Toxin expression during staphylococcus aureus infection imprints host immunity to inhibit vaccine efficacy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _Staphylococcus aureus_ infections are a major public health issue, and a vaccine is urgently needed. Despite a considerable promise in preclinical models, all vaccines tested thus
far have failed to protect humans against _S. aureus_. Unlike laboratory mice, humans are exposed to _S. aureus_ throughout life. In the current study, we hypothesized that prior exposure to
_S. aureus_ “imprints” the immune response to inhibit vaccine-mediated protection. We established a mouse model in which _S. aureus_ skin and soft tissue infection (SSTI) is followed by
vaccination and secondary SSTI. Unlike naïve mice, _S. aureus_-sensitized mice were incompletely protected against secondary SSTI by vaccination with the inactivated α-hemolysin (Hla) mutant
HlaH35L. Inhibition of protection was specific for the HlaH35L vaccine and required _hla_ expression during primary SSTI. Surprisingly, inhibition occurred at the level of vaccine-elicited
effector T cells; _hla_ expression during primary infection limited the expansion of T cells and dendritic cells and impaired vaccine-specific T cell responses. Importantly, the T
cell-stimulating adjuvant CAF01 rescued inhibition and restored vaccine-mediated protection. Together, these findings identify a potential mechanism for the failure of translation of
promising _S. aureus_ vaccines from mouse models to clinical practice and suggest a path forward to prevent these devastating infections. SIMILAR CONTENT BEING VIEWED BY OTHERS
PATHOBIONT-INDUCED SUPPRESSIVE IMMUNE IMPRINTS THWART T CELL VACCINE RESPONSES Article Open access 16 December 2024 ENHANCED _STAPHYLOCOCCUS AUREUS_ PROTECTION BY UNCOUPLING OF THE
Α-TOXIN-ADAM10 INTERACTION DURING MURINE NEONATAL VACCINATION Article Open access 08 October 2024 A SPA+LUKAB VACCINE TARGETING _STAPHYLOCOCCUS AUREUS_ EVASION FACTORS RESTRICTS INFECTION IN
TWO MINIPIG INFECTION MODELS Article Open access 20 April 2025 INTRODUCTION Skin and soft tissue infections (SSTI) are a major public health problem with a national economic burden of $15
billion/year1. _Staphylococcus aureus_ is the most common cause of SSTI, and recurrent infections are common2,3. Antibiotic treatment is the mainstay of therapy, but antibiotic-resistant _S.
aureus_ isolates have emerged4. Therefore, preventative strategies are urgently needed. Unfortunately, despite considerable effort, no vaccine is currently licensed to prevent _S. aureus_
infections5. It is not clear whether “natural” immune responses against _S. aureus_ protect against infection. The high rates of recurrent infection in individuals with SSTI—up to 50% within
a year—suggest that protection is incomplete at best3,6,7. Given the failures of all vaccine efforts to date8, it is imperative to determine the nature of protective immunity against _S.
aureus_. Paradoxically, most individuals have detectable levels of _S. aureus_-specific antibodies9 and memory T cells10,11, consistent with the notion that exposure to _S. aureus_ is
ubiquitous and persists throughout the lifespan. These findings support the hypothesis that natural exposure to _S. aureus_ “imprints” the immune system resulting in resistance to
vaccination. Indeed, Tsai et al. recently reported that _S. aureus_ infection may imprint non-protective antibody responses that interfere with protective antibodies elicited by
vaccination12. Natural exposure to pathogens is thought to be a challenge in vaccination against a variety of pathogens. For example, exposure early in life to influenza shapes the immune
system in such a way that subsequent responses to vaccination with a heterologous strain are inhibited at the expense of recall of responses against the original strain13. Francis called
these patterned responses “Original Antigenic Sin”14. Whether human exposure to _S. aureus_ contributes to the failure of vaccine efforts is not yet clear. It is also not clear what
immunologic mechanisms should be targeted with candidate vaccines. Although there is evidence in murine models that both cellular and humoral immune responses are important for protection
against _S. aureus_, human studies suggest that T cells are most important in determining susceptibility to infection15. We and others have identified immune responses against the
staphylococcal a-hemolysin (Hla) as protective against _S. aureus_ SSTI16,17. Although Hla-specific antibody responses are clearly important for protection in mouse models, there is also a
role for T cell responses18,19,20,21,22. We reported that concomitant _S. aureus_ SSTI interferes with vaccine-mediated protective antibody and T cell responses in a mouse model by the
preferable presentation of immunodominant, but not protective, epitopes in a manner dependent on the host major histocompatibility complex, providing one potential mechanism by which _S.
aureus_ may thwart vaccine-mediated protection18. In the current study, we sought to understand how prior exposure to _S. aureus_ could inhibit vaccine-mediated protection. Using a novel
mouse model of _S. aureus_ SSTI in which infection “imprints” host immune responses, we found that prior infection inhibits the ability of vaccination to elicit protection against secondary
infection. Importantly, this inhibition was dependent on _hla_ expression during primary infection and specific to Hla-targeted vaccination. Our findings demonstrate that toxin expression
during infection inhibits vaccine-specific T cell-mediated protection against secondary infection and can be overcome by targeting T cell responses using alternative vaccine adjuvants.
RESULTS _S. AUREUS_ SSTI INHIBITS THE EFFICACY OF HLAH35L VACCINATION AGAINST SECONDARY DERMONECROSIS Based on our findings that _saeRS_ expression during primary SSTI is necessary for
protection against recurrent SSTI23, we developed vaccines comprised of the _sae_-regulated antigens leukotoxin E (LukE), Panton-Valentine leucocidin S (LukS-PV), serine protease B (SplB), a
cysteine protease (SspB), and an α-hemolysin mutant (HlaH35L)18,24. We reported that the 4S (LukE, LukS-PV, SplB, and SspB), and 5S (4S + HlaH35L) vaccines protected against _S. aureus_
dermonecrosis18; 5S demonstrated superior protection compared with 4S or HlaH35L alone by virtue of eliciting complementary antibody and T cell responses. However, in contrast to
experimental infection in naïve mice, most humans have evidence of prior exposure to _S. aureus_. We, therefore, sought to develop a more clinically relevant mouse model in which to test
candidate vaccines. Moreover, we hypothesized that if exposure to _S. aureus_ inhibited the ability to successfully vaccinate against future infections, such inhibition would be circumvented
by the use of a multivalent vaccine. Specifically, we expected that exposure to _S. aureus_ would inhibit the efficacy of vaccination with HlaH35L and 4S, but that vaccination with 5S would
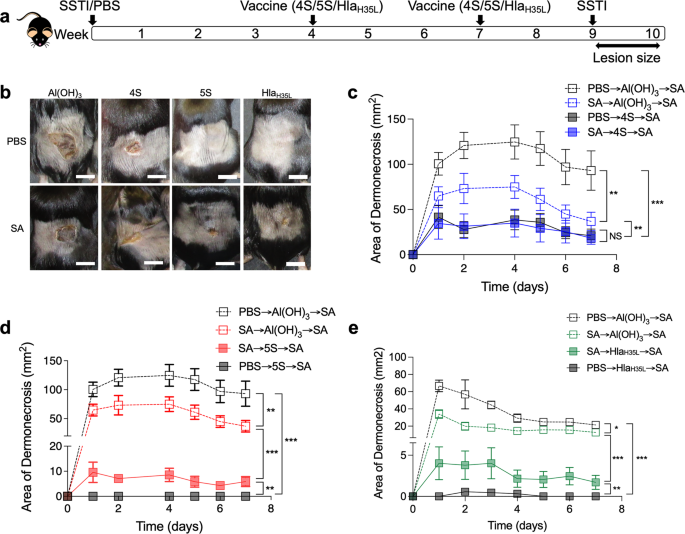
overcome this inhibition. To test this hypothesis, we established a mouse model in which mice were infected with _S. aureus_ (or sham infection with phosphate-buffered saline (PBS))
