Recent advances in single-cell engineered live biotherapeutic products research for skin repair and disease treatment
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The human microbiome has emerged as a key player in maintaining skin health, and dysbiosis has been linked to various skin disorders. Amidst growing concerns regarding the side
effects of antibiotic treatments, the potential of live biotherapeutic products (LBPs) in restoring a healthy microbiome has garnered significant attention. This review aims to evaluate the
current state of the art of the genetically or metabolically engineered LBPs, termed single-cell engineered LBPs (eLBPs), for skin repair and disease treatment. While some studies
demonstrate promising outcomes, the translation of eLBPs into clinical applications remains a significant hurdle. Substantial concerns arise regarding the practical implementation and
scalability of eLBPs, despite the evident potential they hold in targeting specific cells and delivering therapeutic agents. This review underscores the need for further research, robust
clinical trials, and the exploration of current advances in eLBP-based bioengineered bacterial chassis and new outlooks to substantiate the viability and effectiveness of eLBPs as a
transformative approach in skin repair and disease intervention. SIMILAR CONTENT BEING VIEWED BY OTHERS CELLULAR HUMAN TISSUE-ENGINEERED SKIN SUBSTITUTES INVESTIGATED FOR DEEP AND DIFFICULT
TO HEAL INJURIES Article Open access 17 June 2021 UTILIZATION OF EX VIVO TISSUE MODEL TO STUDY SKIN REGENERATION FOLLOWING MICRONEEDLE STIMULI Article Open access 27 October 2022 COMPARATIVE
ASSESSMENT OF COMMERCIALLY AVAILABLE WOUND GELS IN EX VIVO HUMAN SKIN REVEALS MAJOR DIFFERENCES IN IMMUNE RESPONSE-MODULATORY EFFECTS Article Open access 19 October 2022 INTRODUCTION The
human microbiome is a highly complex system that plays a crucial role in maintaining human health such as facilitating digestion, regulating the immune system, and synthesizing essential
vitamins and nutrients1,2. Dysbiosis, an imbalance in the microbiome in many body parts, has been linked to various pathological conditions, including skin disorders such as acne vulgaris,
psoriasis, and atopic dermatitis (AD)3,4,5. Antibiotic treatments for skin conditions have resulted in detrimental side effects such as antibiotic resistance, quorum cheater development,
commensals depletion, and recurrent infections6,7,8. Live biotherapeutic products (LBPs) offer a potential solution by reestablishing the equilibrium of a healthy microbiome and enhancing
overall health, including the skin9,10,11,12,13,14. Single-cell engineered LBPs (eLBPs) are genetically and/or metabolically engineered microorganisms that provide targeted therapeutics
directly at the disease site9. The microbial engineering strategy primarily uses bacteria as synthetic biology and bioengineered chassis has shown promise in treating a range of skin
conditions, including wound healing, skin regeneration, and cancer treatment15,16. Despite increasing interest in microbiome engineering, previous reviews have mostly focused on microbial
living materials, and only a few have considered the use of whole intact cells2,9,17,18,19. A wide array of LBPs and probiotics for the skin have also been thoroughly reviewed, but the
majority barely discussed eLBPs concerning skin therapy. The present review focuses on the current strategies and advancements in the development of eLBP-based treatments for skin repair and
disease through evidence obtained from clinical and preclinical testing over the past 10 years (2014-2023), providing a comprehensive overview of the current state of the art of eLBP
research for skin therapy and its future outlook. SKIN MICROBIOME The human skin is a complex system that comprises a rich variety of microorganisms known as the skin microbiome. In general,
skin microenvironments which comprised moist, dry, and sebaceous sites harbor distinct microbial ecosystems, yet exhibit similarities in terms of the species present. Sebaceous sites are
primarily dominated by _Cutibacterium_ and _Staphylococci_, while moist sites are predominantly inhabited by _Corynebacterium_ and _Staphylococcus_ species20. Dry skin areas contain
comparatively lower numbers of bacteria, but exhibit a more diverse composition, including a wide array of Proteobacteria, in addition to skin commensal species21. The resilience of the
deeper layers of the core skin microbiome is influenced by genetics, diet, and personal hygiene routines. Despite exposure to cleaning and cosmetic products, the surface microbial community
remains relatively stable22,23. Applying lotions on dry skin improves hydration and skin components but does not significantly change the composition of commensal microorganisms such as _C.
acnes_, _S. epidermidis_, and _S. hominis_24. Dysbiosis of the skin microbiome is topographically specific and is commonly associated with pathological conditions or diseases as shown in
Supplementary Table 13,25,26,27. This review will highlight instances where natural LBPs have been utilized as skin disease intervention and subsequently introduce the concept of eLBPs and
discuss recent preclinical and clinical trials involving eLBPs. Considering the current advancement of synthetic biology and biological engineering especially in bacterial strain
modification, this review delves deeper into the recent development of non-commensal bioengineered bacteria towards expanding the repertoire of bacterial chassis tailored for desired
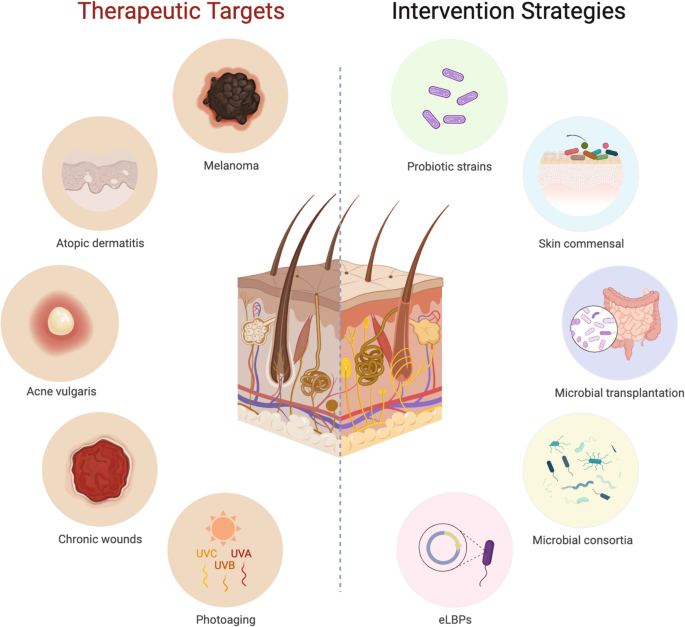
biological and biotherapeutic applications. SKIN DISEASE INTERVENTION USING LIVE BIOTHERAPEUTIC PRODUCTS One prevalent strategy to modulate the composition and/or function of the human
microbiome (Fig. 1) involves the use of probiotics28,29,30. There is a growing body of evidence to suggest that probiotics can effectively impact the composition and metabolic activities of
the human microbiome including the skin12,13,14,31. Conventional oral probiotic treatments have been proven to significantly reduce the severity of AD, particularly in adults, by promoting
immune response and gut impermeability32. Yet, evolution in current probiotics revealed that topically applied probiotics are equally effective in improving anti-oxidation and lowering
inflammation, apart from regulating other age-related conditions through the equilibration of commensal microbes and alteration of functional metabolisms33,34. Beneficial skin commensals
have a prospective biotherapeutic role, particularly for the repair and differentiation of the epidermal barrier35. For instance, certain strains of commensal _S. epidermidis_ can express
serine protease glutamyl endopeptidase and β-defensins, as well as activate Gamma delta T cells and upregulate Perforin-2, all of which inhibit the formation of pathogenic _S. aureus_
biofilms in AD36,37,38,39, and induce interleukin (IL)-8 and neutrophils to combat inflammation in acne40,41. Additionally, scientists discovered that _S. epidermidis_ (strain MO34 and
MO38), which produces 6-N-hydroxyaminopurine (6-HAP), a molecule that hinders DNA polymerase activity, holds promise for providing defense against neoplasia. This breakthrough has unveiled a
fresh perspective on the role of skin commensals in host protection against cancer42. In another case, the clinical trial reports of AD patients treated with the commensal _Roseomonas
mucosa_ showed better skin epithelial barrier function and decreased _S. aureus_ load due to glycerophospholipids synthesis, which activated tissue-repair pathways43,44. Skin commensals
offer the potential for a more robust skin microbiome engineering, such as skin microbiome transplantation (SMT) to treat dysbiosis. SMT involves transplanting healthy skin microbiomes to
the dysbiotic area45. A proof-of-concept study showed that unidirectional SMT, which transferred DNA markers partially from the forearm to the back of the same individual, was feasible46. As
a result, SMT is being proposed as a solution to address underarm odour by replacing odour-causing commensals with new commensals obtained from a non-odorous donour47. Notably, ongoing
research on SMT remains in its early phases, and there is yet to be an established, standardized procedure for its implementation. Contrary to SMT, fecal microbiome transplantation (FMT)
stands as a well-established procedure and has shown promise in treating skin diseases, particularly through its influence on the gut-skin axis48,49,50. A recent clinical efficacy report of
FMT on AD patients revealed a significant reduction in the average Scoring Atopic Dermatitis Score (SCORAD) from baseline, with a remarkable 75% decrease observed after just four
