A regulatory network involving Rpo, Gac and Rsm for nitrogen-fixing biofilm formation by Pseudomonas stutzeri
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Biofilm and nitrogen fixation are two competitive strategies used by many plant-associated bacteria; however, the mechanisms underlying the formation of nitrogen-fixing biofilms remain
largely unknown. Here, we examined the roles of multiple signalling systems in the regulation of biofilm formation by root-associated diazotrophic P. stutzeri A1501. Physiological analysis,
construction of mutant strains and microscale thermophoresis experiments showed that RpoN is a regulatory hub coupling nitrogen fixation and biofilm formation by directly activating the
transcription of pslA, a major gene involved in the synthesis of the Psl exopolysaccharide component of the biofilm matrix and nifA, the transcriptional activator of nif gene expression.
Genetic complementation studies and determination of the copy number of transcripts by droplet digital PCR confirmed that the regulatory ncRNA RsmZ serves as a signal amplifier to trigger
biofilm formation by sequestering the translational repressor protein RsmA away from pslA and sadC mRNAs, the latter of which encodes a diguanylate cyclase that synthesises c-di-GMP.
Moreover, RpoS exerts a braking effect on biofilm formation by transcriptionally downregulating RsmZ expression, while RpoS expression is repressed posttranscriptionally by RsmA. These
findings provide mechanistic insights into how the Rpo/Gac/Rsm regulatory networks fine-tune nitrogen-fixing biofilm formation in response to the availability of nutrients.
The term ‘biofilm’ can be defined as a community of microbes adhering to biotic or abiotic surfaces that is protected from environmental stresses by a self-produced extracellular matrix1,2.
The extracellular matrix, often referred to as extracellular polymeric substances, is composed of exopolysaccharides, proteins and extracellular DNA present in various concentrations
depending on the bacterial species3,4. The biofilm state provides potential advantages over the planktonic state, including increased resistance to antimicrobial agents, protection from
environmental stresses, and improved adaptation to nutrient deprivation5. Numerous investigations in recent decades have demonstrated that bacterial biofilm formation is a sequential process
governed by complex regulatory networks that differ from one bacterial species to another1,6. It is now well accepted that microbial biofilms are the most widely distributed and predominant
mode of life on Earth, influencing our lives tremendously in both positive and negative ways6,7,8,9.
In general, as established in the model bacterium Pseudomonas aeruginosa, biofilm development usually begins with attachment to a surface, followed by microcolony formation and production of
the extracellular matrix responsible for the biofilm architecture10,11,12,13,14. Biofilm formation has been studied intensively in the genus Pseudomonas, with an emphasis on genetic
elements and molecular mechanisms; Gac/Rsm, c-di-GMP signalling and quorum-sensing (QS) pathways were reported as the main mechanisms leading to biofilm formation15,16. The Gac/Rsm
signalling pathway involves the GacS/GacA two-component regulatory system, the RNA-binding protein RmsA, and its cognate regulatory non-coding RNAs (ncRNAs)17,18. The GacS/GacA two-component
system activates the transcription of one or several genes for Rsm ncRNAs, which contain multiple GGA motifs in exposed stem loops of their predicted secondary structures19. The GGA motifs
allow Rsm ncRNAs to bind the RNA-binding proteins that act as global posttranscriptional repressors, e.g., CsrA (in Escherichia coli) and RsmA (in P. aeruginosa), controlling important
cellular processes, such as secondary metabolism (e.g., metabolism of pyocyanine or the QS signal N-butyryl-homoserine lactone in P. aeruginosa), motility, and biofilm formation17,20. RsmA
specifically recognises and binds to conserved GGA motifs in the 5′-untranslated region (5′-UTR) of target mRNAs, thereby preventing ribosome access and protein translation17,21. RsmA
controls biofilm formation through direct repression of various target genes, such as pslA (involved in the synthesis of the exopolysaccharide Psl) and sadC (involved in c-di-GMP
synthesis)22,23. As a key biofilm regulatory molecule, the second messenger c-di-GMP is synthesised by diguanylate cyclases (DGCs) that bear a GGDEF domain and is degraded by
phosphodiesterases (PDEs) that harbour EAL or HD-GYP domains. P. aeruginosa encodes several DGCs and PDEs; for example, WspR/SadC/RoeA (DGC) and RocR/BifA (PDE), are absent in the P.