followed by vaccination with 4S, 5S, or HlaH35L (4 and 7 weeks post-SSTI) and secondary SSTI 2 weeks following vaccination (Fig. 1a). This approach allows for the clearance of bacteria from
the skin lesions prior to vaccination. Consistent with our previous report, vaccination with 4S, 5S, and HlaH35L protected naïve mice against SSTI (Fig. 1b–e). We were surprised to find no
impact of prior SSTI on the efficacy of 4S vaccination; there were no significant differences in lesion size or colony-forming units (CFUs) during secondary infection of mice that received
PBS or SSTI prior to 4S vaccination (Fig. 1b, c and Supplementary Fig. 1). In contrast, whereas naïve 5S-vaccinated mice had no dermonecrosis, SSTI-sensitized 5S-vaccinated mice unexpectedly
all had dermonecrotic lesions, although there were no significant differences in bacterial CFUs from the lesions (Fig. 1b, d and Supplementary Fig. 1). This suggested that the ability of
SSTI to inhibit vaccine efficacy was specific to Hla-mediated protection. Indeed, there were larger dermonecrotic lesions in SSTI-exposed HlaH35L-vaccinated mice during secondary SSTI,
compared with naïve HlaH35L-vaccinated mice (Fig. 1b, e). Together, these findings suggest that prior SSTI specifically inhibits Hla vaccine-mediated protection. _S. AUREUS_ SSTI INTERFERES
WITH HLAH35L VACCINE-ELICITED T CELL, BUT NO ANTIBODY RESPONSES Vaccination with HlaH35L elicits antibody-mediated protection against SSTI25,26. We, therefore, hypothesized that SSTI
inhibits the ability of vaccination to elicit Hla-specific antibodies. To test this, mice were infected with _S. aureus_ SSTI or PBS, followed by vaccination with HlaH35L or Al(OH)3 alone
(Fig. 2a). Anti-Hla antibody levels were quantified by enzyme-linked immunosorbent assay (ELISA) 2 weeks following vaccination. Surprisingly, there were no significant differences in
Hla-specific IgG levels among vaccinated mice, regardless of whether they had previously been infected with SSTI (Fig. 2b). This suggested that SSTI did not inhibit Hla vaccine-specific
antibody responses. Th1 and Th17-polarized immune responses also contribute to protection in mouse models of _S. aureus_ infection17,21,22,27. Therefore, we quantified antigen-specific
effector T cell responses by interleukin-17A (IL-17A) and interferon-γ (IFNγ) ELISpot in splenocytes following vaccination. Surprisingly, mice that received HlaH35L vaccination following
SSTI had fewer Hla-specific IL-17A staining cells, compared with mice that received PBS prior to vaccination (Fig. 2c). Similarly, SSTI-primed Hla-vaccinated mice had a trend toward fewer
Hla-specific IFNγ staining cells, compared with sham-infected Hla-vaccinated mice, although the differences were not significant (Fig. 2d). These findings suggested that exposure to _S.
aureus_ during SSTI interfered with HlaH35L vaccination through inhibition of vaccine-specific T cell responses, but not antibody responses. We next sought to determine whether SSTI elicited
antibodies that interfered with the ability to respond to vaccination. To test this, serum from convalescent C57BL/6 mice (following _S. aureus_ SSTI) was transferred to naïve mice 1 day
prior to HlaH35L vaccination, followed 3 weeks later by secondary SSTI (Supplementary Fig. 2A). Passive transfer of serum from _S. aureus_-infected mice did not interfere with the efficacy
of HlaH35L vaccination; lesion sizes in mice that received serum from _S. aureus_ infected mice prior to vaccination were similar to those received serum from naïve mice (Supplementary Fig.
2B, C). We next tested whether SSTI-elicited T cells could interfere with the ability to respond to vaccination by transfer of T cells from convalescent C57BL/6 mice to naïve mice 1-day
prior HlaH35L vaccination, followed by secondary _S. aureus_ SSTI (Supplementary Fig. 2D). As we observed with serum, transfer of T cells from _S. aureus_-infected mice did not interfere
with the efficacy of HlaH35L vaccination; lesion sizes in mice that received T cells from _S. aureus_ infected mice prior to vaccination were similar to those that received serum from naïve
mice (Supplementary Fig. 2E, F). These results suggest that infection-elicited antibodies or T cells do not mediate the inhibition of vaccine efficacy. BACTERIAL PERSISTENCE DURING PRIMARY
SSTI IS REQUIRED FOR THE INHIBITION OF VACCINE EFFICACY We next tested whether bacterial persistence during primary infection was necessary to inhibit HlaH35L vaccine efficacy. We reported
that vancomycin treatment enhanced bacterial clearance and resolved skin lesions28. Following primary _S. aureus_ SSTI, mice were treated with vancomycin (or PBS) every day for 7 days. Mice
were vaccinated with HlaH35L followed by secondary SSTI (Fig. 3a). Mice that were treated with vancomycin following primary SSTI had smaller lesions (Fig. 3b–d) and fewer bacteria in the
skin lesions (Fig. 3e) following vaccination, compared with untreated previously infected vaccinated mice. Therefore, treatment of primary SSTI with vancomycin restored HlaH35L vaccine
efficacy. We next tested whether vancomycin treatment would rescue vaccine-specific T cell and antibody responses. Consistent with our previous findings, anti-Hla antibody levels were not
different among vaccinated mice, regardless of prior SSTI or vancomycin treatment (Fig. 3f). However, Hla-vaccine-specific IL-17A and IFNγ T cell responses were rescued in mice that received
treatment of primary SSTI with vancomycin prior to vaccination; T cell responses were similar to those of naïve vaccinated mice and increased compared with infected untreated mice (Fig. 3g,
h). Together, these findings suggest that bacterial persistence during primary SSTI is necessary to inhibit vaccine efficacy and vaccine-specific T-cell responses. _HLA_ EXPRESSION DURING
PRIMARY SSTI INTERFERES WITH HLAH35L VACCINE-SPECIFIC T-CELL RESPONSES AND PROTECTIVE EFFICACY The requirement for bacterial persistence suggested that expression of a bacterial
protein-mediated vaccine inhibition. Lee et al. reported that _hla_ expression limits the expansion of local and systemic T cell populations during SSTI29. Our findings suggested that
inhibition of vaccine efficacy was specific for T cells; we, therefore, hypothesized that Hla inhibited vaccine-elicted T cell responses. We adapted our model by performing primary SSTI with
WT or _Δhla_, followed by vaccination with HlaH35L and secondary SSTI with WT _S. aureus_ (Fig. 4a). Consistent with our hypothesis, primary SSTI with _Δhla_ rendered mice strongly
protected by vaccination against dermonecrosis, compared with those that received primary SSTI with WT _S. aureus_ (Fig. 4b–d). The reduction in lesion size was accompanied by enhanced
bacterial clearance from the skin lesions 7 days after infection (Fig. 4e). Therefore, _hla_ expression during primary SSTI inhibited HlaH35L vaccine efficacy. We next tested whether _hla_
expression during primary SSTI inhibits vaccine-specific antibody and T-cell responses. Consistent with our previous findings, anti-Hla IgG levels were not different among vaccinated mice,
regardless of whether primary SSTI was performed with WT or _Δhla_ (Fig. 4f). However, there were more Hla-vaccine-specific IL-17A staining cells in mice that received primary SSTI with
_Δhla_ prior to vaccination, compared with those that received WT _S. aureus_ prior to vaccination (Fig. 4g). Similarly, there was a trend toward stronger Hla-specific IFNγ responses in mice