treatments50. Besides single-cell bacteriotherapy and microbiome transplantation, there is growing evidence that specific groups of microorganisms, when isolated and enriched, can manipulate
the physiological functions of the host. For instance, an 11-member commensal consortium extracted from the fecal matter of healthy donors has been shown to stimulate the production of
interferon-γ-producing CD8 T cells in the intestine which completely ablate and inhibit the metastasis of adenocarcinoma and melanoma cells51. Another _Lactobacilli_ consortium showed
promise in reducing inflammatory lesions by reducing the abundance of _Staphylococci_ and _C. acnes_ in a placebo-controlled study34. While microbial consortia may have greater impacts in
manipulating host physiological functions, single-cell LBPs are relatively simpler to monitor and exploit, therefore are more practical for treating cutaneous diseases. Examples of LBPs
formulated as oral and topical applications to treat skin conditions and diseases are presented in Supplementary Table 2. Single-cell eLBPs are a cutting-edge field of research that utilizes
genetically or metabolically engineered live microorganisms to perform specific functions, such as producing therapeutic compounds or targeting specific pathogen9. eLBPs have the potential
to revolutionize the way we approach skin repair and disease treatment, as they can be tailored to target specific sites, cells and pathways in the body17,18. There is limited information
available regarding the use of eLBPs for skin treatment, and most studies have only tested their efficacy using in vitro or in vivo models (Table 1). This review primarily focuses on two
leading areas of eLBP research: 1) cutaneous wound treatments, and 2) malignant melanoma therapeutics (Fig. 2). CUTANEOUS WOUND TREATMENTS In wound healing, the skin undergoes overlapping
phases of hemostasis, inflammation, proliferation, and remodeling. In the inflammation stage, immune cells gather at the site of injury due to distress signals, cytokines, and chemokines
produced by damaged cells15. Chemokines like CXCL12, have been shown to have beneficial effects in healing cutaneous wounds and bind to CXCR4 receptors on immune cells and keratinocytes. To
translate this theoretical framework, Ilya Pharma developed a first-in-class drug candidate, which is a recombinant CXCL12-expressing _Lactobacillus reuteri_ R2LC (ILP100)15,52. Experiments
on mice and minipigs in both healthy and hyperglycemic conditions showed faster wound healing due to increased CXCL12 availability and increased TGF-β expression in macrophages, therefore
hastening the formation of granulation tissue and thin epithelial layers. In the Phase 1 clinical trial, ILP100 shortened the time to initial healing among patients by an average of 6 days
and by 10 days at the highest dose53. It additionally elevated the density of CXCL12+ cells within the wounds and enhanced local blood perfusion at the wound site. The ILP100 has progressed
to its Phase 2 clinical trial (Identifier: NCT05608187) and is actively enrolling patients with diabetic foot ulcers. This trial spans 26 weeks and includes a long-term follow-up period of 5
years to assess both the safety and biological efficacy of ILP100 in promoting wound healing among subjects. On the contrary, Zhao et al.54 bolstered wound healing mechanisms by applying
CXCL12-expressing _Lactococcus lactis_ in tandem with yellow light-emitting diodes (LEDs). Prior research has demonstrated that LED light of varying wavelengths exerts distinct effects on
skin repair and regeneration. Specifically, yellow light within the range of 570–600 nm can stimulate collagen synthesis, resulting in skin tightening55. This synergy expedited wound
closure, facilitated tissue remodeling, spurred re-epithelialization and hair follicle regeneration, and mitigated over-inflammation. Additionally, it upregulated pivotal proteins within the
Wnt and Notch signaling pathways as well as curtailed inflammatory factors such as interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α). Remarkably, this combined treatment
effectively reduced skin pathogens _Ralstonia_ and _Acinetobacter_, substantially diminishing the risk of infection. In another instance, Li et al.56 devised an inventive approach by
combining CXCL12-expressing _L. lactis_ with a photosynthetic bacteria, _Synechococcus elongatus_ PCC7942, enclosed within a hydrogel matrix. The engineered _L. lactis_ feeds on sucrose
produced by _S. elongatus_ through photosynthesis, creating a synergistic effect that substantially accelerates the wound-healing process. Impressively, this topical hydrogel-encapsulated
microbial consortium led to faster wound closure in mice, reducing the wound area ratio to a mere 13.2% by the 14th day, as compared to control treatments. In addition to inducing
chemokines, another strategy employed to expedite wound healing involves augmenting the presence of growth factors, specifically vascular endothelial growth factor (VEGF). This biomolecule
is essential in the process of angiogenesis57. However, applying VEGF directly has not yielded conclusive benefits in clinical trials58. Lu et al.31 found that thermosensitive hydrogel
containing probiotic _L. lactis_ NZ9000 was able to improve the microenvironment of diabetic wounds and promote wound healing by regulating lactic acid levels. They later developed an
engineered _L. lactis_ carrying a VEGF-encoding gene and embedded it in a heparin-poloxamer hydrogel59. This approach enhanced the stability of VEGF in the oxidative environment of chronic
wounds and enabled the living system to produce and protect VEGF. As a result, it promoted the growth and movement of endothelial cells, and shifted M1 and M2 macrophages toward an
anti-inflammatory phenotype, leading to successful angiogenesis in diabetic wounds59. A prolonged or impaired wound healing may be exacerbated by the biofilm-forming pathogens infection like
_S. aureus_, which can be challenging to treat due to their reduced susceptibility to both the immune system and topical antimicrobial agents60,61. To address this issue, previous research
has explored magnetic hyperthermia as a potential therapeutic modality. Magnetic hyperthermia employed magnetic nanoparticles that absorbed energy from an alternating magnetic field, leading
to highly localized heat transmission that inactivated _S. aureus_ within cutaneous abscesses in murine models62. Building upon this innovative approach, Chen et al.63 modified a bacterial
system comprising the magneto-ovoid strain MO-1 (closely related to _Magnetococcus_ species) which contained magnetosomes and was coated with a polyclonal antibody. In a murine model
experiment, this system significantly improved wound healing by promoting the formation of MO-1-_S. aureus_ aggregates and eradicates the pathogen by hyperthermia63. MALIGNANT MELANOMA
THERAPEUTICS Melanoma is a type of skin cancer that is known for its complexity, aggressive nature, high metastasis rate, and frequent relapses. A commonly used method to treat melanoma is
phototreatment, where near-infrared (NIR) light and a photosensitizer (PTS) interact to destroy tumor spheroids64. However, due to the restricted penetration of NIR light and the low
specificity of PTS, melanoma tumors located deep within the skin and phototreatment margins often lead to quick relapse and metastasis. Therefore, Peng et al.16 developed a system of
transgenic _Escherichia coli_ to deliver recombinant human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. The nanosized outer membrane vesicles (OMVs) produced by the
engineered _E. coli_ which have been modified with αvβ3 integrin targeting ligand and indocyanine green (ICG) were able to penetrate the stratum corneum and specifically target melanoma
cells. When exposed to NIR irritation, these engineered OMVs exhibited photothermal and photodynamic responses against primary melanoma spheroids and activated TRAIL-induced apoptosis in
disseminated tumor cells. This resulted in the complete eradication of melanoma16. A different approach utilized to ablate melanoma involved the metabolic engineering of anaerobic oncolytic
bacteria, specifically _Clostridium butyricum_65. A study conducted nearly five decades ago involving _C. butyricum_ revealed its promising oncolytic activity against carcinomas,
particularly when used in conjunction with radio-frequency therapy to briefly raise tumor temperatures to the range of 42 to 44 °C66. Since then, numerous related studies have been carried
out, with recent investigations utilizing a metabolic labeling substrate to enhance its mechanistic action. This substrate was developed by coupling the metabolic substrate of _C.