stutzeri A1501 genome, except for SadC and BifA, which modulate the level of c-di-GMP and influence ‘surface-associated behaviours’ by controlling polysaccharide syntheses16,24,25,26,27. The
P. aeruginosa biofilm matrix contains several polysaccharide components, including alginate, pellicle (Pel) and Psl exopolysaccharides28. It has been shown that pslA is the first gene in
the psl operon, which comprises 15 cotranscribed genes that are involved in the synthesis of Psl29. Although current data relating to the roles of Psl are limited, Psl is a critical
component of the P. aeruginosa biofilm matrix, which functions as a scaffold, holding biofilm cells together to initiate biofilm development30. In addition, evidence demonstrates that
biofilm formation is controlled positively by RpoN but negatively by RpoS, suggesting global antagonism between RpoN and RpoS, although there are contradictory reports31,32,33,34,35.
Microbial biofilms are common on plant surfaces and have been associated with phytopathogenic infections and colonisation by nitrogen-fixing rhizobacteria36,37. Because of dynamically
fluctuating conditions in the rhizosphere, the ability of diazotrophic bacteria to form nitrogen-fixing biofilms may confer many ecological advantages and thereby facilitate their
physiological and metabolic adaptation to successfully survive in the rhizosphere, a nitrogen-limited environment. An early study compared biofilm formation by a nitrogen-fixing strain of
Klebsiella pneumoniae with that of two other members of Enterobacteriaceae, Salmonella enteritidis and E. coli, and showed that the nitrogen-fixing strain formed the densest and most
metabolically active biofilms38. Many nitrogen-fixing bacteria, such as those of the genera Rhizobium, Gluconacetobacter and Azospirillum, produce biofilms containing various
exopolysaccharides39,40,41,42. For instance, Sinorhizobium meliloti produces two symbiosis-promoting exopolysaccharides, succinoglycan and galactoglucan, which function in host specificity
and participate in early stages of a host plant infection, biofilm formation, and, most importantly, protection from environmental stresses43,44,45. Azospirillum cells are also capable of
forming biofilms on both abiotic surfaces and in association with host plants46. Previous studies have demonstrated that two response regulator proteins, TyrR and FlcA, were found to be
involved in the transcriptional regulation of biofilm formation by A. brasilense Sp7 via the production of capsular polysaccharides42,47.
The root-associated bacterium P. stutzeri A1501 is a rare example of a Pseudomonas strain with nitrogen fixation ability48. P. stutzeri A1501 can survive in the soil, colonise the root
surface, and endophytically invade the root tissues of host plants. During evolution, A1501 acquired a nitrogen fixation island with a nif-specific regulatory system from a diazotrophic
common ancestor48. Similar to many other Pseudomonas species, the nitrogen regulatory cascade in A1501 comprises the AmtB–GlnK–NtrBC-RpoN global nitrogen regulation proteins and a set of
regulatory ncRNAs that control the expression of nif genes and the consequent optimal nitrogen fixation in response to nutrient stress49,50,51,52. Comparative genomics analysis showed that
A1501 does not possess the well-known QS systems and does not produce alginate, but it contains genes possibly involved in cellulose biosynthesis and an incomplete psl operon4,48. It was
previously shown that a nonpolar mutation of the fleQ gene, encoding FleQ (the main regulator of flagella synthesis), impaired motility and root colonisation but enhanced biofilm formation
by P. stutzeri A150153. Additionally, Wang et al. investigated the effect of physiological conditions on the formation and architecture of nitrogen-fixing biofilms by P. stutzeri A150141.
However, the composition of the polysaccharide matrix remains unknown. To date, studies on biofilm formation by nitrogen-fixing rhizobacteria have focused on ecological, physiological and
architectural analyses. Despite its importance to microbial adaptation and survival, there is surprisingly little information about the genetics of nitrogen-fixing biofilm formation.