that were infected with _Δhla_ prior to vaccination, but the differences were not significant (Fig. 4h). Together, these findings demonstrate that _hla_ expression during primary SSTI
inhibits vaccine efficacy and suggests that inhibition is mediated by impaired vaccine-specific T-cell responses. _HLA_ EXPRESSION LIMITS THE EXPANSION OF T CELLS AND DCS Our findings and
those of Lee et al.29 suggested that _hla_ expression during SSTI mediates inhibition of vaccine efficacy by limiting the expansion of potentially vaccine-responsive T cells. We, therefore,
quantified T cell and dendritic cell (DC) populations by flow cytometry in draining and distal LNs 1 or 4 weeks following SSTI with WT _S. aureus_ or an isogenic _hla_ deletion mutant
(_Δhla_) (gating strategy, Supplementary Fig. 3). Consistent with their findings and a role for Hla in limiting the expansion of immune cells, we found that the total number of CD3+, CD4+,
CD8+, γδ T cells, and CD11c+ DCs 1 week after infection were higher in draining LNs in the mice infected with _Δhla_, compared with WT (Fig. 5a–e). However, these differences were limited to
locally draining LN, because there were no significant differences in these cell populations in distal LNs (Fig. 5f–j). Importantly, the differences in APC and T cell populations in
draining LNs persisted for at least 4 weeks after infection (i.e., the time of vaccination) (Fig. 5a–e), but there remained no differences in distal LNs (Fig. 5f–j). To better understand the
cytokine profiles of the impacted T cells, we quantified IL-17A+ and IFNγ+ CD4+, CD8+, and γδ T cells after 1 week in dLNs. We found more IL-17A+ and IFN-γ+ CD4+ T cells after 1 week in
dLNs in the mice group that were infected with _Δhla_, compared with WT (Supplementary Fig. 4A). There were also higher numbers of IFNγ+ CD8+ and γδ T cells following infection with _Δhla_,
compared with WT, but no differences in IL-17A+ CD8+ or γδ T cells (Supplementary Fig. 4B, C). To determine whether the preferential expansion of immunosuppressive regulatory T cells (Tregs)
might contribute to vaccine resistance, we quantified Foxp3+ CD4+ T cells after 1 and 4 weeks in dLNs and distal LNs. However, as we observed with other T cell subsets, there were higher
numbers of Foxp3+ CD4+ T cells after 1 and 4 weeks in dLNs in the mice group that were infected with _Δhla_, compared with WT (Supplementary Fig. 4D). There were also no significant
differences in the numbers of Foxp3+ CD4+ T cells in distal LNs, regardless of the infecting isolate (Supplementary Fig. 4E). To confirm that Hla specifically mediated impairment of T cell
expansion, we next transferred Hla-specific antiserum to naïve mice 1 day before SSTI with WT _S. aureus_ followed by quantification of T cell populations 1 week after infection
(Supplementary Fig. 5A). In support of a role for Hla in impairing expansion of T cell populations, there were higher numbers of CD3+, CD4+, CD8+, and γδ T cells in dLNs of mice that
received Hla-specific antiserum prior to infection, compared with those that received naïve serum (Supplementary Fig. 5B–E). Consistent with our results with WT vs. _Δhla_, there were more
IL-17+ CD4+ T cells in mice that received Hla-specific antiserum prior to infection, compared with those that received naïve serum (Supplementary Fig. 6A), but there were no significant
differences in other IL-17+ or IFNγ+ T cell populations (Supplementary Fig. 6B, C). These results confirmed that antibody-mediated neutralization of Hla rescued T cell expansion during SSTI.
Finally, to confirm that Hla interacted with its cellular receptor ADAM10 (A Disintegrin and metalloproteinase domain-containing protein 10)30,31,32 to drive the toxin-mediated impaired
expansion of T cells, mice received the ADAM10 inhibitor (GI254023X) 1 h before SSTI (Supplementary Fig. 5A). Consistent with our previous findings, we found higher numbers of CD3+, CD4+,
CD8+, and γδ T cells in dLNs 1 week after infection in mice that received ADAM10i prior to infection, compared with those that received vehicle (DMSO) alone (Supplementary Fig. 5F–I).
However, there were no significant differences in IL-17+ or IFNγ+ T cell populations among these groups (Supplementary Fig. 6D–F). Taken together, these findings demonstrate that expression
of _hla_ during primary SSTI limits the expansion of T cells and DCs, suggesting a potential mechanism by which infection impairs the ability to respond to subsequent vaccination. T CELL
RESPONSES CONTRIBUTE TO HLAH35L VACCINE-MEDIATED PROTECTION We and others have reported that HlaH35L elicits antibody-mediated protection18,26. However, our results suggested that _hla_
expression during primary SSTI mediates vaccine resistance by inhibiting vaccine-specific IL-17A and IFNγ T cell responses. To confirm the importance of IL-17A and IFNγ in mediating
vaccine-elicited protection against dermonecrosis, vaccinated mice were treated with IL-17A and IFNγ neutralizing antibodies prior to secondary SSTI (Fig. 6a). Surprisingly, there were no
significant differences in lesion size or bacterial clearance from the skin lesions between sham-vaccinated mice that received isotype control antibodies or αIL-17A/IFNγ antibodies (Fig.
6b–e). However, neutralization of IL-17A and IFNγ in Hla-vaccinated mice resulted in larger dermonecrotic skin lesions, compared with vaccinated mice that received isotype control antibodies
(Fig. 6b–d). Similarly, neutralization of IL-17A and IFNγ resulted in decreased bacterial clearance from the skin lesions (Fig. 6e). Therefore, IL-17A and IFNγ were important in mediated
vaccine-elicited protection against dermonecrosis and complemented antibody-mediated protection. THE T CELL-STIMULATING ADJUVANT CAF01 RESTORES HLAH35L VACCINE EFFICACY AND T CELL RESPONSES
Taken together, our findings suggested that _hla_ expression during primary SSTI patterns immune responses, thereby inhibiting vaccine responsiveness by impairing the ability to generate
protective vaccine-specific T-cell responses. Because Al(OH)3 elicits relatively weak T cell responses33, we hypothesized that the use of a potent T cell-stimulating adjuvant would
circumvent this inhibition. To test this, we used Cationic Adjuvant Formulation (CAF01) as an adjuvant because it potently elicits Th1/Th17 responses34. The CAF platform consists of the
quaternary ammonium surfactant _N,N-_dimethyl_-N,N-_dioctadecylammonium (DDA) formulated into liposomes and TBD is inserted into DDA bilayers. We modified our model by vaccinating previously
infected mice with Al(OH)3 or CAF01-adjuvanted HlaH35L, followed by secondary SSTI (Fig. 7a). In naïve mice, there were no significant differences in protection elicited by HlaH35L
vaccination using Al(OH)3 or CAF01, as assessed by lesion size (Fig. 7b–d) or bacterial clearance from the skin lesions on day 7 (Fig. 7e). However, consistent with our hypothesis,
vaccination with CAF01/HlaH35L resulted in superior protection against dermonecrosis in mice that were previously infected, compared with Al(OH)3/HlaH35L (Fig. 7b–d). Protection against
dermonecrosis was accompanied by enhanced bacterial clearance from the skin lesions in previously infected CAF01/HlaH35L vaccinated mice, compared with Al(OH)3/HlaH35L vaccinated mice (Fig.