butyricum_, D-alanine, with a photosensitizer known as TPApy, which exhibited aggregation-induced emission. This new metabolic substrate was incorporated into bacterial peptidoglycan to
create engineered _C. butyricum_. Once injected into melanoma, this bacterium colonized the hypoxia region and activated the intratumoral immune system thus eradicating the tumor masses.
Following this, the peripheral area, which has more oxygen content, caused the bacteria to die off while the photosensitizer on the bacteria exerted a photodynamic effect under light
irradiation, further removing any remaining melanoma65. Alternatively, chemotherapy drugs, such as doxorubicin (DOX), can induce immunogenic cell death in tumor cells but have severe
long-term side effects due to non-specific drug distribution67,68. Natural polysaccharide polymers, like glycogen (GLY), can be used as nanocarriers for drug delivery, providing targeted
delivery and controlled drug release, as well as reduced DOX toxicity69. On that account, a recent innovative system (GDOX@HSEc) has been developed using engineered _E. coli_ to secret
heparin sulfatase-1 (HSulf-1) within, and anchoring DOX-linked GLY nanoparticles (GDOX NPs) on the surface67. The GDOX@HSEc combination demonstrated a spatiotemporally intratumoral
distribution of therapeutic agents. In this context, HSulf-1 was upregulated to restrict angiogenesis and metastasis. Meanwhile, GDOX nanoparticles successfully infiltrated tumor cells,
inducing intracellular DNA damage70. Specific constituents of the skin commensals can trigger a robust T cell reaction when they colonize the skin, such as CD8+ T cells elicited by _S.
epidermidis_71. These cells are recognized for their role in fostering skin homeostasis and expediting wound closure72. In light of this, Chen et al73. engineered _S. epidermidis_ NIHLM087
to express various versions of ovalbumin (OVA)-derived MHC class I (OT1) or MHC class II-restricted (OT2) peptides. The most effective approach combined soluble OT1 peptide with cell
wall-anchored OT2 peptides, proving highly efficient in triggering immune responses against OVA-expressing B16F10 melanomas in mouse skin. Notably, the absence of either OT1 or OT2 from _S.
epidermidis_, the absence of OVA from B16F10 melanomas, or the depletion of CD8+ T lymphocytes thwarted the observed anticancer effects, underlining the therapeutic potential of a cellular
immune response targeting shared antigens between bacteria and cancer cells74. In previous applications, _Salmonella typhimurium_ served as the foundational bacterial chassis for targeting
tumors. To enhance safety and ameliorate potential toxicity concerns, a strategic attenuation approach was employed, involving the targeted deletion of specific genes, _purI_ and _msbB_,
resulting in the creation of a modified strain denoted as VNP2000975. While these genetic modifications effectively introduced adenine dependency and mitigated lipopolysaccharide-related
toxicity, the clinical utilization of VNP20009 revealed a dose-dependent elevation in proinflammatory cytokines. As a result, this immunological response precipitated adverse effects in
patients, encompassing thrombocytopenia, anemia, bacteremia, hyperbilirubinemia, diarrhea, vomiting, and nausea. Presently, efforts are directed towards reducing its toxicity and enhancing
tumor localization. In one example, an attenuated _S. typhimurium_ expressing recombinant interferon-gamma (IFN-γ) successfully invaded melanoma cells and induced cytotoxicity in
melanoma-bearing mice, while showing minimal toxicity to normal cells76. A modified strain expressing a radiation-sensitizing microRNA vector encoding the inhibin alpha gene (INHA) was also
able to exert cytotoxicity in combination with radiotherapy by enhancing ROS production77. Additionally, combining programmed death 1 (PD-1) knockdown with small interfering RNA (siRNA) and
pimozide drug therapy effectively suppressed melanoma through caspase 3-mediated apoptosis, as compared to bacteriotherapy alone78. STATUS QUO IN CLINICAL TRANSLATION OF SKIN ELBPS Despite
substantial progress in eLBP research, only a small fraction of eLBPs for skin therapy has progressed to clinical trials, and they are still in the early stages. Azitra Inc., a pioneering
skin eLBP company, is set to enter Phase 1b clinical trials in the first half of 2023 for their leading proprietary drug candidate, ATR-12. This innovative treatment incorporates engineered
_S. epidermidis_ to produce the serine protease inhibitor Lympho-epithelial Kazal-type related inhibitor (LEKTI) and is aimed at addressing Netherton syndrome (Identifier number pending). In
a similar vein, their product ATR-04 is expected to commence Phase 1b clinical trials in the first half of 2024, featuring lyophilized _S. epidermidis_ engineered to be auxotrophic to
D-alanine as the active ingredient. This product seeks to alleviate the severity of papulopustular rosacea associated with epidermal growth factor receptor inhibitor (EGFRI) therapy
(Identifier: NCT04731259). Additionally, Ilya Pharma is currently conducting a Phase 2 clinical trial using _L. reuteri_ as a chassis to secrete CXCL12, a short-lived human cytokine, to
improve wound healing. The progression of these eLBPs toward clinical trials is a crucial milestone in becoming a viable component of biomedical strategies for addressing human skin
diseases. Crown Aesthetics, a forefront aesthetic manufacturer, has made an impressive addition to its product lineup with the introduction of the BIOJUVE skin biome products, protected by a
patent under the Xycrobe® technology (WO2017147507A1). These innovative products primarily consist of engineered _C. acnes_ subsp. defendens strain XYCW42 to express human cytokines (such
as IL-10, IL-6, IL-7, and IL-8) through an inducible promoter located in front of the _ftsAZ_ operon, enabling precise control over bacterial cell division79. In their clinical study
involving 121 subjects, a specifically designed skincare regimen was rigorously followed including the XYCM42 Ferment Based Serum, Live XYCM42 Gel, and Prebiotic Activator. According to the
results, participants demonstrated enhanced skin health with sustained pH balance, optimal transepidermal water loss levels, increased skin moisture, reduced redness, improved skin
elasticity, and a noticeable reduction in surface sebum, with particularly pronounced effects in the forehead and nose areas79. This significant progress represents a noteworthy milestone
for eLBPs within the domain of skin health and beauty products, reinforcing their potential not only in skin repair and therapy but also in advancing towards clinical trials and subsequent
commercialization. CHALLENGES TOWARDS COMMERCIALIZATION OF ELBPS To date, the majority of the development of eLBPs has been rooted in probiotics sourced from the gut microbiome, encompassing
lactic acid bacteria and commensal strains80. This trend can be attributed to the promising potential of eLBPs as biotherapeutics, stemming from their superior safety profile when compared
to conventional chemical drugs, especially for extended periods of use80,81. eLBPs not only serve as effective therapeutic agents for chronic diseases by colonizing damaged cells but also
widen the possibilities for tailored tumor-targeted treatments. The use of eLBPs in treating skin diseases is still in its early stages and must address specific limitations, as detailed by
Charbonneau et al.18 and Pedrolli et al.82, concerning regulatory, application, manufacturing, safety, stability and efficacy. In both the United States and Europe, the development of eLBPs
necessitates the establishment of quality by ensuring safety, reliability, robustness, and batch consistency. Nevertheless, regulatory guidance under the purview of the US Food and Drug
Administration (FDA) for LBPs remains broad28, and there is currently no published directive specifying toxicology requirements tailored to LBPs18. Therefore, the development pathway for a
specific clinical candidate must involve discussions with the relevant regulatory authorities in the region or country where the product is intended for development and use. Biocontainment
