEM.
LAB cell-free supernatants also inhibited the viability of PA14 cells embedded within biofilms, as assessed by XTT dye assay38 (Fig. 2b). L. plantarum and LGG supernatants diluted by 8× or
less were able to inhibit PA14 biofilm viability such that no viable cells could be detected relative to the control. When we plotted the viability against the pH of LAB culture dilutions,
we found that the transition to viable biofilms correlates with the increase in pH caused by dilution into PBS (Fig. 2c). Fresh MRS buffered to pH 5 and below completely inhibited biofilm
viability—a finding in agreement with our previous finding that this medium has an innate capacity to inhibit PA14 growth when adjusted to a lower pH (Fig. 1b). Interestingly, MRS adjusted
to pH 6.25 and 5.5 also inhibited PA14 biofilm viability more than the diluted LAB cultures of a similar pH, indicating innate anti-PA14 biofilm activity in the MRS. However, we found the
major driver of decreased biofilm viability to be low pH. Generally, PA14 biofilms treated with solutions with a pH ≤ 4.5 were nonviable, while solutions with a pH ≥ 5.2 were viable. We also
found that for a given pH, undiluted spent LAB supernatant is more inhibitory compared to that diluted in PBS.
P. aeruginosa biofilms are predominantly composed of an array of polysaccharides (Alg or alginate, Psl, Pel), and eDNA, and the specific composition is dependent upon the genetic background
and environment39. The strain P. aeruginosa PA14, a burn wound isolate, does not contain a functional operon to produce Psl, and does not produce alginate as a biofilm component40,41.
Instead, PA14 produces biofilms predominantly composed of Pel polysaccharide and eDNA42. P. aeruginosa biofilms containing these components were previously shown to be sensitive to enzymatic
degradation by solutions containing DNase, cellulase, or native glycoside hydrolases produced by P. aeruginosa to release biofilm cells and transition to planktonic growth16,43,44. We
constructed broad host range LAB expression vectors for secretion of the cellulase EngZ, a processive endoglucanse from the Gram-positive Clostridium cellulovorans; NucA, a thermostable
nuclease from Staphylococcus aureus, a pathogen found to infect similar sites as P. aeruginosa; and PelAh (the hydrolase domain of PelA) a native glycoside hydrolase from P. aeruginosa that
hydrolyzes the Pel polysaccharide.
We validated the expression and secretion of the biofilm degrading enzymes from L. plantarum and LGG using SDS–PAGE of induced culture supernatants and enzyme activity assays. LAB cultures
that contained NucA, EngZ, or PelAh expression vectors had protein bands and/or enzymatic activity in the filtered supernatants, which indicates successful secretion of the intended enzymes.
Specifically, the supernatants of NucA-expressing and PelAh-expressing LAB contained protein bands of the appropriate size (Fig. 3a) but we saw no visible band for EngZ. The larger
molecular weight of EngZ compared to NucA and PelAh puts it in a region where numerous other protein bands in the gel make it difficult to resolve individual proteins, so we also checked for
enzymatic activity. We confirmed that the supernatants of LAB secreting EngZ had CMCase activity (Fig. 3b), whereas the supernatants of LAB secreting NucA had DNase activity (Fig. 3c).
a Silver stained SDS–PAGE gel of supernatants from induced LAB cultures; Lanes 1−4 L. plantarum containing control (empty vector pTCC210, ─), NucA (N), EngZ (E), and PelAh (P) plasmids; Lane
5 Ladder; Lanes 6−9 LGG containing control (─), NucA (N), EngZ (E), and PelAh (P) plasmids. Arrows denote bands for NucA (~19 kDa) and PelAh (~31 kDa). b CMCase plate assay of
EngZ-expressing L. plantarum and LGG. c DNase plate assay of NucA expressing L. plantarum and LGG. d Degradation of PA14 biofilms with the cultures and supernatants of L. plantarum
containing Control, NucA, EngZ, and PelAh expression plasmids. L. plantarum culture pH following induction was 5.0 ± 0.0, 5.1 ± 0.1, 5.3 ± 0.1, and 5.3 ± 0.1 for Control, NucA, EngZ, and
PelAh, respectively. n = 12 from four separate experiments for each condition. * Denotes significant difference as determined by one-way ANOVA (α = 0.05) and Tukey HSD comparing samples of
same type (e.g. cultures or supernatants); p 0.05).
a Degradation of PA14 biofilms with supernatants of L. plantarum containing control, NucA, EngZ, and PelAh plasmids buffered to pH 4.0, pH 7.0, and pH 9.0. Workflow is the same given in Fig.
3. * Denotes significant difference as determined by one-way ANOVA (α = 0.05) and Tukey HSD comparing samples of same pH; p