7e). Interestingly, CAF01 alone rescued protection in previously infected mice, albeit not as strongly as CAF01/HlaH35L (Fig. 7b–e). This argues that a T cell-polarized adjuvant may be
beneficial even in the absence of Hla-specific responses. Next, we tested whether the superior protection elicited using CAF01 as an adjuvant was accompanied by stronger vaccine-specific
antibody or T-cell responses. Consistent with our previous findings, there were no significant differences in anti-Hla IgG levels following vaccination, regardless of adjuvant or whether
mice had been previously infected prior to vaccination (Fig. 7f). However, consistent with CAF01 eliciting stronger T cell responses, vaccination with of naïve mice with CAF01/HlaH35L
elicited more Hla-specific IL-17A and IFNγ staining cells, compared with Al(OH)3/HlaH35L (Fig. 7g, h). Importantly, consistent with the superior protection observed, vaccination of
previously infected mice with CAF01/HlaH35L elicited dramatically more Hla-specific IL-17A and IFNγ staining cells than did vaccination with Al(OH)3/HlaH35L (Fig. 7g, h). Taken together,
these findings demonstrate that infection-elicited inhibition of vaccine efficacy and vaccine-specific T cell responses can be circumvented by the use of the potent T cell-stimulating
adjuvant CAF01. DISCUSSION Despite a recognition of the importance of _S. aureus_ infections and considerable effort, there remains no licensed vaccine. A potential reason for the failure to
translate vaccines that successfully protect in experimental models to clinical practice may be the immune history of the vaccine recipient; vaccines are tested in naïve mice before
application in humans who invariably have evidence of immune responses against _S. aureus_. In this study, we established a tractable mouse model in which mice are infected with _S. aureus_
prior to vaccination. Unlike naïve mice, _S. aureus_-sensitized (previously infected) mice are incompletely protected against secondary SSTI following vaccination with the inactivated Hla
mutant HlaH35L. Inhibition of protection was specific for the HlaH35L vaccine and required _hla_ expression during primary SSTI. Surprisingly, inhibition occurred at the level of
vaccine-elicited effector T cells; antibody levels were not impacted. _Hla_ expression during primary infection impaired the expansion of T cells and DCs, a major antigen-presenting cell
responsible for driving memory T cell generation. Optimal vaccine-mediated protection required robust T-cell responses, and the use of the T-cell-stimulating adjuvant CAF01 circumvented the
inhibition of vaccine efficacy elicited by prior infection. Together, these results demonstrate that prior immune history is an important driver of vaccine efficacy and reveal mechanisms by
which patterned immune responses can inhibit vaccine responsiveness. Importantly, they also suggest a path toward overcoming patterned immune responses by polarizing vaccine-specific immune
responses toward the Th1/Th17 pathways. We found that _hla_ expression during primary infection drives interference with vaccine efficacy. Our findings that _hla_ expression impaired the
expansion of T cells and DCs in dLNs during primary SSTI (within 7 days) are in agreement with Lee et al., who reported that SSTI with _Δhla_ resulted in greater expansion of
antigen-specific memory T cells and DCs 4–7 days post-infection29. Based on these findings, it is tempting to speculate that Hla directly kills T cells and APCs leading to a reduction in
potentially vaccine-responsive APCs and T cells. This hypothesis is supported by several reports demonstrating that Hla kills APCs and T cells30,35,36. The specificity of Hla in mediating
impairment of T cell responses is further supported by our findings that administration of anti-Hla antiserum and chemical inhibition of the Hla receptor ADAM10 rescued T cell numbers in the
dLN. The current study extends these findings by demonstrating that the decreased numbers of T cells and DCs in dLNs persist for at least one month following SSTI, suggesting that
toxin-patterned inhibition of memory T cell responses is durable beyond recovery from infection. Our study also demonstrates that Hla-mediated impairment of T cells and DC expansion may have
long-term consequences in determining vaccine responsiveness. Interestingly, however, there were no differences in APC or T cell numbers in LNs distant from the site of infection, including
the dLNs proximal to the site of vaccination. Thus, at the time of vaccination, there are decreased numbers of DCs and T cells in local dLNs, but not those distant from the site of
infection, in vaccine-resistant mice. Therefore, it is not clear that the depletion of vaccine-responsive APCs or T cells is the mechanism that is driving vaccine inhibition. Future studies
will reconcile the difference between local and distant T cell memory compartments and will seek to identify the location and phenotype of vaccine-responsive APCs/T cells. An alternative
possibility that might explain the ability of SSTI to inhibit vaccine efficacy would be an expansion of an immunosuppressive immune cell population. For example, regulatory T cells (Treg)
constrain inflammatory T cell responses37. However, we found that Treg expansion was similarly impaired following infection with WT _S. aureus_ and rescued after infection with _Δhla_. _S.
aureus_ infection has also been reported to expand a population of immunosuppressive cells called myeloid-derived suppressor cells (MDSC);38 therefore, future studies will determine whether
the expansion of MDSCs in our model might contribute to the inhibition of vaccine responsiveness. T cell-mediated immunity is a critical determinant of protection against _S. aureus_
infections in humans because individuals with defects in specific T cell pathways are at high risk of _S. aureus_ infections. For example, patients with hyper-immunoglobulin E syndrome, in
which there are defects in IL-17-mediated defense in the skin and lung, are highly susceptible to recurrent mucocutaneous _S. aureus_ infection15,39. Similarly, patients with poorly
controlled Human Immunodeficiency Virus infection and CD4+ T cell lymphopenia have high rates of _S. aureus_ infections40,41, although it is not clear that this is specifically due to
impaired T cell immunity. In contrast, individuals with B cell deficiencies do not appear to be at increased risk of _S. aureus_ infection42,43,44. Importantly, all antibody-based
vaccination strategies have failed in clinical trials45,46,47,48,49, although the reasons for these failures remain unclear. In contrast to human studies, many reports have documented that
antibodies and T cells are each important in defense against _S. aureus_ infection in mouse models17,20,21,22,29. Therefore, our findings that SSTI only interfered with vaccine-specific T
cell responses, but not antibody responses, suggest that this model may be more applicable to human infection. Specifically, vaccination provided partial protection even to _S.