stands as a significant hurdle in the progression of eLBP research. To address this challenge, emerging methods are being explored, with auxotrophies being one notable example. These
auxotrophies aim to curtail the proliferation of engineered strains outside their designated environments9. For instance, a biocontained _Saccharomyces boulardii_ strain, engineered with
_THI6_ and _BTS1_ gene knockouts, exhibited constrained growth in the absence of thiamine concentrations exceeding 1 ng/mL and experienced severe growth impairment at temperatures below 20
°C83. Apart from auxotrophies, biocontainment strategies encompass ‘deadman’ and ‘passcode’ kill switches in which the eLBPs are programmed to respond exclusively to precise environmental
signals. These switches can suppress the transcription of essential genes in the absence of specific triggers or initiate self-destruction of engineered strains through toxin production,
effectively preventing unintended cell proliferation84. Furthermore, beyond traditional exo- and endonucleases, CRISPR-associated nucleases (Cas) have been deployed in _E. coli_ to design
effective kill switches85. These kill switch gene circuits leverage Cas386 or Cas987, successfully achieving biocontainment with minimal escape frequencies. A notable advantage of Cas-based
kill switches lies in their use of guide RNAs (gRNAs) to selectively target specific DNA sequences or microorganism strains, enabling the precise elimination of the target strain from the
microbiome87,88. Such innovative biocontainment strategies not only address critical safety concerns but also pave the way for responsible and controlled advancements in the field of eLBPs.
The significance of robust biocontainment in eLBPs research cannot be overstated. Beyond averting unintended consequences and ecological disruptions, it highlights the ethical and safety
imperatives essential for the responsible development and utilization of these innovative therapeutic agents. Consequently, the ongoing exploration and enhancement of biocontainment
techniques bear profound implications for the progression and acceptance of eLBPs in clinical applications89. Apart from clearance or biocontainment, a major future challenge will be
addressing issues such as the potential spread of genetically modified LBPs into other bacterial or mammalian cell genomes, how to establish stable colonization of targeted sites,
interactions with commensal flora, and tissue targeting. These questions highlight the importance of investigating genetic stability in eLBPs under normal physiological conditions. Although
challenging, advancements in biological technologies will enhance the depth and breadth of disease prevention and treatment strategies through the use of newly available bacterial tools and
upgraded therapeutic approaches for eLBPs, ultimately alleviating safety concerns. On a more intricate note, biopharmaceutical manufacturers grapple with a host of challenges when
translating LBP concepts (both engineered and non-engineered products) into mass production and commercialization. These challenges span formulation and development, regulatory approval,
production and packaging, shipping and storage, patient application, and efficacy. Comprehensive discussions by Vargason and Anselmo et al.14 have shed light on the complex landscape
confronting biopharmaceutical manufacturers in this pursuit. To make eLBPs viable products that cater to patient needs, it is vital to address these multifaceted challenges. A core necessity
lies in foundational research on delivering foreign microbiota to the skin, encompassing queries about formulation design, the impact of common topical formulations on eLBP viability during
storage, and the quest for ideal formulations that balance manufacturing practicalities with patient usability. Moreover, analyzing clinical data reveals variations in LBP colonization on
the skin, prompting questions about the specific LBP and formulation parameters dictating microbial adherence, competition, growth, and long-term persistence90. These complexities
necessitate the development of delivery devices and formulations capable of sustaining LBP growth and persistence, even in challenging skin environments, aiming to minimize variability and
enhance the overall potential of LBPs in addressing chronic, recurrent, and difficult-to-treat skin conditions, while paving the way for successful mass production and commercialization. The
utilization of eLBPs also brings forth potential risks concerning human safety that demand careful consideration. One critical aspect entails the evaluation of virulence factors within
bioengineered strains, necessitating a meticulous screening process to identify genes capable of inducing pathogenicity91. Equally vital is the scrutiny of genes responsible for producing
enzymes involved in synthesizing toxic or allergenic compounds, or their precursors92. The introduction of foreign DNA may lead to the production of novel substances, either as proteins or
metabolic byproducts, which can exhibit harmful or allergenic properties. Assessing these newly expressed proteins involves examining amino acid sequences for potential homology and
evaluating their stability under various conditions, including heat, processing, and degradation. Despite these measures, precise tests for predicting allergenic responses in humans remain
elusive91. Moreover, the safety of eLBPs hinges not only on their composition but also on factors such as the mode of application and the genetic profile of the consumers. Certain vulnerable
subpopulations, such as immunocompromised individuals, infants, and the elderly, can exhibit heightened sensitivity, necessitating vigilant post-market surveillance of novel biotherapeutics
to avert potential adverse effects. The need for prolonged monitoring after introducing such biotherapeutic products to the market is evident, albeit complicated by technical challenges
arising from inconsistent intake. Similarly, in the case of engineered bacteria, vigilant and ongoing monitoring post-release into the market is advisable to preempt delayed adverse effects
and guarantee safe consumption93. Overall, the safety considerations surrounding the use of eLBPs accentuate the importance of comprehensive risk assessment and vigilant surveillance to
safeguard human health. As we explore the potential benefits of these innovative approaches, it is imperative to remain cognizant of the intricate web of factors that can impact their safety
profiles and to prioritize ongoing monitoring to ensure the long-term well-being of consumers. PROSPECTS FOR NON-COMMENSAL BACTERIAL CHASSIS AS NEW ELBPS Given the progress made in
microbial systems biology and synthetic biology94, leveraging non-pathogenic skin commensals seems promising for initiating the development of new bacterial chassis dedicated to advancing
skin treatment in the field of eLBP. Skin commensal bacterium, _S. epidermidis_ is an emerging bioengineered chassis as eLBP in skin disease treatment given the innate anti-staphylococcal
activity36,37 as well as the successful development of genetic tools for delivery therapeutic proteins such as recombinant filaggrin and LEKTI proteins important for treating AD and
microbial dysbiosis81,95,96. Nevertheless, genetic manipulation of beneficial strains derived from human-associated microbes presents notable challenges, primarily stemming from the
necessity to navigate innate restriction-modification systems. Consequently, these strains are often regarded as genetically intractable when compared to well-established models or known
bacterial systems2,97. The discovery of antimicrobial activities of a subset of commensal and non-commensal _Corynebacterium_ spp. suggests the possibility of utilizing these bacteria as
alternative bacterial chassis for biotherapeutic or biodiagnostic purposes, particularly in targeting skin pathogens like _S. aureus_98,99,100,101,102. A recent _Staph_-targeted study has
showcased the use of an engineered non-commensal _C. glutamicum_ which has been modified to respond to the quorum sensing (QS) molecule known as autoinducing peptide (AIP) produced by _S.