In this work, physiological conditions leading to nitrogen-fixing biofilm formation by the root-associated bacterium P. stutzeri A1501 were further investigated. We found that conditions
favouring biofilm formation differ between diazotrophic and non-diazotrophic P. stutzeri strains, although both strains contain the same set of regulatory genes involved in biofilm formation
in other systems. Thus, we systematically characterised genetic elements and molecular mechanisms involved in nitrogen-fixing biofilm formation. Genome-wide identification of putative genes
involved in biofilm formation and mutant construction led to the identification of a complex regulatory circuitry involving the alternative sigma factors RpoN and RpoS and the Gac/Rsm
regulators, and to the proposal of a model that integrates multiple levels of positive and negative regulation.
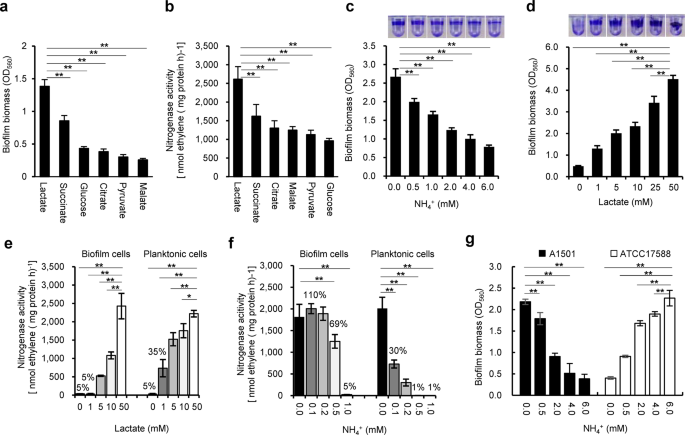
It was previously shown that when lactate was the sole carbon source, P. stutzeri A1501 tended to form biofilms rather than maintain a planktonic state under nitrogen-deficient conditions41.
To further examine this behaviour, the ability of A1501 to form mature biofilms and fix nitrogen was assayed 48 h after inoculation using carbon sources other than lactate and different
concentrations of NH4Cl. Among the carbon substrates tested at 50 mM, lactate was the best for both biofilm formation and nitrogen fixation (Fig. 1a, b). The ability of A1501 to form
biofilms gradually decreased with increasing NH4+ concentration (Fig. 1c) but was enhanced with increasing lactate concentration (Fig. 1d). In addition, ~35% of the maximum nitrogenase
activity was observed in planktonic growth at a low lactate concentration (1.0 mM), but very low nitrogenase activity was detected in biofilm growth (Fig. 1e), indicating that biofilm cells
were incapable of fixing nitrogen unless supplied with an adequately available carbon source. These results indicated that nitrogen-fixing biofilm growth requires a sufficient supply of
carbon sources, as both biofilm formation and nitrogen fixation are energetically expensive and highly regulated processes16,54.
a Effect of different carbon substrates on the mature biofilm biomass determined 48 h after inoculation by the crystal violet (CV) method: bacteria were inoculated in minimal medium K devoid
of a nitrogen source and containing the carbon substrates at 50 mM. b Effect of the different carbon substrates on the nitrogenase activity under the same conditions as in a. c Effect of
NH4+ concentration (0–6.0 mM) on biofilm biomass under the same conditions as in a. CV staining of the biofilm obtained is shown at the top. d Effect of lactate concentration (0–50 mM) on
biofilm biomass under the same conditions as in a. CV staining of the biofilm obtained is shown at the top. e Effect of lactate concentration (0–50 mM) on nitrogenase activity under
planktonic growth (with shaking at 220 r.p.m. and 0.5% oxygen) and biofilm growth conditions (without shaking in air). f Effect of NH4+ concentration on the nitrogenase activity of the
planktonic and biofilm cells in minimal medium K containing 50 mM lactate. g Effect of NH4+ concentration on biofilm formation by diazotrophic and non-diazotrophic P. stutzeri strains in
minimal medium K containing 50 mM lactate. Unlike A1501, the non-diazotrophic strain ATCC17588 favoured biofilm formation under nitrogen-sufficient conditions. Each error bar indicates the
standard deviation of three independent experiments. Asterisks indicate statistical significance by one-way ANOVA with LSD multiple-comparison test: *p