aureus_-sensitized mice. We propose that the more modest protection observed in these mice is mediated by vaccine-elicited antibodies, but the ability of naïve mice to respond to vaccination
with both strong antibody and T-cell responses resulted in superior protection. It should be noted that we quantified Hla-specific IgG levels; it is, therefore, possible that the
vaccine-specific antibodies were functionally different in naïve and experienced vaccinated mice. Future studies will address the epitope-specificity and function of vaccine-elicited
antibodies to confirm that primary infection does not interfere with humoral immune responses. This is of particular importance because Tsai et al. recently reported that prior _S. aureus_
infection inhibits the efficacy of vaccination with IsdB in mouse models12. In their study, they found that inhibition was mediated by the recall by vaccination of non-protective
IsdB-specific antibodies originally elicited by infection. Therefore, it is apparent that _S. aureus_ has evolved multiple mechanisms by which exposure early in life may inhibit the ability
to vaccinate later in life. Taken together, these studies suggest that the use of mouse models of vaccination in experienced mice, and the associated immune response, may better recapitulate
human infection. The Th17/IL-17A pathway is important in defense against _S. aureus_ infections, particularly SSTI17,21,39,50. It should be noted that γδ T cells are also a potential source
of IL-1721,51,52, but our studies did not address their role in mediating protection. The magnitude of the IL-17A response may help determine the fate of infection; high levels of IL-17A
promote clearance of _S. aureus_ SSTI17,53. Along these lines, _hla_ expression during _S. aureus_ SSTI may limit IL-17A secretion, and infection with _Δhla_ promotes the rapid expansion of
Th1 and Th17 cells54. Similarly, there is emerging evidence that the Th1/IFNγ pathway is also important in defense against _S. aureus_ infection22. While the mechanisms of Th17 and
Th1-mediated protection against _S. aureus_ infection have not been fully elucidated, it is likely that they work in concert to potentiate neutrophil and macrophage-mediated proinflammatory
bacterial clearance55,56. In the current study, we found that IL-17+ and IFNγ+ T cells (mainly CD4+) are expanded in higher numbers following infection with _Δhla_, compared with WT. We also
found that vaccination with HlaH35L elicited IL-17+ and IFNγ+ T cells. However, we found that _hla_ expression during primary SSTI interfered with the expansion of vaccine-specific IL-17+ T
cells, and, to a lesser extent, IFNγ+ T cells. Importantly, our findings that neutralization of IL-17A and IFNγ inhibits vaccine-mediated protection support a role for targeting both
cytokines to achieve optimal vaccine efficacy, particularly in _S. aureus_-experienced individuals. The use of T cell-targeted vaccine adjuvants such as CAF01 is one promising approach to
accomplish this. Adjuvants are used in vaccines to enhance immunogenicity but can also be leveraged to polarize the immune response toward or away from desired antibody and T cell
pathways57. We demonstrated that “reactivating” the T cell response with the Th1/Th17 stimulating adjuvant CAF01 can polarize the host immune response toward IFNγ and IL-17A and restore
vaccine efficacy in _S. aureus_-experienced mice. CAF01 has been tested in vaccines against tuberculosis, HIV, and malaria58,59,60,61. Indeed, CAF01 rescued vaccine efficacy in _S.
aureus_-experienced mice by virtue of enhanced vaccine-specific T-cell responses. Interestingly, CAF01 alone partially rescued protection in previously infected mice. The mechanisms
underlying this protection are not clear, but possibilities include restored Hla-independent T-cell responses or augmentation of trained immunity by CAF0162; future studies will address this
“non-specific” protection. These results are particularly important in light of the fact that most of the _S. aureus_ vaccines used in clinical trials are considered self-adjuvanted due to
pathogen-associated molecular patterns in the vaccines. For example, V710, StaphVAX, and SA4Ag were unadjuvanted5,8. V710 failed to prevent postoperative _S. aureus_ infections and was
associated with increased mortality in patients who developed _S. aureus_ infection48. Importantly, mortality was associated with low endogenous levels of IL-17 in patients before receiving
the vaccine63. Together with the findings of Tsai et al12, it is likely that several mechanisms may contribute to the difficulty in vaccinating previously exposed individuals. Specifically,
prior exposure may both (i) imprint non-protective antibodies that may be recalled upon vaccination, and (ii) establish non-protective T cell memory that interferes with vaccine-elicited
protective T cell responses. Our results suggest that the use of a T cell-stimulating adjuvant such as CAF01 to re-activate the suppressed T cell response could be combined with protective
epitope-specific approaches to optimize vaccine efficacy in _S. aureus_-experienced individuals. Importantly, while we agree with Lee et al. that toxin-imprinted immune impairment may
justify leveraging the childhood vaccine infrastructure to ensure population-wide immunity29, our studies suggest that an alternative targeted approach using novel adjuvants may also enhance
protection in older individuals with a lifetime of exposure to _S. aureus_. There are several important limitations to this study. First, it remains unclear if mouse models reflect the
pathogenesis and associated immune response in human _S. aureus_ infections. We propose that the use of _S. aureus_-experienced mice better recapitulates human infection, but further
confirmatory studies are necessary. Second, the mechanisms by which _hla_ expression during primary SSTI suppresses vaccine-specific IL-17, and IFN-γ T cell responses are not entirely clear.
We found that _hla_ expression durably reduces the number of DCs and CD4+, CD8+, and γδ T cells in dLNs during and following infection, but it is not clear how these local effects impact
systemic vaccine responsiveness. Third, these findings are limited to the C57BL/6 genetic background; future studies will be necessary to determine the impact of host genetics on these
findings. Finally, these studies were performed using our SSTI model. Therefore, it is not clear if these findings are generalizable to other infectious syndromes, such as pneumonia or
bacteremia. In conclusion, we developed a mouse model in which _S. aureus_ infection “imprints” host immune responses to inhibit the efficacy of subsequent vaccination. These findings
highlight the importance of toxin expression in the evasion of protective immunity, specifically protective T-cell responses. This inhibition was circumvented by the use of a T cell-specific
adjuvant. Together, these findings suggest that understanding the mechanisms of _S. aureus_-mediated immune evasion can advance vaccine efforts. METHODS BACTERIA _S. aureus_ isolates 923
(USA300 isolated from a patient with SSTI), and an isogenic _hla_ deletion mutant (_∆hla_) have been previously reported23. Bacteria were revived and cultured on tryptic soy agar overnight
at 37 °C. The following day, one colony was transferred into tryptic soy broth (TSB) and cultured in a shaking incubator (250 rpm) overnight at 37 °C. On the day of inoculation, the
overnight cultures were diluted 1:100 in fresh TSB and cultured at 37 °C for 3 h (approximate optical density at 600 nm [OD600] of 1.8). The bacteria were washed in sterile PBS and adjusted
to 2 × 107 CFU/50 µl PBS. MICE All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Abigail Wexner Research Institute at Nationwide
Children’s Hospital (protocol no. AR17-00072) and adhered to the standards of NIH Guide for the Care and Use of Laboratory Animals. For all experiments, female C57BL/6 mice were purchased
from Taconic. Primary infection or vaccination was performed when the mice were 7–8 weeks old. MOUSE MODEL OF _S. AUREUS_ SSTI Prior to inoculation, mice were sedated with isoflurane, and
their flanks were shaved with a fine hair clipper and cleaned with Nair™ hair removal cream and ethanol. Mice were subcutaneously inoculated with 2 × 107 CFU of WT or ∆_hla S. aureus_ in a
volume of 50 µl PBS17. Mice were observed to awaken and be given access to food and water throughout the experiment. Mice were treated with 150 mg/kg vancomycin (Medline) or PBS via
intraperitoneal injection 3 h post-primary infection, and treatment was continued every day for 7 days. The secondary infection was performed on the opposite flank 7 weeks following the
primary infection (2 weeks after the second dose of vaccination). To neutralize Hla or its receptor, mice were treated with 200 µl Hla-specific antiserum retro-orbitally 1 day before SSTI or
20 µM GI254023X (ADAM10 inhibitor) (Sigma-Aldrich) subcutaneously at the infection site, 3 h before SSTI. To assess the severity of the skin infection, lesions were photographed daily for 7
days. Lesion sizes were calculated digitally and compared with a 100 mm2 standard. To quantify the lesion bacterial burden, mice were euthanized 7 days after SSTI, and lesions were
aseptically dissected and homogenized in PBS. Serial dilutions of the homogenate were plated on mannitol salt agar for colony-forming unit enumeration. VACCINATION AND CYTOKINE
NEUTRALIZATION Our methods for protein purification and vaccination have been reported18. Briefly, _lukE_, _splB_, and _ssB_ were amplified by a polymerase chain reaction and cloned in
pET28a (Novagen). The resulting plasmid was expressed in _Escherichia coli_ (DE3, BL21; Invitrogen). The proteins were purified with chromatography using a His-Bind kit (Novagen). Plasmids
for _hla__H35L_ and _lukS-PV_ purification were generously provided by Juliane Bubeck Wardenburg (Washington University, St. Louis). _hla__H35L_ was cloned in pET24b and purified from _E.