aureus_100. The expression of accessory regulatory proteins agrAC in tandem with a recombinant red fluorescent protein (RFP) conferred AIP-stimulated protein production in engineered _C.
glutamicum_ pResponse strain100. Given the inherent ability of _C. glutamicum_ to hinder the growth of _S. aureus_100, this model bacterium can be subjected to additional manipulation to
mount a response and combat _Staph_ infections, following a strategy akin to that demonstrated by Guan et al.96. In this approach, _S. epidermidis_ was engineered to produce lysostaphin
biomolecules, which effectively inhibit the growth of _S. aureus_96. Compared to _S. epidermidis_ and other closely related commensal _Corynebacterium_ spp., _C. glutamicum_ has been more
readily used in the food, feed and biopharmaceutical industry with prior approval from the FDA103. Metabolic engineering of AIP-responsive _C. glutamicum_ to produce important skin
biomolecules such as cobamide104 and arginine105 will aid in stimulating the production of filaggrin-derived natural moisturizing factors as well as reducing pathogenic _Staph_ growth in the
skin microbiome. With the expanded availability of CRISPR-Cas genome editing tools106,107, unwanted genes that may interfere with the host system can be accurately modified and modulated
hence providing a promising means for development of _C. glutamicum_ strains as eLBPs. Importantly, the increased interest in the employment of bioengineering and synthetic biology
approaches in developing new strains as eLBPs should bring about timely technological development in the race against infectious pathogens following the rules and regulations in countries
all over the world. FUTURE PERSPECTIVES In summary, recent advancements in the development of eLBPs and their clinical trials for tackling skin diseases have ignited significant enthusiasm
for delving into the therapeutic potential of these agents. All of these endeavours have been conducted with the highest level of diligence, ensuring the implementation of stringent safety
assessment protocols. The substantial body of evidence supporting the efficacy of non-engineered LBPs in maintaining a healthy skin microbiome and their potential as chassis organisms
underscores the promising prospects for utilizing eLBPs in skin disease intervention. Nonetheless, it is imperative to acknowledge the multifaceted challenges associated with advancing
eLBPs, encompassing issues related to manufacturing scalability, ensuring stability throughout the production process, and the implementation of robust biocontainment strategies. These
formidable challenges emphasize the need for comprehensive research and development efforts to effectively address them, facilitating a seamless transition into the clinical phase. The
significant progress achieved through the bioengineering of skin commensals, particularly _S. epidermidis_, and non-commensals like _L. lactis_ has established a promising foundation for
expanding eLBP development. This pioneering work serves as a blueprint for harnessing other non-pathogenic and non-commensal bacteria, precisely tailored to combat skin pathogens and
alleviate inflammatory responses. By leveraging microbial engineering and synthetic biology approaches, this emerging platform holds tremendous potential for revolutionizing the field of
skin disease intervention through the development of innovative eLBPs. REFERENCES * Mohajeri, M. H. et al. The role of the microbiome for human health: from basic science to clinical
applications. _Eur. J. Nutr._ 57, 1–14 (2018). Article PubMed PubMed Central Google Scholar * Aggarwal, N. et al. Microbiome and human health: current understanding, engineering and
enabling technologies. _Chem. Rev._ 123, 31–72 (2023). Article CAS PubMed Google Scholar * Fyhrquist, N. et al. Microbe-host interplay in atopic dermatitis and psoriasis. _Nat. Commun._
10, 1–15 (2019). Article Google Scholar * Chilicka, K., Dzieńdziora-Urbińska, I., Szyguła, R., Asanova, B. & Nowicka, D. Microbiome and probiotics in acne vulgaris—A narrative review.
_Life_ 12, 1–11 (2022). Article Google Scholar * Tett, A. et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. _npj Biofilms
Microbiomes_ 3, 14 (2017). Article PubMed PubMed Central Google Scholar * Mohsen, S., Dickinson, J. A. & Somayaji, R. Update on the adverse effects of antimicrobial therapies in
community practice. _Can. Fam. Phys._ 66, 651–659 (2020). Google Scholar * Patangia, D. V., Anthony Ryan, C., Dempsey, E., Paul Ross, R. & Stanton, C. Impact of antibiotics on the human
microbiome and consequences for host health. _Microbiologyopen_ 11, 1–23 (2022). Article Google Scholar * He, L. et al. Antibiotic treatment can exacerbate biofilm-associated infection by
promoting quorum cheater development. _npj Biofilms Microbiomes_ 9, 26 (2023). Article CAS PubMed PubMed Central Google Scholar * Rutter, J. W., Dekker, L., Owen, K. A. & Barnes,
C. P. Microbiome engineering: engineered live biotherapeutic products for treating human disease. _Front. Bioeng. Biotechnol_. 10, (2022). * Paquet, J. C. et al. Entering first-in-human
clinical study with a single-strain live biotherapeutic product: Input and feedback gained from the EMA and the FDA. _Front. Med._ 8, 1–9 (2021). Article Google Scholar * Heavey, M. K.,
Durmusoglu, D., Crook, N. & Anselmo, A. C. Discovery and delivery strategies for engineered live biotherapeutic products. _Trends Biotechnol._ 40, 354–369 (2022). Article CAS PubMed
Google Scholar * Ağagündüz, D. et al. Recent developments in the probiotics as live biotherapeutic products (LBPs) as modulators of gut brain axis related neurological conditions. _J.
Transl. Med._ 20, 1–26 (2022). Article Google Scholar * Schemczssen-Graeff, Z. & Pileggi, M. Probiotics and live biotherapeutic products aiming at cancer mitigation and patient
recover. _Front. Genet._ 13, 1–13 (2022). Article Google Scholar * Vargason, A. M. & Anselmo, A. C. Live biotherapeutic products and probiotics for the skin. _Adv. NanoBiomed Res._ 1,
2100118 (2021). Article CAS Google Scholar * Öhnstedt, E. et al. Accelerated wound healing in minipigs by on-site production and delivery of CXCL12 by transformed lactic acid bacteria.