coli_ (BL21)64. _lukS-PV_ was cloned in pGEX and purified from _E. coli_ (BL21)64. Endotoxin was removed using an endotoxin removal kit (Sigma). Mice were vaccinated with one of three
vaccines (10 µg of each protein): HlaH35L, 4S (LukE, LukS-PV, SplB, and SspB), or 5S (4S + HlaH35L). The protein concentration was measured by Bio-Rad protein assay dye reagent concentrate
(Bio-Rad). The vaccines were adjuvanted with Al(OH)3 (Alhydrogel; Brenntag) or CAF01 ((N,N′-dimethyl-N,N′-dioctadecylammonium bromide (DDA) (Sigma) and α,α′-trehalose-6,6′-dibehenate (TDB)
(Invivogen))65 at a final concentration of 0.1% or the ratio 5:1, DDA to TDB in a total volume of 200 µl. First and second doses of vaccinations were administered subcutaneously distal from
the infection site (between the shoulder blades at the base of the neck) 3 and 5 weeks after the primary infection. For cytokine neutralization, mice were treated intraperitoneally with 500
µg anti-IL-17A (clone 17F3, catalog number BP0173) and anti-IFN-γ (clone XMG1.2, catalog number BP0055) or isotype controls (clone MOPC-21; catalog number BP0083, HRPN; catalog number
PB0088, BioXcell) one day prior to SSTI. ADOPTIVE TRANSFER Mice were euthanized 8 weeks after secondary SSTI or PBS by CO2 inhalation, and sera and spleens were harvested. T cells were
isolated from the single-cell suspension of spleens by negative selection using the Pan T cell Isolation Kit II (1:5; Miltenyi Biotec, catalog number 130-095-130). Briefly, non-target cells
were labeled by using a cocktail of biotin-conjugated antibodies against CD14, CD15, CD16, CD19, CD34, CD36, CD56, CD123, and CD235a. Then, non-target cells were magnetically labeled with
anti-Biotin microbeads and purified in a magnetic field. Mouse blood was collected by cardiac puncture, and sera were harvested by centrifugation of mouse whole blood. Totally, 200 µl serum
or T cells (9 × 106 cells) were transferred retro-orbitally to each recipient mouse 1 day prior to vaccination. QUANTIFICATION OF ANTIBODY RESPONSES Ninety-six-well ELISA plates (Costar,
Corning Inc.) were coated with purified HlaH35L (5 µg/ml). Mouse serum was prepared from whole blood using serum separator tubes (BD Biosciences) and diluted 1:100 in PBS, following which
serum was added to the wells. Detection of antigen-specific IgG was performed using alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (1:5000; AffiniPure, Jackson ImmunoResearch,
catalog number 115-055-003) and AP substrate p-nitrophenyl phosphate (Sigma-Aldrich) following the manufacturer’s recommendations. Absorbance was measured using a GENios spectrophotometer
(Tecan). QUANTIFICATION OF T CELL RESPONSES BY ELISPOT Enzyme-linked immunosorbent spot (ELISpot) 96-well plates were coated with anti–IL-17 or anti–IFN-γ antibody (1:500; Becton Dickinson
Biosciences, catalog numbers 551309, 555068) overnight at 4 °C. Splenocytes were harvested from mice and plated at 8 × 105 or 4 × 105/well for IL-17 or IFN-γ detection, respectively. The
splenocytes were incubated with purified HlaH35L (10 µg/ml) for 24 h at 37 °C with 5% CO2. Following washing, biotin-labeled IFN-γ and IL17 detection antibodies (1:500; Becton Dickinson
Biosciences, catalog numbers 555067, 551506)were added to the wells, followed by horseradish peroxidase-conjugated anti-biotin (1:250; eBioscience, catalog number 18-41-00-51). Spots were
counted following the addition of the substrate solution (BD Biosciences) using an ImmunoSpot series 1 analyzer (Cellular Technology). QUANTIFICATION OF T CELL RESPONSES BY FLOW CYTOMETRY
Lymphocytes were isolated from draining lymph nodes following SSTI and processed into single-cell suspensions. Cells were stained for flow cytometry using AquaFluor LiveDead (Life
Technologies) solution to exclude dead cells. Two different panels were used for surface and intracellular staining. For surface staining, antibodies against CD8 PerCP Cy 5.5 (0.12 µg,
clone:53-6.7, Biolegend, catalog number 100734), CD3 FITC (0.25 µg, clone:145-2C11, Invitrogen, catalog number 11-0031-95), CD4 BUV395 (0.12 µg, clone: GK1.5, BD, catalog number 563790),
CD11c eflur450 (0.06 µg, clone: N418, Invitrogen, catalog number 48-0114-82), and γδ TCR PE (0.06 µg, clone: GL3, Biolegend, catalog number 118108) were used. Cells were incubated with
antibodies for 30 min on ice in the dark. For intracellular staining, cells were fixed and permeabilized using a Fixing/Permeabilization solution for 30 min in the dark at room temperature,
following two subsequent washes with stain buffer [PBS and 2% fetal calf serum] after surface staining. Cells were then washed twice with Permeabilization Wash Buffer and incubated with
intracellular antibodies against IFN-γ BV785 (0.12 µg, clone: KMG1.1, Biolegend, catalog number 554412), IL-17 APC (0.12 µg, clone: Bio17B, ebioscience, catalog number 505838) and Foxp3 PE
Cy7 (0.06 µg, clone: FJK-16s, Invitrogen, catalog number 17-6988-82) for 45 min on ice. Counting beads (25,000 beads) were added to every sample to normalize the total number of cells. Flow
cytometry was performed on an LSRII or Fortessa (BD Biosciences) cytometer and analyzed with FlowJo software. DATA ANALYSIS Data were compared using one-way analysis of variance (ANOVA) with
the Tukey post-test, or two-way ANOVA with repeated measures and the Tukey post-test, where appropriate. For cell numbers and bacterial CFU, values were log10-transformed prior to analysis.
Differences were considered significant when _p_ < 0.05. All data were analyzed using GraphPad Prism. REPORTING SUMMARY Further information on research design is available in the Nature
Research Reporting Summary linked to this article. DATA AVAILABILITY All data generated or analyzed during this study are presented in the article, and materials are available from the
corresponding author upon reasonable request. REFERENCES * Kaye, K. S., Petty, L. A., Shorr, A. F. & Zilberberg, M. D. Current epidemiology, etiology, and burden of acute skin infections
in the United States. _Clin. Infect. Dis._ 68, S193–S199 (2019). Article Google Scholar * Ray, G. T., Suaya, J. A. & Baxter, R. Microbiology of skin and soft tissue infections in the
age of community-acquired methicillin-resistant _Staphylococcus aureus_. _Diagn. Microbiol. Infect. Dis._ 76, 24–30 (2013). Article Google Scholar * Fritz, S. A. et al. A serologic
correlate of protective immunity against community-onset _Staphylococcus aureus_ infection. _Clin. Infect. Dis._ 56, 1554–1561 (2013). Article CAS Google Scholar * McGuinness, W. A.,
Malachowa, N. & DeLeo, F. R. Vancomycin resistance in _Staphylococcus aureus_. _Yale J. Biol. Med._ 90, 269–281 (2017). CAS Google Scholar * Miller, L. S., Fowler, V. G., Shukla, S.