_Pharmaceutics_ 14, 1–21 (2022). Article Google Scholar * Peng, L. H. et al. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy
against melanoma. _Sci. Adv._ 6, eaba2735 (2020). Article CAS PubMed PubMed Central Google Scholar * Riglar, D. T. & Silver, P. A. Engineering bacteria for diagnostic and
therapeutic applications. _Nat. Rev. Microbiol._ 16, 214–225 (2018). Article CAS PubMed Google Scholar * Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new
class of engineered live bacterial therapeutics to treat human diseases. _Nat. Commun._ 11, 1–11 (2020). Article Google Scholar * Omer, R. et al. Engineered bacteria-based living materials
for biotherapeutic applications. _Front. Bioeng. Biotechnol._ 10, 1–15 (2022). Article Google Scholar * Grice, E. A. et al. Topographical and temporal diversity of the human skin
microbiome. _Science (80-)_ 324, 1190–1192 (2009). Article CAS Google Scholar * Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. _Nat. Rev. Microbiol._ 16, 143–155
(2018). Article CAS PubMed Google Scholar * Bouslimani, A. et al. The impact of skin care products on skin chemistry and microbiome dynamics. _BMC Biol._ 17, 1–20 (2019). Article Google
Scholar * Kapono, C. A. et al. Creating a 3D microbial and chemical snapshot of a human habitat. _Sci. Rep._ 8, 1–12 (2018). Article CAS Google Scholar * Murphy, B. et al. Alteration of
barrier properties, stratum corneum ceramides and microbiome composition in response to lotion application on cosmetic dry skin. _Sci. Rep._ 12, 1–11 (2022). Article Google Scholar *
Khadka, V. D. et al. The skin microbiome of patients with atopic dermatitis normalizes gradually during treatment. _Front. Cell. Infect. Microbiol._ 11, 1–10 (2021). Article Google Scholar
* Olejniczak-Staruch, I. et al. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. _Int. J. Mol. Sci._ 22, 3998 (2021). Article CAS PubMed PubMed Central
Google Scholar * Brandwein, M., Steinberg, D. & Meshner, S. Microbial biofilms and the human skin microbiome. _npj Biofilms Microbiomes_ 2, 0–1 (2016). Article Google Scholar * FDA.
Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information. _Guid. Ind_. 1–20 (2016). * Cordaillat-Simmons, M., Rouanet, A. & Pot, B. Live
biotherapeutic products: the importance of a defined regulatory framework. _Exp. Mol. Med._ 52, 1397–1406 (2020). Article CAS PubMed PubMed Central Google Scholar * Martín, R. &
Langella, P. Emerging health concepts in the probiotics field: streamlining the definitions. _Front. Microbiol._ 10, 1047 (2019). Article PubMed PubMed Central Google Scholar * Fang, Z.
et al. _Bifidobacteria adolescentis_ regulated immune responses and gut microbial composition to alleviate DNFB-induced atopic dermatitis in mice. _Eur. J. Nutr._ 59, 3069–3081 (2020).
Article CAS PubMed Google Scholar * Umborowati, M. A. et al. The role of probiotics in the treatment of adult atopic dermatitis: a meta-analysis of randomized controlled trials. _J.
Heal. Popul. Nutr._ 41, 1–11 (2022). Google Scholar * Zhou, X. et al. Nicotinamide mononucleotide combined with Lactobacillus fermentum TKSN041 reduces the photoaging damage in murine skin
by activating AMPK signaling pathway. _Front. Pharmacol._ 12, 1–17 (2021). Google Scholar * Lebeer, S. et al. Selective targeting of skin pathobionts and inflammation with topically applied
lactobacilli. _Cell Rep. Med._ 3, 100521 (2022). Article CAS PubMed PubMed Central Google Scholar * Uberoi, A. et al. Commensal microbiota regulates skin barrier function and repair
via signaling through the aryl hydrocarbon receptor. _Cell Host Microbe_ 29, 1235–1248.e8 (2021). Article CAS PubMed PubMed Central Google Scholar * Iwase, T. et al. _Staphylococcus
epidermidis_ Esp inhibits _Staphylococcus aureus_ biofilm formation and nasal colonization. _Nature_ 465, 346–349 (2010). Article CAS PubMed Google Scholar * Pastar, I. et al.
_Staphylococcus epidermidis_ boosts innate immune response by activation of Gamma Delta T cells and induction of Perforin-2 in human skin. _Front. Immunol._ 11, 10–12 (2020). Article Google
Scholar * Dubin, G. et al. Molecular cloning and biochemical characterization of proteases from _Staphylococcus epidermidis_. _Biol. Chem._ 382, 1575–1582 (2001). Article CAS PubMed
Google Scholar * Marito, S., Keshari, S. & Huang, C. M. Peg-8 laurate fermentation of _Staphylococcus epidermidis_ reduces the required dose of clindamycin against _Cutibacterium
acnes_. _Int. J. Mol. Sci._ 21, 1–11 (2020). Article Google Scholar * Marito, S. et al. Electricity-producing _Staphylococcus epidermidis_ counteracts _Cutibacterium acnes_. _Sci. Rep._
11, 1–11 (2021). Article Google Scholar * Yang, A. J. et al. A microtube array membrane (MTAM) encapsulated live fermenting _Staphylococcus epidermidis_ as a skin probiotic patch against
_Cutibacterium acnes_. _Int. J. Mol. Sci._ 20, 14 (2019). Article Google Scholar * Nakatsuji, T. et al. A commensal strain of _Staphylococcus epidermidis_ protects against skin neoplasia.
_Sci. Adv._ 4, eaao4502 (2018). Article PubMed PubMed Central Google Scholar * Myles, I. A. et al. First-in-human topical microbiome transplantation with _Roseomonas mucosa_ for atopic
dermatitis. _JCI insight_ 3, e120608 (2018). Article PubMed PubMed Central Google Scholar * Myles, I. A. et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may
involve lipid-mediated TNF-related epithelial repair. _Sci. Transl. Med._ 12, 1–29 (2020). Article Google Scholar * Callewaert, C., Knödlseder, N., Karoglan, A., Güell, M. & Paetzold,
B. Skin microbiome transplantation and manipulation: current state of the art. _Comput. Struct. Biotechnol. J._ 19, 624–631 (2021). Article CAS PubMed PubMed Central Google Scholar *
Perin, B., Addetia, A. & Qin, X. Transfer of skin microbiota between two dissimilar autologous microenvironments: a pilot study. _PLoS One_ 14, 1–17 (2019). Article Google Scholar *
Callewaert, C., Lambert, J. & Van de Wiele, T. Towards a bacterial treatment for armpit malodour. _Exp. Dermatol._ 26, 388–391 (2017). Article PubMed Google Scholar * Kumar, P. et al.
The cure from within? a review of the microbiome and diet in melanoma. _Cancer Metastasis Rev._ 41, 261–280 (2022). Article PubMed PubMed Central Google Scholar * Pessemier et al.
Gut–skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. _Microorganisms_ 9, 1–33 (2021). Article Google Scholar * Mashiah, J. et al.
Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. _Immun. Inflamm. Dis._ 10, 1–10 (2022). Article Google Scholar * Tanoue,
T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. _Nature_ 565, 600–605 (2019). Article CAS PubMed Google Scholar * Vågesjö, E. et al. Accelerated
wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. _Proc. Natl Acad. Sci._ 115, 1895–1900 (2018). Article PubMed PubMed Central Google
Scholar * Öhnstedt, E. et al. Engineered bacteria to accelerate wound healing: an adaptive, randomised, double-blind, placebo-controlled, first-in-human phase 1 trial. _eClinicalMedicine_
60, 1–10 (2023). Article Google Scholar * Zhao, X. et al. Combination of an engineered _Lactococcus lactis_ expressing CXCL12 with light-emitting diode yellow light as a treatment for
scalded skin in mice. _Microb. Biotechnol._ 14, 2090–2100 (2021). Article CAS PubMed PubMed Central Google Scholar * Oh, P. S. & Jeong, H. J. Therapeutic application of light
emitting diode: Photo-oncomic approach. _J. Photochem. Photobiol. B Biol._ 192, 1–7 (2019). Article CAS Google Scholar * Li, L. et al. Hydrogel-encapsulated engineered microbial
consortium as a photoautotrophic “living material” for promoting skin wound healing. _ACS Appl. Mater. Interfaces_ 15, 6536–6547 (2023). Article CAS PubMed Google Scholar * Apte, R. S.,
Chen, D. S. & Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. _Cell_ 176, 1248–1264 (2019). Article CAS PubMed PubMed Central Google Scholar * Crawford,
Y. & Ferrara, N. VEGF inhibition: Insights from preclinical and clinical studies. _Cell Tissue Res._ 335, 261–269 (2009). Article CAS PubMed Google Scholar * Lu, Y. et al.
Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing. _Adv. Funct. Mater._ 31, 1–13 (2021). Article CAS Google Scholar * Del Giudice, P. Skin
infections caused by _Staphylococcus aureus_. _Acta Derm. Venereol._ 100, 208–215 (2020). Article Google Scholar * Wang, Z. F. et al. A phage lysin fused to a cell-penetrating peptide
kills intracellular methicillin-resistant _Staphylococcus aureus_ in keratinocytes and has potential as a treatment for skin infections in mice. _Appl. Environ. Microbiol._ 84, 1–13 (2018).
Article Google Scholar * Kim, M. H. et al. Magnetic nanoparticle targeted hyperthermia of cutaneous _Staphylococcus aureus_ infection. _Ann. Biomed. Eng._ 41, 598–609 (2013). Article
PubMed Google Scholar * Chen, C. et al. Killing of _Staphylococcus aureus_ via magnetic hyperthermia mediated by magnetotactic bacteria. _Appl. Environ. Microbiol._ 82, 2219–2226 (2016).
Article CAS PubMed PubMed Central Google Scholar * Honors, C. N., Kruger, C. A. & Abrahamse, H. Photodynamic therapy for metastatic melanoma treatment: a review. _Technol. Cancer
Res. Treat._ 17, 1–15 (2018). Google Scholar * Shi, L. et al. Living bacteria-based immuno-photodynamic therapy: metabolic labeling of _Clostridium butyricum_ for eradicating malignant
melanoma. _Adv. Sci._ 9, 1–8 (2022). Article Google Scholar * Dietzel, F., Gericke, D. & König, W. Tumor hyperthermia using high frequency for increase of oncolysis by _Clostridium
butyricum_ (M 55). _Strahlentherapie_ 152, 537–541 (1976). CAS PubMed Google Scholar * Yang, M. et al. Engineered bacteria combined with doxorubicin nanoparticles suppress angiogenesis
and metastasis in murine melanoma models. _Acta Biomater._ 158, 734–746 (2023). Article CAS PubMed Google Scholar * Zhao, N., C Woodle, M. & Mixson, A. J. Advances in delivery
systems for doxorubicin. _J. Nanomed. Nanotechnol._ 09, 678–687 (2018). Article Google Scholar * Yang, S. et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential
immune response with minimal doxorubicin-related toxicity. _Biomaterials_ 272, 120791 (2021). Article CAS PubMed Google Scholar * Huang, T. et al. Immunogenic cell death effects induced
by doxorubicin improved chemo-immunotherapy via restoration of granzyme B activity. _Nano Res_. (2023) * Naik, S. et al. Compartmentalized control of skin. _Science_ 337, 1115–1120 (2012).
Article CAS PubMed PubMed Central Google Scholar * Harrison, O. J. et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. _Science (80-)_ 363, eaat6280
(2019). Article CAS Google Scholar * Chen, Y. E. et al. Engineered skin bacteria induce antitumor T cell responses against melanoma. _Science_ 380, 203–210 (2023). Article CAS PubMed
Google Scholar * Kepp, O., Zitvogel, L. & Kroemer, G. Prevention and treatment of cancers by tumor antigen-expressing _Staphylococcus epidermidis_. _Oncoimmunology_ 12, 10–12 (2023).
Article Google Scholar * Toso, J. F. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. _J. Clin. Oncol._ 20,
142–152 (2002). Article PubMed Google Scholar * Yoon, W. et al. Application of genetically engineered _Salmonella typhimurium_ for interferon-gamma–induced therapy against melanoma.
_Eur. J. Cancer_ 70, 48–61 (2017). Article CAS PubMed Google Scholar * Yoon, W., Park, Y., Kim, S., Park, Y. & Kim, C. Y. Combined therapy with microRNA-expressing _Salmonella_ and
irradiation in melanoma. _Microorganisms_ 9, 2408 (2021). Article CAS PubMed PubMed Central Google Scholar * Zhao, T. et al. PD-1-siRNA delivered by attenuated _Salmonella_ enhances the
antimelanoma effect of pimozide. _Cell Death Dis._ 10, 164 (2019). Article PubMed PubMed Central Google Scholar * Rhee, M. S. et al. Characterization of a live _Cutibacterium acnes_
subspecies defendens strain XYCM42 and clinical assessment as a topical regimen for general skin health and cosmesis. _J. Cosmet. Dermatol._ 22, 1031–1045 (2023). Article PubMed Google
Scholar * Börner, R. A., Kandasamy, V., Axelsen, A. M., Nielsen, A. T. & Bosma, E. F. Genome editing of lactic acid bacteria: Opportunities for food, feed, pharma and biotech. _FEMS
Microbiol. Lett._ 366, 1–12 (2019). Article Google Scholar * Dodds, D. et al. Controlling the growth of the skin commensal _Staphylococcus epidermidis_ using d-alanine auxotrophy.
_mSphere_ 5, 1–13 (2020). Article Google Scholar * Pedrolli, D. B. et al. Engineering microbial living therapeutics: the synthetic biology toolbox. _Trends Biotechnol._ 37, 100–115 (2019).
Article CAS PubMed Google Scholar * Hedin, K. A., Kruse, V., Vazquez-Uribe, R. & Sommer, M. O. A. Biocontainment strategies for in vivo applications of _Saccharomyces boulardii_.
_Front. Bioeng. Biotechnol._ 11, 1–13 (2023). Article Google Scholar * Chan, C. T. Y., Lee, J. W., Cameron, D. E., Bashor, C. J. & Collins, J. J. ‘Deadman’ and ‘Passcode’ microbial
kill switches for bacterial containment. _Nat. Chem. Biol._ 12, 82–86 (2016). Article CAS PubMed Google Scholar * Pavão, G., Sfalcin, I. & Bonatto, D. Biocontainment techniques and
applications for yeast biotechnology. _Fermentation_ 9, 1–16 (2023). Article Google Scholar * Caliando, B. J. & Voigt, C. A. Targeted DNA degradation using a CRISPR device stably
carried in the host genome. _Nat. Commun._ 6, 6989 (2015). Article CAS PubMed Google Scholar * Rottinghaus, A. G., Ferreiro, A., Fishbein, S. R. S., Dantas, G. & Moon, T. S.