K., Rose, W. E. & Proctor, R. A. Development of a vaccine against _Staphylococcus aureus_ invasive infections: evidence based on human immunity, genetics and bacterial evasion
mechanisms. _FEMS Microbiol. Rev._ 44, 123–153 (2020). Article CAS Google Scholar * Miller, L. G. et al. _Staphylococcus aureus_ skin infection recurrences among household members: an
examination of host, behavioral, and pathogen-level predictors. _Clin. Infect. Dis._ 60, 753–763 (2015). Article CAS Google Scholar * Vella, V. et al. _Staphylococcus aureus_ skin and
soft tissue infection recurrence rates in outpatients: a retrospective database study at 3 US Medical Centers. _Clin. Infect. Dis._ 73, e1045–e1053 (2021). Article Google Scholar * Clegg,
J. et al. _Staphylococcus aureus_ vaccine research and development: the past, present and future, including novel therapeutic strategies. _Front. Immunol._ 12, 705360 (2021). Article CAS
Google Scholar * Meyer, T. C. et al. A comprehensive view on the human antibody repertoire against _Staphylococcus aureus_ antigens in the general population. _Front. Immunol._ 12, 651619
(2021). Article CAS Google Scholar * Hendriks, A. et al. _Staphylococcus aureus_-specific tissue-resident memory CD4(+) T cells are abundant in healthy human skin. _Front. Immunol._ 12,
642711 (2021). Article CAS Google Scholar * Kolata, J. B. et al. The Fall of a Dogma? Unexpected high T-cell memory response to _Staphylococcus aureus_ in humans. _J. Infect. Dis._ 212,
830–838 (2015). Article CAS Google Scholar * Tsai, C. M. et al. Non-protective immune imprint underlies failure of _Staphylococcus aureus_ IsdB vaccine. _Cell Host Microbe_ 30,
1163–1172.e1166 (2022). Article CAS Google Scholar * Henry, C., Palm, A. E., Krammer, F. & Wilson, P. C. From original antigenic sin to the universal influenza virus vaccine. _Trends
Immunol._ 39, 70–79 (2018). Article CAS Google Scholar * Thomas Francis, J. On the doctrine of original antigenic sin. _Proc. Am. Philos. Soc._ 104, 7 (1960). Google Scholar * Milner, J.
D. et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. _Nature_ 452, 773–776 (2008). Article CAS Google Scholar * Sampedro, G. R. et al.
Targeting _Staphylococcus aureus_ alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. _J. Infect. Dis._ 210, 1012–1018 (2014). Article CAS
Google Scholar * Montgomery, C. P. et al. Protective immunity against recurrent _Staphylococcus aureus_ skin infection requires antibody and interleukin-17A. _Infect. Immun._ 82, 2125–2134
(2014). Article Google Scholar * Si, Y. et al. Inhibition of protective immunity against _Staphylococcus aureus_ infection by MHC-restricted immunodominance is overcome by vaccination.
_Sci. Adv._ 6, eaaw7713 (2020). Article CAS Google Scholar * Karauzum, H. & Datta, S. K. Adaptive immunity against _Staphylococcus aureus_. _Curr. Top. Microbiol Immunol._ 409,
419–439 (2017). CAS Google Scholar * Lin, L. et al. Th1-Th17 cells mediate protective adaptive immunity against _Staphylococcus aureus_ and _Candida albicans_ infection in mice. _PLoS
Pathog._ 5, e1000703 (2009). Article Google Scholar * Cho, J. S. et al. IL-17 is essential for host defense against cutaneous _Staphylococcus aureus_ infection in mice. _J. Clin. Invest._
120, 1762–1773 (2010). Article Google Scholar * Brown, A. F. et al. Memory Th1 cells are protective in invasive _Staphylococcus aureus_ infection. _PLoS Pathog._ 11, e1005226 (2015).
Article Google Scholar * Zhao, F. et al. Proteomic identification of saeRS-dependent targets critical for protective humoral immunity against _Staphylococcus aureus_ skin infection.
_Infect. Immun._ 83, 3712–3721 (2015). Article CAS Google Scholar * Bubeck Wardenburg, J. & Schneewind, O. Vaccine protection against _Staphylococcus aureus_ pneumonia. _J. Exp. Med._
205, 287–294 (2008). Article Google Scholar * Brady, R. A. et al. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of _Staphylococcus aureus_
infection. _PLoS One_ 8, e63040 (2013). Article CAS Google Scholar * Kennedy, A. D. et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin
infection in a mouse model. _J. Infect. Dis._ 202, 1050–1058 (2010). Article Google Scholar * Ferraro, A. et al. Role and plasticity of Th1 and Th17 responses in immunity to
_Staphylococcus aureus_. _Hum. Vaccines Immunother._ 15, 2980–2992 (2019). Article Google Scholar * Kleinhenz, M., Beesetty, P., Yang, C., Li, Z. & Montgomery, C. P. Antibiotic
treatment of _Staphylococcus aureus_ infection inhibits the development of protective immunity. _Antimicrob. Agents Chemother_. 66, e0227021 (2022). * Lee, B., Olaniyi, R., Kwiecinski, J. M.
& Wardenburg, J. B. _Staphylococcus aureus_ toxin suppresses antigen-specific T cell responses. _J. Clin. Invest._ 130, 1122–1127 (2020). Article CAS Google Scholar * Becker, R. E.,
Berube, B. J., Sampedro, G. R., DeDent, A. C. & Bubeck Wardenburg, J. Tissue-specific patterning of host innate immune responses by _Staphylococcus aureus_ alpha-toxin. _J. Innate
Immun._ 6, 619–631 (2014). Article CAS Google Scholar * Inoshima, I. et al. A _Staphylococcus aureus_ pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice.
_Nat. Med._ 17, 1310–1314 (2011). Article CAS Google Scholar * Inoshima, N., Wang, Y. & Bubeck Wardenburg, J. Genetic requirement for ADAM10 in severe _Staphylococcus aureus_ skin
infection. _J. Invest. Dermatol._ 132, 1513–1516 (2012). Article CAS Google Scholar * Hogenesch, H. Mechanism of immunopotentiation and safety of aluminum adjuvants. _Front. Immunol._ 3,
406 (2012). Google Scholar * Pedersen, G. K., Andersen, P. & Christensen, D. Immunocorrelates of CAF family adjuvants. _Semin. Immunol._ 39, 4–13 (2018). Article CAS Google Scholar *
Berube, B. J. & Bubeck Wardenburg, J. _Staphylococcus aureus_ alpha-toxin: nearly a century of intrigue. _Toxins_ 5, 1140–1166 (2013). Article Google Scholar * Nygaard, T. K. et al.
Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. _PLoS ONE_ 7, e36532 (2012). Article CAS Google Scholar * Josefowicz, S. Z.,
Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. _Annu. Rev. Immunol._ 30, 531–564 (2012). Article CAS Google Scholar * Tebartz, C. et al. A
major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during _Staphylococcus aureus_ infection. _J. Immunol._ 194, 1100–1111 (2015).
Article CAS Google Scholar * Minegishi, Y. et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. _J.
Exp. Med._ 206, 1291–1301 (2009). Article CAS Google Scholar * Hemmige, V., McNulty, M., Silverman, E. & David, M. Z. Predictors of skin and soft tissue infections in HIV-infected
outpatients in the community-associated methicillin-resistant _Staphylococcus aureus_ era. _Eur. J. Clin. Microbiol. Infect. Dis._ https://doi.org/10.1007/s10096-014-2237-1 (2014). * Vyas,
K. J., Shadyab, A. H., Lin, C. D. & Crum-Cianflone, N. F. Trends and factors associated with initial and recurrent methicillin-resistant _Staphylococcus aureus_ (MRSA) skin and
soft-tissue infections among HIV-infected persons: an 18-year study. _J. Int. Assoc. Prov. AIDS Care_ 13, 206–213 (2014). Article Google Scholar * Chan, H. Y. et al. Clinical
characteristics and outcomes of primary antibody deficiency: a 20-year follow-up study. _J. Formos. Med. Assoc._ 113, 340–348 (2014). Article Google Scholar * Hausser, C., Virelizier, J.