Genetically stable CRISPR-based kill switches for engineered microbes. _Nat. Commun._ 13, 1–17 (2022). Article Google Scholar * Rottinghaus, A. G., Vo, S. & Moon, T. S. Computational
design of CRISPR guide RNAs to enable strain-specific control of microbial consortia. _Proc. Natl Acad. Sci._ 120, 2017 (2023). Article Google Scholar * Liu, Y., Feng, J., Pan, H., Zhang,
X. & Zhang, Y. Genetically engineered bacterium: principles, practices, and prospects. _Front. Microbiol._ 13, 1–15 (2022). Google Scholar * Oh, J. et al. Biogeography and individuality
shape function in the human skin metagenome. _Nature_ 514, 59–64 (2014). Article CAS PubMed PubMed Central Google Scholar * Plavec, T. V. & Berlec, A. Safety aspects of genetically
modified lactic acid bacteria. _Microorganisms_ 8, 297 (2020). Article CAS PubMed PubMed Central Google Scholar * Mathipa, M. G. & Thantsha, M. S. Probiotic engineering: towards
development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. _Gut Pathog._ 9, 1–17 (2017). Article Google Scholar * de Simone,
C. The unregulated probiotic market. _Clin. Gastroenterol. Hepatol._ 17, 809–817 (2019). Article PubMed Google Scholar * Leggieri, P. A. et al. Integrating systems and synthetic biology
to understand and engineer microbiomes. _Annu. Rev. Biomed. Eng._ 23, 169–201 (2021). Article CAS PubMed PubMed Central Google Scholar * Koh, L. F., Ong, R. Y. & Common, J. E. Skin
microbiome of atopic dermatitis. _Allergol. Int._ 71, 31–39 (2022). Article CAS PubMed Google Scholar * Guan, C. et al. Engineering a “detect and destroy” skin probiotic to combat
methicillin-resistant _Staphylococcus aureus_. _PLoS One_ 17, e0276795 (2022). Article CAS PubMed PubMed Central Google Scholar * Costa, S. K., Donegan, N. P., Corvaglia, A. R.,
François, P. & Cheung, A. L. Bypassing the restriction system to improve transformation of _Staphylococcus epidermidis_. _J. Bacteriol._ 199, 1–14 (2017). Article Google Scholar *
Ramsey, M. M., Freire, M. O., Gabrilska, R. A., Rumbaugh, K. P. & Lemon, K. P. _Staphylococcus aureus_ shifts toward commensalism in response to _Corynebacterium_ species. _Front.
Microbiol._ 7, 1230 (2016). Article PubMed PubMed Central Google Scholar * Menberu, M. A. et al. _Corynebacterium accolens_ has antimicrobial activity against _Staphylococcus aureus_ and
methicillin-resistant _S. aureus_ pathogens isolated from the sinonasal niche of chronic rhinosinusitis patients. _Pathogens_ 10, 207 (2021). Article CAS PubMed PubMed Central Google
Scholar * Ruslan, U. S. et al. Development of _Corynebacterium_ glutamicum as _Staphylococcal_-targeting chassis via the construction of autoinducing peptide (AIP)-responsive expression
system. _Sains Malays._ 52, 431–439 (2023). Article CAS Google Scholar * Hardy, B. L. et al. Corynebacterium pseudodiphtheriticum exploits _Staphylococcus aureus_ virulence components in
a novel polymicrobial defense strategy. _MBio_ 10, e02491–18 (2019). Article CAS PubMed PubMed Central Google Scholar * Bomar, L., Brugger, S. D., Yost, B. H., Davies, S. S. &
Lemon, K. P. _Corynebacterium accolens_ releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. _MBio_ 7, e01725–15 (2016). Article CAS PubMed
PubMed Central Google Scholar * Wolf, S. et al. Advances in metabolic engineering of _Corynebacterium glutamicum_ to produce high-value active ingredients for food, feed, human health, and
well-being. _Essays Biochem._ 65, 197–212 (2021). Article CAS PubMed PubMed Central Google Scholar * Swaney, M. H., Sandstrom, S. & Kalan, L. R. Cobamide sharing is predicted in
the human skin microbiome. _mSystems_ 7, e0067722 (2022). Article PubMed Google Scholar * Leung, M. H. Y. et al. Skin microbiome differentiates into distinct cutotypes with unique
metabolic functions upon exposure to polycyclic aromatic hydrocarbons. _Microbiome_ 11, 1–14 (2023). Article Google Scholar * Kim, G. Y. et al. Synthetic biology tools for engineering
_Corynebacterium glutamicum_. _Comput. Struct. Biotechnol. J._ 21, 1955–1965 (2023). Article CAS PubMed PubMed Central Google Scholar * Wang, Q. et al. Advances and perspectives for
genome editing tools of _Corynebacterium glutamicum_. _Front. Microbiol._ 12, 654058 (2021). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work
was financially supported by Universiti Kebangsaan Malaysia through Dana Impak Perdana 2.0 grant (DIP-2022-004). The funder played no role in the study design, data collection, analysis and
interpretation of data, or the writing of this manuscript. Figures 1 and 2 were created with BioRender.com. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Systems Biology
(INBIOSIS), Universiti Kebangsaan Malaysia, 43600 UKM, Bangi, Selangor, Malaysia Muhamad Aidilfitri Mohamad Roslan, Mohd Norfikri Omar & Ahmad Bazli Ramzi * Department of Biological
Sciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 UKM, Bangi, Selangor, Malaysia Nur Azlina Mohd Sharif & Nurul Hanun Ahmad Raston *
Department of Fundamental Dental & Medical Sciences, Kulliyyah of Dentistry, International Islamic University Malaysia, 25200, Kuantan, Pahang, Malaysia Mohd Hafiz Arzmi * Melbourne
Dental School, The University of Melbourne, 3053, Melbourne, Victoria, Australia Mohd Hafiz Arzmi * UKM Medical Molecular Biology Institute (UMBI), Universiti Kebangsaan Malaysia, 56000,
Cheras, Kuala Lumpur, Malaysia Hui-Min Neoh Authors * Muhamad Aidilfitri Mohamad Roslan View author publications You can also search for this author inPubMed Google Scholar * Mohd Norfikri
Omar View author publications You can also search for this author inPubMed Google Scholar * Nur Azlina Mohd Sharif View author publications You can also search for this author inPubMed
Google Scholar * Nurul Hanun Ahmad Raston View author publications You can also search for this author inPubMed Google Scholar * Mohd Hafiz Arzmi View author publications You can also search
for this author inPubMed Google Scholar * Hui-Min Neoh View author publications You can also search for this author inPubMed Google Scholar * Ahmad Bazli Ramzi View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.A.M.R. and A.B.R. are responsible for the design, writing and organization of this review. M.N.O., N.A.M.S., N.H.A.R.,
M.H.A., and N.H.M. contributed to the content of this work. Figures were designed and created by M.A.M.R. CORRESPONDING AUTHOR Correspondence to Ahmad Bazli Ramzi. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIALS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Roslan, M.A.M., Omar, M.N., Sharif, N.A.M. _et al._ Recent advances in single-cell
engineered live biotherapeutic products research for skin repair and disease treatment. _npj Biofilms Microbiomes_ 9, 95 (2023). https://doi.org/10.1038/s41522-023-00463-8 Download citation
* Received: 14 July 2023 * Accepted: 20 November 2023 * Published: 08 December 2023 * DOI: https://doi.org/10.1038/s41522-023-00463-8 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