L., Buriot, D. & Griscelli, C. Common variable hypogammaglobulinemia in children. Clinical and immunologic observations in 30 patients. _Am. J. Dis. Child_ 137, 833–837 (1983). Article
CAS Google Scholar * Hermaszewski, R. A. & Webster, A. D. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. _Q. J. Med._ 86, 31–42 (1993). CAS
Google Scholar * Shinefield, H. et al. Use of a _Staphylococcus aureus_ conjugate vaccine in patients receiving hemodialysis. _N. Engl. J. Med._ 346, 491–496 (2002). Article Google Scholar
* Bloom, B. et al. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low
birth weight infants. _Pediatr. Infect. Dis. J._ 24, 858–866 (2005). Article Google Scholar * DeJonge, M. et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of
nosocomial staphylococcal bloodstream infection in premature infants. _J. Pediatr._ 151, 260–265 (2007). Article CAS Google Scholar * Fowler, V. G. et al. Effect of an investigational
vaccine for preventing _Staphylococcus aureus_ infections after cardiothoracic surgery: a randomized trial. _J. Am. Med. Assoc._ 309, 1368–1378 (2013). Article CAS Google Scholar * Inoue,
M. et al. Safety, tolerability, and immunogenicity of a novel 4-antigen _Staphylococcus aureus_ vaccine (SA4Ag) in healthy Japanese adults. _Hum. Vaccines Immunother._ 14, 2682–2691 (2018).
Google Scholar * Spellberg, B. & Daum, R. A new view on development of a _Staphylococcus aureus_ vaccine: insights from mice and men. _Hum. Vaccines_ 6, 857–859 (2010). Article CAS
Google Scholar * Sutton, C. E., Mielke, L. A. & Mills, K. H. IL-17-producing gammadelta T cells and innate lymphoid cells. _Eur. J. Immunol._ 42, 2221–2231 (2012). Article CAS Google
Scholar * Marchitto, M. C. et al. Clonal Vgamma6(+)Vdelta4(+) T cells promote IL-17-mediated immunity against _Staphylococcus aureus_ skin infection. _Proc. Natl Acad. Sci. USA_ 116,
10917–10926 (2019). Article CAS Google Scholar * Valeri, M. & Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. _Pathog. Dis._ 74
https://doi.org/10.1093/femspd/ftw111 (2016). * Tkaczyk, C. et al. _Staphylococcus aureus_ alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis
model. _PLoS ONE_ 8, e75103 (2013). Article CAS Google Scholar * van Kessel, K. P., Bestebroer, J. & van Strijp, J. A. Neutrophil-mediated phagocytosis of _Staphylococcus aureus_.
_Front. Immunol._ 5, 467 (2014). Google Scholar * Pidwill, G. R., Gibson, J. F., Cole, J., Renshaw, S. A. & Foster, S. J. The role of macrophages in _Staphylococcus aureus_ infection.
_Front. Immunol._ 11, 620339 (2020). Article CAS Google Scholar * De Gregorio, E., Caproni, E. & Ulmer, J. B. Vaccine adjuvants: mode of action. _Front. Immunol._ 4, 214 (2013).
Article Google Scholar * van Dissel, J. T. et al. A novel liposomal adjuvant system, CAF01, promotes long-lived _Mycobacterium tuberculosis_-specific T-cell responses in human. _Vaccine_
32, 7098–7107 (2014). Article Google Scholar * Fomsgaard, A. et al. Development and preclinical safety evaluation of a new therapeutic HIV-1 vaccine based on 18 T-cell minimal epitope
peptides applying a novel cationic adjuvant CAF01. _Vaccine_ 29, 7067–7074 (2011). Article CAS Google Scholar * Karlsson, I. et al. Adjuvanted HLA-supertype restricted subdominant
peptides induce new T-cell immunity during untreated HIV-1-infection. _Clin. Immunol._ 146, 120–130 (2013). Article CAS Google Scholar * Dejon-Agobe, J. C. et al. Controlled human malaria
infection of healthy adults with lifelong malaria exposure to assess safety, immunogenicity, and efficacy of the asexual blood stage malaria vaccine candidate GMZ2. _Clin. Infect. Dis._ 69,
1377–1384 (2019). Article CAS Google Scholar * Williams, S. J. Sensing lipids with mincle: structure and function. _Front. Immunol._ 8, 1662 (2017). Article Google Scholar * McNeely,
T. B. et al. Mortality among recipients of the Merck V710 _Staphylococcus aureus_ vaccine after postoperative _S. aureus_ infections: an analysis of possible contributing host factors. _Hum.
Vaccines Immunother._ 10, 3513–3516 (2014). Article Google Scholar * Bubeck Wardenburg, J., Bae, T., Otto, M., Deleo, F. R. & Schneewind, O. Poring over pores: alpha-hemolysin and
Panton-Valentine leukocidin in _Staphylococcus aureus_ pneumonia. _Nat. Med._ 13, 1405–1406 (2007). Article Google Scholar * Davidsen, J. et al. Characterization of cationic liposomes
based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6’-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. _Biochim.
Biophys. Acta_ 1718, 22–31 (2005). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Juliane Bubeck Wardenburg (Washington University, St. Louis) for the gift of
plasmids for the purification of HlaH35L and LukS-PV. FUNDING This study was funded by the National Institute of Allergy and Infectious Diseases (AI125489 to C.P.M.) and the Abigail Wexner
Research Institute at Nationwide Children’s Hospital. AUTHOR INFORMATION Author notes * Omid Teymournejad Present address: Department of Pathology, College of Medicine, University of
Illinois at Chicago, Chicago, IL, US * Pavani Beesetty Present address: Toronto General Hospital Research Institute, University Health Network, Toronto, Ontario, Canada * Ching Yang Present
address: Veterinary Biomedical Sciences, College of Veterinary Medicine, Long Island University, Brookville, NY, US AUTHORS AND AFFILIATIONS * Center for Microbial Pathogenesis, Abigail
Wexner Research Institute at Nationwide Children’s Hospital, Columbus, OH, US Omid Teymournejad, Zhaotao Li, Pavani Beesetty, Ching Yang & Christopher P. Montgomery * Department of
Pediatrics, The Ohio State University College of Medicine, Columbus, OH, US Christopher P. Montgomery * Division of Critical Care Medicine, Nationwide Children’s Hospital, Columbus, OH, US
Christopher P. Montgomery Authors * Omid Teymournejad View author publications You can also search for this author inPubMed Google Scholar * Zhaotao Li View author publications You can also
search for this author inPubMed Google Scholar * Pavani Beesetty View author publications You can also search for this author inPubMed Google Scholar * Ching Yang View author publications
You can also search for this author inPubMed Google Scholar * Christopher P. Montgomery View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
O.T., P.B., and C.P.M. conceived and designed the experiments. O.T., Z.L., P.B., and C.Y. performed the experiments. O.T., P.B., and C.P.M. analyzed the data. O.T. and C.P.M. wrote the
paper. CORRESPONDING AUTHOR Correspondence to Omid Teymournejad. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENT REPORTING SUMMARY RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Teymournejad, O.,
Li, Z., Beesetty, P. _et al._ Toxin expression during _Staphylococcus aureus_ infection imprints host immunity to inhibit vaccine efficacy. _npj Vaccines_ 8, 3 (2023).
https://doi.org/10.1038/s41541-022-00598-3 Download citation * Received: 21 September 2022 * Accepted: 05 December 2022 * Published: 24 January 2023 * DOI:
https://doi.org/10.1038/s41541-022-00598-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
